Introduction
Oral lichen planus (OLP), a chronic inflammatory
disease, has the potential for malignant transformation, with a
high malignancy risk of erosive OLP (EOLP) (1). Previous studies demonstrated that
angiogenesis served a major role in chronic inflammatory diseases,
including Crohn's Disease, rheumatoid arthritis and atherosclerosis
(1-3).
Although the etiology of OLP remains unknown (4), the role of angiogenesis in the
development of OLP is of considerable concern. A previous study has
stated that angiogenesis is significantly increased in OLP compared
with normal oral mucosa (5). To the
best of our knowledge, a previous clinical study was the first to
report that anti-angiogenic therapy exerted a positive effect on
the management of OLP (6).
Therefore, an in-depth understanding of angiogenesis is needed to
provide an insight into the pathogenesis and progression of OLP,
which may provide insight for clinicians for the management of
OLP.
Previous studies have demonstrated that activated
fibroblasts, also termed myofibroblasts (MFs), are involved in the
development of neoplasms and certain inflammatory processes, such
as cardiac fibrosis and cirrhosis (7-9).
Portal myofibroblasts promote vascular remodeling underlying
cirrhosis formation by releasing microparticles (10). A previous study suggested that
OLP-associated fibroblasts acquired the characteristics of
myofibroblasts (termed OLP/MFs) (11). Immunophenotypically, myofibroblasts
are characterized by the expression of abundant pericellular matrix
proteins, including vimentin, α-smooth muscle actin (α-SMA) and
fibroblast activation protein (FAP) (11). It was hypothesized that fibroblasts
in OLP connective tissues affected by the inflammatory environment
may be involved in the molecular pathogenesis underlying OLP.
In recent years, previous studies have reported that
IL-6 produced by carcinoma-associated fibroblasts (CAFs) is
critical for tumor angiogenesis (12,13).
However, the role of OLP/MFs in angiogenesis and whether IL-6
promotes angiogenesis in OLP and the underlying mechanisms have not
yet been reported. The present study investigated the expression of
various vascular markers in OLP. Additionally, OLP/MFs were
isolated and cultured in vitro and, to the best of our
knowledge, the present study was the first to report the biological
characteristics of OLP/MFs. This strategy aimed to elucidate
whether the putative effects of IL-6 released by OLP/MFs were
critical for OLP angiogenesis.
Materials and methods
Tissue collection
Human OLP specimens from patients diagnosed with OLP
without epithelial dysplasia and normal oral squamous epithelium
tissues from patients undergoing plastic surgery were collected
from the Stomatological Hospital of Southern Medical University
(Guangzhou, China) from January 2018 to January 2019. Patients were
excluded if they had severe systemic diseases, received drug
treatment in the past 4 weeks, or suffered other oral mucosal
diseases. The OLP tissues were divided into EOLP and NEOLP groups.
The protocol was reviewed by the Institutional Ethics Committee of
this hospital [approval no. (2019)26]. Each patient signed an
informed consent form. A total of 15 normal epithelium tissues and
25 OLP samples were utilized for immunohistochemistry (IHC) and the
clinical features of the participants are presented in Table I. All surgically-resected tissues
were collected for histopathological confirmation.
 | Table IClinical features of patients. |
Table I
Clinical features of patients.
| | Sex |
|---|
| Patient group | Number | Age, years (mean ±
SD) | Female | Male |
|---|
| OLP | 25 | | 16 | 9 |
|
EOLP | 14 | 46.50±3.54 | 10 | 4 |
|
NEOLP | 11 | 46.82±2.71 | 6 | 5 |
| Normal | 15 | 43.53±3.44 | 8 | 7 |
| Total | 40 | 45.48±12.05 | 24 | 16 |
| F-value | 0.303 | | 0.560 | |
| P-value | 0.741 | | 0.576 | |
IHC analysis and microvessel density
(MVD) analysis
The tissue samples were fixed in 4% formalin at room
temperature for 24 h. Sections were deparaffinized in xylene,
rehydrated in an alcohol gradient and embed with epoxy resin. The 3
µm thick mucosal sections were processed for histology. The antigen
retrieval was performed using the wet autoclaving method in the
presence of citrate buffer pH 6.0. The sections were blocked with
1% BSA/PBS at 37˚C for 30 min. Immunostaining for vascular cell
adhesion molecule 1 (VCAM-1, also known as CD106; 1:60; cat. no.
ab134047; Abcam), intercellular adhesion molecular 1 (ICAM-1, also
known as CD54; 1:100; cat. no. ab222736; Abcam), VEGF (1:100; cat.
no. ab46154; Abcam) and CD34 (1:500; cat. no. ab81289; Abcam) was
performed using mouse monoclonal antibodies with the DakoCytomation
EnVision® system (Dako; Agilent Technologies, Inc.),
according to the manufacturer's protocols. The tissue was incubated
with the primary antibodies overnight at 4˚C. Subsequently, the
sections were incubated with anti-rabbit-HRP (1:50; cat. no.
PV-9000; ZSGB-BIO.) or anti-mouse-HRP (1:50; cat. no. PV-9000;
ZSGB-BIO.) at room temperature for 30 min. The stained tissue
samples were incubated with 10% normal goat serum at room
temperature for 30 min. The DAB REAL EnVision Detection System
(cat. no. K5007; DAKO; Agilent Technologies, Inc.) was used for
color development. Slides were counterstained with modified Harris
hematoxylin (Thermo Fisher Scientific, Inc). Excess dye solution
was then washed away and the slides were mounted using neutral gum
mounting film. Images were captured using an inverted light
microscope (magnification, x200 and x400; BX51; Olympus
Corporation). Immunoreactivity was scored as: i) 0, absent; ii) 1,
≤25% positive cells; iii) 2, 26-75% positive cells; or iv) 3,
>75% positive cells. OLP were assessed from ≥10 randomly
selected fields using a high-power microscope (magnification, x400)
and the % of positive cells was calculated. All slides were
interpreted by two investigators. Immunostaining for CD34 (1:500;
cat. no. ab81289; Abcam) was used to examine microvessels in normal
or OLP tissue sections. Statistical analysis was performed as
previously described (14).
Dual immunofluorescence staining
The tissues were fixed in 4% formalin at room
temperature for 24 h. The 3 µm thick tissue sections were
deparaffinized in xylene and rehydrated in an alcohol gradient.
These sections were blocked with 1% BSA/PBS at 37˚C for 30 min. For
dual immunofluorescence staining of tissue samples, sections were
first reacted with human α-SMA (1:500; cat. no. ab5694; Abcam) and
human IL-6 antibodies (1:500; cat. no. ab6672; Abcam) overnight at
4˚C. Subsequently, the sections were treated with donkey
anti-mouse-555 (1:300; cat. no. ab150110; Abcam) for 1 h in the
dark at room temperature. DAPI (Abcam) was applied and the slides
were examined with a fluorescence (DM4000; Leica Microsystems,
Inc.) or confocal microscope (magnification, x200 and x400; TCS
SP8; Leica Microsystems, Inc.).
Cell cultivation and associated
assays
NEOLP or EOLP lesions were isolated and cultured for
OLP-MFs. 8 Primary OLP-MFs and 6 normal fibroblasts (NFs) were
obtained according to methods described by a previous study
(12). Cultured cells at passages
3-6 were used. OLP-MFs and NFs were examined using anti-cytokeratin
(1:100; cat. no. AM06387SU-N; OriGene Technologies, Inc.),
anti-vimentin (1:200; cat. no. ab92547; Abcam), anti-α-SMA (1:100;
cat. no. ab5694; Abcam) and anti-FAP (1:100; cat. no. ab53066;
Abcam) antibodies. Growth and viability of OLP-MFs were assessed
using a Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies,
Inc.) at 24, 48 and 72 h, according to the manufacturer's protocol.
The cells were rinsed with PBS and fixed overnight in 3%
glutaraldehyde at 4˚C. Subsequently, the samples were dehydrated
using the following ethanol gradient: 30, 50, 70, 95 and 100%. The
samples were then dehydrated with xylene, air-dried at room
temperature and embed with epoxy resin. The specimens were coated
with gold. The ultrastructure of OLP-MFs and NFs was compared using
a transmission electron microscope (magnification, x1.2k) and
scanning electron microscopy (magnification, x700), respectively.
The images were processed using Adobe Photoshop CS6 (Version
13.0.1; Adobe Systems, Inc.).
Wound healing assays
Wound healing assays were used to detect the
migration of OLP-MFs and NFs at different time points (0, 24 and 48
h). Cells were grown in 24-well plates (at a seeding density of
5.0x106 cells/well) in medium (DMEM, high glucose;
Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS.
After the cells grow into a monolayer, the medium was discarded and
a sterile pipette tip was used to scratch the center of the
monolayer at the bottom of the 24-well plate, perpendicular to the
marking line. The samples were rinsed with PBS solution 3 times and
1% serum media, as aforementioned, was added. Images were captured
using an inverted light microscope (TE2000; Nikon Corporation) at
the aforementioned time points. The acquired images were used to
calculate the migration rate of cells using the image analyzing
software, ImageJ (version 1.46r; National Institutes of Health).
The % closure (% of the surface area of the migrated cells into the
defined wound area) was used to indirectly measure the cells
migration rate. Closure (%)=(migrated cell surface area/total
surface area) x100. A total of 3 replicates of the OLP-MFs and 3
NFs cell lines were selected for reverse transcription-quantitative
PCR (RT-qPCR) and western blot analysis.
RT-qPCR
Cells were grown in 24-well plates (at a seeding
density of 1.0x105 cells/well) were treated with 100
ng/ml Toll-like receptor ligand pg-lipopolysaccharide (LPS;
Sigma-Aldrich; Merck KGaA) for 1-4 or 5 days and the corresponding
supernatants were collected to evaluate the expression of IL-6. The
subgroups were as follows: NFs, NFs-LPS stimulation, OLP/MFs and
OLP/MFs-LPS. To compare the expression level of each gene, RT-qPCR
was performed. Cells (seeding density, 1x105 cells/well)
were seeded into 6-well plates. DNA was harvested from 3 OLP-MFs
and 3 NFs primary cell lines. Total RNA from each sample was
isolated using TRIzol® Universal reagent (Tiangen
Biotech Co., Ltd.), according to the manufacturer's protocol.
RT-qPCR was performed using the GoScript™ Reverse Transcription
system (A5001; Promega Corporation) and GoTaq® qPCR
Master Mix (Promega Corporation). The following thermocycling
conditions were used: Initial denaturation at 95˚C for 30 sec;
followed by 40 cycles of 95˚C for 5 sec and 60˚C for 30 sec. The
primers used to amplify α-SMA, IL-6, VEGF, VCAM-1 and ICAM-1 are
listed in Table II. β-actin was
used as the internal control. Experiments were performed in
triplicate. The 2-ΔΔCq method was used for
quantification (15).
 | Table IIPrimer sequences of the investigated
genes. |
Table II
Primer sequences of the investigated
genes.
| Gene | Primer sequences
(5'-3') |
|---|
| α-SMA | F:
GTGTTGCCCCTGAAGAGCAT |
| | R:
GCTGGGACATTGAAAGTCTCA |
| IL-6 | F:
AATCTGGATTCAATGAGGAGACTT |
| | R:
TCTGGCTTGTTCCTCACTACTCTC |
| VEGF | F:
GAGGGCAGAATCATCACGAAG |
| | R:
TGTACTCGATCTCATCAGGGTACTC |
| VCAM-1 | F:
CATAAGAAACTGGAAAAGGGAATC |
| | R:
AGGGGGTACACGCTAGGAAC |
| ICAM-1 | F:
ATGCCCAGACATCTGTGTCC |
| | R:
GGGGTCTCTATGCCCAACAA |
| β-actin | F:
CCTGGCACCCAGCACAAT |
| | R:
GCTGATCCACATCTGCTGGAA |
Protein preparation and Simple Western
analysis
A total of 3 OLP-MFs and 3 NFs were selected for
Simple Western analysis. Total protein expression of α-SMA and IL-6
were assessed using an automated capillary
electrophoresis-sized-based Simple Western system using a Wes
machine (ProteinSimple). Simple Western is a gel-free, blot-free,
capillary-based, automated protein immunodetection system that
automates all the steps following sample preparation, including
sample loading, size-based protein separation, immunoprobing,
washing, detection and data analysis (16,17).
All procedures were performed using the manufacturer's reagents and
according to protocol. Data were analyzed with Compass software
(version 4.0; ProteinSimple). Anti-α smooth muscle Actin antibody
(1:2,000; cat. no. ab5694; Abcam), anti-IL6 antibody (1:2,000; cat.
no. ab6672; Abcam) and GAPDH (clone 6C5; 1:5,000; cat. no.
MAB374-AF647; Chemicon International; Thermo Fisher Scientific,
Inc.) were used, with GAPDH being used as the loading control.
ELISA
IL-6, VEGF, VCAM-1 and ICAM-1 expression levels in
each cultured OLP-MFs and NFs were evaluated using ELISAs. NFs and
OLP-MFs cell lines were seeded into 6-well plates at a density of
4x105 cells/ml. Following overnight incubation at 37˚C,
cell lines were exposed to the conditions described in the
following section and incubated with 10% FBS in DMEM. After 12, 24,
36 or 48 h, culture supernatants were collected, centrifuged at
1,000 x g at room temperature for 20 min to pellet any detached
cells and measured using IL-6 ELISA Kit (cat. no. CSB-E04638h;
Cusabio Technology LLC), VEGF (cat. no. DVE00; R&D Systems,
Inc.), VCAM-1 (cat. no. DVC00; R&D Systems, Inc.) and ICAM-1
(cat. no. DCIM00; R&D Systems, Inc.) ELISA kits, according to
the manufacturer's protocols.
Effect of IL-6 on
angiogenesis-associated cytokine expression
Cells were treated with 1 ng/ml IL-6 (PeproTech,
Inc.), 40 mg/ml soluble Interleukin-6 receptor (sIL-6R;
Prospec-Tany TechnoGene, Ltd.) and 10 µg/ml tocilizumab (MRA;
anti-human IL-6 receptor antibody; Selleck Chemicals) for 24 h
(RT-qPCR) or 48 h (ELISA) at 37˚C. VEGF, VCAM-1 and ICAM-1 mRNA
expression levels were measured using RT-qPCR, as aforementioned.
VEGF, VCAM-1 and ICAM-1 expression levels in cell culture
supernatants were collected and measured using ELISA, as
aforementioned.
Cell migration
NFs or OLP-MFs were seeded (seeding density,
1x106 cells) in serum-free medium (DMEM/F12; Gibco;
Thermo Fisher Scientific, Inc.) into the upper chamber of 0.8 µm
Transwell inserts (Corning, Inc.). Medium (DMEM, high glucose;
Gibco, Inc.) supplemented with 10% FBS was added to the lower
chamber as the chemoattractant. Following incubation for 12 h at
37˚C, non-migrated-cells were carefully removed from the upper
membrane surface using a cotton tip and cells that passed through
the filter were fixed with 4% paraformaldehyde for 20 min and
stained with hematoxylin for 30 min at room temperature. Cells were
observed under a light microscope (x100) and images were captured
using a digital camera.
Tube formation assay
Human umbilical vein endothelial cells (HUVECs) were
purchased from Procell Life Science & Technology Co., Ltd.. A
96-well plate pre-coated with Matrigel was incubated at 37˚C for 2
h. Following this, 2x104 HUVECs/well suspended in 100 µl
conditioned medium were added with IL-6/sIL-6R and MRA by groups,
and the groups were as follow: NFs/OLP-MFs + HUVECs, NFs/OLP-MFs +
HUVECs + IL-6/sIl-6R, NFs/OLP-MFs + HUVECs + IL-6/sIl-6R + MRA.
Following incubation at 37˚C for 24 h, three fields were chosen at
random, the number of capillary-like tubes were counted, and the
number of branch points, number of branches and tube length were
measured. Images were captured using an inverted microscope (x100)
(TE2000; Nikon Corporation).
Statistical analysis
Data were analyzed using SPSS software (version
22.0; IBM Corp.). Student's t-test and one-way ANOVAs were used to
evaluate differences between sample groups when appropriate and
Fisher's least significant difference (LSD) test was used as the
post hoc test. Data are presented as the mean ± SEM. P<0.05 was
considered to indicate a statistically significant difference.
Results
VCAM-1, ICAM-1, VEGF and CD34
expression in OLP
To verify the expression of VCAM-1, ICAM-1, VEGF and
CD34 in clinical samples and fully assess their association with
the severity of OLP, 15 oral normal squamous epithelium tissues and
25 OLP tissues were examined. The results of IHC demonstrated that
the expression levels of VCAM-1, ICAM-1, VEGF and CD34 were nearly
undetectable in normal stromal cells (Fig. 1). Notably, low cytoplasmic
expression of ICAM-1 and moderate expression of VCAM-1, VEGF and
CD34 was detected in the normal tissues. Conversely,
moderate-to-intense homogeneous cytoplasmic expression was observed
in the OLP tissues and the positive staining in EOLP was
significantly higher compared with non-erosive OLP (NEOLP), as
shown in Fig. 1. Based on the
number of positive cells, the expression of angiogenesis factors in
OLP tissues was significantly higher compared with normal tissues.
The results of the immunoreactivity assay of ICAM-1, VCAM-1 and
VEGF in normal and OLP tissues is presented in Table III. MVD were observed in the
inflammatory infiltrating area and surrounding areas (Fig. 1). The statistical results are
presented in Table IV. In the
present study, the mean MVD in OLP tissues (NEOLP and EOLP) was
significantly higher compared with normal tissues (P<0.001).
Additionally, the mean MVD of the EOLP group was obviously higher
than that in the NEOLP group (P<0.001).
 | Figure 1ICAM-1, VCAM-1, VEGF and CD34
expression in OLP. In normal tissues, the levels of VCAM-1, ICAM-1
and VEGF were nearly undetectable. In normal oral squamous
epithelium, low cytoplasmic expression of ICAM-1 and moderate
expression of VCAM-1, VEGF and CD34 was detected. In OLP tissues,
there was a moderate-to-intense homogeneous cytoplasmic expression
of ICAM-1, VCAM-1, VEGF and CD34. Microvessel density analysis
demonstrated that the numbers of CD34-stained brown-colored blood
vessels increased gradually from normal tissues to NEOLP and EOLP
tissues. ICAM-1, intercellular adhesion molecular 1; VCAM-1,
vascular cell adhesion molecule 1; VEGF, vascular endothelial
growth factor; CD, cluster of differentiation; OLP, oral lichen
planus; NEOLP, non-erosive OLP; EOLP, erosive OLP. |
 | Table IIIComparison of ICAM-1, VCAM-1 and VEGF
positive cells between groups. |
Table III
Comparison of ICAM-1, VCAM-1 and VEGF
positive cells between groups.
| Group | ICAM-1 | VCAM-1 | VEGF |
|---|
| Normal | 1.99±3.67% | 2.33±4.28% | 3.20±5.40% |
| OLP | 36.96±15.59% | 29.32±6.06% | 41.56±15.99% |
| t-value | -10.74 | -15.09 | -10.99 |
| P-value | P<0.001 | P<0.001 | P<0.001 |
 | Table IVComparison of MVD between groups. |
Table IV
Comparison of MVD between groups.
| Group | MVD (mean ±
SD) | t-value | P-value |
|---|
| Normal tissues
group | 23.05±6.58 |
-7.171a |
P<0.001a |
| OLP tissues
group | 58.82±11.44 |
-4.982b |
P<0.001b |
|
NEOLP
group | 87.89±15.45 | | |
|
EOLP
group | | | |
Characteristics of fibroblasts
isolated from normal and OLP specimens
A total of 8 primary human OLP/MFs lines and 6
primary oral NFs lines were derived from oral mucosa and cultured
successfully. All cultured cells were positive for vimentin;
however, all cells were negative for cytokeratin (Fig. 2A). The expression of α-SMA is a
defining feature of MFs and CAFs (18). Immunostaining for intracellular
cytoskeletal proteins demonstrated an increased proportion of α-SMA
and FAP antibody expression in OLP-MFs. However, neither were
expressed by NFs. Transmission electron microscopy demonstrated
typical morphological structures in OLP-MFs cells, including large
spindle shapes, peripheral filaments and focal density (red arrow),
which were absent in NFs (Fig. 2B).
Moreover, scanning electron microscopy demonstrated that OLP-MFs
exhibited fusiform or polygonal shapes with slab-shaped pseudopods
(white arrow) and a pile of elastic fibers (white arrow) resembling
smooth muscles that are formed in the cytoplasm and usually
arranged in parallel to the cell axis. The biological
characteristics of OLP-MFs were evaluated by CCK-8 assays, which
revealed that the OLP-MFs exhibited a significantly higher
hyperplasia ability compared with NFs at 48 and 72 h post-culture
(Fig. 2C). Furthermore, wound
healing assays were conducted to investigate the migratory ability
of OLP-MFs. The scratch width of OLP-MFs and NFs at 24 and 48 h was
markedly narrower compared with 0 h (Fig. 2D). Additionally, at both time
points, increased numbers of OLP-MFs migrated compared with NFs.
Statistically, the rate of migration of OLP-MFs was 80%, whereas
that of NFs was 60% (Fig. 2E).
These results indicated that certain specific ultrastructures in
OLP-MFs changed and that OLP-MFs exhibited higher proliferative and
migratory abilities compared with NFs.
 | Figure 2Fibroblasts isolated from specimens.
(A) All cultured cells were positive for vimentin and negative for
cytokeratin. OLP-MFs exhibited an elevated level of α-SMA and FAP.
NFs did not express α-SMA or FAP. Magnification, x200. (B)
Transmission electron microscopy (x1.2k) demonstrated typical
morphological structures, including large spindle shapes,
peripheral filaments and focal density in OLP-MFs (red arrow).
These were absent in the NFs. Scanning electron microscopy (x700)
revealed that OLP-MFs exhibited fusiform or polygonal shapes with
slab-shaped pseudopods (white arrow) and a pile of elastic fibers
(red arrow) resembling smooth muscles that are formed in the
cytoplasm and usually arranged parallel to the cell axis, while NFs
present long and thin spindle shape. (C) Cell Counting Kit-8 assays
demonstrated that OLP-MFs had a significantly higher hyperplasia
ability compared with NFs. (D) Wound healing assays revealed that
increased numbers of OLP-MFs migrated compared with NFs.
Magnification, x40. (E) The migration rate of OLP-MFs was higher
compared with NFs. *P<0.05, **P<0.01,
***P<0.001 vs. NFs. OLP, oral lichen planus; MFs,
myofibroblasts; α-SMA, α-smooth muscle actin; FAP, fibroblast
activation protein; NFs, normal fibroblasts; OD, optical density;
ns, not significant; TEM, transmission electron microscopy; SEM,
scanning electron microscopy. |
Increased IL-6 is released from MFs
compared with NFs in vitro
Dual immunofluorescence staining of normal tissues
demonstrated that IL-6 was not expressed in the epithelium or
stroma, whereas IL-6 was notably detected in the epithelium and
stroma in OLP tissues (Fig. 3A).
Certain α-SMA-positive, non-blood vessel and spindle-shaped cells
(white arrows) were positioned beneath the squamous epithelium in
OLP tissues. Moreover, α-SMA-positive vascular endothelial cells
(yellow arrows) formed as vessels. The combined images confirmed
this result. Furthermore, regions of normal specimens were scarcely
stained for IL-6 and α-SMA was rarely expressed in the lamina
propria. Immunofluorescence staining demonstrated that most OLP-MFs
were positive for IL-6 and α-SMA, whereas NFs were weakly stained
for IL-6 and α-SMA (Fig. 3B).
 | Figure 3IL-6 is primarily released from
fibroblasts in vitro. (A) In OLP tissues, IL-6 was intensely
detected in the epithelium and stroma, and certain α-SMA positive,
non-blood vessel, spindle-shaped cells (white arrows) were
positioned beneath the squamous. In normal tissues, IL-6 was mainly
negative in the epithelium and stroma, and α-SMA-positive vascular
endothelial cells (yellow arrows) formed as vessels. Magnification,
x200. (B) The same field of view revealed that all OLP-MFs were
positive for IL-6 and α-SMA, whereas NFs were weakly stained for
IL-6 and slightly positive for α-SMA. Magnification, x400. In
OLP-MFs, the relative mRNA expression of (C) IL-6 and (D) α-SMA was
higher compared with NFs. (E) The results of Simple Western
analysis demonstrated that the expression of α-SMA (48 kDa) and
IL-6 (58 kDa) in OLP-MFs was detected. NFs exhibited weak IL-6
expression and rare expression of α-SMA protein. IL-6, interleukin
6; OLP, oral lichen planus; α-SMA, α-smooth muscle actin; MFs,
myofibroblasts; NFs, normal fibroblasts; E, epithelium; S, stroma;
bm, basement membrane. |
Moreover, gene expression and secretion of IL-6 in
NFs and OLP-MFs were evaluated (n=3). RT-qPCR reported that the
relative mRNA expression of IL-6 in OLP-MFs was 5-6 times higher
compared with NFs (Fig. 3C),
whereas that of α-SMA in OLP-MFs was 30-40-fold higher as compared
with NFs (Fig. 3D). Simple Western
analysis demonstrated 48-kDa and 58-kDa bands in OLP-MFs, which
were consistent with the size of α-SMA and IL-6, respectively
(Fig. 3E). In NFs, weak IL-6 bands
were expressed, whereas α-SMA bands were barely detected. As α-SMA
is an indicator of MFs, these results revealed that OLP-MFs were a
crucial source of IL-6.
Inflammation promotes IL-6 expression
by stromal fibroblasts
ELISA results revealed that the expression of IL-6
peaked on day 3 or 4 in the four subgroups, NFs, NFs-LPS
stimulation, OLP/MFs and OLP/MFs-LPS, and then declined (Fig. 4A). LPS is known to promote the
inflammation and secretion of inflammatory factors (5). The secretion of IL-6 by NFs was
increased following LPS stimulation, which was similar to the
expression level of OLP-MFs (Fig.
4A). These data indicated that inflammation increased the
secretion of IL-6 in fibroblasts. Irrespective of the stimulation
of OLP-MFs by LPS, the overall expression level of IL-6 was higher
compared with the NFs groups. RT-qPCR results demonstrated that the
expression of IL-6 mRNA in OLP-MFs was significantly higher
compared with NFs at the four time points (0, 12, 24 and 48 h;
Fig. 4B) and protein expression
levels revealed similar results (Fig.
4C). Therefore, RT-qPCR and Simple Western analysis results
reported that the expression of IL-6 in OLP-MFs decreased with time
and that NFs exhibited non-significant, weak IL-6 expression. Thus,
IL-6 was primarily derived from OLP-MFs and inflammatory reactions,
and was gradually secreted into the culture supernatants.
IL-6 promotes VCAM-1, ICAM-1 and VEGF
expression by fibroblasts, particularly by OLP-MFs
To further clarify whether IL-6 promoted
angiogenesis, NFs and OLP-MFs were grouped for the treatment with
IL-6/sIL-6R and MRA. The relative mRNA expression of VCAM-1, ICAM-1
and VEGF was significantly increased in OLP-MFs and NFs stimulated
by IL-6/sIL-6R, compared with the control group. However, the
expression of VCAM-1, ICAM-1 and VEGF was decreased in OLP-MFs and
NFs following treatment with 10 µg/ml MRA, compared with the
IL-6/sIL-6R treatment (Fig. 5A). In
terms of changes in overall expression levels, the relative mRNA
expression of angiogenesis-associated cytokines in the NFs group
treated with IL-6/sIL-6R and MRA was similar to the OLP/MFs group;
however, the expression of VCAM-1, ICAM-1 and VEGF in the OLP-MFs
group was still significantly higher than the NFs group, when cells
were treated with IL-6/sIL-6R (Fig.
5A). ELISA results revealed that IL-6 significantly promoted
the secretion of VCAM-1, ICAM-1 and VEGF in OLP-MFs and NFs
compared with the control group (Fig.
5B). MRA significantly decreased the secretion of
vascular-related growth factors by OLP-MFs compared with the
control group (Fig. 5B).
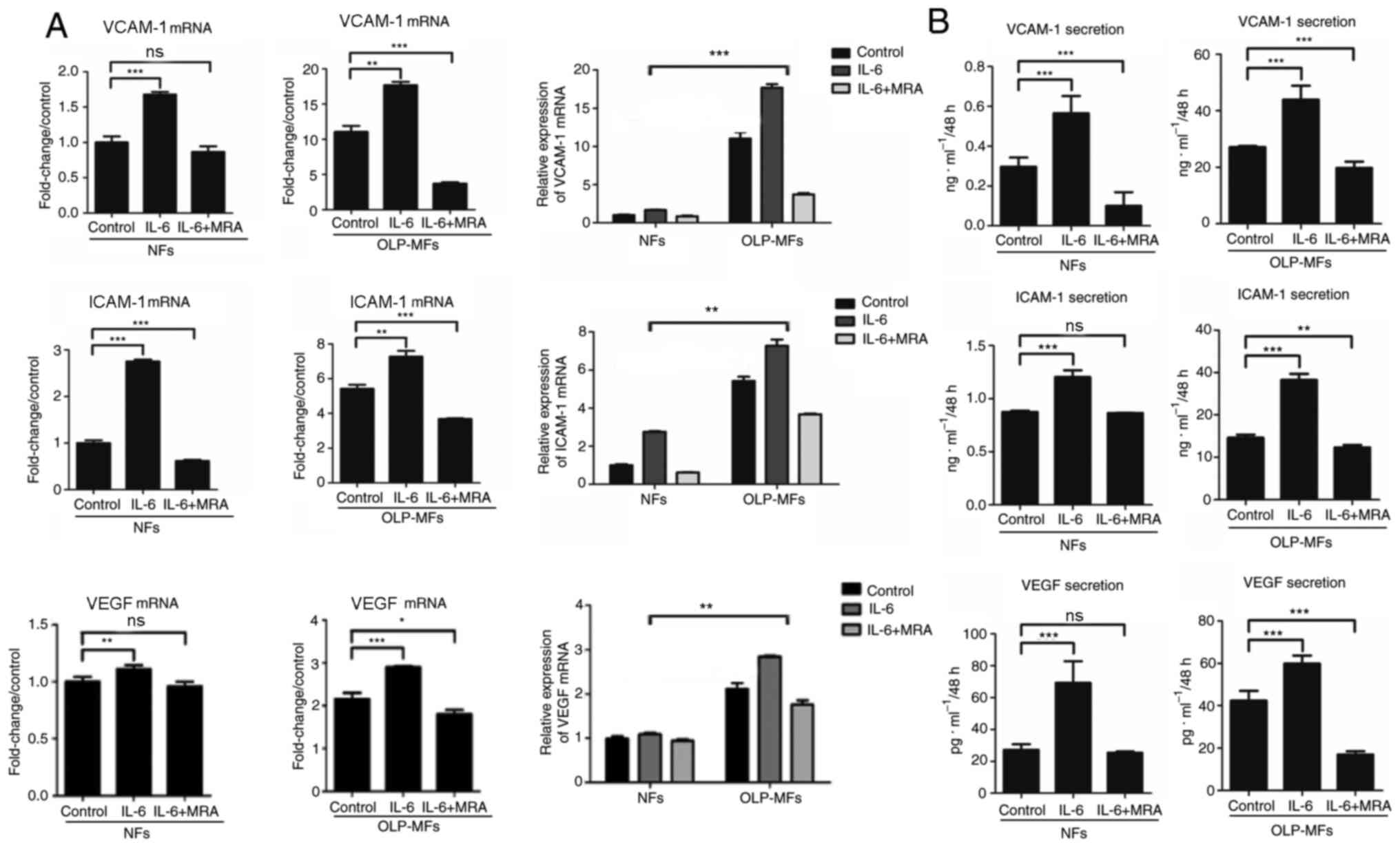 | Figure 5IL-6 promotes VCAM-1, ICAM-1 and VEGF
expression. (A) Reverse transcription-quantitative PCR and (B)
ELISA demonstrated that IL-6 significantly promoted the expression
of VCAM-1, ICAM-1, and VEGF in OLP-MFs and NFs. MRA significantly
decreased the secretion of vascular-related growth factors in the
OLP-MFs group compared with the control group. The expression of
angiogenesis-associated cytokines in the OLP-MFs group were
significantly higher compared with the NFs group. Control group,
cells without treatment; IL-6 group, cells were treated with
IL-6/sIL-6R; IL-6+MRA group, cells were treated with IL-6/sIL-6R
and MRA. *P<0.05, **P<0.01 and
***P<0.001. IL-6, interleukin 6; VCAM-1, vascular
cell adhesion molecule 1; ICAM-1, intercellular adhesion molecular
1; VEGF, vascular endothelial growth factor; OLP, oral lichen
planus; MFs, myofibroblasts; NFs, normal fibroblasts; MRA,
tocilizumab; ns, not significant. |
IL-6 signaling by OLP-MFs promotes the
angiogenic potential of HUVECs
Cell migration assays demonstrated that IL-6
promoted the migratory ability of OLP-MFs and NFs (Fig. 6A). Notably, OLP-MFs exhibited
stronger migratory ability compared with NFs. Moreover, MRA weakly
suppressed the migratory ability of OLP-MFs and NFs compared with
the control group. Statistically, the numbers of migrated OLP-MF
and NF cells were significantly increased in the
IL-6/sIL-6R-treated group compared with the control group
(P<0.01) (Fig. 6B). However,
there was no significant difference in numbers of migrated cells
following MRA treatment compared with the IL-6/siL-R-treated group
in both cell groups. Numbers of migrated OLP-MFs and NFs in the
MRA-treated group did not differ significantly with the control
group (Fig. 6C).
 | Figure 6IL-6 promotes the angiogenic
potential of HUVECs. (A) Cell migration assays demonstrated that
OLP-MFs exhibited a stronger migratory ability compared with NFs.
Magnification, x100. (B) Numbers of migrated NF and OLP-MF cells in
the IL-6/sIL-6R treated group were significantly higher compared
with the control group. (C) MRA inhibited the migratory ability of
NFs and OLP-NFs. The results of the Matrigel capillary germination
assays demonstrated that following treatment with IL-6/sIL-6R, (D)
abundant HUVECs had formed into blood vessels and the (E) numbers
of capillary branch points, (F) numbers of blood vessel branches
and (G) tube lengths formed by HUVECs were significantly higher
compared with the blank group. Magnification, x100. The number and
density of blood vessels formed by HUVECs were significantly
reduced following MRA treatment compared with the IL-6/sIL-6R
treatment group. **P<0.01 and
***P<0.001. IL-6, interleukin 6; HUVECs, human
umbilical vein endothelial cells; OLP, oral lichen planus; MFs,
myofibroblasts; NFs, normal fibroblasts; ns, not significant; MRA,
tocilizumab; sIL-6R, soluble interleukin-6 receptor. |
Matrigel capillary germination assays were used to
analyze the effects of IL-6/sIL-6 and MRA on the angiogenic
potential of HUVECs in vitro. The results demonstrated that
HUVECs formed capillary buds at 3 h (Fig. 6D). Furthermore, numerous HUVECs
formed into blood vessels post-IL-6/sIL-6R treatment and markedly
blood vessel density was measured. The formation of blood vessels
was highest at 6 h. Moreover, the number and density of blood
vessels formed by HUVECs were markedly reduced following treatment
with MRA. Numbers of capillary branch points (Fig. 6E) and blood vessel branches
(Fig. 6F), and tube lengths
(Fig. 6G) formed by
IL-6/sIL-6R-treated HUVECs were significantly higher compared with
the control group. Following the addition of MRA, the three indexes
were significantly lower compared with the IL-6/sIL-6R treatment
group.
Discussion
OLP has been regarded as a non-specific inflammatory
condition due to its pathological and micrographic features,
including band-like infiltration of superficial lymphocytes in the
lamina propria and infiltration of inflammatory cytokines (19). Angiogenesis is of considerable
concern during the development of chronic inflammatory disorders
(20-23).
Previous IHC-based studies reported a strong involvement of the
angiogenic phenomenon in the malignant transformation of
precancerous lesions, including OLP (24-26).
VEGF, ICAM-1 and VCAM-1 have been regarded as critical angiogenic
factors that directly mediate OLP angiogenesis (27,28).
Additionally, the current IHC data demonstrated the high expression
of angiogenic factors in OLP tissues according to the percentage of
stained cells. Additionally, MVD in OLP tissue increased with the
deterioration of the lesion. Previous results, together with those
from the present study, indicated that increased angiogenesis may
serve a vital role in the malignant transformation of OLP (29,30).
However, the mechanism underlying this phenomenon remains unclear.
The dynamic interplay between multiple cell types, including MFs,
immune cells, endothelial cells and adipocytes, in the
microenvironment of the lesion has gained interest as a promising
target for the treatment of inflammatory diseases (31). Previous studies have demonstrated
that MFs and NFs produce significant amounts of IL-6 in the
presence of cancer cells (32,33).
Furthermore, IL-6 stimulation significantly increased the
expression of VEGF in stroma fibroblasts. Therefore, it was
hypothesized that IL-6 released by MFs is critical for OLP
angiogenesis.
In order to verify this hypothesis, eight primary
OLP-MF lines were isolated and cultured. OLP-MFs acquired
characteristics and biological behavior of MFs, including the
expression of α-SMA and FAP markers (34). To identify these stromal cells dual
immunofluorescence staining was used and combined with observation
of the morphology of the isolated cells. Notably, OLP-MFs exhibited
a significantly high expression of IL-6 at the mRNA and protein
levels as compared with NFs. Additionally, fibroblasts produced
IL-6 under inflammatory conditions and markedly upregulated the
level of IL-6 following treatment with LPS. To the best of our
knowledge, this is the first report of the expression of IL-6 in
OLP stroma fibroblasts. Furthermore, the present study demonstrated
that IL-6 facilitated OLP angiogenesis via the induction of
angiogenesis-related proteins in OLP-MFs. The secreted angiogenic
factors from OLP-MFs subsequently induced angiogenesis. The current
results indicated that IL-6-stimulated OLP-MFs significantly
facilitated the migration of HUVECs and tube length formation.
Based on this model, it was concluded that IL-6-induced
inflammation in OLP-MFs induced the surrounding activated
fibroblasts to express VEGF, ICAM-1 and VCAM-1. Consequently,
angiogenesis was induced. In a previous study, anti-human IL-6
receptor antibodies were used to remove the blockade of the IL-6
receptor (35). MRA successfully
inhibited cell migration and tube formation. IL-6 was observed to
be one of the primary inductors of OLP angiogenesis; however, there
are still other associated mechanisms that require further
research. Therefore, OLP-MFs serve a major role in mediating
IL-6-induced OLP angiogenesis. Thus, we hypothesized that the
modulation between OLP-MFs and angiogenesis may have a primary role
in the pathological process underlying OLP.
Taken together, these data indicated that increased
angiogenesis was a common event and served a critical role in OLP
pathogenesis. OLP-MFs and NFs produced significant amounts of IL-6
in the presence of inflammatory factors. Therefore, the anti-IL-6
receptor antibodies and the target OLP-MFs may serve as novel
therapeutic agents capable of inhibiting the malignant
transformation of OLP.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant no. 81500850) and the Nonprofit
Industry Research Specific Fund of National Health and Family
Planning Commission of China (grant no. 201502018).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XHX and YL contributed to the conception and design
of the present study, performed the data acquisition and
interpretation, and drafted and critically revised the manuscript.
LF contributed to the conception, data acquisition and analysis,
and drafted and critically revised the manuscript. YSY contributed
to the data acquisition and interpretation, and drafted and
critically revised the manuscript. SGL, WG and HXZ contributed to
the data acquisition and analysis, and critically revised the
manuscript. ZQL and LZ ccontributed to the data acquisition and
critically revised the manuscript. WXM contributed to the
conception, design, data analysis and interpretation, and drafted
and critically revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was reviewed by the Institutional
Ethics Committee of the Stomatological Hospital, Southern Medical
University, Guangzhou, China [approval no. (2019)26]. Each patient
signed the informed consent form.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rode M and Kogoi-Rode M: Malignant
potential of the reticular form of oral lichen planus over a
25-year observation period in 55 patients from Slovenia. J Oral
Sci. 44:109–111. 2002.PubMed/NCBI
|
|
2
|
Brown RS, Bottomley WK, Puente E and
Lavigne GJ: A retrospective evaluation of 193 patients with oral
lichen planus. J Oral Pathol Med. 22:69–72. 1993.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lanfranchi-Tizeira HE, Aguas SC and Sano
SM: Malignant transformation of atypical oral lichen planus: A
review of 32 cases. Med Oral. 8:2–9. 2003.PubMed/NCBI(In English, Spanish).
|
|
4
|
Malekzadeh H, Robati M, Yousefimanesh H,
Ghafourian Boroujerdnia M and Nadripour R: Salivary interferon
gamma and interleukin-4 levels in patients suffering from oral
lichen planus. Cell J. 17:554–558. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Al-Hassiny A, Friedlander LT, Parachuru
VPB, Seo B, Hussaini HM and Rich AM: Upregulation of angiogenesis
in oral lichen planus. J Oral Pathol Med. 47:173–178.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mahmoud MM and Afifi MM: Anti-angiogenic
therapy (bevacizumab) in the management of oral lichen planus. Eur
J Oral Sci. 124:119–126. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Meng W, Wu Y, He X, Liu C, Gao Q, Ge L, Wu
L, Liu Y, Guo Y, Li X, et al: A systems biology approach identifies
effective tumor-stroma common targets for oral squamous cell
carcinoma. Cancer Res. 74:2306–2315. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
She G, Ren YJ, Wang Y, Hou MC, Wang HF,
Gou W, Lai BC, Lei T, Du XJ and Deng XL: KCa3.1 channels
promote cardiac fibrosis through mediating inflammation and
differentiation of monocytes into myofibroblasts in angiotensin
II-treated rats. J Am Heart Assoc. 8(e010418)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cadamuro M, Brivio S, Mertens J, Vismara
M, Moncsek A, Milani C, Fingas C, Cristina Malerba M, Nardo G,
Dall'Olmo L, et al: Platelet-derived growth factor-D enables liver
myofibroblasts to promote tumor lymphangiogenesis in
cholangiocarcinoma. J Hepatol. 70:700–709. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lemoinne S, Cadoret A, Rautou PE, El
Mourabit H, Ratziu V, Corpechot C, Rey C, Bosselut N, Barbu V,
Wendum D, et al: Portal myofibroblasts promote vascular remodeling
underlying cirrhosis formation through the release of
microparticles. Hepatology. 61:1041–1055. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang L, Yang Y, Xiong X, Yu T, Wang X,
Meng W, Wang H, Luo G and Ge L: Oral lichen-planus-associated
fibroblasts acquire myofibroblast characteristics and secrete
pro-inflammatory cytokines in response to Porphyromonas gingivalis
lipopolysaccharide stimulation. BMC Oral Health. 18(197)2019.
|
|
12
|
Karakasheva TA, Lin EW, Tang Q, Qiao E,
Waldron TJ, Soni M, Klein-Szanto AJ, Sahu V, Basu D, Ohashi S, et
al: IL-6 mediates cross-talk between tumor cells and activated
fibroblasts in the tumor microenvironment. Cancer Res.
78:4957–4970. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Mirkeshavarz M, Ganjibakhsh M, Aminishakib
P, Farzaneh P, Mahdavi N, Vakhshiteh F, Karimi A, Gohari NS, Kamali
F, Kharazifard MJ, et al: Interleukin-6 secreted by oral
cancer-associated fibroblast accelerated VEGF expression in tumor
and stroma cells. Cell Mol Biol (Noisy-le-grand). 63:131–136.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis-correlation in invasive breast
carcinoma. N Engl J Med. 324:1–8. 1991.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tabebordbar M, Zhu K, Cheng JKW, Chew WL,
Widrick JJ, Yan WX, Maesner C, Wu EY, Xiao R, Ran FA, et al: In
vivo gene editing in dystrophic mouse muscle and muscle stem cells.
Science. 351:407–411. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Schiattarella GG, Altamirano F, Tong D,
French KM, Villalobos E, Kim SY, Luo X, Jiang N, May HI, Wang ZV,
et al: Nitrosative stress drives heart failure with preserved
ejection fraction. Nature. 568:351–356. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Holm Nielsen S, Willumsen N, Leeming DJ,
Daniels SJ, Brix S, Karsdal MA, Genovese F and Nielsen MJ:
Serological assessment of activated fibroblasts by alpha-smooth
muscle actin (α-SMA): A noninvasive biomarker of activated
fibroblasts in lung disorders. Transl Oncol. 12:368–374.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Casparis S, Borm JM, Tektas S, Kamarachev
J, Locher MC, Damerau G, Grätz KW and Stadlinger B: Oral lichen
planus (OLP), oral lichenoid lesions (OLL), oral dysplasia, and
oral cancer: Retrospective analysis of clinicopathological data
from 2002-2011. Oral Maxillofac Surg. 19:149–156. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Varricchi G, Granata F, Loffredo S,
Genovese A and Marone G: Angiogenesis and lymphangiogenesis in
inflammatory skin disorders. J Am Acad Dermatol. 73:144–153.
2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Balogh E, Biniecka M, Fearon U, Veale DJ
and Szekanecz Z: Angiogenesis in inflammatory arthritis. Isr Med
Assoc J. 5:345–352. 2019.PubMed/NCBI
|
|
22
|
Shu DY and Lovicu FJ: Myofibroblast
transdifferentiation: The dark force in ocular wound healing and
fibrosis. Prog Retin Eye Res. 60:44–50. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Villalobos E, Criollo A, Schiattarella GG,
Altamirano F, French KM, May HI, Jiang N, Nguyen NUN, Romero D, Roa
JC, et al: Fibroblast primary cilia are required for cardiac
fibrosis. Circulation. 139:2342–2357. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kordbacheh F, Bhatia N and Farah CS:
Patterns of differentially expressed genes in oral mucosal lesions
visualised under autofluorescence (VELscope(™)). Oral Dis.
22:285–296. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Scardina GA, Ruggieri A, Messina P and
Maresi E: Angiogenesis of oral lichen planus: A possible
pathogenetic mechanism. Med Oral Patol Oral Cir Bucal.
14:e558–e562. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mittal N, Shankari GM and Palaskar S: Role
of angiogenesis in the pathogenesis of oral lichen planus. J Oral
Maxillofac Pathol. 16:45–48. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Seyedmajidi M, Shafaee S, Bijani A and
Bagheri S: VCAM1 and ICAM1 expression in oral lichen planus. Int J
Mol Cell Med. 2:34–40. 2013.PubMed/NCBI
|
|
28
|
Mardani M, Ghabanchi J, Fattahi MJ and
Tadbir AA: Serum level of vascular endothelial growth factor in
patients with different clinical subtypes of oral lichen planus.
Iran J Med Sci. 37:233–237. 2012.PubMed/NCBI
|
|
29
|
Scardina GA, Ruggieri A, Maresi E and
Messina P: Angiogenesis in oral lichen planus: An in vivo and
immunohistological evaluation. Arch Immunol Ther Exp (Warsz).
59:457–462. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hazzaa HH, El-Wakeel NM, Attia EA and Abo
Hager EA: ALK1 expression in oral lichen planus: A possible
relation tomicrovessel density. J Oral Pathol Med. 45:373–380.
2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liao YP, Schaue D and McBride WH:
Modification of the tumor microenvironment to enhance immunity.
Front Biosci. 12:3576–3600. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Al-Ansari MM and Aboussekhra A: ATR
suppresses the pro-tumorigenic functions of breast stromal
fibroblasts. Oncotarget. 9:34681–34690. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hendrayani SF, Al-Harbi B, Al-Ansari MM,
Silva G and Aboussekhra A: The inflammatory/cancer-related
IL-6/STAT3/NF-κB positive feedback loop includes AUF1 and maintains
the active state of breast myofibroblasts. Oncotarget.
7:41974–41985. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Meyer K, Hodwin B, Ramanujam D, Engelhardt
S and Sarikas A: Essential role for premature senescence of
myofibroblasts in myocardial fibrosis. J Am Coll Cardiol.
67:2018–2028. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nagasaki T, Hara M, Nakanishi H, Takahashi
H, Sato M and Takeyama H: Interleukin-6 released by colon
cancer-associated fibroblasts is critical for tumour angiogenesis:
Anti-interleukin-6 receptor antibody suppressed angiogenesis and
inhibited tumour-stroma interaction. Br J Cancer. 110:469–478.
2014.PubMed/NCBI View Article : Google Scholar
|




















