Introduction
It is well-known that reactive oxygen species (ROS)
serve a critical role in a number of biological processes,
including disease and aging (1).
Oxidative stress is defined as cellular damage from ROS exposure
that occurs when ROS production exceeds the capability of the
cellular antioxidant defense system (1,2). This
ultimately leads to the modification and degradation of proteins,
damage to the mitochondria and cell death (1,2).
Ionizing radiation (IR) causes damage to biological tissues by
inducing ROS production and can cause oxidative injury to cellular
macromolecules like DNA, lipids and proteins, resulting in the
impairment of organs and systems and even mortality (3,4). IR
can arise from occupational exposure, medical procedures or
exposure to nuclear explosion (5-7).
The lens is an avascular, encapsulated and
transparent tissue containing organelle-free, terminally
differentiated fiber cells at the center, where a single layer of
epithelial cells covers the anterior surface of the organ (8). The lens is one of the most
radiosensitive tissues in the body, where the dividing epithelial
cells near the equator serve a critical role in IR-induced cataract
(9). ROS-induced by IR can cause
oxidative damage to proteins, resulting in protein aggregation and
cataract, which is the leading cause of blindness worldwide
(1). However, the lens has evolved
several antioxidant systems to defend against ROS damage, including
ROS scavenger systems and enzyme protective systems (1). Several antioxidant enzymes, such as
superoxide dismutase (SOD) and catalase, are regulated by the key
transcription factor, nuclear factor erythroid 2-related factor 2
(Nrf2) (10). Nrf2 is normally
sequestered by the Kelch-like ECH-associated protein 1 (Keap1)
protein in the cytoplasm, which serves as a master regulator of the
antioxidant response element (ARE)-driven cellular defense system
against oxidative stress (10).
Upon activation by ROS, the Nrf2-Keap1 complex is disrupted and the
free Nrf2 subsequently translocates into the nucleus and binds to
ARE, which in turn activates the expression of downstream
antioxidant and detoxification genes to combat ROS and boost cell
survival (11). Previous studies
have reported that the onset of cataract as a result of IR may be
associated with compromised antioxidant capacity in the lens
(12,13). However, the effects of varying IR
doses on the Nrf2-regulated antioxidant defense systems of lens and
underlying molecular mechanisms remain poorly understood.
In present study, animal models were used to
simulate ocular injury caused by space radiation experienced by
astronauts whilst participating in missions on the International
Space Station (ISS). For an ISS-type orbit, estimates of neutron
contribution to an astronaut's total radiation dose range is 30-60%
(14). The quality factor for the
neutron tends to be 4-5X greater compared with that in the charged
particles from space radiation (15). Therefore, the impact of neutron
radiation on ocular injuries in space travel serves an important
role. Furthermore, little has been investigated regarding neutron
radiation on the lens. Therefore, the present study chose neutrons
as the radiation source, which aimed to investigate the effects of
different doses of neutron radiation on the status of the Nrf2
antioxidant defense system and severity of oxidative stress in rat
lenses in vivo. Results from the present study hopes to
provide a deeper understanding of the effects of neutron radiation
on the lens and the role of Nrf2 in the regulation of the
antioxidant defense systems following radiation, which is
indispensable for the prevention and treatment of IR-induced
cataract. In addition, it is hoped that data from the present study
facilitate the development of management strategies for even other
oxidative stress-associated diseases and the field of radiation
protection.
Materials and methods
Animals and neutron radiation
A total of 24 male Sprague-Dawley rats (age, 6
weeks; weight, 200±8 g) were purchased from the Central Animal
House of Qinglongshan Institute (Nanjing, China). All procedures
involving animals and corresponding experimental protocols were
approved by The Ethics Committee of Jinling Hospital (approval no.
2020JLHGKJDWLS-109; Nanjing, China). Animals were allowed free
access to water and normal pellet diet and were housed in
polypropylene cages bedded with sterilized rice husk under 12-h
light/dark cycles with a temperature of 24±1˚C and humidity of
50±10%. The rats were randomly divided into the following four
groups (n=6 rats in each group): i) Control group; ii) 0.4 Sv
group; iii) 1.2 Sv group; and iv) 3.6 Sv group.
Neutron radiations were performed at the
Radiological Research Center of Nanjing University of Aeronautics
and Astronautics (Nanjing, China). Neutrons were generated by the
reaction of deuterium on a tritium target (16), and rats were anesthetized
(intraperitoneal injection of 90 mg/kg ketamine and xylazine 10
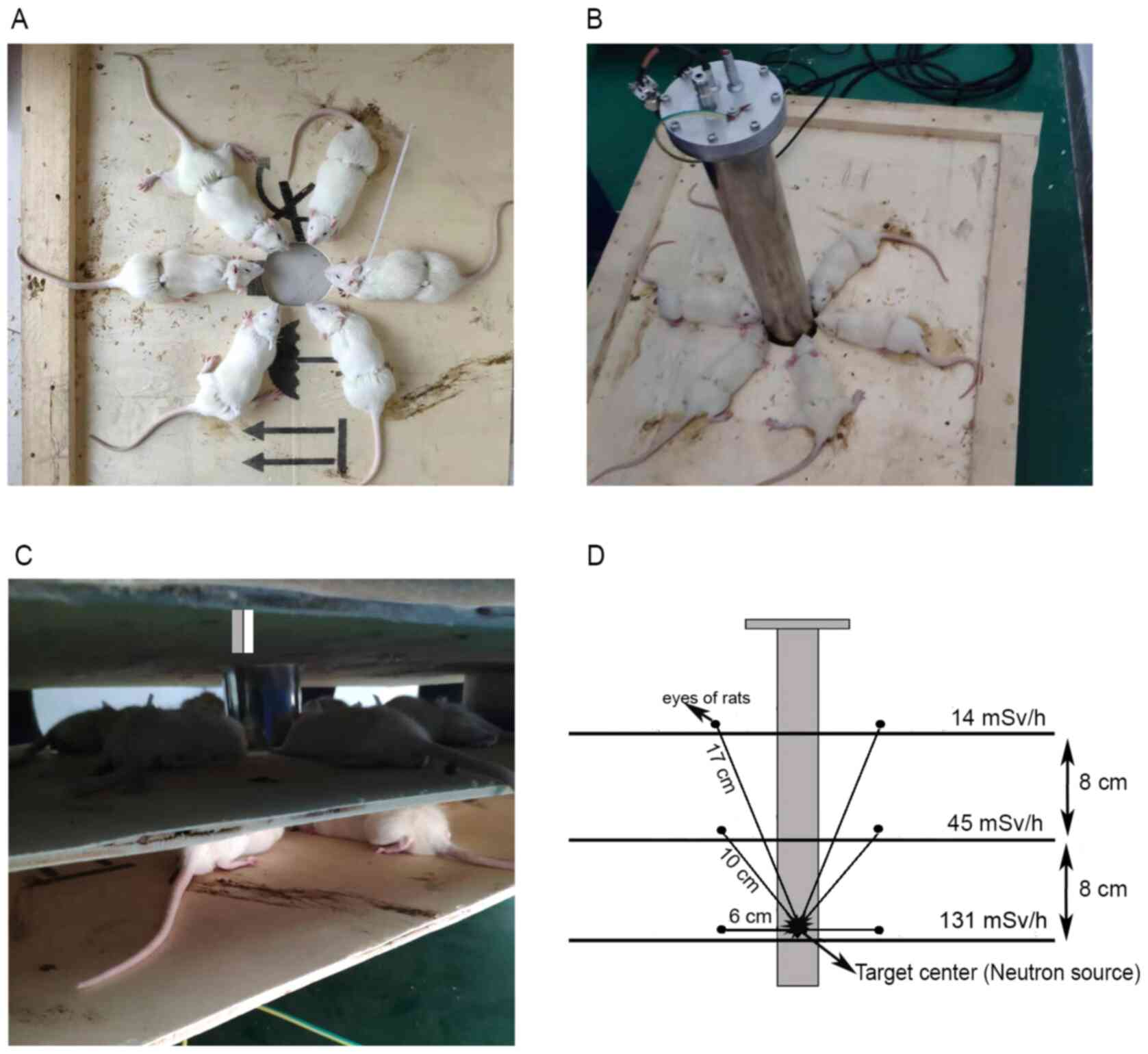
mg/kg) and immobilized by jigs surrounding the neutron generation
device (Fig. 1A-C) (17). Rats in the control group were sham
irradiated, that is, rats were immobilized like the other 3 groups
but were irradiated by 0 Sv. The eyes of the rats the other three
groups were 17, 10 and 6 cm from the target center (Fig. 1D), where the dose rates were 14, 45
and 131 mSv/h, respectively. Radiation was delivered in four
fractions in 4 successive days (once per day) and the total doses
were 0.4, 1.2 and 3.6 Sv, respectively. Sham-radiated control
animals were treated similarly to radiated animals but the
radiation source was not activated. The rats were anesthetized with
an intraperitoneal injection of pentobarbital sodium (30 mg/kg body
weight) and subsequently sacrificed by cervical dislocation 7 days
after the final radiation. Their eyes were then enucleated, where
their lenses were collected for histopathological, biochemical and
western blot analyses.
Histological analysis
The eyeballs were fixed in 10% formalin for 24 h at
room temperature and dehydrated using a gradient alcohol series
(70, 80, 90, 95 and 100% ethanol) followed by two xylene
treatments. The samples were embedded in paraffin and then cut into
5-µm-thick sections. Tissue sections were subsequently
deparaffinized in xylene and then rehydrated with a gradient
alcohol series (100, 95, 80 and 75% ethanol) and washed in PBS. The
sections were then stained with hematoxylin for 7 min and eosin for
3 min at room temperature. For histopathological analysis, the
slides were observed under a light microscope (magnification, x400;
Olympus BX41).
Biochemical assays
The lens tissues were homogenized in cold PBS (10%
w/v) and centrifuged at 3,000 x g for 10 min at 4˚C. The
supernatant was used for biochemical analyses, as previously
described (18-20).
Malondialdehyde (MDA) concentration in the
homogenate was determined based on the thiobarbituric acid reactive
substances assay (TBARS), using the MDA Assay kit (cat. no. A003-1;
Nanjing Jiancheng Bioengineering Institute). Briefly, TBA reacted
with MDA to form red products, the absorbance of which can be
measured at a wavelength of 532 nm. MDA concentration was expressed
as nmol of MDA per milligram of protein (nmol/mg protein).
Reduced glutathione (GSH) concentration was analyzed
based on the dithionitrobenzoic acid (DTNB) reaction, using the GSH
Assay kit (Nanjing Jiancheng Bioengineering Institute; cat. no.
A006-2-1). DTNB reacts with reduced GSH to form yellow products,
where the absorbance of which was measured at a wavelength of 405
nm. GSH concentration was expressed as µg of GSH per milligram of
protein (µg/mg protein).
Superoxide dismutase (SOD) activity was assessed
using the SOD Assay kit (cat. no. A001-3; Nanjing Jiancheng
Bioengineering Institute), based on the xanthine and xanthine
oxidase systems, where absorbance was measured at a wavelength of
450 nm. In total, 1 unit (U) of SOD activity was defined as the
amount of enzyme causing 50% inhibition of the xanthine and
xanthine oxidase reaction systems. SOD activity was expressed as
units per milligram protein (U/mg protein).
Western blotting
Total protein and nuclear protein of lens tissue
were extracted using the Whole Cell Lysis Assay (cat. no.
KGP250/KGP2100) and Nuclear Protein Extraction (cat. no.
KGP150/KGP1100) kits (both from Nanjing KeyGen Biotech Co., Ltd.),
according to the manufacturer's protocols. For total protein
extraction, lenses were homogenized in ice-cold lysis buffer to
obtain tissue homogenate. The homogenate was subsequently
centrifuged at 12,000 x g for 5 min at 4˚C and the supernatant was
collected. For nuclear protein extraction, lenses were homogenized
in ice-cold lysis buffer and the homogenate was centrifuged at
3,000 x g for 10 min at 4˚C. Following centrifugation, the pellets
were sonicated (3,000 rpm for 15 sec at 4˚C) with appropriate
volumes of nuclear extraction buffer and re-centrifuged at 14,000 x
g for 30 min at 4˚C. The supernatant was collected for nuclear
fractions. The protein samples were quantified using bicinchoninic
acid kit (cat. no. KGPBCA; Nanjing KeyGen Biotech Co., Ltd.). In
total, 50 µg protein samples were separated by 10% SDS-PAGE and
transferred onto polyvinylidene fluoride membranes (EMD Millipore).
The membranes were blocked with 5% non-fat milk in TBST for 2 h at
room temperature and incubated with primary antibodies at 4˚C
overnight against: Nrf2 (1:1,000; Abcam; cat. no. ab137550),
glutamate-cysteine ligase catalytic subunit (GCLC; 1:1,000; Abcam;
cat. no. ab207777), heme oxygenase 1 (HO-1; 1:1,000; Abcam; cat.
no. ab189491), β-actin (1:5,000; Abcam; cat. no. ab8226) and
Histone H2A (1:1,000; Abcam; cat. no. ab177312). Following primary
antibody incubation, membranes were incubated with goat anti-rabbit
HRP-conjugated secondary antibody (1:10,000; cat. no. BL003A;
Biosharp) for 1 h at 37˚C. Protein bands were visualized with New
Super ECL (cat. no. KGP1127-KGP1128; Nanjing KeyGen Biotech Co.,
Ltd.) using G:BOX chemiXR5 (Syngene Europe), whilst the densities
were determined using ImageJ software (version 2.1.4.7; National
Institutes of Health). The band densities of each sample were
normalized to β-actin or Histone H2A.
Terminal deoxynucleotidyl transferase
dUTP nick-end labeling (TUNEL) assay
The eyeballs were fixed in 10% formalin for 24 h at
room temperature, paraffin-embedded, then sectioned at a thickness
of 5 µm. TUNEL analysis was performed using One Step TUNEL
Apoptosis Assay Kit (cat. no. KGA7071; Nanjing KeyGen Biotech Co.,
Ltd.), according to the manufacturer's protocol. Briefly,
paraffin-embedded lens sections were deparaffinized in xylene,
rehydrated with a gradient ethanol series, digested with protein K
for 30 min at 37˚C and incubated with the TUNEL reaction mixture
for 1 h at 37˚C. Nuclei were counterstained with 2 µg/ml DAPI (cat.
no. KGA215; Nanjing KeyGen Biotech Co., Ltd.) in the dark for 5 min
at room temperature, and then rinsed with PBS. Antifade Mounting
Medium (cat. no. KGF028; Nanjing KeyGen Biotech Co., Ltd.) was used
for mounting. TUNEL-positive cells were identified via green
fluorescence. For quantitative analysis, the number of lens
epithelial cell nuclei and the number of TUNEL-positive epithelial
nuclei were manually counted. The TUNEL index was expressed as the
percentage of the number of TUNEL-positive epithelial cells in
relation to the total number of epithelial cells in five randomly
selected fields under a fluorescence microscope (magnification,
x200), as previously described (21).
Statistical analysis
Statistical analysis was performed using SPSS 17.0
software (SPSS, Inc.). All experiments were performed in triplicate
and data are presented as the mean ± standard deviation. One-way
ANOVA was used to compare differences among multiple groups
followed by Tukey's post hoc test, whilst the unpaired Student's
t-test was used to compare differences between two groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of neutron radiation on lens
morphology
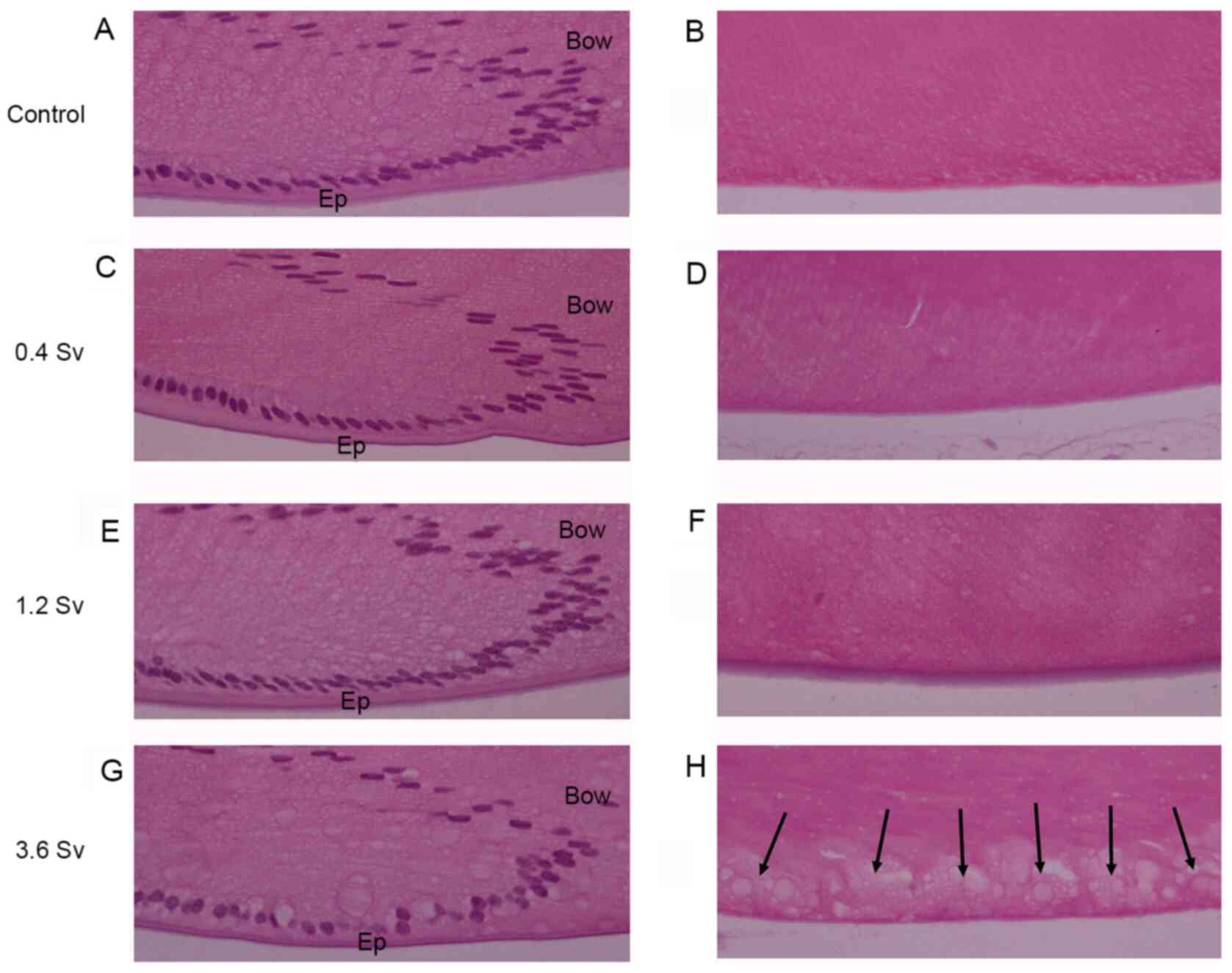
The morphological features of the lenses remained
intact in the 0.4 Sv, 1.2 Sv and control groups, including the
epithelial cells, lens bow pattern, fiber cells and posterior pole
(Fig. 2). However, lenses that were
exposed to 3.6 Sv exhibited injury, with reduced density and
abnormal alignments in the epithelial cells, slight distortion in
the lens bow configuration, swollen cortical fibers and
vacuolization near the posterior pole of the lens (Fig. 2).
Effects of neutron radiation on
oxidative stress and SOD activity in rat lens
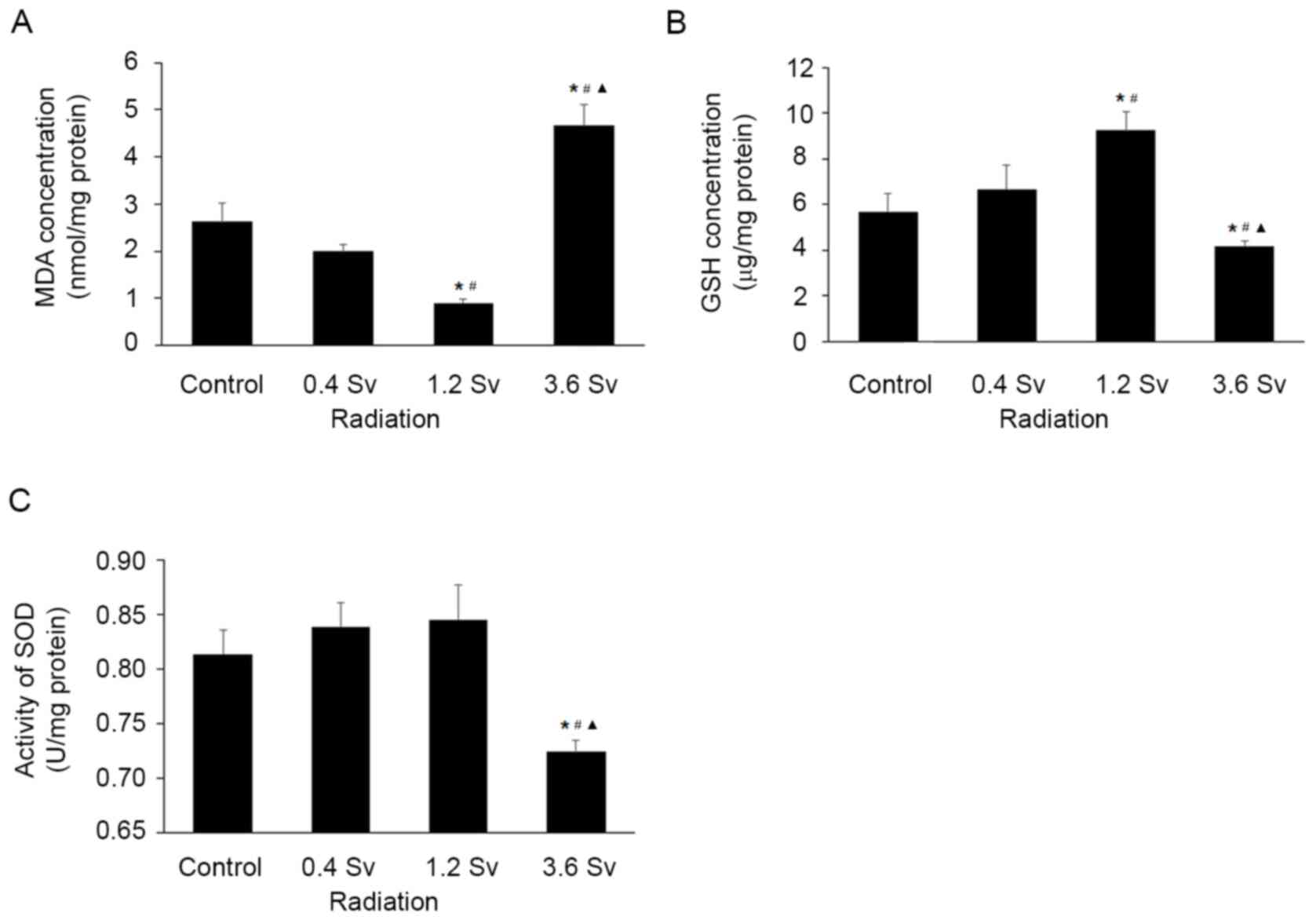
Compared with those in the control group, MDA levels
were found to be significantly lower in the 1.2 Sv group
(P<0.05; Fig. 3A) but
significantly higher in the 3.6 Sv group (P<0.05; Fig. 3A).
Conversely, GSH levels were significantly increased
in the 1.2 Sv groups (P<0.05; Fig.
3B) and significantly decreased in the 3.6 Sv group, compared
with those in the control group (P<0.05; Fig. 3B).
Although SOD activity was increased in the 0.4 Sv
and 1.2 Sv groups, the differences was not found to be
statistically significant (Fig.
3C). Conversely, SOD activity was significantly decreased in
the 3.6 Sv group compared with that in the control group
(P<0.05; Fig. 3C).
Effects of neutron radiation on Nrf2
and downstream antioxidant enzymes
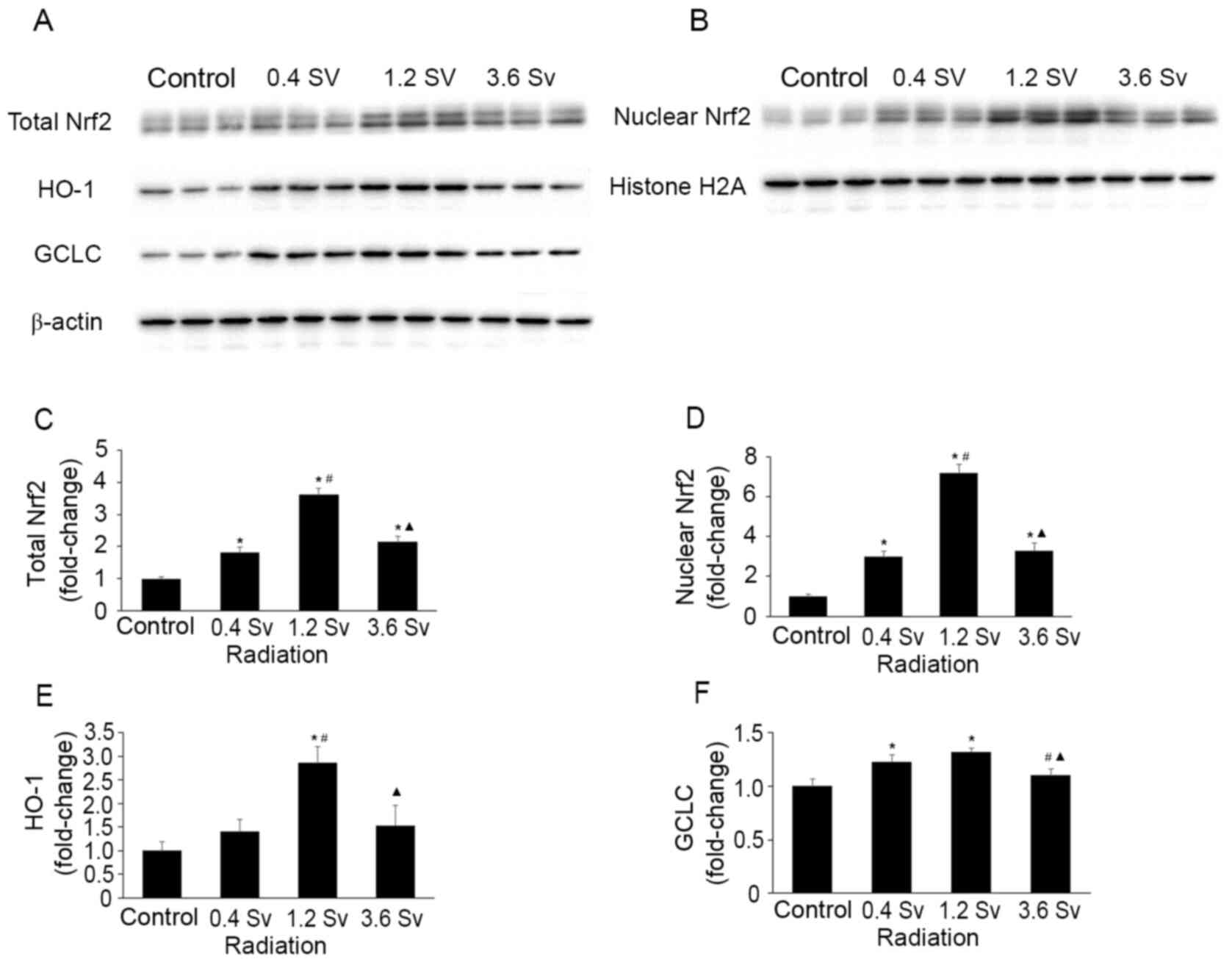
The total and nuclear protein levels of Nrf2 were
increased following 0.4, 1.2 and 3.6 Sv neutron radiation compared
with the control group (all P<0.05; Fig. 4). However, the levels were
significantly lower in the 3.6 Sv group compared with that in the
1.2 Sv group (P<0.05; Fig. 4C
and D). The protein levels of HO-1
and GCLC, downstream antioxidant enzymes of Nrf2(4), exhibited similar trends (P<0.05 in
the 1.2 Sv group vs. control group for HO-1; P<0.05 in the 0.4
Sv and 1.2 Sv groups vs. control group for GCLC; Fig. 4E and F).
Apoptosis of lens epithelial
cells
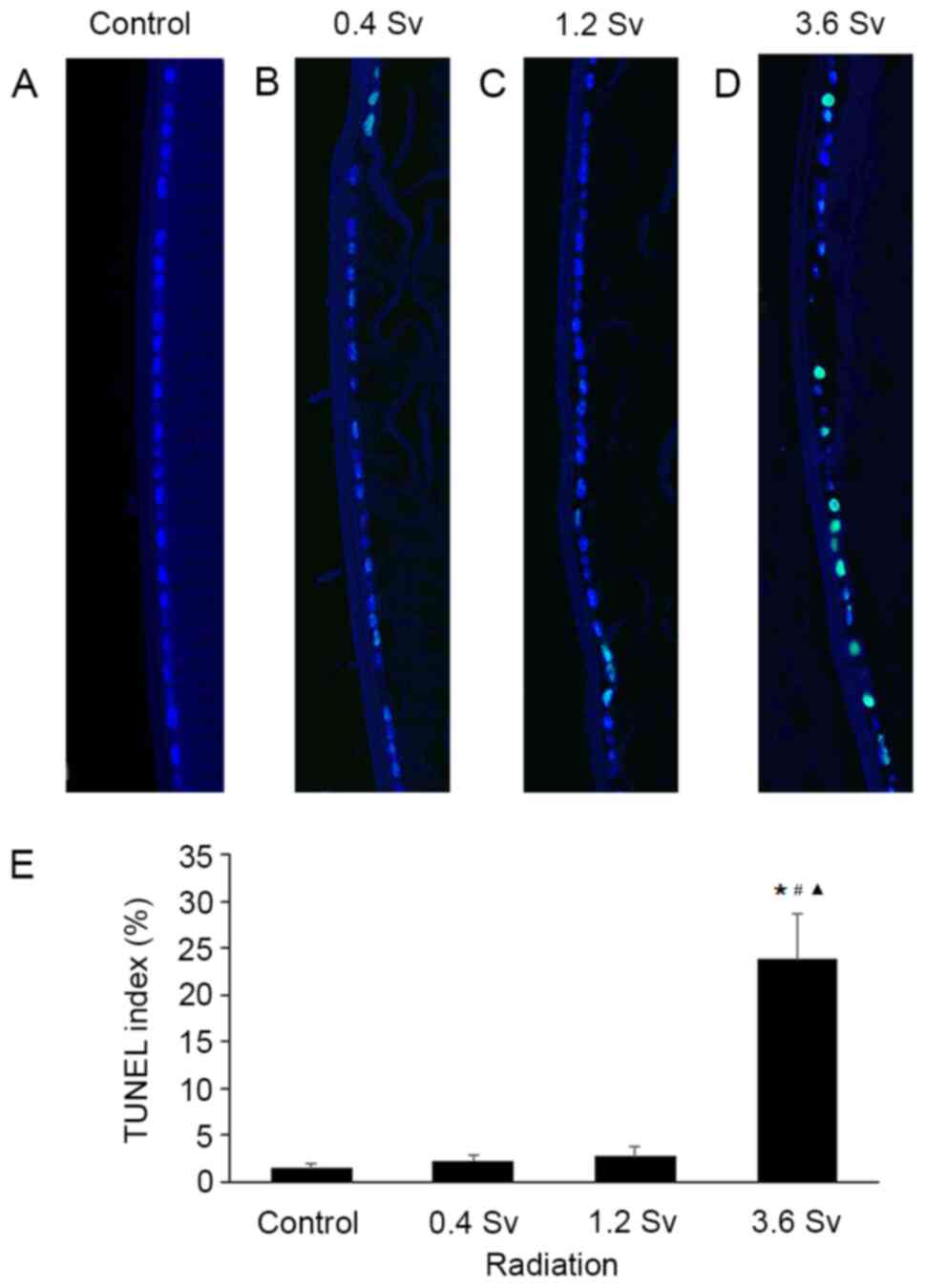
The results of the TUNEL assay demonstrated that the
apoptotic cells were sparse following radiation with 0.4 Sv and 1.2
Sv (Fig. 5B and C). However, 3.6 Sv neutron radiation
significantly induced cell apoptosis compared with that in control
(Fig. 5D and E). In addition, quantitative assessment
demonstrated that the TUNEL index was significantly higher
following radiation with 3.6 Sv compared with that in the control
group (P<0.05; Fig. 5E).
Notably, the difference was not statistically significant following
radiation with 0.4 Sv and 1.2 Sv compared with that in the control
group (Fig. 5E).
Discussion
IR-induced damage is primarily attributed to ROS,
which serves an important role in the effects of radiation on
biological tissues and organisms (22). Organisms have antioxidant defense
systems for scavenging ROS, which are reported to be regulated by
the Nrf2/ARE signaling pathway (10,23).
The present study investigated the effect of neutron radiation on
the status of oxidative stress and Nrf2-regulated antioxidant
defense systems in rat lenses at different radiation doses (0.4,
1.2 and 3.6 Sv).
Several protective systems have evolved in the
ocular lens, including the antioxidant enzymatic and nonenzymatic
systems, to combat oxidative stress induced by ROS (1). SOD is one of these antioxidant
enzymes. When the SOD activity increases, the capability of
eliminating ROS enhances (24). MDA
is commonly used to assess the active oxygen damage (25). In the present study, SOD activity
increased following radiation with 0.4 and 1.2 Sv compared with
that in the control group, but the difference was not statistically
significant. Conversely, MDA levels were reduced in the 0.4 and 1.2
Sv groups compared with those in the control group. This phenomenon
of beneficial biological effects caused by low-dose radiation was
previously termed as ‘radiation hormesis’ (26). A number of previous studies have
addressed the beneficial effects of low-dose radiation, which
demonstrated induction of antioxidant enzymes by low-dose radiation
both in vitro and in vivo. For example, Yamaoka et
al (24) assessed changes in
SOD activity and lipid peroxide (TBARS/MDA) levels in brain, lungs,
liver, thymus, spleen and bone marrow of rats following exposure to
whole-body low-dose X-radiation, which reported that SOD activity
increased in immune organs following exposure to radiation at doses
of 0.05-0.50 Gy for 4 h, whilst the levels of MDA reduced. In
another study, Pathak et al (27) demonstrated that SOD activity
increased in rodent kidneys by 37% following exposure to whole-body
low-dose γ-radiation (10-50 cGy) for 12 h. However, the levels of
MDA were enhanced, which differed from the results of the present
study and the study by Yamaoka et al (24). This variation may be due to species
differences in each animal model, radiation source, radiation dose,
dose rate, time and organs selected for the measurement of several
parameters.
The lens also has a non-enzymatic antioxidant
defense system to cope with oxidative stress (28). One aspect of this mechanism is by
redox balancing by GSH (28).
Lenses contain high concentrations of GSH, which maintain the thiol
groups in their reduced forms (29). Reduced GSH levels have been reported
in human lens following aging and those with cataract (29). GSH serves a vital role in the
maintenance of cellular redox balance by acting as a radical
scavenger (30). Yamaoka et
al (24) previously
demonstrated that GSH levels significantly increased in the kidneys
following radiation at varying doses (10-50 cGy) for 12 h.
Consistent with these findings, the results of the present study
demonstrated that GSH levels were higher in the lower dose groups
(0.4 Sv and 1.2 Sv) compared with those in the control group. This
increase in GSH levels may be due to the activation of protective
responses in the lenses to counteract ROS accumulation (28). However, GSH levels and SOD activity
were reduced in the lenses of the high dose group, whilst the MDA
levels increased. Taken together, these results suggest that
low-dose neutron radiation can increase both the non-enzymatic and
enzymatic antioxidant defense systems to overcome IR-induced ROS in
the lenses; as the radiation dose increases and ROS content exceeds
the capacity of the antioxidant defense systems, lenses may become
damaged eventually leading to cataractogenesis. In the present
study, the lenses remained intact in the 0.4 Sv, 1.2 Sv and control
groups. However, lenses that were exposed to 3.6 Sv exhibited
injury, including reduced density and abnormal alignment of
epithelial cells, apoptotic epithelial cells, slight distortion in
lens bow, swollen cortical fiber cells and vacuolization near the
posterior pole of the lens.
Nrf2 is a redox-sensitive transcription factor that
regulates the expression of antioxidant enzymes and phase II
metabolic enzymes (31). Nrf2 is
normally sequestered in the cytoplasm, which can be activated by
signals, such as ROS, which subsequently translocates into the
nucleus to regulate expression of downstream antioxidant and
detoxificationgenes that counteract ROS (31). These target genes include
glutathione peroxidase, SOD, HO-1, quinone oxidoreductase (NQO1)
and GCLC (4,32). Activation of the Nrf2 signaling
pathway is one of the critical defensive mechanisms against
oxidative stress in a number of tissues, including heart, retina,
liver and kidney (23). Purbey
et al (33) previously
reported that Nrf2 activation by ROS is highly selective to
radiation exposure compared with other environmental insults, such
as a microbial inducer of inflammation. Furthermore, Tsukimoto
et al (34) demonstrated
that low-dose γ-radiation induces Nrf2 activation in mouse
macrophage RAW264.7 cells, whilst McDonald et al (35) reported that single doses of IR from
2-8 Gy activate ARE-dependent transcription in breast cancer cells.
These findings are consistent with the results of the present
study, which demonstrated that neutron radiation increased the
level of Nrf2 and induced nuclear translocation of Nrf2. Therefore,
a two-phase Nrf2 expression was observed following neutron
radiation, namely, an increasing phase from 0.4-1.2 Sv and a
decreasing phase from 1.2-3.6 Sv. A previous study demonstrated
Nrf2-medidated antioxidant defense in two phases in mouse embryonic
fibroblasts isolated from p53 and p21 wild-type and knockout
pregnant female mice at embryonic day 13(36). However, McDonald et al
(35) previously demonstrated that
radiation activated Nrf2 in a dose-dependent manner, which differed
from the results of the present study. The difference may be due to
the different experimental subjects, radiation sources, dose and
dose rates.
The results of the present study demonstrated that
the levels of the downstream antioxidant enzymes of Nrf2, GCLC and
HO-1, increased following radiation at doses of 0.4 and 1.2 Sv.
These enzymes serve vital roles in the detoxification and
antioxidant processes in the body (37). Zhao et al (38) demonstrated that RNAi-mediated
reduction of Nrf2 expression significantly decreases the expression
levels of GCLC and HO-1 in radiated lung cancer cells. Notably,
Tsukimoto et al (34)
reported that HO-1 expression increases following radiation with
>0.1 Gy of γ-rays for 24 h in mouse macrophage RAW264.7 cells.
However, the present study demonstrated that high-dose neutron
radiation would deplete Nrf2, HO-1 and GCLC protein levels and
weaken anti-oxidative stress mechanisms, leading to
histopathological changes in the lenses, such as vacuolation under
posterior capsule. This is in accordance with the study performed
by Liu et al (39), who
demonstrated that lower fluences of ultraviolet A rays enhance Nrf2
expression, along with its downstream enzymes HO-1 and NQO1, whilst
higher fluences of UVA downregulate Nrf2 and its downstream
antioxidant enzymes in corneal endothelial cells.
A limitation of the present study is that neutron
radiation [high-linear energy transfer (LET)] was not compared with
low-LET radiation, such as X-rays or γ-rays, or non-ionizing
radiation, such as UV. The aim of the present study was to simulate
lens injury from space radiation experienced by astronauts
participating in missions on the ISS in animal models. Another
reason was that, among other types of radiation, neutrons can
produce more severe damage compared with χ-rays, γ-rays or UV
(40). A number of studies have
studied the effects of other radiations on lens. Bahia et al
(41) exposed human lens epithelial
cells (HLE) to χ-rays at different doses and demonstrated that HLE
exhibits a bi-phasic response in terms of cell viability and ROS.
Similar to the Bahia et al study, the findings of the
present study also reported a two-phase response. Several studies
have demonstrated that excessive UV can induce oxidative damage to
the lens and cause cataract (42,43).
Similarly, results of the present study demonstrated that high-dose
neutron radiation caused lens damage. Another limitation of the
present study was that Nrf2-knockout rats were not used.
Prospective studies with Nrf2-knockout rats are required to
validate the role of the Nrf2 pathway and to identify agents that
can prevent or delay IR-induced cataract by activating the Nrf2
pathway.
In conclusion, results of the present study
demonstrated that Nrf2-regulated antioxidant systems are affected
by neutron radiation in two phases. Low-dose neutron radiation
upregulates Nrf2 and its downstream enzymes to combat oxidative
damage in the lenses. However, as the radiation dose increases
further, Nrf2-mediated antioxidant mechanisms are compromised,
which causes oxidative damage in the lens and eventually leads to
cataract. Taken together, these findings suggest that activation
and enhancement of the Nrf2-mediated antioxidant defense systems
may be useful in preventing and delaying IR-induced cataract or
even for other oxidative stress-associated diseases, in addition to
the field of radiation protection.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by financial support
from the Fundamental Research Funds for the Central Universities
(grant no. 3082019NT2019017). The Space Medical Experiment Project
of China Manned Space Program (HYZHXM02004) and the Fundamental
Research Funds for the Central Universities (Grant No.
NJ2020017-3)
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC, JL, HZ, HL performed the experiments. JF, CX, WG
designed the experiments. YC performed the data analysis and the
interpretation. YC and CX confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures involving animals and the
corresponding experimental protocols were approved by the Ethics
Committee of Jinling Hospital (Nanjing, China) (no.
2020JLHGKJDWLS-109).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brennan LA, McGreal RS and Kantorow M:
Oxidative stress defense and repair systems of the ocular lens.
Frontiers in bioscience (Elite Ed). 4:141–155. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Li Q, Bai D, Qin L, Shao M, Zhang S, Yan
C, Yu G and Hao J: Protective effect of d-tetramannuronic acid
tetrasodium salt on UVA-induced photo-aging in HaCaT cells. Biomed
Pharmacother. 126(110094)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Spitz DR and Hauer-Jensen M: Ionizing
radiation-induced responses: Where free radical chemistry meets
redox biology and medicine. Antioxid Redox Signal. 20:1407–1409.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Liu K, Singer E, Cohn W, Micewicz ED,
McBride WH, Whitelegge JP and Loo JA: Time-dependent measurement of
Nrf2-regulated antioxidant response to ionizing radiation Toward
identifying potential protein biomarkers for acute radiation
injury. Proteomics Clin Appl. 13(e1900035)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rafnsson V, Olafsdottir E, Hrafnkelsson J,
Sasaki H, Arnarsson A and Jonasson F: Cosmic radiation increases
the risk of nuclear cataract in airline pilots: A population-based
case-control study. Arch Ophthalmol. 123:1102–1105. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mao XW, Boerma M, Rodriguez D,
Campbell-Beachler M, Jones T, Stanbouly S, Sridharan V, Wroe A and
Nelson GA: Acute effect of low-dose space radiation on mouse retina
and retinal endothelial cells. Radiat Res. 190:45–52.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hamada N and Fujimichi Y: Role of
carcinogenesis related mechanisms in cataractogenesis and its
implications for ionizing radiation cataractogenesis. Cancer Lett.
368:262–274. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Markiewicz E, Barnard S, Haines J, Coster
M, van Geel O, Wu W, Richards S, Ainsbury E, Rothkamm K, Bouffler S
and Quinlan RA: Nonlinear ionizing radiation-induced changes in eye
lens cell proliferation, cyclin D1 expression and lens shape. Open
Biol. 5(150011)2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ainsbury EA, Barnard S, Bright S, Dalke C,
Jarrin M, Kunze S, Tanner R, Dynlacht JR, Quinlan RA, Graw J, et
al: Ionizing radiation induced cataracts: Recent biological and
mechanistic developments and perspectives for future research.
Mutat Res. 770:238–261. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kensler TW, Wakabayashi N and Biswal S:
Cell survival responses to environmental stresses via the
Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 47:89–116.
2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lee JM, Li J, Johnson DA, Stein TD, Kraft
AD, Calkins MJ, Jakel RJ and Johnson JA: Nrf2, a multi-organ
protector? FASEB J. 19:1061–1066. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Meyer LM, Lofgren S, Ho YS, Lou M, Wegener
A, Holz F and Soderberg P: Absence of glutaredoxin1 increases lens
susceptibility to oxidative stress induced by UVR-B. Exp Eye Res.
89:833–839. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Taysi S, Memisogullari R, Koc M, Yazici
AT, Aslankurt M, Gumustekin K, Al B, Ozabacigil F, Yilmaz A and
Tahsin Ozder H: Melatonin reduces oxidative stress in the rat lens
due to radiation-induced oxidative injury. Int J Radiat Biol.
84:803–808. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Badhwar GD, Keith JE and Cleghorn TF:
Neutron measurements onboard the space shuttle. Radiat Meas.
33:235–241. 2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Benton ER and Benton EV: Space radiation
dosimetry in low-Earth orbit and beyond. Nucl Instrum Methods Phys
Res B. 184:255–294. 2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jing S, Guo H, Qi Y, Yang G and Huang Y: A
portable fast neutron irradiation system for tumor therapy. Appl
Radiat Isot. 160(109138)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ozgen SC, Dokmeci D, Akpolat M, Karadag
CH, Gunduz O, Erbas H, Benian O, Uzal C and Turan FN: The
protective effect of curcumin on ionizing radiation-induced
cataractogenesis in rats. Balkan Med J. 29:358–363. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li C, Yang X, Xu Y, Li L and Wang Y:
Cadmium detoxification induced by salt stress improves cadmium
tolerance of multi-stress-tolerant Pichia kudriavzevii.
Environ Pollut. 242:845–854. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang M, Feng L, Gu J, Ma L, Qin D, Wu C
and Jia X: The attenuation of Moutan Cortex on oxidative stress for
renal injury in AGEs-induced mesangial cell dysfunction and
streptozotocin-induced diabetic nephropathy rats. Oxid Med Cell
Longev. 2014(463815)2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ma N, Li C, Dong X, Wang D and Xu Y:
Different effects of sodium chloride preincubation on cadmium
tolerance of Pichia kudriavzevii and Saccharomyces
cerevisiae. J Basic Microbiol. 55:1002–1012. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang Y, Huo Y, Zhao L, Lu F, Wang O, Yang
X, Ji B and Zhou F: Cyanidin-3-glucoside and its phenolic acid
metabolites attenuate visible light-induced retinal degeneration in
vivo via activation of Nrf2/HO-1 pathway and NF-κB suppression. Mol
Nutr Food Res. 60:1564–1577. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Buonanno M, de Toledo SM, Pain D and Azzam
EI: Long-term consequences of radiation-induced bystander effects
depend on radiation quality and dose and correlate with oxidative
stress. Radiat Res. 175:405–415. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Tu W, Wang H, Li S, Liu Q and Sha H: The
anti-inflammatory and anti-oxidant mechanisms of the Keap1/Nrf2/ARE
signaling pathway in chronic diseases. Aging Dis. 10:637–651.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yamaoka K, Edamatsu R and Mori A:
Increased SOD activities and decreased lipid peroxide levels
induced by low dose X irradiation in rat organs. Free Radic Biol
Med. 11:299–306. 1991.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Macotpet A, Suksawat F, Sukon P, Pimpakdee
K, Pattarapanwichien E, Tangrassameeprasert R and Boonsiri P:
Oxidative stress in cancer-bearing dogs assessed by measuring serum
malondialdehyde. BMC Vet Res. 9(101)2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sharma S, Singla N, Chadha VD and Dhawan
DK: A concept of radiation hormesis: Stimulation of antioxidant
machinery in rats by low dose ionizing radiation. Hell J Nucl Med.
22:43–48. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Pathak CM, Avti PK, Kumar S, Khanduja KL
and Sharma SC: Whole body exposure to low-dose gamma radiation
promotes kidney antioxidant status in Balb/c mice. J Radiat Res.
48:113–120. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ganea E and Harding JJ:
Glutathione-related enzymes and the eye. Curr Eye Res. 31:1–11.
2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Donma O, Yorulmaz E, Pekel H and Suyugül
N: Blood and lens lipid peroxidation and antioxidant status in
normal individuals, senile and diabetic cataractous patients. Curr
Eye Res. 25:9–16. 2002.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ali SS, Ahsan H, Zia MK, Siddiqui T and
Khan FH: Understanding oxidants and antioxidants: Classical team
with new players. J Food Biochem. 44(e13145)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kaspar JW, Niture SK and Jaiswal AK:
Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol
Med. 47:1304–1309. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang W, Zhao H and Chen B: DJ-1 protects
retinal pericytes against high glucose-induced oxidative stress
through the Nrf2 signaling pathway. Sci Rep.
10(2477)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Purbey PK, Scumpia PO, Kim PJ, Tong AJ,
Iwamoto KS, McBride WH and Smale ST: Defined sensing mechanisms and
signaling pathways contribute to the global inflammatory gene
expression output elicited by ionizing radiation. Immunity.
47:421–434.e3. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tsukimoto M, Tamaishi N, Homma T and
Kojima S: Low-dose gamma-ray irradiation induces translocation of
Nrf2 into nuclear in mouse macrophage RAW264.7 cells. J Radiat Res.
51:349–353. 2010.PubMed/NCBI View Article : Google Scholar
|
|
35
|
McDonald JT, Kim K, Norris AJ, Vlashi E,
Phillips TM, Lagadec C, Della Donna L, Ratikan J, Szelag H, Hlatky
L and McBride WH: Ionizing radiation activates the Nrf2 antioxidant
response. Cancer Res. 70:8886–8895. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chen W, Jiang T, Wang H, Tao S, Lau A,
Fang D and Zhang DD: Does Nrf2 contribute to p53-mediated control
of cell survival and death? Antioxid Redox Signal. 17:1670–1675.
2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Tan XL and Spivack SD: Dietary
chemoprevention strategies for induction of phase II
xenobiotic-metabolizing enzymes in lung carcinogenesis: A review.
Lung cancer. 65:129–137. 2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhao Q, Mao A, Yan J, Sun C, Di C, Zhou X,
Li H, Guo R and Zhang H: Downregulation of Nrf2 promotes
radiation-induced apoptosis through Nrf2 mediated Notch signaling
in non-small cell lung cancer cells. Int J Oncol. 48:765–773.
2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Liu C, Vojnovic D, Kochevar IE and
Jurkunas UV: UV-A irradiation activates Nrf2-regulated antioxidant
defense and induces p53/Caspase3-dependent apoptosis in corneal
endothelial cells. Invest Ophthalmol Vis Sci. 57:2319–2327.
2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hamada N and Sato T: Cataractogenesis
following high-LET radiation exposure. Mutat Res. 770:262–291.
2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bahia S, Blais E, Murugkar S, Chauhan V
and Kumarathasan P: Oxidative and nitrative stress-related changes
in human lens epithelial cells following exposure to X-rays. Int J
Radiat Biol. 94:366–373. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Varma SD, Kovtun S and Hegde KR: Role of
ultraviolet irradiation and oxidative stress in cataract
formation-medical prevention by nutritional antioxidants and
metabolic agonists. Eye Contact Lens. 37:233–245. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Tülüce Y, Ozkol H and Koyuncu I:
Photoprotective effect of flax seed oil (Linum usitatissimum
L.) against ultraviolet C-induced apoptosis and oxidative stress in
rats. Toxicol Ind Health. 28:99–107. 2012.PubMed/NCBI View Article : Google Scholar
|