Introduction
Myocardial ischemia-reperfusion injury (MIRI) is one
of the major diseases threatening human health with a high
morbidity and mortality rate worldwide (1,2). A
number of pathological processes and mediators, including cell
apoptosis, oxidative stress injury, intracellular calcium overload
and inflammatory response activation have been proposed to be
crucial in ischemia-reperfusion (I/R)-related myocardial cell
injury (3,4). Among them, oxidative stress and
inflammation are considered important mechanisms implicated in the
pathogenesis of MIRI (5,6). During the phase of MIRI, oxidative
stress occurs when there are imbalances between reactive oxygen
species (ROS) generation and antioxidant defense systems, leading
to the activation of signal transduction cascades and the
production of various inflammatory mediators, which results in
damage of the viable tissue surrounding the infarct and accelerated
cell death programs (7-9).
Despite the advances in the understanding of MIRI mechanisms
(1), novel effective strategies for
MIRI remain to be explored.
Numerous traditional Chinese herbs have been proven
to exert cardioprotective effects, and their role in ameliorating
MIRI has been investigated (10-12).
Schizandrin B, the most abundant active dibenzocyclooctadiene
derivative isolated from a traditional Chinese herb [Schisandra
chinensis (Turcz) Baill], possesses diverse pharmacological
activities such as anti-apoptosis, antioxidative, anti-inflammatory
and cardioprotective properties (13-15).
A previous in vivo study demonstrated that schizandrin B
might protect myocardial tissue from I/R injury via the
phosphoinositide 3-kinase/Akt signaling pathway in rats (15). In addition, a previous study
demonstrated that schizandrin B has a high antioxidative activity
and protects against MIRI (16).
However, the potential mechanism of schizandrin B-induced
cardioprotection against MIRI remains elusive.
Adenosine monophosphate-activated protein kinase
(AMPK) is a major regulator of cellular homeostasis, and its
activation can reduce oxidative stress injury and the inflammatory
response (17,18). Increasing evidence indicated that
AMPK regulates a variety of biological processes and is a highly
effective therapeutic target for protecting against MIRI (19-21).
Nuclear factor erythroid 2-related factor 2 (Nrf2) is essential for
the transcription and expression of key antioxidant enzymes,
including heme oxygenase-1 (HO-1) and NAD(P)H dehydrogenase quinone
1 (NQO-1) (22,23). Under normal conditions, the
transcriptional activity of the Nrf2 protein is inhibited by the
negative regulator Kelch-like ECH-associated protein 1 (Keap1);
however, upon excess oxidative stress, Nrf2 translocates to the
nucleus, where it binds to the antioxidant responsive element
(24,25). Numerous studies revealed that Nrf2
served as an important downstream target of AMPK signaling by
increasing resistance to oxidative damage and inflammatory reaction
(11,26). The AMPK/Nrf2 signaling pathway plays
an important role in cellular defense against oxidative stress and
inflammatory injury by activating antioxidant cascades (27,28).
Notably, previous studies confirmed that the mechanisms of these
antioxidative and anti-inflammatory activities of schizandrin B are
mediated, at least in part, via activation of Nrf2 and Nrf2-driven
antioxidant responses (29,30). However, the potential roles of the
AMPK/Nrf2 signaling pathway in the cardioprotection of schizandrin
B in MIRI remain to be elucidated.
Hence, in the present study, a cell model of MIRI
was established to investigate whether schizandrin B attenuates
oxidative stress and inflammatory response via the AMPK/Nrf2
signaling pathway, resulting in cardioprotection against MIRI, and
provide a theoretical foundation for its clinical application.
Materials and methods
Materials and reagents
Dulbecco's modified Eagle's medium (DMEM; cat. no.
11965118) and fetal bovine serum (FBS; cat. no. 16140071) were
supplied by Gibco; Thermo Fisher Scientific, Inc. Annexin
V/fluorescein isothiocyanate (FITC) apoptosis detection kit (cat.
no. 556547) was purchased from BD Biosciences. Cell counting kit-8
(CCK-8; cat. no. C0038), JC-1 mitochondrial membrane potential
detection kit (cat. no. C2006) and lactate dehydrogenase (LDH)
cytotoxicity assay kit (cat. no. C0016) were purchased from
Beyotime Institute of Biotechnology. 2', 7'-dichlorofluorescein
acetyl acetate (DCFH-DA; cat. no. D6883) kit was supplied by
Sigma-Aldrich; Merck KGaA. Rabbit polyclonal antibodies against
B-cell lymphoma 2 (Bcl-2; cat. no. ab32124) and Bcl-2-associated X
protein (Bax; cat. no. ab32503) were purchased from Abcam. Rabbit
monoclonal antibody against NAPDH oxidase 2 (NOX2; cat. no.
ALX-350-100-C050) was purchased from Enzo Life Sciences, Inc.
Rabbit monoclonal antibodies against histone H3 (cat. no. 7631),
Keap 1 (cat. no. 8047), AMPKα (cat. no. 5832), phospho (p)-AMPKα
(Ser485; cat. no. 2537), Nrf2 (cat. no. 12721) and GAPDH (cat. no.
5174) were obtained from Cell Signaling Technology, Inc.
Cell culture and hypoxia/reoxygenation
(H/R) injury model establishment
The H9c2 cardiomyocyte cell line (rat embryonic
cardiomyoblasts) was purchased from Shanghai Institutes for
Biological Sciences, and was maintained in DMEM supplemented with
10% (v/v) FBS and 1% (v/v) penicillin-streptomycin solution in a
humidified atmosphere containing 95% air and 5% CO2 at
37˚C. The medium was replaced every 2 days. To induce H/R injury,
H9c2 cells were cultured with serum-free medium in an anaerobic
chamber containing 1% O2, 5% CO2 and 94%
N2 at 37˚C for 6 h to establish hypoxia. Subsequently,
the cells were cultured with normoxic medium in the presence of 95%
air and 5% CO2 at 37˚C for 12 h in a humidified
atmosphere to establish reoxygenation (31).
Cell viability assay
Cell viability was determined using a CCK-8 assay
kit according to the manufacturer's instructions. Briefly, H9c2
cells were seeded in a 96-well plate at a density of
3x104 cells/well. After treatment as described above,
CCK-8 solution (10 µl) was added to each well and further incubated
at 37˚C for 3 h. Subsequently, the optical density was measured at
a wavelength of 450 nm using a PerkinElmer microplate reader
(PerkinElmer, Inc.).
Small interfering RNA (siRNA)
transfection
H9c2 cells were transfected with Nrf2-specific siRNA
(si-Nrf2; 100 nM; cat. no. sc-37030), AMPK-specific siRNA (si-AMPK;
100 nM; cat. no. sc-29673) or scrambled siRNA (100 nM; cat. no.
sc-37007) as the negative control (Santa Cruz Biotechnology, Inc.)
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocols. The sequences of
the siRNA were as follows: Nrf2 siRNA sense,
5'-GAGGAUGGGAAACCUUACUTT-3' and antisense,
5'-AGUAAGGUUUCCCAUCCUCTT-3'; AMPK siRNA sense,
5'-CCCAUAUUAUUUGCGUGUADTDT-3' and antisense,
5'-UACACGCCAAAUAAUAUGGGCTCT-3' and scramble siRNA sense,
5'-UUCUCCGAACGUGUCACGUTT-3' and antisense,
5'-ACGUGACACGUUCGGAGAATT-3'. Briefly, cells were plated in six-well
plates prior to transfection. After growing to 60-70% confluence,
siRNA (100 nM) was prepared in Opti-MEM™ I (100 ml; Invitrogen;
Thermo Fisher Scientific, Inc.; cat. no. 51985091) and then added
to Opti-MEM™ I (100 ml) containing Lipofectamine™ Stem Transfection
Reagent (14 ml), and the mixture was incubated for 10 min at room
temperature. The mixture was then added to the cells and incubated
for 48 h prior to experimentation. Knockdown efficacy was
determined by reverse transcription-quantitative PCR (RT-qPCR).
RNA isolation and RT-qPCR assay
Total RNA from cells of the control, H/R, Sch B +
H/R + si-Scram and Sch B + H/R + si-Nrf2 groups was isolated using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) following the manufacturer's protocol. RT of RNA (1 µg) to
cDNA was performed using the M-MLV Reverse Transcriptase system
(Promega Corporation) according to the manufacturer's instructions.
The mRNA levels of AMPK and Nrf2 were detected by RT-qPCR using the
SYBR Green PCR master mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.) on an ABI PRISM 7900 Sequence Detection system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The following
primer pairs were used for the qPCR: Nrf2 forward,
5'-ACTGTCCCCAGCCCAGAGGC-3'and reverse, 5'-CCAGGCGGTGGGTCTCCGTA-3';
HO-1 forward, 5'-GCTGGTGATGGCTTCCTTGTA-3' and reverse,
5'-ACCTCGTGGAGACGCTTTACAT-3'; NOQ1 forward,
5'-ACGACAACGGTCCTTTCCAGA-3' and reverse,
5'-CAGAAACGCAGGATGCCACT-3'; GAPDH forward,
5'-GGACCTGACCTGCCGTCTAG-3' and reverse, 5'-GTAGCCCAGGATGCCCTTGA-3'.
The following thermocycling conditions were used for the qPCR:
Initial denaturation at 95˚C for 5 min; 40 cycles of denaturation
at 95˚C for 10 sec, annealing at 58˚C for 45 sec and elongation at
72˚C for 1 min; and a final extension at 72˚C for 5 min. The
relative expressions of Nrf2, HO-1 and NOQ1 mRNA were quantified
using the 2-ΔΔCq method and normalized to GAPDH
(32). Each sample was measured in
triplicate.
Flow cytometric analysis of cell
apoptosis
H9c2 cells were seeded at 5x105
cells/well into a 6-well plate and treated as described above for
24 h prior to digestion with 0.05% trypsin-EDTA to harvest the
cells. In total, 1x105 treated cells were resuspended in
binding buffer (500 µl), and incubated with Annexin V-FITC (5 µl)
and propidium iodide (PI; 5 µl) for 15 min at room temperature
according to the manufacturer's instructions (BD Pharmingen; BD
Biosciences). Subsequently, apoptosis was analyzed by flow
cytometry (BD FACSCalibur; BD Biosciences) with CellQuest software
(version 3.3; BD Biosciences). Apoptotic cells were counted and
represented as a percentage of the total cell count.
ROS generation analysis
The measurement of intracellular ROS production was
dependent on the ROS-mediated conversion of non-fluorescent DCFH-DA
to DCFH. Following treatment, H9c2 cells were harvested and
co-incubated with serum-free medium containing DCFH-DA (50 µmol/l)
for 20 min at 37˚C. Subsequently, the cells were rinsed three times
with PBS, and the fluorescence intensity in each group was measured
by flow cytometry (BD FACSCalibur; BD Biosciences) with CellQuest
software (version 3.3;BD Biosciences).
LDH release, malondialdehyde (MDA)
content, superoxide dismutase (SOD) and glutathione peroxidase
(GSH-Px) activity measurement
LDH release and MDA content were measured using LDH
activity assay kit (cat. no. C0017) and lipid peroxidation MDA
assay kit (cat. no. S0131M; each, Beyotime Institute of
Biotechnology), respectively, according to manufacturer's protocol.
SOD and GSH-Px activities were detected by SOD (cat. no. A001-3-2)
and GSH-Px (cat. no. A005-1-2) assay kits purchased from Nanjing
Jiancheng Bioengineering Institute Co., Ltd. according to the
manufacturer's instructions. MDA content was determined using the
thiobarbituric acid method, SOD activity was measured using the
xanthine oxidase method and GSH-Px activity was determined using
the dithio-dinitrotoluidine method.
Western blot analysis
Cells were rinsed twice with cold PBS and nuclear
and total proteins were extracted using a Cell Nuclear and
Cytoplasmic Protein Extraction kit (cat. no. P0027) and cell lysis
buffer (cat. no. P0013) for western blots containing protease
inhibitors (phenylmethylsulfonyl fluoride; cat. no. ST505) and a
protease inhibitor cocktail (cat. no P1009) all from Beyotime
Institute of Biotechnology. Protein concentration was measured
using the bicinchoninic acid method (Beyotime Institute of
Biotechnology). Equal amounts of protein lysate (30 µg) were
separated by 12% SDS-PAGE and then transferred to polyvinylidene
fluoride membranes [Roche Diagnostics (Shanghai) Co., Ltd.]. Upon
blocking in 5% non-fat milk for 2 h at room temperature, the
membranes were incubated overnight with primary antibodies against
AMPK, p-AMPK, Nrf2, Bax, Bcl-2, NOX2 and GAPDH (all dilutions were
1:1,000) at 4˚C. After washing with TBS-Tween 20 solution, the
membranes were then incubated with secondary antibodies (cat. no.
7074) for 2 h at 37˚C (horseradish peroxidase-conjugated AffiniPure
Goat Anti-Rabbit IgG; 1:5,000; Cell Signaling Technology, Inc.).
Protein bands were visualized using an enhanced chemiluminescent
agent (Beyotime Institute of Biotechnology). The results were
determined by GraphPad Prism 5.0 software (GraphPad Software,
Inc.), and the expression of total protein was expressed relative
to that of GAPDH. The expression of Nrf2 in the nucleus was
expressed relative to that of histone H3.
Statistical analysis
All data are represented as the mean ± SD.
Differences between groups were determined by one-way ANOVA
followed by Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Schizandrin B inhibits myocardial
injury following myocardial H/R in H9c2 cells
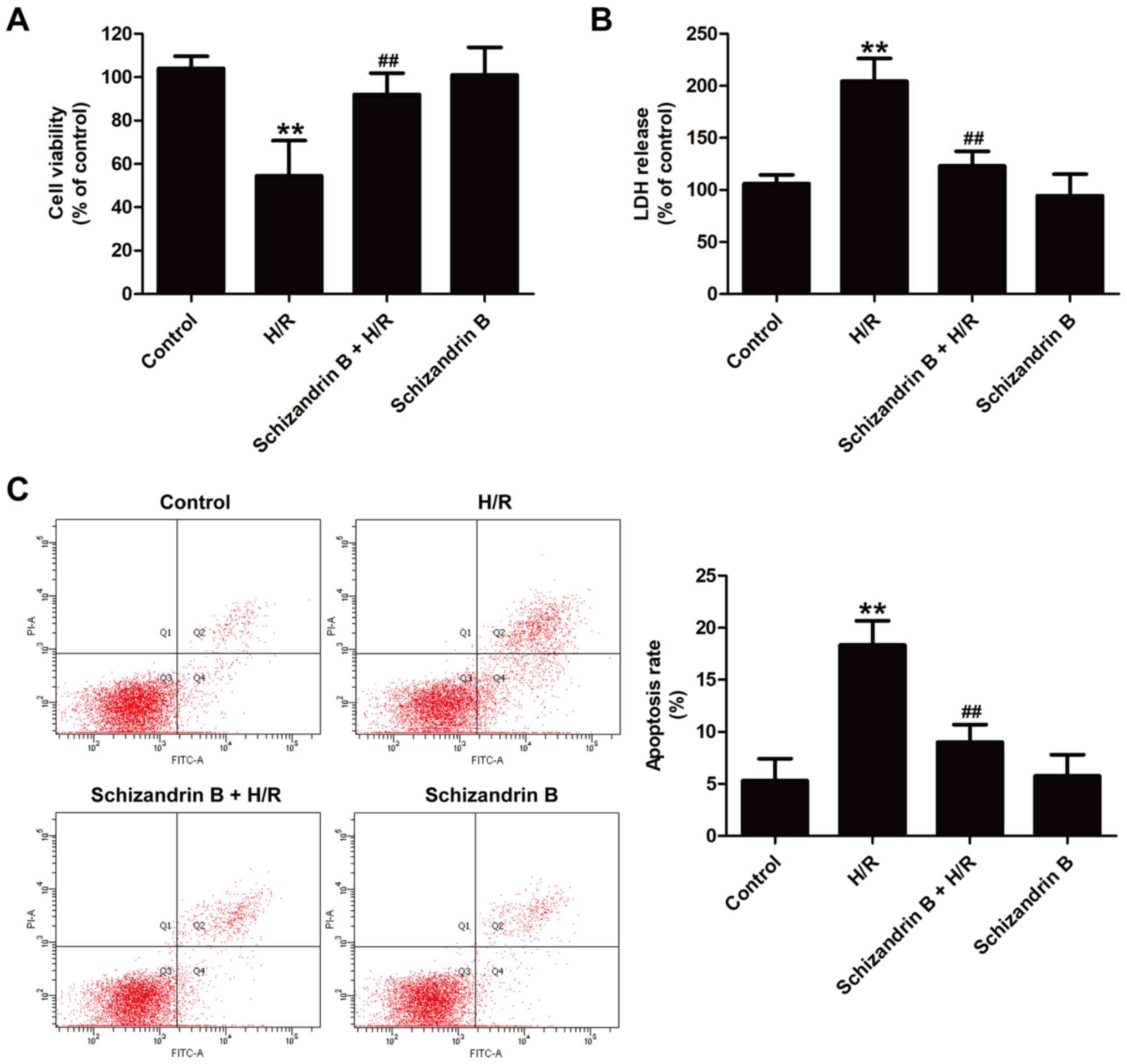
To investigate the cardioprotective effects of
schizandrin B against MIRI in vitro, cytotoxicity and
apoptosis after H/R injury in the presence or absence of
schizandrin B were examined. The CCK-8 assay results showed that
schizandrin B pretreatment significantly increased cell viability
compared with H/R treatment (Fig.
1A). The LDH release results revealed that H/R induced the
upregulation of LDH release, which was reversed by schizandrin B
pretreatment (Fig. 1B).
Additionally, annexin V-FITC/PI double staining results indicated
that H/R resulted in a significant increase in the apoptosis rate
compared with controls, which was reversed by schizandrin B
treatment (Fig. 1C). Schizandrin B
treatment alone had no effects on cell survival or apoptosis
compared with controls. These results indicated that schizandrin B
protects H9c2 cells against H/R injury.
Schizandrin B attenuates oxidative
stress in H9c2 cells subjected to H/R
Evidence has shown the contribution of oxidative
stress to MIRI (8). Thus, the
present study investigated the effects of schizandrin B on
oxidative stress-related biomarkers and the antioxidant defense
system in H/R-treated H9c2 cells. As illustrated in Fig. 2, compared with the control groups,
H/R treatment increased intracellular ROS generation (Fig. 2A), MDA content (Fig. 2B) and NOX2 expression (Fig. 2C and D) in H9c2 cells. However, these effects
were reversed by schizandrin B pretreatment. In addition, compared
with the control groups, H/R led to a decrease in the enzymatic
activities of SOD (Fig. 2E) and
GSH-Px (Fig. 2F), which was
prevented by schizandrin B pretreatment. Schizandrin B treatment
alone had no effect on oxidative stress. Taken together, these data
indicated that the cardioprotection of schizandrin B is
antioxidant-dependent.
Schizandrin B reduces the inflammatory
response in H9c2 cells after H/R injury
Eliminating ROS production is important for
controlling inflammatory response (33). To assess the anti-inflammatory
effects of schizandrin B in MIRI, the levels of pro-inflammatory
and anti-inflammatory cytokines in H9c2 cells were evaluated. As
shown in Fig. 3, compared with the
control groups, H/R treatment significantly increased the levels of
pro-inflammatory IL-1β (Fig. 3A),
TNF-α (Fig. 3B), IL-6 (Fig. 3C) and IL-8 (Fig. 3D), while these effects were all
reversed by schizandrin B. In addition, the release of
anti-inflammatory cytokines TGF-β and IL-10 was also detected. H/R
significantly reduced the levels of TGF-β (Fig. 3E) and IL-10 (Fig. 3F) compared with the control groups.
However, these effects were also effectively reversed by
schizandrin B. Schizandrin B treatment alone did not affect the
inflammatory response. Taken together, these results demonstrated
that schizandrin B inhibits the inflammatory response in
H/R-treated H9c2 cells.
Schizandrin B activates the Nrf2
signaling pathway in H/R-treated H9c2 cells
Nrf2, a basic leucine zipper transcription factor,
modulates the levels of numerous ROS detoxifying and antioxidant
genes such as NQO-1 and HO-1 (20,21).
To investigate whether schizandrin B protects H9c2 cells from H/R
injury by activating the Nrf2 signaling pathway, Nrf2 expression
and transcription efficiency of Nrf2 were assessed. Western blot
analysis results (Fig. 4A)
indicated that H/R significantly reduced the expression of Nrf2
(Fig. 4B) in the nucleus compared
with controls, while schizandrin B pretreatment reversed this
effect. The present study further evaluated HO-1 and NQO-1
expression to investigate the activation of Nrf2 signaling. The
results showed that H/R significantly decreased the expression of
HO-1 (Fig. 4A and C) and NQO-1 (Fig. 4A and D) in H9c2 cells compared with the control
groups. However, these effects were reversed by schizandrin B
pretreatment. In addition, H/R-induced upregulation of Keap1, an
Nrf2 repressor that leads to Nrf2 ubiquitination and degradation,
was significantly attenuated by schizandrin B (Fig. 4E and F). Schizandrin B treatment alone had no
impact on the Nrf2 signaling pathway. Taken together, these results
indicated that schizandrin B might exert beneficial effects in H/R
injury by activating the Nrf2 signaling pathway.
Schizandrin B exhibits
cardioprotective effects in an Nrf2-dependent manner in H/R-treated
H9c2 cells
si-Nrf2 was used to knockdown Nrf2 expression and
further verify the role of Nrf2 signaling in schizandrin B-induced
cardioprotection. Knockdown efficiency was first determined by
RT-qPCR, and the results showed that si-Nrf2 transfection
significantly reduced the levels of Nrf2 mRNA compared with
scramble siRNA transfection (Fig.
5A). The mRNA levels of the Nrf2-dependent antioxidant genes
HO-1 and NQO-1 were also significantly reduced by si-Nrf2
transfection compared with scramble siRNA transfection in
schizandrin B-treated cells (Fig.
5B). These results indicated that inhibition of the Nrf2
signaling pathway was induced by si-Nrf2 transfection. In addition,
si-Nrf2 transfection inhibited schizandrin B-induced upregulation
of cell viability in H/R compared with scramble siRNA transfection
(Fig. 5C). Si-Nrf2 transfection
also reversed the schizandrin B-induced decrease in apoptosis rate
compared with scrambled siRNA transfection (Fig. 5D and E). Furthermore, si-Nrf2 transfection
blocked the anti-inflammatory effect of schizandrin B in H/R
injury, as evidenced by the fact that si-Nrf2 transfection reversed
schizandrin B-induced downregulation of pro-inflammatory cytokines
(IL-1β, TNF-α and IL-8) and the upregulation of the
anti-inflammatory cytokine IL-10 (Fig.
5F). Furthermore, si-Nrf2 transfection blocked the decrease in
ROS generation (Fig. 5G) and the
increase in SOD and GSH-Px (Fig.
5H) activities induced by schizandrin B in H/R-treated H9c2
cells compared with scramble siRNA transfection. Taken together,
these data indicated that schizandrin B protects H9c2 cells against
H/R injury via enhancing Nrf2 signaling pathway activation.
 | Figure 5Nrf2 signaling pathway mediates the
cardioprotection of schizandrin B in H/R-treated H9c2 cells. H9c2
cells were transfected with si-Nrf2 or si-Scram for 48 h, followed
by treatment with schizandrin B (20 µM) prior to H (6 h)/R (12 h).
(A) The levels of Nrf2 mRNA were in si-Nrf2- and
si-Scram-transfected cells. NSP>0.05 vs. control group;
***P<0.001 vs. si-Scram group. (B) The levels of
Nrf2, HO-1 and NQO-1 mRNA were measured by reverse
transcription-quantitative PCR. (C) Cell viability was determined
by performing a Cell Counting Kit-8 assay. The results were
expressed as a percentage of the untreated control. (D) The
apoptosis rate was determined by Annexin V-FITC/PI double staining
followed by flow cytometry. (E) Quantitative analysis of apoptosis
rates. (F) The levels of pro-inflammatory cytokines were detected
by ELISA. (G) Intracellular ROS generation was assessed using a 2',
7'-dichlorofluorescein acetyl acetate kit followed by flow
cytometry. (H) SOD and GSH-Px activities were detected with SOD and
GSH-Px assay kits, respectively. Values are expressed as the
mean±standard deviation from three independent experiments.
*P<0.05 and **P<0.01 vs. control group;
#P<0.05 and ##P<0.01 vs. H/R group;
&P<0.05 and &&P<0.01 vs.
schizandrin B + H/R + si-Nrf2 group. H/R, hypoxia/reperfusion;
Nrf2, nuclear factor erythroid 2-related factor 2; HO-1, heme
oxygenase-1; NQO-1, NAD(P)H: Quinone oxidoreductase; si-Nrf2,
Nrf2-specific small interfering RNA; si-Scram, scrambled siRNA; Sch
B, schizandrin B; IL, interleukin; TNF-α, tumor necrosis factor-α;
SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; PI,
propidium iodide. |
Nrf2-dependent cardioprotective
effects of schizandrin B are mediated by the AMPK pathway
Previous studies demonstrated that AMPK regulates a
variety of biological processes and is a highly effective
therapeutic target for protecting against MIRI (21,34).
Notably, AMPK can stimulate the nuclear accumulation of Nrf2
(26,27). The present study further
investigated whether AMPK may be responsible for the activation of
Nrf2 in the protective effect of schizandrin B. The results showed
that schizandrin B pretreatment increased the phosphorylation of
AMPK in H/R-treated H9c2 cells compared with the H/R group
(Fig. 6A and B). Cells were transfected with si-AMPK,
and the western blot results revealed that si-AMPK transfection
significantly reduced the expression of AMPK compared with
scrambled siRNA transfection (Fig.
6C and D). si-AMPK transfection
also significantly decreased the expression of Nrf2 (Fig. 6C and D) compared with scrambled siRNA
transfection, indicating that inhibition of the AMPK/Nfr2 signaling
pathway was induced by AMPK knockdown. Schizandrin B-mediated
increased cell viability (Fig. 6E)
and decreased LDH release (Fig. 6F)
were reversed by si-AMPK transfection. These results demonstrated
that the cardioprotection of schizandrin B in MIRI is dependent on
the AMPK/Nfr2 signaling pathway.
 | Figure 6AMPK pathway contributes to
Nrf2-dependent cardioprotective effects of schizandrin B in
H/R-treated H9c2 cells. H9c2 cells were treated with schizandrin B
(20 µM) prior to H (6 h)/R (12 h). (A) The expression of P-AMPK and
AMPK were measured by western blotting. (B) Densitometric analysis
for P-AMPK expression normalized to total AMPK. Values are
expressed as the mean±standard deviation from three independent
experiments. **P<0.01 vs. control group;
#P<0.05vs. H/R group. H9c2 cells weretransfected with
si-AMPK or si-Scram for 48 h. (C) The expression of AMPK and Nrf2
was analyzed by western blot analysis. (D) Densitometric analysis
of AMPK and Nrf2 expression. **P<0.01 vs.si-Scram
groups. H9c2 cells were transfected with si-AMPK or si-Scram for 48
h, followed by treatment with schizandrin B (20 µM) prior to H (6
h)/R (12 h). (E) Cell viability in the different groups was
evaluated by a Cell Counting Kit-8 assay. (F) LDH release was
measured using an LDH cytotoxicity assay kit. Values are expressed
as the mean±standard deviation from three independent experiments.
**P<0.01 vs. control group; ##P<0.01
vs. H/R group; &P<0.05 and
&&P<0.01 vs. schizandrin B + H/R + si-AMPK
group. AMPK, adenosine monophosphate-activated protein kinase;
p-AMPK, phospho-AMPK; H/R, hypoxia/reperfusion; Nrf2, nuclear
factor erythroid 2-related factor 2; si-AMPK, AMPK-specific small
interfering RNA; si-Nrf2, Nrf2-specific siRNA; si-Scram, scrambled
siRNA; Sch B, schizandrin B; LDH, lactate dehydrogenase. |
Discussion
The cytoprotective function of schizandrin B in
MIRI, in which a decrease in oxidation and ER stress-induced
apoptosis has been reported (14,15).
The beneficial effects of schizandrin B on ameliorating MIRI are
dependent on its anti-apoptotic (14) and antioxidative activities (35), and mitochondrial functional
enhancement (36). Despite
significant advances in the understanding of the mechanisms
accounting for the myocardial protective mechanism of schizandrin B
(2,37), the protective mechanism of
schizandrin B on MIRI remains to be elucidated. The present study
aimed to investigate whether schizandrin B protected H9c2 cells
against H/R injury via regulating the AMPK/Nrf2 signaling
pathway.
Schizandrin B is an active dibenzocyclooctadiene
lignan isolated from the fruit of Schisandra chinensis (a
traditional Chinese herb), and was found to possess antioxidant,
anti-apoptotic, anti-inflammatory and cardioprotective activities
in vitro and in vivo (38,39).
Studies indicated that schizandrin B could improve cardiac function
and mitigate myocardial infarct size in a MIRI model via inhibiting
cell apoptosis and attenuating oxidative stress (15,36).
Consistent with these studies, our findings also revealed that
schizandrin B pretreatment mitigated H/R-induced cytotoxicity and
apoptosis.
There are several mechanisms involved in the
development of MIRI, including increased oxidative stress and
inflammatory response (40,41). Oxidative stress, which is an
imbalance between endogenous ROS generation and antioxidant
systems, is involved in the etiology of I/R-induced myocardial
injury (8,42) and inflammatory response (33). The participation of oxidative stress
and inflammation in the pathologies of MIRI has been reported
(43,44). Recent evidence has suggested that
certain drugs, such as lipopolysaccharide and myeloperoxidase, that
serve anti-oxidative and anti-inflammatory functions are considered
a therapeutic strategy for limiting MIRI (39,44).
The present results showed that schizandrin B pretreatment
significantly reduced oxidative stress in H/R-treated H9c2 cells,
as evidenced by the decrease in intracellular ROS generation, lipid
peroxide (MDA) levels and NOX2 expression, and the increase in
antioxidant enzymatic activities, including SOD and GSH-Px, in H9c2
cells. Inflammation is a hallmark of MIRI (45). Previous studies have confirmed that
the imbalance between pro-inflammatory (IL-1β, TNF- α, IL-6 and
IL-18) and anti-inflammatory cytokines (TGF-β, IL-10 and IL-13)
contribute to the development of MIRI (46,47).
The results of the present study found that schizandrin B
attenuated H/R-induced upregulation of pro-inflammatory cytokines,
including IL-1β, TNF-α, IL-6 and IL-8, and the downregulation of
anti-inflammatory cytokines, including TGF-β and IL-10, in H9c2
cells, indicating the inhibition of schizandrin B on inflammatory
response. These above results are consistent with the anti-oxidant
and anti-inflammatory activities of schizandrin B reported in
previous studies (1,29). Taken together, these results
indicated that schizandrin B protects H9c2 cells against H/R injury
via inhibiting oxidative stress and inflammation.
Nrf2 is known to play a central role in cellular
defense against oxidative stress via regulating the transcriptional
expression of downstream antioxidant enzymes such as HO-1 and NOQ1
(22,48). Researches confirm that inhibition of
the Nrf2 axis exacerbates oxidative stress, inflammation and
induced cell apoptosis (11,49,50).
Numerous studies have shown that Nrf2 is one of the essential
signaling pathways that can mitigate myocardial infarct size and
preserve cardiac function following MIRI, which is dependent on the
coordinated upregulation of antioxidant, anti-inflammatory and
autophagic mechanisms (25,51). Oxidative stressors and several
anti-inflammatory traditional Chinese medicines, such as
Arctigenin, Nardochinoid C and azafrin, promote the nuclear
translocation of Nrf2 and activate the transcription of antioxidant
genes, including HO-1 and NOQ1, leading to beneficial protection on
various diseases (40,52-54).
Notably, schizandrin B was also shown to reduce oxidative stress
and possess strong anti-inflammatory properties, at least in part
via the induction of Nrf2 and Nrf2-driven antioxidant responses
(55). In the present study,
schizandrin B pretreatment also enhanced Nrf2 expression in the
nucleus, and HO-1 and NOQ1 expression in H9c2 cells. Notably, Nfr2
knockdown induced by si-Nrf2 remarkably attenuated schizandrin
B-induced inhibition against cytotoxicity, apoptosis, oxidative
stress and inflammation in H/R-treated H9c2 cells. These results
showed that the Nfr2 signaling pathway contributed to the
cardioprotection of schizandrin B in MIRI.
AMPK activation was verified to confer
cardioprotection against MIRI by regulating processes such as
survival and cellular longevity, apoptosis, inflammation, ROS
reduction and mitochondrial function (17,56,57).
AMPK activation is known to promote myocardial resistance to I/R
and oxidative stress of different magnitudes by upregulating Nrf2
(58,59). However, the role of the AMPK pathway
in the beneficial function of schizandrin B remains to be
elucidated. In the present study, the results showed that
schizandrin B enhanced AMPK activation in H/R-treated H9c2 cells.
In addition, AMPK knockdown induced by si-AMPK transfection reduced
the expression of Nfr2, HO-1 and NOQ1, which is consistent with
previous studies where inhibition of AMPK by relative inhibitor or
specific siRNA decreased Nrf2 expression in MIRI (59,60).
Meanwhile, si-AMPK transfection reversed schizandrin B-induced
protection on H/R injury. Taken together, these results showed that
the Nrf2-dependent cardioprotective effects of schizandrin B are
mediated by the AMPK pathway.
In conclusion, the present results demonstrated that
schizandrin B protects H9c2 cells against H/R injury by suppressing
oxidative stress and inflammation. The present results supported
the notion that the AMPK/Nrf2 signaling pathway may play a role in
the cardioprotection of schizandrin B against MIRI. These results
provide a better understanding of the molecular mechanisms
associated with the cardioprotection of schizandrin B and may
provide a new insight into a better design of myocardial protective
agents against MIRI.
However, since the present experiments were only
performed in vitro, in vivo animal experiments and
clinical trials with human subjects should be performed in further
studies. In addition, the endogenous hydrogen sulfide-regulated
Nrf2 signaling pathway was involved in myocardial protection
against I/R injury (61,62). It was also confirmed that Nrf2
regulates hundreds of genes, of which many are either directly or
indirectly involved in modulating ferroptosis, which serves as a
cardioprotective strategy for cardiomyopathy prevention (63,64).
Hence, the role of these above related signaling pathways in the
cardioprotection of schizandrin B warrants further study.
Additionally, since the method of the present study of checking IRI
is a two-step process but presented as a final step, it is also
worth checking ischemia and reperfusion as two distinct paths in
subsequent experiments.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BZ and GPL designed and performed the experiments
and wrote the manuscript. JJP, LHR and LCL analyzed the data. HMY
and ZYW designed the experiments and analyzed the data. SZ
performed the experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chi HJ, Chen ML, Yang XC, Lin XM, Sun H,
Zhao WS, Qi D, Dong JL and Cai J: Progress in therapies for
myocardial ischemia reperfusion injury. Curr Drug Targets.
18:1712–1721. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Neri M, Riezzo I, Pascale N, Pomara C and
Turillazzi E: Ischemia/reperfusion injury following acute
myocardial infarction: A critical issue for clinicians and forensic
pathologists. Mediators Inflamm. 2017(7018393)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhu N, Cai C, Zhou A, Zhao X, Xiang Y and
Zeng C: Schisandrin B prevents hind limb from
ischemia-reperfusion-induced oxidative stress and inflammation via
MAPK/NF-κB pathways in rats. Biomed Res Int.
2017(4237973)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zheng Q, Huang YY, Zhu PC, Tong Q, Bao XY,
Zhang QH, Zheng GQ and Wang Y: Ligustrazine exerts cardioprotection
in animal models of myocardial ischemia/reperfusion injury:
Preclinical evidence and possible mechanisms. Front Pharmacol.
9(729)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liu J, Wang H and Li J: Inflammation and
inflammatory cells in myocardial infarction and reperfusion injury:
A double-edged sword. Clin Med Insights Cardiol. 10:79–84.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yang CF: Clinical manifestations and basic
mechanisms of myocardial ischemia/reperfusion injury. Ci Ji Yi Xue
Za Zhi. 30:209–215. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gielis JF, Beckers PAJ, Briede JJ, Cos P
and Van Schil PE: Oxidative and nitrosative stress during pulmonary
ischemia-reperfusion injury: From the lab to the OR. Ann Transl
Med. 5(131)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
González-Montero J, Brito R, Gajardo AI
and Rodrigo R: Myocardial reperfusion injury and oxidative stress:
Therapeutic opportunities. World J Cardiol. 10:74–86.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
He F and Zuo L: Redox roles of reactive
oxygen species in cardiovascular diseases. Int J Mol Sci.
16:27770–27780. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li ZM, Xu SW and Liu PQ: Salvia
miltiorrhizaBurge (Danshen): A golden herbal medicine in
cardiovascular therapeutics. Acta Pharmacol Sin. 39:802–824.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li J, Zheng X, Ma X, Xu X, Du Y, Lv Q, Li
X, Wu Y, Sun H, Yu L and Zhang Z: Melatonin protects against
chromium(VI)-induced cardiac injury via activating the AMPK/Nrf2
pathway. J Inorg Biochem. 197(110698)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yu LM, Dong X, Xue XD, Zhang J, Li Z, Wu
HJ, Yang ZL, Yang Y and Wang HS: Naringenin improves mitochondrial
function and reduces cardiac damage following ischemia-reperfusion
injury: The role of the AMPK-SIRT3 signaling pathway. Food Funct.
10:2752–2765. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xin DQ, Hu ZM, Huo HJ, Yang XJ, Han D,
Xing WH, Zhao Y and Qiu QH: Schisandrin B attenuates the
inflammatory response, oxidative stress and apoptosis induced by
traumatic spinal cord injury via inhibition of p53 signaling in
adult rats. Mol Med Rep. 16:533–538. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang W, Sun Z and Meng F: Schisandrin B
ameliorates myocardial ischemia/reperfusion injury through
attenuation of endoplasmic reticulum stress-induced apoptosis.
Inflammation. 40:1903–1911. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhao X, Xiang Y, Cai C, Zhou A, Zhu N and
Zeng C: Schisandrin B protects against myocardial
ischemia/reperfusion injury via the PI3K/Akt pathway in rats. Mol
Med Rep. 17:556–561. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yim TK and Ko KM: Schisandrin B protects
against myocardial ischemia-reperfusion injury by enhancing
myocardial glutathione antioxidant status. Mol Cell Biochem.
196:151–156. 1999.PubMed/NCBI
|
|
17
|
Steinberg GR and Schertzer JD: AMPK
promotes macrophage fatty acid oxidative metabolism to mitigate
inflammation: Implications for diabetes and cardiovascular disease.
Immunol Cell Biol. 92:340–345. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang M, Yang D, Gong X, Ge P, Dai J, Lin
L and Zhang L: Protective benefits of AMP-activated protein kinase
in hepatic ischemia-reperfusion injury. Am J Transl Res. 9:823–829.
2017.PubMed/NCBI
|
|
19
|
Daskalopoulos EP, Dufeys C, Beauloye C,
Bertrand L and Horman S: AMPK in cardiovascular diseases. Exp
Suppl. 107:179–201. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kanugula AK and Thodeti CK: AMP-activated
kinase 'Keaps' ischemia/reperfusion-induced necroptosis under
control. Int J Cardiol. 259:168–169. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Qi D and Young LH: AMPK: Energy sensor and
survival mechanism in the ischemic heart. Trends Endocrinol Metab.
26:422–429. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
da Costa RM, Rodrigues D, Pereira CA,
Silva JF, Alves JV, Lobato NS and Tostes RC: Nrf2 as a potential
mediator of cardiovascular risk in metabolic diseases. Front
Pharmacol. 10(382)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu L, Locascio LM and Doré S: Critical
role of Nrf2 in experimental ischemic stroke. Front Pharmacol.
10(153)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Schmidlin CJ, Dodson MB, Madhavan L and
Zhang DD: Redox regulation by NRF2 in aging and disease. Free Radic
Biol Med. 134:702–707. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shen Y, Liu X, Shi J and Wu X: Involvement
of Nrf2 in myocardial ischemia and reperfusion injury. Int J Biol
Macromol. 125:496–502. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhou F, Wang M, Ju J, Wang Y, Liu Z, Zhao
X, Yan Y, Yan S, Luo X and Fang Y: Schizandrin A protects against
cerebral ischemia-reperfusion injury by suppressing inflammation
and oxidative stress and regulating the AMPK/Nrf2 pathway
regulation. Am J Transl Res. 11:199–209. 2019.PubMed/NCBI
|
|
27
|
Fan X, Lv H, Wang L, Deng X and Ci X:
Isoorientin ameliorates APAP-induced hepatotoxicity via activation
Nrf2 antioxidative pathway: The involvement of AMPK/Akt/GSK3β.
Front Pharmacol. 9(1334)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yang Q, Han L, Li J, Xu H, Liu X, Wang X,
Pan C, Lei C, Chen H and Lan X: Activation of Nrf2 by phloretin
attenuates palmitic acid-induced endothelial cell oxidative stress
via AMPK-dependent signaling. J Agric Food Chem. 67:120–131.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mou Z, Feng Z, Xu Z, Zhuang F, Zheng X, Li
X, Qian J and Liang G: Schisandrin B alleviates diabetic
nephropathy through suppressing excessive inflammation and
oxidative stress. Biochem Biophys Res Commun. 508:243–249.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wu Y, Li ZC, Yao LQ, Li M and Tang M:
Schisandrin B alleviates acute oxidative stress via modulation of
the Nrf2/Keap1-mediated antioxidant pathway. Appl Physiol Nutr
Metab. 44:1–6. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cheng J, Wu Q, Lv R, Huang L, Xu B, Wang
X, Chen A and He F: MicroRNA-449a inhibition protects H9C2 cells
against hypoxia/reoxygenation-induced injury by targeting the
Notch-1 signaling pathway. Cell Physiol Biochem. 46:2587–2600.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Doridot L, Jeljeli M, Chêne C and Batteux
F: Implication of oxidative stress in the pathogenesis of systemic
sclerosis via inflammation, autoimmunity and fibrosis. Redox Biol.
25(101122)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Park JS, Leem YH, Park JE, Kim DY and Kim
HS: Neuroprotective effect of β-lapachone in MPTP-induced
parkinson's disease mouse model: Involvement of astroglial
p-AMPK/Nrf2/HO-1 signaling pathways. Biomol Ther (Seoul).
27:178–184. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chen N and Ko M: Schisandrin B-induced
glutathione antioxidant response and cardioprotection are mediated
by reactive oxidant species production in rat hearts. Biol Pharm
Bull. 33:825–829. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ko KM and Chiu PY: Structural determinants
of schisandrin B which enhance mitochondrial functional ability and
glutathione status as well as heat shock protein expression in rat
hearts and H9c2 cells. Mol Cell Biochem. 276:227–234.
2005.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lakota J: Molecular mechanism of
ischemia-reperfusion injury after myocardial infarction and its
possible targeted treatment. Int J Cardiol. 220:571–572.
2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Leong PK and Ko KM: Schisandrin B: A
double-edged sword in nonalcoholic fatty liver disease. Oxid Med
Cell Longev. 2016(6171658)2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Shao M, Yang W and Han G: Protective
effects on myocardial infarction model: Delivery of schisandrin B
using matrix metalloproteinase-sensitive peptide-modified,
PEGylated lipid nanoparticles. Int J Nanomedicine. 12:7121–7130.
2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Qiu Z, He Y, Ming H, Lei S, Leng Y and Xia
ZY: Lipopolysaccharide (LPS) aggravates high glucose- and
hypoxia/reoxygenation-induced injury through activating
ROS-dependent NLRP3 inflammasome-mediated pyroptosis in H9C2
cardiomyocytes. J Diabetes Res. 2019(8151836)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yao BJ, He XQ, Lin YH and Dai WJ:
Cardioprotective effects of anisodamine against myocardial
ischemia/reperfusion injury through the inhibition of oxidative
stress, inflammation and apoptosis. Mol Med Rep. 17:1253–1260.
2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Yang J, Yin HS, Cao YJ, Jiang ZA, Li YJ,
Song MC, Wang YF, Wang ZH, Yang R, Jiang YF, et al: Arctigenin
attenuates ischemia/reperfusion induced ventricular arrhythmias by
decreasing oxidative stress in rats. Cell Physiol Biochem.
49:728–742. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yang MY, Wang YB, Han B, Yang B, Qiang YW,
Zhang Y, Wang Z, Huang X, Liu J, Chen YD, et al: Activation of
aldehyde dehydrogenase 2 slows down the progression of
atherosclerosis via attenuation of ER stress and apoptosis in
smooth muscle cells. Acta Pharmacol Sin. 39:48–58. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ndrepepa G: Myeloperoxidase-A bridge
linking inflammation and oxidative stress with cardiovascular
disease. Clin Chim Acta. 493:36–51. 2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Rohrbach S, Troidl C, Hamm C and Schulz R:
Ischemia and reperfusion related myocardial inflammation: A network
of cells and mediators targeting the cardiomyocyte. IUBMB Life.
67:110–119. 2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Vinten-Johansen J, Jiang R, Reeves JG,
Mykytenko J, Deneve J and Jobe LJ: Inflammation, proinflammatory
mediators and myocardial ischemia-reperfusion Injury. Hematol Oncol
Clin North Am. 21:123–145. 2007.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wallert M, Ziegler M, Wang X, Maluenda A,
Xu X, Yap ML, Witt R, Giles C, Kluge S, Hortmann M, et al:
α-Tocopherol preserves cardiac function by reducing oxidative
stress and inflammation in ischemia/reperfusion injury. Redox Biol.
26(101292)2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Chen QM and Maltagliati AJ: Nrf2 at the
heart of oxidative stress and cardiac protection. Physiol Genomics.
50:77–97. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Lv Y, Bing Q, Lv Z, Xue J, Li S, Han B,
Yang Q, Wang X and Zhang Z: Imidacloprid-induced liver fibrosis in
quails via activation of the TGF-β1/Smad pathway. Sci Total
Environ. 705(135915)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Han B, Li S, Lv Y, Yang D, Li J, Yang Q,
Wu P, Lv Z and Zhang Z: Dietary melatonin attenuates
chromium-induced lung injury via activating the Sirt1/Pgc-1α/Nrf2
pathway. Food Funct. 10:5555–5565. 2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Jakobs P, Serbulea V, Leitinger N, Eckers
A and Haendeler J: Nuclear factor (Erythroid-Derived 2)-like 2 and
thioredoxin-1 in atherosclerosis and ischemia/reperfusion injury in
the heart. Antioxid Redox Signal. 26:630–644. 2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Hun Lee J, Shu L, Fuentes F, Su ZY and
Tony Kong AN: Cancer chemoprevention by traditional chinese herbal
medicine and dietary phytochemicals: Targeting nrf2-mediated
oxidative stress/anti-inflammatory responses, epigenetics, and
cancer stem cells. J Tradit Complement Med. 3:69–79.
2013.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Luo JF, Shen XY, Lio CK, Dai Y, Cheng CS,
Liu JX, Yao YD, Yu Y, Xie Y, Luo P, et al: Activation of Nrf2/HO-1
pathway by nardochinoid C inhibits inflammation and oxidative
stress in lipopolysaccharide-stimulated macrophages. Front
Pharmacol. 9(911)2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Yang S, Chou G and Li Q: Cardioprotective
role of azafrin in against myocardial injury in rats via activation
of the Nrf2-ARE pathway. Phytomedicine. 47:12–22. 2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Chiu PY, Chen N, Leong PK, Leung HY and Ko
KM: Schisandrin B elicits a glutathione antioxidant response and
protects against apoptosis via the redox-sensitive ERK/Nrf2 pathway
in H9c2 cells. Mol Cell Biochem. 350:237–250. 2011.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Potenza MA, Sgarra L, Nacci C, Leo V, De
Salvia MA and Montagnani M: Activation of AMPK/SIRT1 axis is
required for adiponectin-mediated preconditioning on myocardial
ischemia-reperfusion (I/R) injury in rats. PLoS One.
14(e0210654)2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zhang Y, Wang Y, Xu J, Tian F, Hu S, Chen
Y and Fu Z: Melatonin attenuates myocardial ischemia-reperfusion
injury via improving mitochondrial fusion/mitophagy and activating
the AMPK-OPA1 signaling pathways. J Pineal Res.
66(e12542)2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Duan J, Guan Y, Mu F, Guo C, Zhang E, Yin
Y, Wei G, Zhu Y, Cui J, Cao J, et al: Protective effect of butin
against ischemia/reperfusion-induced myocardial injury in diabetic
mice: Involvement of the AMPK/GSK-3β/Nrf2 signaling pathway. Sci
Rep. 7(41491)2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Tanaka M, Kishimoto Y, Sasaki M, Sato A,
Kamiya T, Kondo K and Iida K: Terminalia bellirica (Gaertn.) Roxb.
Extract and gallic acid attenuate LPS-induced inflammation and
oxidative stress via MAPK/NF-κB and Akt/AMPK/Nrf2 pathways. Oxid
Med Cell Longev. 2018(9364364)2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Hou X, Fu M, Cheng B, Kang Y and Xie D:
Galanthamine improves myocardial ischemia-reperfusion-induced
cardiac dysfunction, endoplasmic reticulum stress-related
apoptosis, and myocardial fibrosis by suppressing AMPK/Nrf2 pathway
in rats. Ann Transl Med. 7(634)2019.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Huang S, Li H and Ge J: A cardioprotective
insight of the cystathionine γ-lyase/hydrogen sulfide pathway. Int
J Cardiol Heart Vasc. 7:51–57. 2015.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Peake BF, Nicholson CK, Lambert JP, Hood
RL, Amin H, Amin S and Calvert JW: Hydrogen sulfide preconditions
the db/db diabetic mouse heart against ischemia-reperfusion injury
by activating Nrf2 signaling in an Erk-dependent manner. Am J
Physiol Heart Circ Physiol. 304:H1215–H224. 2013.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Fang XX, Wang H, Han D, Xie E, Yang X, Wei
J, Gu S, Gao F, Zhu N, Yin X, et al: Ferroptosis as a target for
protection against cardiomyopathy. Proc Natl Acad Sci USA.
116:2672–2680. 2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Dodson M, Castro-Portuguez R and Zhang DD:
NRF2 plays a critical role in mitigating lipid peroxidation and
ferroptosis. Redox Biol. 23(101107)2019.PubMed/NCBI View Article : Google Scholar
|