Introduction
Hepatitis A and E are common acute, self-limited
viral infections produced by hepatitis A virus (HAV) and hepatitis
E virus (HEV), respectively, generally acquired by the fecal-oral
route, via either person-to-person contact or ingestion of
contaminated food or water (1,2). A
rare modality of transmission is blood transfusion, in the case of
contaminated blood or when the donor is in the viremic prodromal
phase of the infection at the time of blood donation which is more
frequently encountered in the transmission of hepatitis B or C
(3). HAV infection affects 120
million people annually worldwide, especially children (4). By contrast, World Health Organization
(WHO) estimated that HEV causes 20 million new infections annually
and over 55,000 deaths (5).
Identified for the first time in 1983, in sporadic cases of
infections in low-income countries from Asia and Africa, the number
of cases of HEV infections has continuously increased over the last
15 years, especially in developed countries (6). Currently, HEV infection represents a
worldwide public health issue (7).
Netherlands reported a 5-fold increased incidence of HEV infections
in 2014 compared to previous years, while the number of cases in
Germany went up 40-fold in the last 10 years (8,9).
In both HAV and HEV infections, the levels of
endemicity are strongly correlated with hygienic and sanitary
conditions of each geographic area and are divided into areas with
high, intermediate or low endemicity as defined by results of
age-seroprevalence surveys (10,11). A
special interest is given to middle-income countries with varied
epidemiological profiles but are an important factor in the spread
of fecal-oral transmitted infections through international economic
and social globalization (12,13).
This process of socio-economic interdependence may lead to
significant changes in the epidemiological patterns of infections.
Located in southeastern Europe, Romania is part of this
globalization process due to a growing number of foreign visitors
or international food trade. Romania has an intermediate endemicity
of HAV infection (14). On the
other hand, the seroprevalence of HEV infection in Romania is still
unknown. Based on studies of seroprevalence of IgG HEV antibodies,
an incidence of 12% was noted in 2009. Further studies revealed an
increased number of cases in Romania by 14% among medical staff and
up to 28% among the general population (6,14).
In this context, the aim of the present study was to
evaluate the most recent epidemiological, clinical, biological and
therapeutic data concerning HAV and HEV infections based on
clinical practice at ‘Sf. Parascheva’ Clinic Hospital of Infectious
Diseases (Iasi, Romania).
Patients and methods
Patients
This was a retrospective analysis of hospital-based
medical records of patients with a diagnosis of hepatitis A and
hepatitis E viral infections hospitalized between 2018 and 2019 at
‘Sf. Parascheva’ Clinic Hospital of Infectious Diseases from Iaşi.
The inclusion criteria for the study consist of in-patients with a
diagnosis of HAV and HEV infections confirmed by blood tests and
serological tests. Patients with hepatic disease (other than HAV or
HEV) were excluded from the study.
Data collection
The following data were collected: Demographic data,
medical and medication history, clinical data, blood and urine
tests, serological tests, treatment administered and outcome. All
blood and urine tests were performed by the central hospital's
laboratory. The District Public Health Directorate, Iasi, Romania,
performed the serology tests (IgM HVA and IgM HVE,
respectively).
Statistical analysis
Only cases with a complete dataset were included in
the statistical analysis. Data were analyzed by descriptive
statistics, where applicable. Correlation between demographic
parameters, clinical data and outcome was performed using Pearson
test in XLSTAT version 2019 software. Kendall's Τau correlation
coefficients were calculated (11).
Statistical analysis was performed using Statistical Software for
Excel (XLSTAT) version 2019.
Results
Our analysis consisted of 272 HAV- and HEV-infected
patients with complete dataset, of which 98.9% were HAV infections
and only 1.1% were HEV infection cases. There were no recorded
cases of HAV and HEV coinfection. Patients were hospitalized at
‘Sf. Parascheva’ Clinic of Infectious Diseases in Iasi between 2018
and 2019.
Patient characteristics
A wide range of age was noted for the HAV-infected
patients (between 11 months and 47 years), but as expected, most
patients were aged 8-15 years (53% cases) (Fig. 1).
Most of the patients were male (53.9% cases) with
epidemiological context of outbreaks of HAV infections from rural
areas (87.73% cases) (Table I).
 | Table ICharacteristics of the HAV and HEV
patients (N=272). |
Table I
Characteristics of the HAV and HEV
patients (N=272).
| Characteristics | HAV infection | HEV infection |
|---|
| Mean age ± SD
(years) | 13.31±8.56 | 54.33±3.06 |
| Male/female
ratio | 145 (53.9%)/124
(46.1%) | 3 (100%)/0 (0%) |
| Residence area -
rural, n (%) | 236 (87.73%) | 2 (66.67%) |
| Symptomatology, n
(%) | 168 (62.45%) | 3 (100%) |
| Comorbidities, n
(%) | 12 (4.46%) | 2 (66.67%) |
Comorbidities were recorded in 12 cases (6.3%) of
HAV infections, the majority represented by digestive disorders
(41.66%, 5 cases). Hypertension (24.99%, 3 cases), chronic
peripheral venous insufficiency (8.33%, 1 case), chronic kidney
disease (8.33%, 1 case), urinary infection (8.33%, 1 case) and
oncological pathology (8.33%, 1 case) were also noted in this group
of patients (Fig. 2).
In regards to HEV infection, only 3 male patients
with a mean age of 54.3 years were identified in the hospital
database (Table I). None of the
patients had international travel history within the last 2 months,
but only one patient described previous contact with farm animals
and possible contaminated water.
Clinical findings
Regarding the onset symptomatology, most of the
HAV-infected patients (57.24%, 154 cases) had digestive
disturbances (abdominal pain, nausea) and/or jaundice (52.78%, 142
cases) at clinical examination. Flu-like onset was presented in 31
patients (11.52%), fatigue and neurasthenia in 17 patients (6.31%)
and pruritus was found in 7 patients (2.6%) (Fig. 3).
Regarding the HEV cases, the patients described a
typical onset of infection with digestive symptoms (nausea,
vomiting, abdominal pain) in 2 cases or with flu-like associated
with somnolence and asthenia in the third case.
Laboratory findings
In the HAV cases, biological investigations revealed
cholestasis in 59.65% of the cases. Based on the intensity of
cholestasis syndrome, it was found that most patients had the
moderate form of the disease, with a slight significant difference
between adults (67.19%) (Fig. 4)
and children (54.43% cases) (Fig.
5).
During an average hospitalization period of 12 days
(maximum period of 36 days), the evolution was favorable with a
mean decrease of 83% for alanine transaminase (ALT) and 60% for
bilirubin.
In HEV, jaundice with significant cytolysis
(transaminases increased up to 100-fold) and moderate cholestatic
syndrome were noted in all 3 cases.
HEV infection had a self-limited evolution under
hepatoprotective treatment with jaundice remission and significant
decrease in cytolysis and cholestasis in up to 16 days of
hospitalization (Fig. 6).
Extrahepatic manifestations were not identified in all 3 cases and
associated disorders (diabetes mellitus, hypertension, pre-renal
failure) were not aggravated by viral infection.
Evolution
After hospital isolation, diet, strict hygiene,
hepatoprotective treatment and isoprinosine administration (in 2.5%
of cases), over 95% of the HAV-infected patients showed a favorable
evolution, with only 5 cases of relapse in the first 3 months
post-first infection.
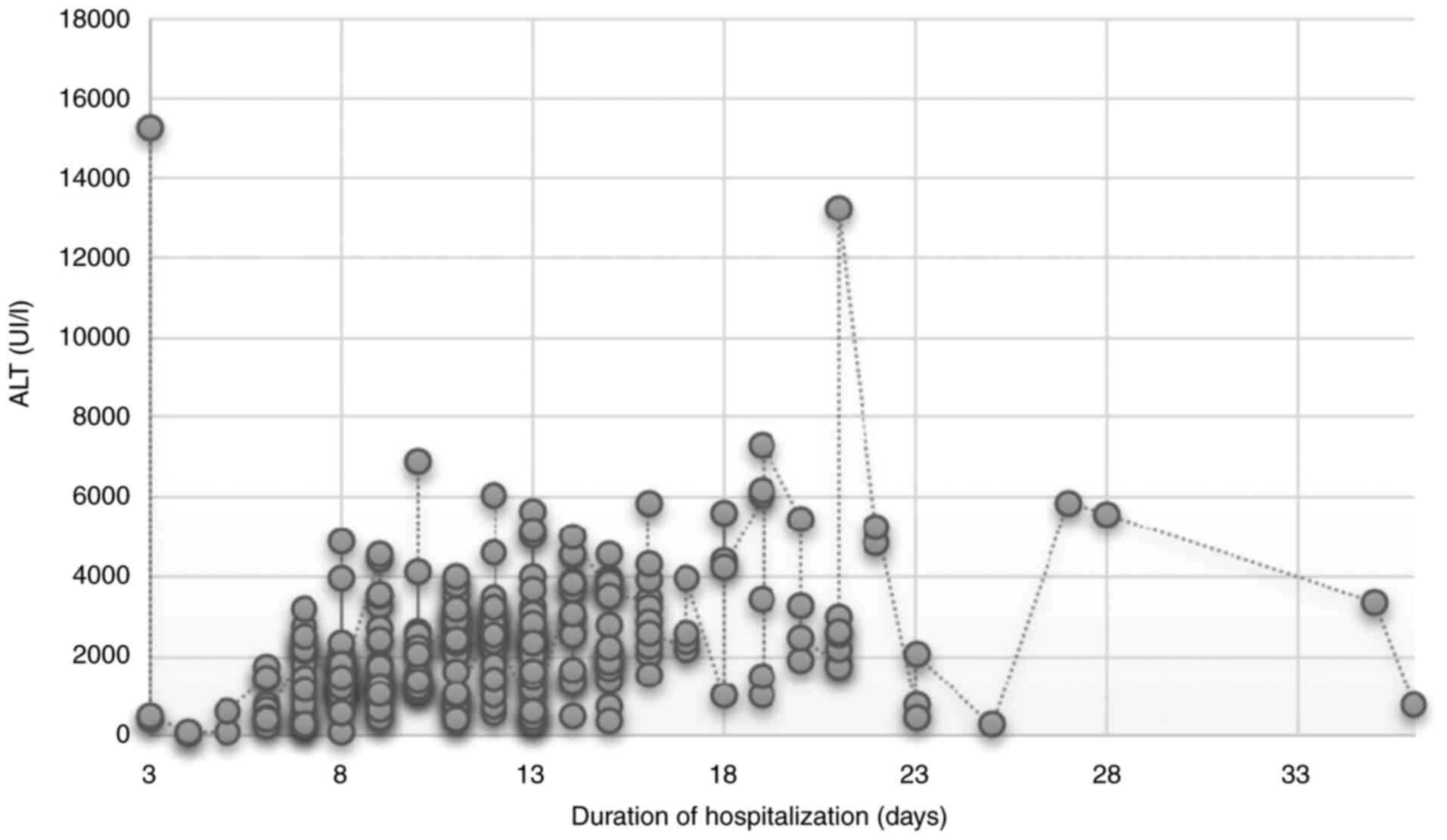
There was a weak association between duration of
hospitalization and age (Kendall's tau=0.198; Fig. 7) but a stronger correlation with
severity of liver dysfunction (Kendall's tau=0.297, Fig. 8).
The cases diagnosed with HEV, found in adult men,
had a favorable evolution, with a hospital stay of ~2 weeks.
Discussion
In this retrospective study, we evaluated the
epidemiological and clinical-biological evolution of patients with
HAV and HEV infections hospitalized at ‘Sf. Parascheva’ Clinic
Hospital of Infectious Diseases in Iaşi in northeastern Romania.
Only laboratory-confirmed cases with clinical criteria were
included. Of the total number of patients hospitalized in our
clinic during the analyzed period, 272 cases were included in our
study. The majority of these cases originated from rural areas with
poor sanitary conditions and/or bad hygienic habits. Moreover, in
some cases with HAV disease, a familial aggregation was noted.
Hepatitis A infection occurs worldwide, sporadically
or in an epidemic form. Globally, an estimated 1.4 million cases
occur each year (15). The US
reported an increase in the incidence of HAV by 294% during
2016-2018 compared to 2013-2015 and in 2017, more than 650 people
in the state of California were infected with hepatitis A
(including 417 hospitalizations and 21 deaths) (16).
International outbreaks have occurred via
importation of contaminated food from areas where HAV is endemic
(17). In some circumstances,
seemingly sporadic occurrences may reflect cases from
geographically distant outbreaks. In one report, for example, 213
cases of hepatitis A were detected in 23 schools in Michigan and 29
cases in 13 schools in Maine; all were related to contaminated
frozen strawberries from a common source (18).
The epidemiological characteristics of HAV disease
recorded in our study are in line with other Southeastern European
countries (19,20). Tsankova et al found in
Bulgaria recurrent outbreaks with time and geographic fluctuations.
Similar with our data, male gender and children aged less than 9
years are the main risk factors associated with spread of the
disease (20).
However, adult patients appear to be more frequently
affected in the last few years since we found 9% of cases in 2018
and ~15% of cases aged over 25 years. Even though we have noted
this increase in the age of HAV-infected patients during this
period, the incidence of this infection is uncommon in patients
over 50 years. When the serological tests for HAV infection in 3
male patients aged over 50 years were negative, we took into
consideration HEV infection.
In a recent study performed in northwestern Romania,
the mean age of HEV-infected patients was 50.6 years compared to
39.1 years for HAV infection (21).
The diagnosis was based on clinical and serological IgM anti-HEV
tests. We must mention that only three other cases were confirmed
in our clinic in the last 11 years and is not a frequent cause of
viral hepatitis in our country.
The cases of HEV previously reported, diagnosed and
treated in the infectious diseases clinic in Iași were not
numerous, but, nevertheless, recent studies conducted in our region
found a seroprevalence of 32.5% in the general population, which
indicates the fact that most cases are possibly related to zoonotic
transmission (22) similar to the
way of transmission of leptospirosis (23,24).
Other studies show a seroprevalence rate of IgG HEV antibodies up
to 17% in Romania, comparable to other east European countries
(e.g. 20.9% in Bulgaria, 15% in Serbia) (25).
In 2014, research on the evidence of HAE infection
in pigs and humans in the northeastern part of Romania showed a
seroprevalence of 17.14% (12/70) and 12.82% (10/78), in the tested
human sera. The authors also reported that HEV infections were
found in middle-aged adults exposed to the virus through contact
with pig farms (26,27).
The genotype 4 of HEV is the most frequently
detected in the European area and is involved especially in cases
of zoonotic transmission, including undercooked deer meat, wild
boar meat, pig liver sausage, and internal organs of animals
(28,29). Information regarding other possible
routes of transmission (e.g. blood transfusions, organ donors,
vertical transmission) are limited in our region (30).
The risk of infection is increased in travelers in
endemic areas (31). However, we
could not identify a clear epidemiological context for our
HEV-infected patients. Most patients with acute HEV are
asymptomatic or mildly symptomatic, but severe cases were noted in
middle age male or elderly patients, especially with comorbidities.
The HEV infection is often associated with extrahepatic
manifestation, including central nervous system (CNS) disorders,
acute pancreatitis, glomerulonephritis or hematological
abnormalities (32-34)
which can also cause psychological distress and may require the
intervention of a psychologist (35,36).
In conclusion, moderate cases with only hepatic
involvement were found in our hospital. The number of cases of HEV
infection identified in our hospital was extremely low compared to
HAV infections. One reason is that an HAV infection is a nationally
notifiable infectious disease that must be reported from all
medical specialists to the District Public Health directorates,
when detected. By contrast, HEV infections remain underevaluated in
humans in our region. In the meantime, studies performed in farmed
and wild animals confirm that HEV is a ubiquitously pathogen in
swine in northeastern Romania and is a potential reservoir for
HEV-associated infection.
HEV infection is a new threat to global public
health with fatal outcome in sporadic cases. Even in light of this
fact, the evaluation within Europe is still uncertain due to
limitations in surveillance systems, differences between diagnosis
tests and lack of information.
Although the seroprevalence of HEV infection is
increasing in Romania, the use of different methods for the
diagnosis of hepatitis and the increased incidence of HEV infection
among asymptomatic patients, can allow this pathology to go
undiagnosed.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published.
Authors' contributions
IFM, SL and MCL designed the study. IFM and GEL
retrieved the data concerning the HEV infections. IMH, CM and GAL
retrieved the data concerning HAV infections. CM, IAHA and AV were
responsible for the data analyses. GEL, IFM and IMH made
substantial contributions to discussion of the data. IFM, IMH and
GAL drafted the manuscript. MCL, AV, CM and IMH critically revised
the manuscript. All authors read and give final approval of the
version to be submitted for publication.
Ethics approval and consent to
participate
No ethics approval was required as the study was
performed based on medical records, retrospectively. The results of
this study do not include patient names or any personally
identifiable data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gossner CM, Severi E, Danielsson N, Hutin
Y and Coulombier D: Changing hepatitis A epidemiology in the
European union: New challenges and opportunities. Euro Surveill.
20(21101)2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pavio N, Meng XJ and Doceul V: Zoonotic
origin of hepatitis E. Curr Opin Virol. 10:34–41. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Stănculeţ N, Grigoraş A, Predescu O,
Floarea-Strat A, Luca C, Manciuc C, Dorobăţ C and Căruntu ID:
Operational scores in the diagnosis of chronic hepatitis. A
semi-quantitative assessment. Rom J Morphol Embryol. 53:81–87.
2012.PubMed/NCBI
|
|
4
|
Dalton HR, Pas SD, Madden RG and van der
Eijk AA: Hepatitis e virus: Current concepts and future
perspectives. Curr Infect Dis Rep. 16(399)2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
World Health Organization: Hepatitis E
Fact sheet (updated July 2016). http://www.who.int/mediacentre/factsheets/fs280/en/.
Accessed June 20, 2019.
|
|
6
|
Luca MC, Harja-Alexa IA, Luca S,
Leonte-Enache G, Matei A and Vata A: Hepatitis E-effect on the
liver and beyond. Ro J Infect Dis. 21:172–179. 2018.
|
|
7
|
Ditah I, Ditah F, Devaki P, Ditah C,
Kamath PS and Charlton M: Current epidemiology of hepatitis E virus
infection in the United States: Low seroprevalence in the national
health and nutrition evaluation survey. Hepatology. 60:815–822.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Aspinall EJ, Couturier E, Faber M, Said B,
Ijaz S, Tavoschi L, Takkinen J and Adlhoch C: The Country Experts:
Hepatitis E virus infection in Europe. Surveillance and descriptive
epidemiology of confirmed cases, 2005 to 2015. Euro Surveill.
22(30561)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Faber M, Askar M and Stark K: Case-control
study on risk factors for acute hepatitis E in Germany, 2012 to
2014. Euro Surveill. 23:17–00469. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mushahwar IK: Hepatitis E virus: Molecular
virology, clinical features, diagnosis, transmission, epidemiology,
and prevention. J Med Virol. 80:646–658. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gust ID: Epidemiological patterns of
hepatitis A in different parts of the world. Vaccine. 10 (Suppl
1):S56–S58. 1992.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Vâță A, Manciuc C, Dorobăț C, Vâță LG and
Luca CM: Biochemical investigations in the assessment of health
risks for over 35-year-old patients affected by environments with
hepatitis A virus. EEMJ. 17:2749–2754. 2018.
|
|
13
|
Tahaei SM, Mohebbi SR and Zali MR: Enteric
hepatitis viruses. Gastroenterol Hepatol Bed Bench. 5:7–15.
2012.PubMed/NCBI
|
|
14
|
Istrate A and Rădulescu AL: A comparison
of hepatitis E and A in a teaching hospital in Northwestern
Romania. Acute hepatitis E-a mild disease? Med Pharm Rep. 93:30–38.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
World Health Organization. Global Alert
and Response (GAR): Hepatitis A. http://www.who.int/csr/disease/hepatitis/whocdscsredc2007/en/index4.html#estimated.
Accessed April 22, 2020.
|
|
16
|
California Department of Public Health.
Hepatitis A. https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/Immunization/Hepatitis-A.aspx.
Accessed April 22, 2020.
|
|
17
|
Wheeler C, Vogt TM, Armstrong GL, Vaughan
G, Weltman A, Nainan OV, Dato V, Xia G, Waller K, Amon J, et al: An
outbreak of hepatitis A associated with green onions. N Engl J Med.
353:890–897. 2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hutin YJ, Pool V, Cramer EH, Nainan OV,
Weth J, Williams IT, Goldstein ST, Gensheimer KF, Bell BP, Shapiro
CN, et al: A multistate, foodborne outbreak of hepatitis A.
National hepatitis a investigation team. N Engl J Med. 340:595–602.
1999.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mrzljak A, Dinjar-Kujundzic P, Jemersic L,
Prpic J, Barbic L, Savic V, Stevanovic V and Vilibic-Cavlek T:
Epidemiology of hepatitis E in South-East Europe in the ‘one
health’ concept. World J Gastroenterol. 25:3168–3182.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tsankova GS, Todorova TT, Ermenlieva NM,
Popova TK and Tsankova DT: Epidemiological study of hepatitis a
infection in Eastern Bulgaria. Folia Med (Plovdiv). 59:63–69.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Teshale EH, Hu DJ and Holmberg SD: The two
faces of hepatitis E virus. Clin Infect Dis. 51:328–334.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Riveiro-Barciela M, Minguez B, Girones R,
Rodriguez-Frias F, Quer J and Buti M: Phylogenetic demonstration of
hepatitis E infection transmitted by pork meat ingestion. J Clin
Gastroenterol. 49:165–168. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Manciuc DC, Iordan IF, Adavidoaiei AM and
Largu MA: Risks of leptospirosis linked to living and working
environments. EEMJ. 17:749–753. 2018.
|
|
24
|
Manciuc C, Dorobăţ C, Hurmuzache M and
Nicu M: Leptospirosis: Clinical and environmental aspects of the
Iaşi County. EEMJ. 6:133–136. 2007.
|
|
25
|
Guerra JAAA, Kampa KC, Morsoletto DGB,
Junior AP and Ivantes CAP: Hepatitis E: A literature review. J Clin
Transl Hepatol. 5(376)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kamar N, Bendall R, Legrand-Abravanel F,
Xia NS, Ijaz S, Izopet J and Dalton HR: Hepatitis E. Lancet.
379:2477–2488. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Aniţă A, Gorgan L, Aniţă D, Oşlobanu L,
Pavio N and Savuţa G: Evidence of hepatitis E infection in swine
and humans in the East Region of Romania. Int J Infect Dis.
29:232–237. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Murrison LB and Sherman KE: The enigma of
hepatitis E virus. Gastroenterol Hepatol (NY). 13:484–491.
2017.PubMed/NCBI
|
|
29
|
Li TC, Chijiwa K, Sera N, Ishibashi T,
Etoh Y, Shinohara Y, Kurata Y, Ishida M, Sakamoto S, Takeda N and
Miyamura T: Hepatitis E virus transmission from wild boar meat.
Emerg Infect Dis. 11:1958–1960. 2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Teshale EH and Hu DJ: Hepatitis E:
Epidemiology and prevention. World J Hepatol. 3:285–291.
2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Khuroo MS, Kamili S and Jameel S: Vertical
transmission of hepatitis E virus. Lancet. 345:1025–1026.
1995.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hewitt PE, Ijaz S, Brailsford SR, Brett R,
Dicks S, Haywood B, Kennedy IT, Kitchen A, Patel P, Poh J, et al:
Hepatitis E virus in blood components: A prevalence and
transmission study in southeast England. Lancet. 384:1766–1773.
2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kim JH, Nelson KE, Panzner U, Kasture Y,
Labrique AB and Wierzba TF: A systematic review of the epidemiology
of hepatitis E virus in Africa. BMC Infect Dis.
14(308)2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Colson P, Borentain P, Queyriaux B, Kaba
M, Moal V, Gallian P, Heyries L, Raoult D and Gerolami R: Pig liver
sausage as a source of hepatitis E virus transmission to humans. J
Infect Dis. 202:825–834. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
35
|
Manciuc C and Largu A: Impact and risk of
institutionalized environments on the psyho-emotional development
of the HIV-positive youth. Environ Eng Manag J. 13:3123–3129.
2014.
|
|
36
|
Manciuc C, Filip-Ciubotaru F, Badescu A,
Duceag LD and Largu AM: The patient-doctor-psychologist triangle in
a case of severe imunosupression in the HIV infection. Rev Med Chir
Soc Med Nat Iasi. 120:119–123. 2016.PubMed/NCBI
|