Introduction
Endoscopic retrograde cholangiopancreatography
(ERCP) is one of the most important methods for clinical diagnosis
and treatment of biliary and pancreatic diseases (1). ERCP assists in the implementation of
surgeries and the ERCP indications are increasing (2). However, it is a traumatic and invasive
examination that may cause pancreatitis, hyperamylasemia,
hemorrhage, perforation and other complications. Among them,
post-ERCP pancreatitis (PEP) is the most common one, with an
incidence rate of 7.7-10% (3-5).
PEP patients without timely treatment are prone to develop severe
acute pancreatitis, which may even lead to death in severe cases
(6). Therefore, avoiding its
occurrence is of great significance.
Clinically, conventional drugs for the prevention
and treatment of PEP and post-ERCP hyperamylasemia (PEH) include
somatostatin analogs and calcium channel blockers (7,8).
Octreotide is a kind of synthetic somatostatin analogue that
inhibits the secretion of pituitary, pancreas and gastrointestinal
hormones (9). Although some
progress has been made in the prevention of PEP by octreotide, the
effect of the monotherapy is still unsatisfactory (10). As a new proton pump inhibitor (PPI),
lansoprazole is effective in treating the gastroesophageal reflux
disease and peptic ulcer (11);
however, its effect on the prevention and treatment of PEP has
hardly been investigated. In addition, inflammatory factors, such
as interleukin-17 (IL-17) and tumor necrosis factor-α (TNF-α),
increase gradually in the early stages of pancreatitis. TNF-α
activates the lysozyme system, thus damaging pancreatic cells and
participating in the progression of pancreatitis (12,13).
Moreover, surgical trauma and anesthesia may cause disorders of the
immune function, and low immune function is closely related to the
development of pancreatitis (14).
Up to our knowledge, only a few previous studies
have been reported on the preventive and therapeutic effects of
octreotide combined with lansoprazole on PEP. Therefore, in the
present study, the combination therapy was used to treat patients
undergoing ERCP in order to explore its effects on PEP, serum
amylase (AMS), inflammatory factors and immune function.
Patients and methods
General data
In this observational study, a total of 132 patients
who underwent ERCP in Shaoxing People's Hospital (Shaoxing, China)
from March, 2012 to June, 2015 were enrolled and allocated into two
groups: The study group (treated with octreotide plus lansoprazole,
68 cases) and the control group (treated with octreotide alone, 64
cases). In the study group, there were 37 male and 31 female
patients, aged 24-75 years with an average age of 59.4±10.1 years.
In the control group, there were 36 male and 28 female patients,
aged 22-74 years with an average age of 58.1±9.8 years. The study
was approved by the Ethics Committee of Shaoxing People's Hospital
(1536-40-16). Signed written informed consents were obtained from
the patients and/or guardians.
Inclusion and exclusion criteria
Inclusion criteria: Patients who met the ERCP
indications and the study followed the Strengthening the Reporting
of Observational Studies in Epidemiology (STROBE) Statement
guidelines; patients with complete clinical data, and aged 22-75
years. Exclusion criteria: Patients with surgical and anesthetic
intolerance, or contraindications to drugs applied in this
treatment; patients with previous hyperamylasemia or pancreatitis;
patients receiving anti-inflammatory, immunosuppressive and
non-steroidal anti-infection drugs in the past month; patients with
abnormal coagulation function; patients with cognitive dysfunction
and mental diseases; patients complicated with digestive tract
ulcer, heart failure, respiratory failure, malignant tumor,
hemorrhage, perforation, severe cardiovascular and cerebrovascular
diseases, autoimmune diseases, connective tissue diseases, or liver
and kidney dysfunctions.
Treatment methods
All patients received routine intramuscular
injection of 10 mg of scopolamine (H41021048; Zhengzhou Suicheng
Pharmaceutical Co., Ltd.), 50 mg of pethidine (H63020021; Qinghai
Pharmaceutical Co., Ltd.), 10 mg of diazepam (H31021864; Shanghai
Xudong Haipu Pharmaceutical Co., Ltd.) and 50 mg of propofol
(H20030115; Sichuan Guorui Pharmaceutical Co., Ltd.) before ERCP
for intravenous anesthesia. Oxygen inhalation, oxygen saturation
and electrocardiogram monitoring were also performed. The ERCP was
conducted by physicians in accordance with the standard manual.
After ERCP, rehydration, anti-infection, anti-inflammatory and
other supportive treatments were given. A total of 0.1 mg of
octreotide (H120150364; Swiss Novartis Pharma Stein AG) was
administered subcutaneously 1 h after ERCP, followed by 0.1 mg
every 8 h for 3 times. On this basis, the patients in the study
group were given intravenous drip of 30 mg of lansoprazole
(H20100055; Shandong Luoxin Pharmaceutical Co., Ltd.) and 0.9%
normal saline (100 ml) 1 h after ERCP, twice per day. The drip was
completed within 30 min and the treatment was carried out for 7
consecutive days.
Evaluation indices
The incidence rates of PEP and PEH in the two groups
were recorded. According to the diagnostic criteria of Ilone and
Fauzi (15), patients with
abdominal pain and tenderness at 2 h after ERCP, AMS levels >3
times higher than the normal upper limit, typical pancreatitis-like
abdominal pain over 24 h and severe pain requiring hospitalization
were diagnosed with PEP. PEH was diagnosed in patients whose serum
AMS levels exceeded the normal value without the above clinical
signs. The symptom disappearance time and hospital stay in the two
groups were recorded.
Outcome measures
A total of 3 ml of venous blood were drawn before
treatment, and at 6 and 24 h after treatment, respectively, and
placed in vacuum tubes without anticoagulant. Additional 3-ml
samples were collected 24 h after treatment and placed in a vacuum
tube containing EDTA.
DxC 600 automatic biochemical analyzer (Beckman
Coulter, Inc.) was used to detect AMS (702; Beijing Bioassay
Technology Laboratory) levels before treatment, and at 6 and 24 h
after treatment.
The levels of serum IL-17 and TNF-α in the two
groups, before treatment and at 24 h after treatment, were measured
by ELISA (kits purchased from Shanghai Xinfan Biotechnology Co.,
Ltd.; XF-HUMAN-0979 and XF-HUMAN-1140) (16). A standard well, a testing well and a
blank control well (with no sample and ELISA kit) were set up. A
2-fold diluted standard (50 µl) was added into the standard well
and 50 µl of sample were added into the testing well. After the
addition of 50 µl of diluted antibody to each well, the plate was
sealed and incubated for 2 h. Next, the liquid in each well was
discarded, the well was repeatedly washed for 6 times and dried. A
total of 100 µl of diluted horseradish peroxidase-labeled
streptavidin were added to each well and the plate was incubated
for 45 min. The liquid in each well was then discarded, the well
was repeatedly washed for 6 times and dried. Afterwards, 100 µl of
chromogenic substrate TMB solution were added to each well and
incubated in the dark for 5 min. Finally, 100 µl of stop solution
were added. The optical density was measured at a wavelength of 450
nm using Multiskan MK3 microplate reader (Shanghai Thermo Fisher
Scientific, Inc.) and the IL-17 and TNF-α levels were measured.
FACSCanto flow cytometer (BD Biosciences) was
employed to detect the T-lymphocyte population in peripheral blood
before treatment and at 24 h after treatment. A total of 20 µl from
1 ml of EDTA-treated peripheral venous blood was added into an Tru
COUNT tube containing known quantities of freeze-dried standard
fluorescent microspheres. The mixture was incubated with mouse
anti-human monoclonal antibodies conjugated with fluorescein
isothiocyanate (FITC) or phycoerythrin (PE): Anti-CD3-PE,
anti-CD4-FITC, anti-CD8-PE (Shanghai Hengfei Biological Technology
Co., Ltd.; cat. nos. 130-103-130, 130-109-536, 130-098-078, 5 µl
each) in the dark at room temperature for 15 min. Following a
reaction with red blood cell lysate (450 µl) for 15 min, a flow
cytometer was used to measure the sample and CELLQUEST software
(Becton-Dickinson) was used to analyze the percentages of
CD3+, CD4+ and CD8+ cells.
Statistical analysis
SPSS 22.0 software (Guangzhou Coslan Instrument Co.,
Ltd.) was used for the statistical analysis of the data.
Measurement data were expressed as the mean ± standard deviation
(mean ± SD), and the intergroup comparison was conducted by
independent samples t-test, whereas the intragroup comparison was
conducted by paired t-test. Count data were expressed by the number
of cases and percentage [n (%)] and the intergroup comparison
between groups was conducted by Chi-square test. The data of
multiple time points were analyzed by repeated measures analysis of
variance, and LSD-t-test was the post-hoc text used for pairwise
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient general data in the two
groups
There was no significant difference in sex, age,
body mass index (BMI), disease type, ERCP duration, hypertension,
diabetes, duodenal diverticulum, smoking history, drinking history,
balloon dilatation, white blood cells, platelets and residence
between the two groups (P>0.05; Table I).
 | Table IPatient general data in the study and
control groups [n (%), mean ± SD]. |
Table I
Patient general data in the study and
control groups [n (%), mean ± SD].
|
Characteristics | Study group
(n=68) | Control group
(n=64) | t/χ2
value | P-value |
|---|
| Sex | | | 0.045 | 0.832 |
|
Male | 37 (54.41) | 36 (56.25) | | |
|
Female | 31 (45.59) | 28 (43.75) | | |
| Age (years) | 59.4±10.1 | 58.1±9.8 | 0.750 | 0.455 |
| BMI
(kg/m2) | 23.57±3.48 | 23.78±3.22 | 0.359 | 0.720 |
| Disease type | | | 0.870 | 0.351 |
|
Choledocholithiasis | 46 (67.65) | 48 (75.00) | | |
|
Obstructive
jaundice | 22 (32.35) | 16 (25.00) | | |
| ERCP duration
(min) | 31.12±8.13 | 30.27±8.15 | 0.600 | 0.550 |
| Hypertension | | | 0.005 | 0.945 |
|
Yes | 3 (4.41) | 2 (3.12) | | |
|
No | 65 (95.59) | 62 (96.88) | | |
| Diabetes | | | 0.244 | 0.621 |
|
Yes | 2 (2.94) | 4 (6.25) | | |
|
No | 66 (97.06) | 60 (93.75) | | |
| Duodenal
diverticulum | | | 0.495 | 0.482 |
|
Yes | 15 (22.06) | 11 (17.19) | | |
|
No | 53 (77.94) | 53 (82.81) | | |
| Smoking
history | | | 0.238 | 0.625 |
|
Yes | 29 (42.65) | 30 (46.88) | | |
|
No | 39 (57.35) | 34 (53.12) | | |
| Drinking
history | | | 0.535 | 0.465 |
|
Yes | 35 (51.47) | 37 (57.81) | | |
|
No | 33 (48.53) | 27 (42.19) | | |
| Balloon
dilation | | | 1.072 | 0.300 |
|
Dilated | 40 (58.82) | 45 (70.31) | | |
|
Undilated | 28 (41.18) | 19 (29.69) | | |
| White blood cells
(x109/l) | 6.07±2.02 | 5.86±1.72 | 0.641 | 0.523 |
| Platelets
(x109/l) | 153.46±32.69 | 162.58±35.51 | 1.536 | 0.127 |
| Residence | | | 0.189 | 0.664 |
|
Urban | 46 (67.65) | 41 (64.06) | | |
|
Rural | 22 (32.35) | 23 (35.94) | | |
Serum AMS levels in the two groups at
different time points
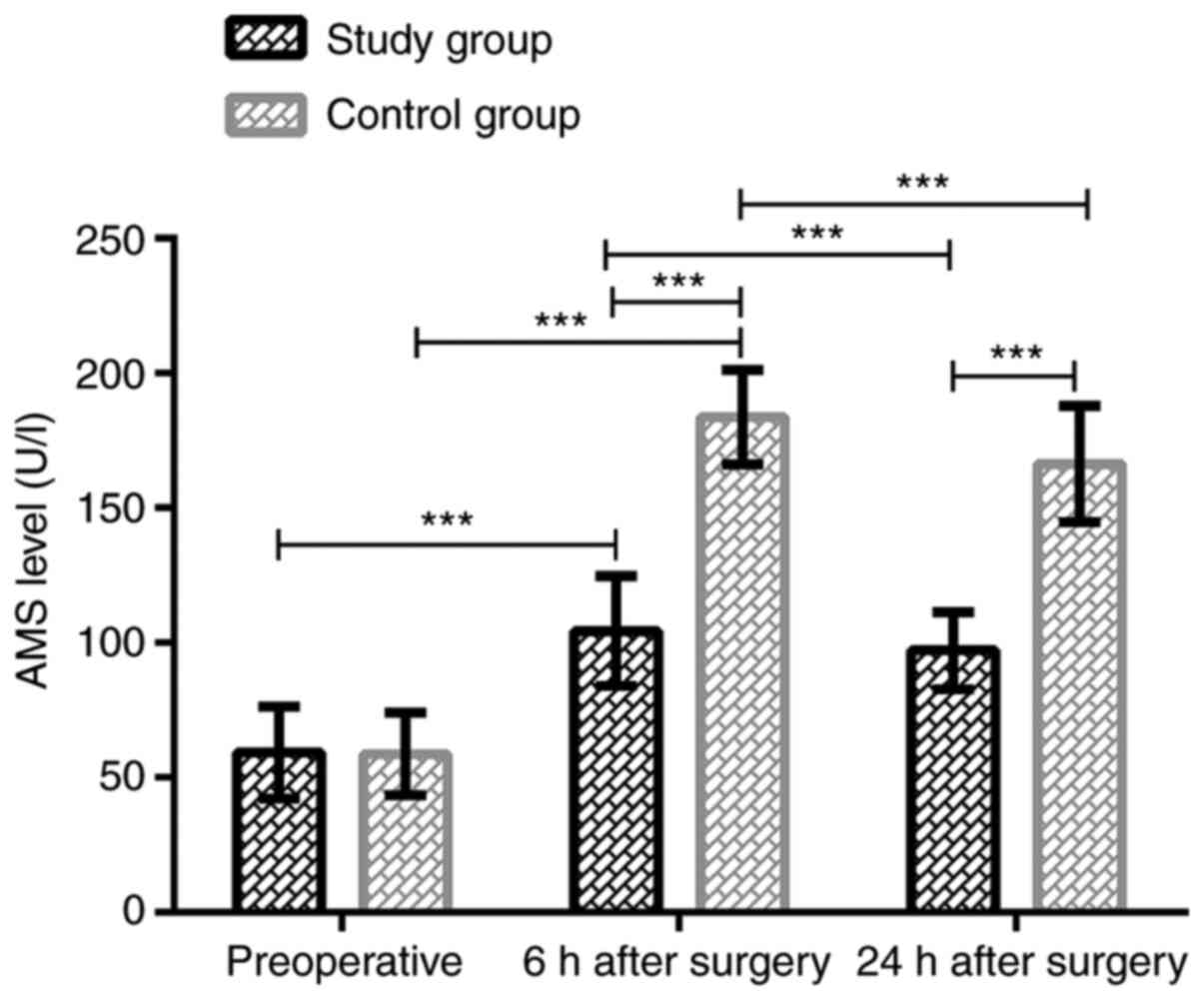
In the study group, the serum AMS levels before
treatment, at 6 and 24 h after treatment were 59.24±17.02,
104.32±20.31 and 97.12±14.32 U/l, respectively, whereas those in
the control group were 58.74±15.32, 183.65±17.43 and 166.32±21.53
U/l, respectively. Thus, the serum AMS levels in the two groups at
6 h after treatment were significantly higher than those before
treatment (P<0.001). The levels decreased significantly at 24 h
after treatment compared with those at 6 h after treatment
(P<0.001); however, they remained higher than those before
treatment (P<0.001). There was no significant difference in
serum AMS levels between the study and the control group before
treatment (P>0.05). AMS expression in the study group was
significantly lower than that in the control group at 6 and 24 h
after treatment (P<0.001). Data are shown in Fig. 1.
Incidence of PEP and PEH in the two
groups
In the study group, PEP occurred in 1 case (1.47%)
and PEH occurred in 12 cases (17.65%). In the control group, PEP
occurred in 6 cases (9.38%) and PEH in 23 cases (35.94%). The
incidence of postoperative PEP and PEH in the study group was
significantly lower than that in the control group (P<0.05).
Data are presented in Table
II.
 | Table IIComparison of incidence of
pancreatitis and hyperamylasemia between the two groups [n
(%)]. |
Table II
Comparison of incidence of
pancreatitis and hyperamylasemia between the two groups [n
(%)].
| Group | n | Pancreatitis |
Hyperamylasemia |
|---|
| Study group | 68 | 1 (1.47) | 12 (17.65) |
| Control group | 64 | 6 (9.38) | 23 (35.94) |
| χ2
value | - | 2.338 | 5.661 |
| P-value | - | 0.021 | 0.017 |
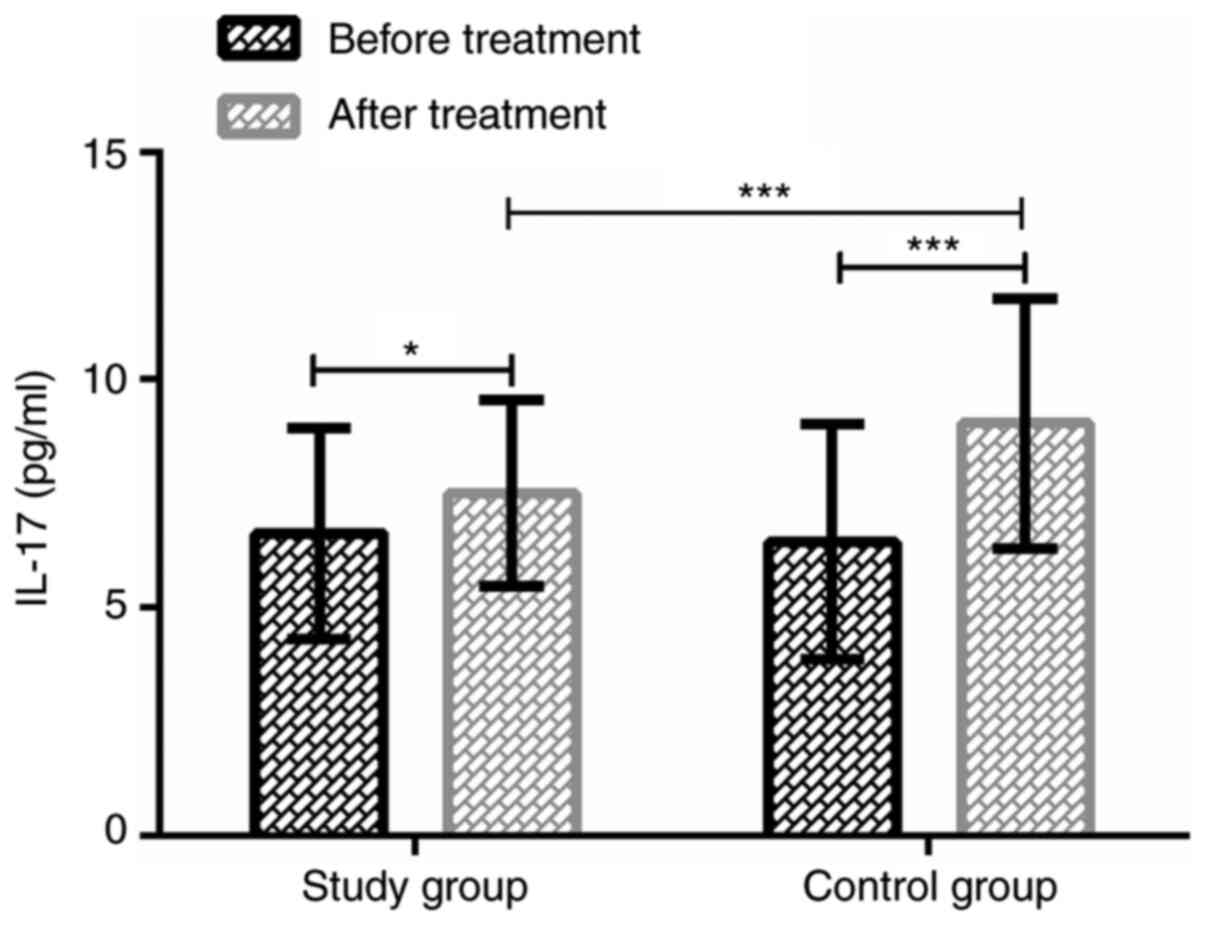
Serum IL-17 levels in the two groups
before and after treatment
The serum IL-17 levels in the study group before and
at 24 h after treatment were 6.62±2.31 and 7.51±2.04 pg/ml,
respectively. In the control group, IL-17 levels were 6.45±2.58 and
9.04±2.75 pg/ml, respectively. There was no significant difference
in serum IL-17 levels between the two groups before treatment
(P>0.05). At 24 h after treatment, the IL-17 expression was
significantly increased in both groups (P<0.05), and in the
study group, IL-17 expression was significantly lower than that in
the control group (P<0.001). Data are shown in Fig. 2.
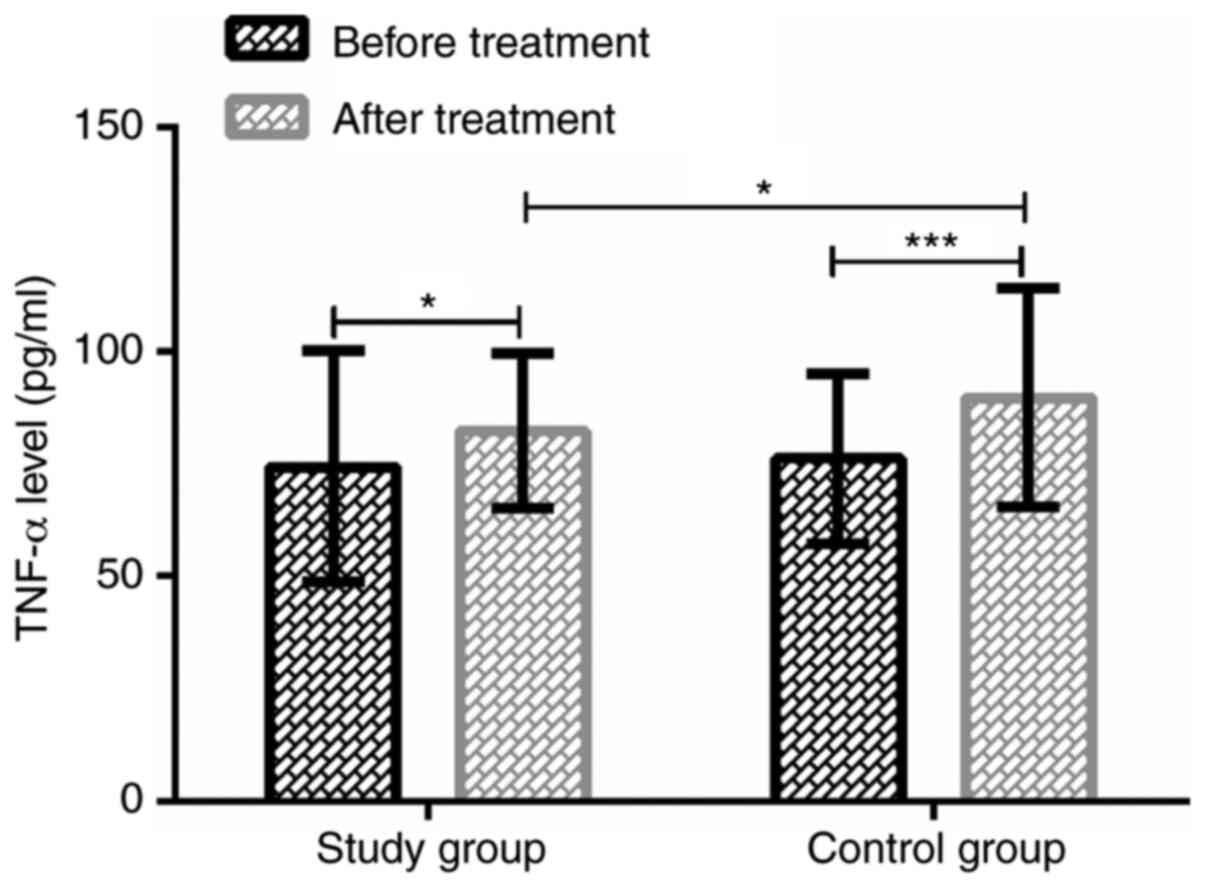
Serum TNF-α levels in the two groups
before and after treatment
The serum TNF-α levels in the study group were
74.35±25.72 pg/ml before treatment and 82.29±17.18 pg/ml at 24 h
after treatment, whereas in the control group were 76.13±18.91 and
89.73±24.32 pg/ml, respectively. There was no significant
difference in serum TNF-α levels between the two groups before
treatment (P>0.05). After treatment, the TNF-α expression was
significantly increased in both groups (P<0.05), and in the
study group, TNF-α expression was significantly lower than that in
the control group (P<0.05). Data are shown in Fig. 3.
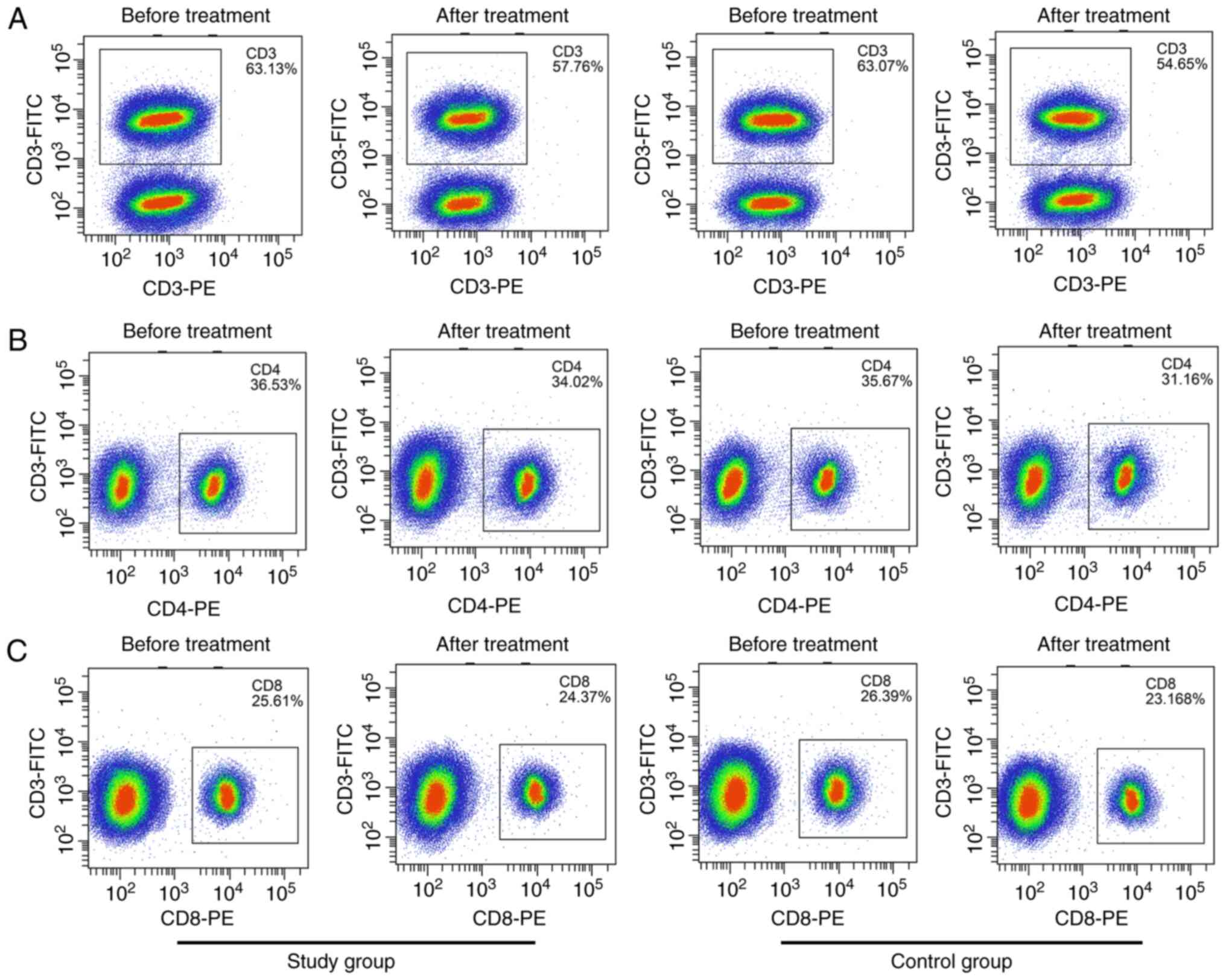
T-lymphocyte population in the two
groups before and after treatment
There was no significant difference in the
percentages of CD3+, CD4+ and CD8+
cells and the CD4+/CD8+ ratio in peripheral
blood between the two groups before treatment (P>0.05). At 24 h
after treatment, the values of CD3+, CD4+,
CD8+ and CD4+/CD8+ in both groups
were significantly decreased (P<0.05), and in the study group
were significantly higher than those in the control group
(P<0.05) (Table III and
Fig. 4).
 | Table IIIComparison of T-lymphocyte
populations in peripheral blood between the two groups (mean ±
SD). |
Table III
Comparison of T-lymphocyte
populations in peripheral blood between the two groups (mean ±
SD).
| Group | Study group
(n=68) | Control group
(n=64) | t value | P-value |
|---|
| CD3+
(%) |
|
Before
treatment | 63.13±4.65 | 63.07±4.75 | 0.073 | 0.942 |
|
After
treatment (24 h) | 57.76±4.66 | 54.65±4.82 | 3.769 | <0.001 |
|
t value | 6.727 | 9.954 | - | - |
|
P-value | <0.001 | <0.001 | - | - |
| CD4+
(%) |
|
Before
treatment | 36.53±4.42 | 35.67±4.31 | 1.131 | 0.260 |
|
After
treatment (24 h) | 34.02±4.15 | 31.16±4.23 | 3.920 | <0.001 |
|
t value | 3.414 | 5.975 | - | - |
|
P-value | <0.001 | <0.001 | - | - |
| CD8+
(%) |
|
Before
treatment | 25.61±3.32 | 26.39±3.15 | 1.383 | 0.168 |
|
After
treatment (24 h) | 24.37±3.17 | 23.16±3.08 | 2.222 | 0.028 |
|
t value | 2.228 | 5.951 | - | - |
|
P-value | 0.028 | <0.001 | - | - |
|
CD4+/CD8+ |
|
Before
treatment | 1.51±0.23 | 1.45±0.23 | 1.498 | 0.137 |
|
After
treatment (24 h) | 1.34±0.18 | 1.25±0.28 | 2.210 | 0.029 |
|
t value | 4.800 | 4.416 | - | - |
|
P-value | <0.001 | <0.001 | - | - |
Symptom disappearance time and
hospital stay in the two groups
The symptom disappearance time and hospital stay in
the study group were significantly lower than those in the control
group (P<0.001) (Table IV).
 | Table IVSymptom disappearance time and
hospital stay in the two groups (mean ± SD). |
Table IV
Symptom disappearance time and
hospital stay in the two groups (mean ± SD).
| Group | n | Symptom
disappearance time (days) | Hospital stay
(days) |
|---|
| Study group | 68 | 1.6±1.3 | 12.7±1.2 |
| Control group | 64 | 3.5±3.2 | 14.6±1.4 |
| t value | - | 4.517 | 8.387 |
| P-value | - | <0.001 | <0.001 |
Discussion
ERCP is an important method for the treatment of
biliary and pancreatic diseases that not only cures the disease,
but also relieves the pain of patients caused by surgical trauma to
the greatest extent (17). However,
even with the continuous development of ERCP technology, patients
still suffer from PEP, PEH and other complications (18).
Previous studies on octreotide in the prevention of
PEP are numerous. For example, Thomopoulos et al (19) have pointed out that octreotide may
reduce the incidence of PEP. Moreover, in a multi-center randomized
controlled trial by Bai et al (20), prophylactic use of somatostatin
(octreotide) was shown to decrease the incidence of PEP. However,
Binmoeller et al (21)
showed that octreotide may have no preventive effect on PEP. The
development of PEP is closely related to the increase of gastric
acid. Abnormally increased gastric acid enters the inner wall of
small intestine and duodenum, resulting in high pressure in
pancreatic duct, leading to edema and necrosis of pancreas
(22). Therefore, inhibiting
gastric acid secretion may also be the key to prevent PEP (23). Lansoprazole is a PPI, another
clinical drug to prevent PEP, that is widely used in the treatment
of peptic ulcer and other acid-related gastrointestinal diseases by
reducing gastric acid secretion (24). Up to our knowledge, there has been
no previous report on the prevention and treatment of PEP by
octreotide combined with lansoprazole. The results of the present
study revealed that the AMS levels in the study group were
significantly lower than those in the control group at 6 and 24 h
after ERCP, and the incidence of PEP and PEH, symptom disappearance
time and hospital stay in the study group were significantly lower
than those in the control group. These indicate that octreotide
combined with lansoprazole reduces AMS levels and the incidence of
PEP, and accelerates patient recovery. The study of Yoo et
al (25) showed that PPIs have
no effect on the clinical progress of acute pancreatitis. In
addition, in the study of Alhazzani et al (26), PPIs and histamine 2 receptor
antagonists were reported to prevent stress ulcer in critically ill
patients. This suggests that lansoprazole inhibits gastric acid
secretion, leading to the reduction of pancreatin and pancreatic
secretion (27), thus decreasing
the incidence of PEP. Therefore, octreotide combined with
lansoprazole plays a preventive role in PEP.
The release and activation of inflammatory cytokines
are not only a significant cause of pancreatitis, but also an
important factor of pancreatic tissue necrosis and organ
dysfunction (28). Pancreatitis
increases the release of some inflammatory cytokines and leads to
the over-release of IL-17, TNF-α and other pro-inflammatory
cytokines (29). Previous studies
have shown that IL-17 and TNF-α are overexpressed in the early
stages of pancreatitis, and their levels are closely related to the
severity of the disease (27,30).
In the present study, the levels of serum IL-17 and TNF-α in the
study and the control groups after treatment were significantly
higher than those before treatment, and the levels in the study
group were significantly lower than those in the control group
after treatment. These results indicate that inhibition of
inflammatory cytokines may be one of the mechanisms of octreotide
combined with lansoprazole in preventing and treating PEP. In the
study of Wang et al (31),
the levels of AMS and inflammatory cytokines TNF-α, IL-6 and IL-8
in serum were significantly increased after ERCP. However, compared
with octreotide monotherapy, the above levels in patients treated
with octreotide combined with indomethacin were significantly
decreased. Hackert et al (32) revealed that PPIs are
anti-inflammatory and can reduce the progression of pancreatitis,
inflammation and the expression of adhesion proteins. Therefore,
inhibition of inflammatory factors may be one of its therapeutic
mechanisms.
Moreover, there is an imbalance in the immune
function of patients with pancreatitis, and the disorder of immune
function can further induce macrophage activation and
pro-inflammatory response in the early stage of pancreatitis
(33). T-lymphocyte subsets are
vital effector cells reflecting the immune function of the body and
changes in their percentages often reflect changes in the immune
function of the patients (34).
T-lymphocyte subsets are separated into CD3+,
CD4+ and CD8+ cells according to the
difference of surface CD molecules. In the present study, the
percentages of CD3+, CD4+, CD8+
cells and the CD4+/CD8+ ratio in the two
groups after treatment were significantly lower than those before
treatment, and in the study group were significantly higher than
those in the control group. These results suggest that octreotide
combined with lansoprazole improved the disordered immune function
induced by ERCP. Vaidya et al (35) reported that octreotide has no effect
on T-lymphocyte subsets in patients with thyroid-associated
ophthalmopathy, whereas the research of Larussa et al
(36) confirmed that lansoprazole
regulates Th1/Th2 immune response of human gastric mucosa, thus
improving the clinical symptoms of gastritis patients. Because
lansoprazole inhibits inflammatory cytokines and reduces their
release, it has a protective effect on the immune function.
However, the mechanism need to be further investigated.
The present study confirmed that octreotide combined
with lansoprazole has preventive and therapeutic effects on PEP and
can improve the inflammatory factors and immune function of
patients. However, there are still several limitations. For
example, no randomized control trials were performed, leading to
biases in our results. Besides, only changes in IL-17 and TNF-α
were measured. In addition, lansoprazole has been reported to
induce side effects, such as hypomagnesemia (37), which was not mentioned in our study.
These limitations will be addressed in future studies to
corroborate the conclusions of the present study.
In conclusion, octreotide combined with lansoprazole
reduces serum AMS levels and the incidence of PEP, and also
alleviates inflammation while improving the patients' immune
function.
Acknowledgements
Not applicable.
Funding
The study is part of the Project ‘Clinical Study of
Indomethacin Combined with Pancreatic Duct Stent in the Prevention
of Hyperamylasemia’ (no. 2017B70034), supported by the Shaoxing
Science and Technology Bureau, Shaoxing People's Hospital and the
Shaoxing Health and Family Planning Commission.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZC, HF, JF and JY conceived and designed the study.
ZC, XZ, BC and TD were responsible for the data acquisition and
analysis. HF, JF and XZ were responsible for the interpretation of
the data and the drafting of the manuscript. ZC and HF revised the
manuscript critically for important intellectual content. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Shaoxing People's Hospital (Shaoxing, China) (1536-40-16). Signed
written informed consents were obtained from the patients and/or
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Parikh MP, Wadhwa V, Thota PN, Lopez R and
Sanaka MR: Outcomes associated with timing of ERCP in acute
cholangitis secondary to choledocholithiasis. J Clin Gastroenterol.
52:e97–e102. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Park JK, Woo YS, Noh DH, Yang JI, Bae SY,
Yun HS, Lee JK, Lee KT and Lee KH: Efficacy of EUS-guided and
ERCP-guided biliary drainage for malignant biliary obstruction:
Prospective randomized controlled study. Gastrointest Endosc.
88:277–282. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kochar B, Akshintala VS, Afghani E,
Elmunzer BJ, Kim KJ, Lennon AM, Khashab MA, Kalloo AN and Singh VK:
Incidence, severity, and mortality of post-ERCP pancreatitis: A
systematic review by using randomized, controlled trials.
Gastrointest Endosc. 81:143–149.e9. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
ASGE Standards of Practice Committee.
Chandrasekhara V, Khashab MA, Muthusamy VR, Acosta RD, Agrawal D,
Bruining DH, Eloubeidi MA, Fanelli RD, Faulx AL, et al: Adverse
events associated with ERCP. Gastrointest Endosc. 85:32–47.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ito K, Fujita N, Noda Y, Kobayashi G,
Horaguchi J, Takasawa O and Obana T: Relationship between post-ERCP
pancreatitis and the change of serum amylase level after the
procedure. World J Gastroenterol. 13:3855–3860. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dumonceau JM, Andriulli A, Elmunzer BJ,
Mariani A, Meister T, Deviere J, Marek T, Baron TH, Hassan C,
Testoni PA, et al: Prophylaxis of post-ERCP pancreatitis: European
society of gastrointestinal endoscopy (ESGE) guideline-updated June
2014. Endoscopy. 46:799–815. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hou YC, Hu Q, Huang J, Fang JY and Xiong
H: Efficacy and safety of rectal nonsteroidal anti-inflammatory
drugs for prophylaxis against post-ERCP pancreatitis: A systematic
review and meta-analysis. Sci Rep. 7(46650)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dumonceau JM, Kapral C, Aabakken L,
Papanikolaou IS, Tringali A, Vanbiervliet G, Beyna T, Dinis-Ribeiro
M, Hritz I, Mariani A, et al: ERCP-related adverse events: European
society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy.
52:127–149. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lamberts SWJ and Hofland L: ANNIVERSARY
REVIEW: Octreotide, 40 years later. Eur J Endocrinol.
181:R173–R183. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li ZS, Pan X, Zhang WJ, Gong B, Zhi FC,
Guo XG, Li PM, Fan ZN, Sun WS, Shen YZ, et al: Effect of octreotide
administration in the prophylaxis of post-ERCP pancreatitis and
hyperamylasemia: A multicenter, placebo-controlled, randomized
clinical trial. Am J Gastroenterol. 102:46–51. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Morgan D, Pandolfino J, Katz PO, Goldstein
JL, Barker PN and Illueca M: Clinical trial: Gastric acid
suppression in Hispanic adults with symptomatic gastro-oesophageal
reflux disease-comparator study of esomeprazole, lansoprazole and
pantoprazole. Aliment Pharmacol Ther. 32:200–208. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Grieco FA, Moore F, Vigneron F, Santin I,
Villate O, Marselli L, Rondas D, Korf H, Overbergh L, Dotta F, et
al: IL-17A increases the expression of proinflammatory chemokines
in human pancreatic islets. Diabetologia. 57:502–511.
2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Paajanens H, Laato M, Jaakkola M, Pulkki
K, Niinikoski J and Nordback I: Serum tumour necrosis factor
compared with C-reactive protein in the early assessment of
severity of acute pancreatitis. Br J Surg. 82:271–273.
1995.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Watanabe T, Kudo M and Strober W:
Immunopathogenesis of pancreatitis. Mucosal Immunol. 10:283–298.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ilone S and Fauzi A: Diagnostic and
prevention approach in post endoscopic retrograde
cholangiopancreatography pancreatitis. Indones J Gastroenterol
Hepatol Dig Endosc. 17:188–193. 2017.
|
|
16
|
Yang R, Masters AR, Fortner KA, Champagne
DP, Yanguas-Casás N, Silberger DJ, Weaver CT, Haynes L and Rincon
M: IL-6 promotes the differentiation of a subset of naive
CD8+ T cells into IL-21-producing B helper
CD8+ T cells. J Exp Med. 213:2281–2291. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kurihara T, Yasuda I, Isayama H,
Tsuyuguchi T, Yamaguchi T, Kawabe K, Okabe Y, Hanada K, Hayashi T,
Ohtsuka T, et al: Diagnostic and therapeutic single-operator
cholangiopancreatoscopy in biliopancreatic diseases: Prospective
multicenter study in Japan. World J Gastroenterol. 22:1891–1901.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Elmunzer BJ, Serrano J, Chak A,
Edmundowicz SA, Papachristou GI, Scheiman JM, Singh VK,
Varadurajulu S, Vargo JJ, Willingham FF, et al: Rectal indomethacin
alone versus indomethacin and prophylactic pancreatic stent
placement for preventing pancreatitis after ERCP: Study protocol
for a randomized controlled trial. Trials. 17(120)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Thomopoulos KC, Pagoni NA, Vagenas KA,
Margaritis VG, Theocharis GI and Nikolopoulou VN: Twenty-four hour
prophylaxis with increased dosage of octreotide reduces the
incidence of post-ERCP pancreatitis. Gastrointest Endosc.
64:726–731. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bai Y, Ren X, Zhang XF, Lv NH, Guo XG, Wan
XJ, Nie ZG, Han ST, Bie P, Tian DA, et al: Prophylactic
somatostatin can reduce incidence of post-ERCP pancreatitis:
Multicenter randomized controlled trial. Endoscopy. 47:415–420.
2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Binmoeller KF, Harris AG, Dumas R,
Grimaldi C and Delmont JP: Does the somatostatin analogue
octreotide protect against ERCP induced pancreatitis? Gut.
33:1129–1133. 1992.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Saunders JH, Cargill JM and Wormsley KG:
Gastric secretion of acid in patients with pancreatic disease.
Digestion. 17:365–369. 1978.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kahl S and Malfertheiner P: Exocrine and
endocrine pancreatic insufficiency after pancreatic surgery. Best
Pract Res Clin Gastroenterol. 18:947–955. 2004.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li Z, Wu C, Li L, Wang Z, Xie H, He X and
Feng J: Effect of long-term proton pump inhibitor administration on
gastric mucosal atrophy: A meta-analysis. Saudi J Gastroenterol.
23:222–228. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yoo JH, Kwon CI, Yoo KH, Yoon H, Kim WH,
Ko KH, Hong SP and Park PW: Effect of proton pump inhibitor in
patients with acute pancreatitis-pilot study. Korean J
Gastroenterol. 60:362–367. 2012.PubMed/NCBI View Article : Google Scholar : (In Korean).
|
|
26
|
Alhazzani W, Alenezi F, Jaeschke RZ,
Moayyedi P and Cook DJ: Proton pump inhibitors versus histamine 2
receptor antagonists for stress ulcer prophylaxis in critically ill
patients: A systematic review and meta-analysis. Crit Care Med.
41:693–705. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Staubli SM, Oertli D and Nebiker CA:
Laboratory markers predicting severity of acute pancreatitis. Crit
Rev Clin Lab Sci. 52:273–283. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yang R, Tenhunen J and Tonnessen TI: HMGB1
and histones play a significant role in inducing systemic
inflammation and multiple organ dysfunctions in severe acute
pancreatitis. Int J Inflam. 2017(1817564)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Dawar FU, Xiong Y, Khattak MNK, Li J, Lin
L and Mei J: Potential role of cyclophilin A in regulating cytokine
secretion. J Leukoc Biol. 102:989–992. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dai SR, Li Z and Zhang JB: Serum
interleukin 17 as an early prognostic biomarker of severe acute
pancreatitis receiving continuous blood purification. Int J Artif
Organs. 38:192–198. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang J, Shen Y, Zhong Z, Wu S and Zheng L:
Risk factors for post-endoscopic retrograde
cholangiopancreatography (ERCP) pancreatitis and the effect of
octreotide combined with nonsteroidal anti-inflammatory drugs on
preventing its occurrence. Med Sci Monit. 24:8964–8969.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hackert T, Tudor S, Felix K, Dovshanskiy
D, Hartwig W, Simon WA and Werner J: Effects of pantoprazole in
experimental acute pancreatitis. Life Sci. 87:551–557.
2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Uehara S, Gothoh K, Handa H, Tomita H and
Tomita Y: Immune function in patients with acute pancreatitis. J
Gastroenterol Hepatol. 18:363–370. 2003.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhang T, Fan Y, Liu K and Wang Y: Effects
of different general anaesthetic techniques on immune responses in
patients undergoing surgery for tongue cancer. Anaesth Intensive
Care. 42:220–227. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vaidya B, Shenton BK, Stamp S, Miller M,
Baister E, Andrews CD, Dickinson AJ, Perros P and Kendall-Taylor P:
Analysis of peripheral blood T-cell subsets in active
thyroid-associated ophthalmopathy: Absence of effect of
octreotide-LAR on T-cell subsets in patients with
thyroid-associated ophthalmopathy. Thyroid. 15:1073–1078.
2005.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Larussa T, Suraci E, Leone I, Nazionale I,
Abenavoli L, Galasso O, Amorosi A, Imeneo M and Luzza F: Short-term
therapy with celecoxib and lansoprazole modulates Th1/Th2 immune
response in human gastric mucosa. Helicobacter. 15:449–459.
2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chrysant SG and Chrysant GS: Adverse
cardiovascular and blood pressure effects of drug-induced
hypomagnesemia. Expert Opin Drug Saf. 19:59–67. 2020.PubMed/NCBI View Article : Google Scholar
|