Introduction
The temporomandibular joint (TMJ) is a bilaterally
linked joint in the jaw that functions as a single entity when
speaking, chewing, swallowing and yawning. More than 25% of the
world's population is affected by temporomandibular joint disorders
(TMDs) (1), leading to mandibular
dysfunction and pain. TMJ replacement is recommended for 2-5% of
TMDs linked to ankylosis, tumors and degenerative diseases
(1). The goal of TMJ replacement is
to reconstruct the anatomy and function of the mandible, enable the
patient to resume a sustainable diet, prevent further morbidity,
reduce pain and improve quality of life. In arthroplasty,
transplantation with either autologous or allogeneic bone has
substantial disadvantages when compared to artificial joint
transplantation. These include limited supplies, limited dimensions
and the risk of infection (bacterial or viral) (2,3).
Therefore, using an artificial TMJ for joint reconstruction is one
of the most effective methods for clinical replacement.
Joint substitute implant materials require unique
properties, including excellent biological properties, such as
compatibility and safety, strong mechanical properties and fatigue
resistance, a Young's modulus similar to that of human bone,
biological inertia and good bio-surface modification capability.
Orthopedic implant materials are typically composed of metals,
inorganic non-metals (or bioceramics), polymers, complexes and
biological derivatives. The most common orthopedic or dental
materials are metals, such as titanium and precious metals that
have excellent environmental resistance and good physical and
biological properties (4). However,
metallic materials may lead to various problems after implantation,
including the release of toxic metal ions, interference with
imaging diagnoses, bone resorption, osteolysis, allergic reactions
and implant shedding (5,6). In dentistry, the clinical use of
precious metals, such as titanium, has resolved certain issues
related to the use of metallic materials; however, the inherently
high elastic modulus of metallic materials frequently leads to
stress shielding due to mismatch with the human bone, resulting in
bone absorption (7). At present,
there are two types of mature TMJ prostheses worldwide, provided by
the companies TMJ Concepts and Biomet Microfixation, whose condylar
prostheses are predominantly made of metal. Therefore, there is
still a requirement to improve the clinical performance of the
prosthesis and reduce the incidence of post-operative complications
by using more suitable biomaterials and subject-specific
designs.
Polyetheretherketone (PEEK) is widely used in
medical fields, such as cranioplasty (8-10)
and spinal fusion (11,12) for its light weight, excellent
biocompatibility, biomechanical properties and stability.
Specifically, its modulus of elasticity is similar to that of
cortical bone, which improves load sharing between the implant and
the surrounding tissue (13).
However, reports on the use of PEEK in TMJ reconstruction are
limited (14), primarily those
evaluating the effect of carbon fiber reinforced-PEEK material in
the reconstruction of TMJ defects in rabbits, and there is no
relevant research on the biomechanical properties of PEEK
materials, to the best of our knowledge. The objective of the
present study was to evaluate the biomechanical behavior of custom
3D-printed PEEK condylar prostheses. For this purpose, a finite
element analysis (FEA) was established to explore the effect of the
clenching tasks on the prosthesis and surrounding bones. The
designed prostheses were prepared using fusion deposition modeling
(FDM) technology and their compression properties were tested using
uniaxial compression experiments.
Materials and methods
Modeling the mandible and custom
condylar prosthesis
In the present study, the skull of the patient was
scanned CT (slice thickness, 0.625 mm; slice interval, 0.5 mm) for
26 dental implants at the Radiology Department of the First
Affiliated Hospital of Xi'an Jiaotong University (Xi'an, China).
The patient presented with a normal occlusion and without TMJ
disease. The patient provided informed consent. The data were
stored and then imported into the Mimics medical imaging processing
software (version 20.0; Materialise, Inc.) for 3D reconstruction.
In this modeling process, the mandible included the cortical bone,
cancellous bone and complete dentition.
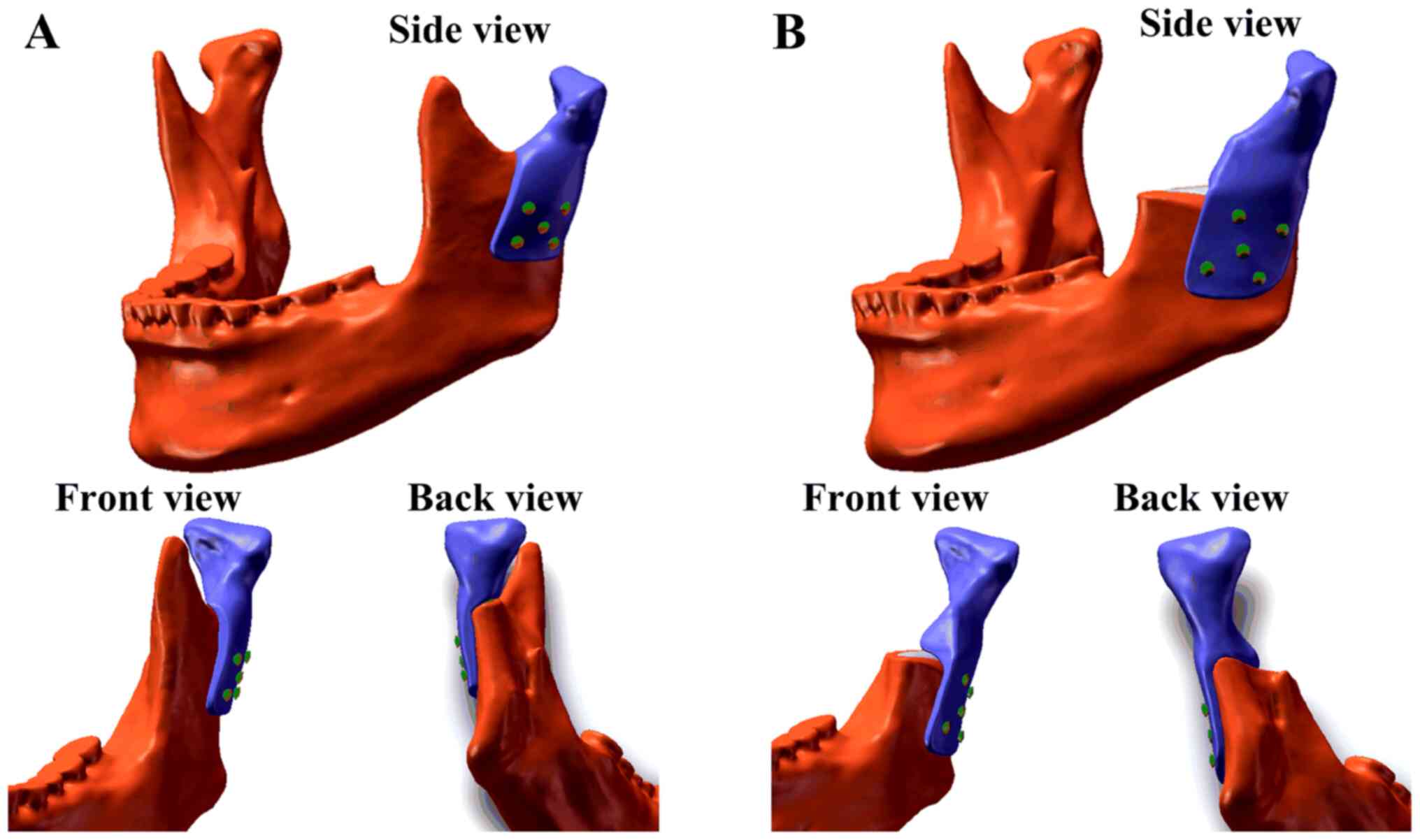
Due to the different extents of surgical osteotomy
in real clinical applications, two models were created. One model
(Model A) had a higher osteotomy position and retained the coracoid
process, whereas the other model (Model B) had a lower osteotomy
position and did not retain the coracoid process (Fig. 1). To design the corresponding
prostheses and create the two models, the 3D reconstruction model
of the mandible was imported into Geomagic Studio (version 2012; 3D
Systems, Inc.). The osteotomy of the mandibular lesion was
simulated according to the surgical requirements and partial bone
grinding (circular blunt) was performed on the buccal side of the
surgical stump to reduce the stress concentration on the
corresponding part of the prostheses. The corresponding data of the
contralateral mandible were symmetrically mapped to the defect area
according to the natural symmetry of the mandibular side. Part of
the condylar prostheses data in the defect area was obtained and
the overall design of the prostheses was achieved (Fig. 1). The TMJ condylar prostheses were
designed on the left side of the mandible and fixed with five
screws (Fig. 1). Each screw was 8
cm in length, 2 mm in diameter and did not have any screw
teeth.
FEA of the PEEK condylar
prostheses
The FEM was used to investigate the biomechanical
properties of the condylar prostheses and mandible. In the present
study, FEA was performed using the ANSYS Workbench finite element
package (version 14.5; ANSYS, Inc.). The parameters of the
materials used in the FEA are presented in Table I (15,16).
To reduce the influence of manufacturing methods and process
parameters, the material properties of the PEEK condylar prostheses
were defined based on the results of tensile, compression and
flexure tests, which were conducted using an electrohydraulic servo
mechanical testing machine (SANS CMT4304; MTS Corp.) according to
ISO 527-1:2012, ISO 604 and ISO 178, respectively. All materials
were assumed to be homogeneous, linearly elastic and isotropic.
 | Table IMechanical parameters of the materials
used in the modeling. |
Table I
Mechanical parameters of the materials
used in the modeling.
| Parameter | Cortical bone | Cancellous bone | Titanium alloy | PEEK |
|---|
| Elastic
modulus/GPa | 13 | 1.6 | 110 | 2.8 |
| Poisson's ratio | 0.3 | 0.3 | 0.3 | 0.3 |
Mesh Model A contained 1,157,007 nodes (778,460
elements) and mesh Model B contained 1,115,658 nodes (751,727
elements). The contact interface between the prosthesis and
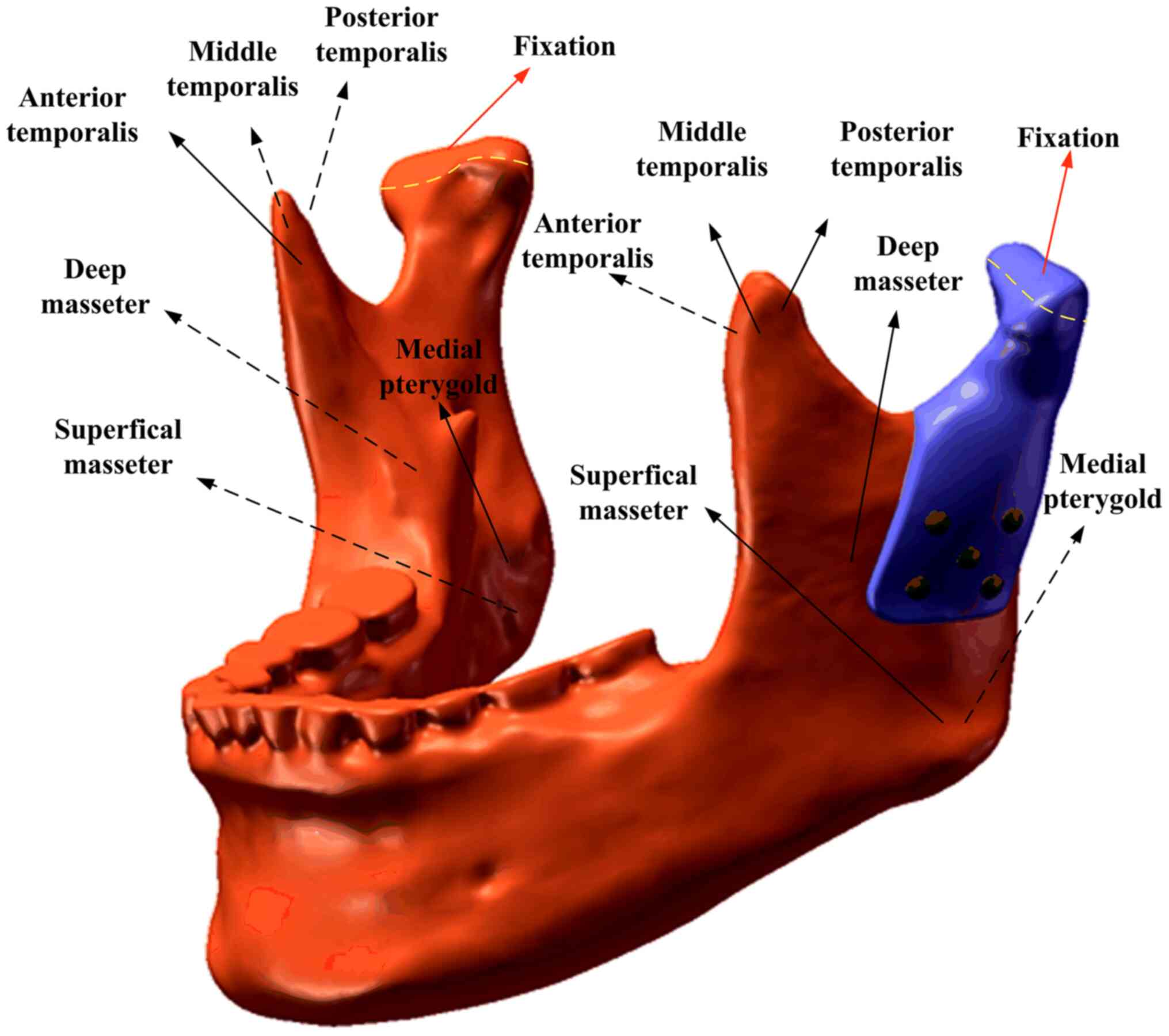
mandible was considered bonded. The loading conditions pertained to
six principal muscles (Fig. 2). A
total of four static clenching tasks were simulated in this study:
The intercuspal position (ICP), incisal clench (INC), left
unilateral molar clench (LMOL) and right unilateral molar clench
(RMOL). The models were constrained in all directions at the top
condyle in all clenching tasks. In addition, the models were
constrained in the incisor regions (INC constraint), the canine and
premolar regions (ICP constraint), the right molars (RMOL
constraint) and the left molars (LMOL constraint). The magnitude
and directions of the six muscular forces were obtained from a
previous study (17). The maximum
stress of the prostheses and screws and the stress and strain of
the cortical bone and cancellous bones during ICP, INC, LMOL and
RMOL were recorded and analyzed.
Preparation and mechanical testing of
the condylar prostheses
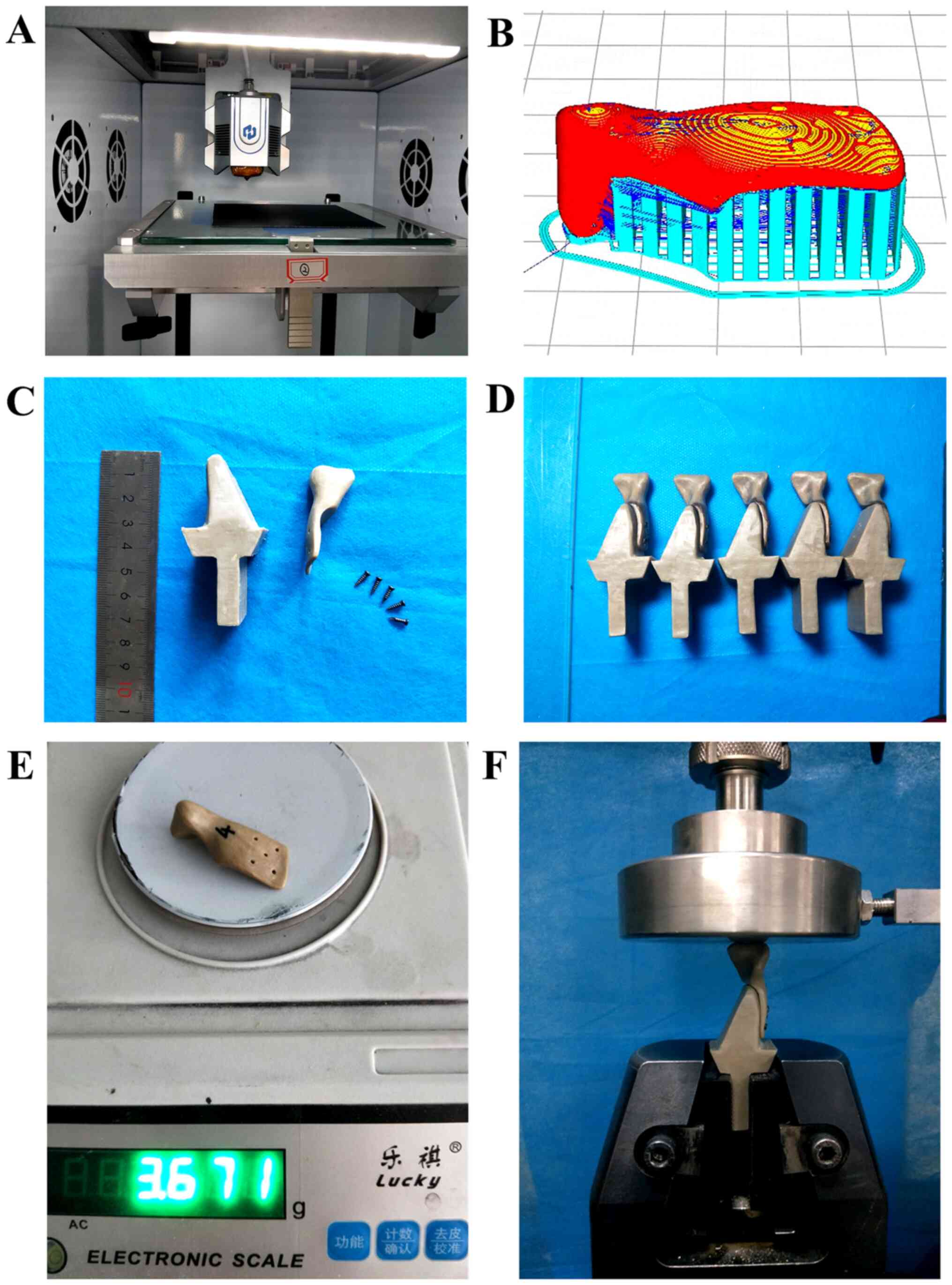
The condylar prostheses were prepared using a
self-developed 3D printer (Fig. 3A)
with a nozzle diameter of 0.4 mm, a printing speed of 40 mm/sec, a
wall thickness of 0.8 mm (printing line width of 0.4 mm), a layer
thickness of 0.2 mm, a nozzle temperature of 420˚C, an infill
percentage of 100% and printing trajectories of tiled scan, buccal
up and lingual down (Fig. 3B).
To evaluate the compression performance of the
printed samples, a compression test was conducted for the
3D-printed condylar prostheses using a universal testing machine.
The fixture was designed and fixed with titanium nails to simulate
an actual clinical prosthesis (Fig.
3C). The fixed end of the fixture was clamped and the speed was
tested at 1 mm/min (Fig. 3F). A
total of five physical models were used for the compression tests
(Fig. 3D). Finally, the results of
the FEA and experimental tests were compared to comprehensively
assess the mechanical properties of the condylar prostheses.
Results
General
In the present study, the following parameters were
evaluated: The von Mises stress of the condylar prosthesis and five
bone screws along with the von Mises stress and von Mises strain of
the cortical bone and cancellous bone in four grasping tasks.
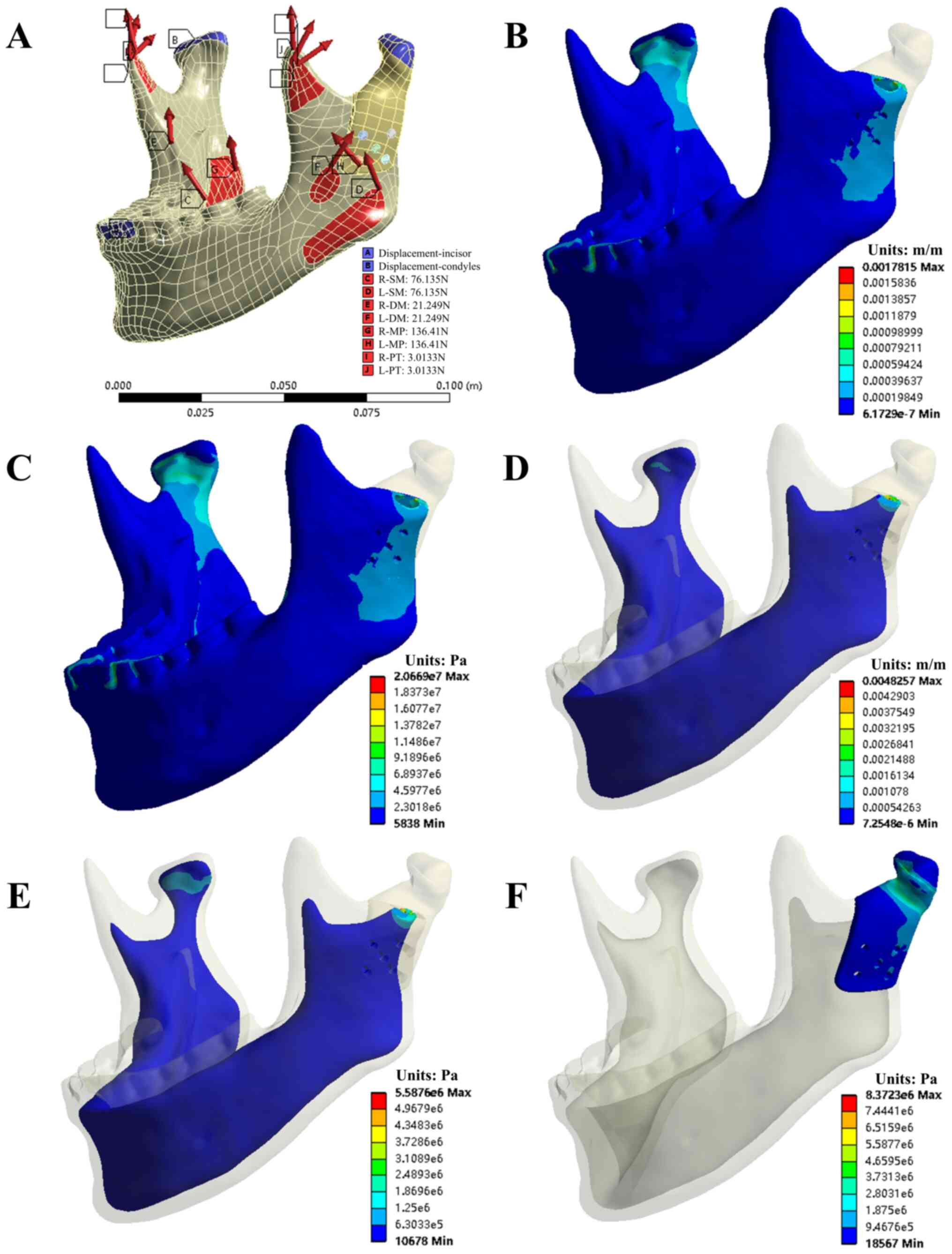
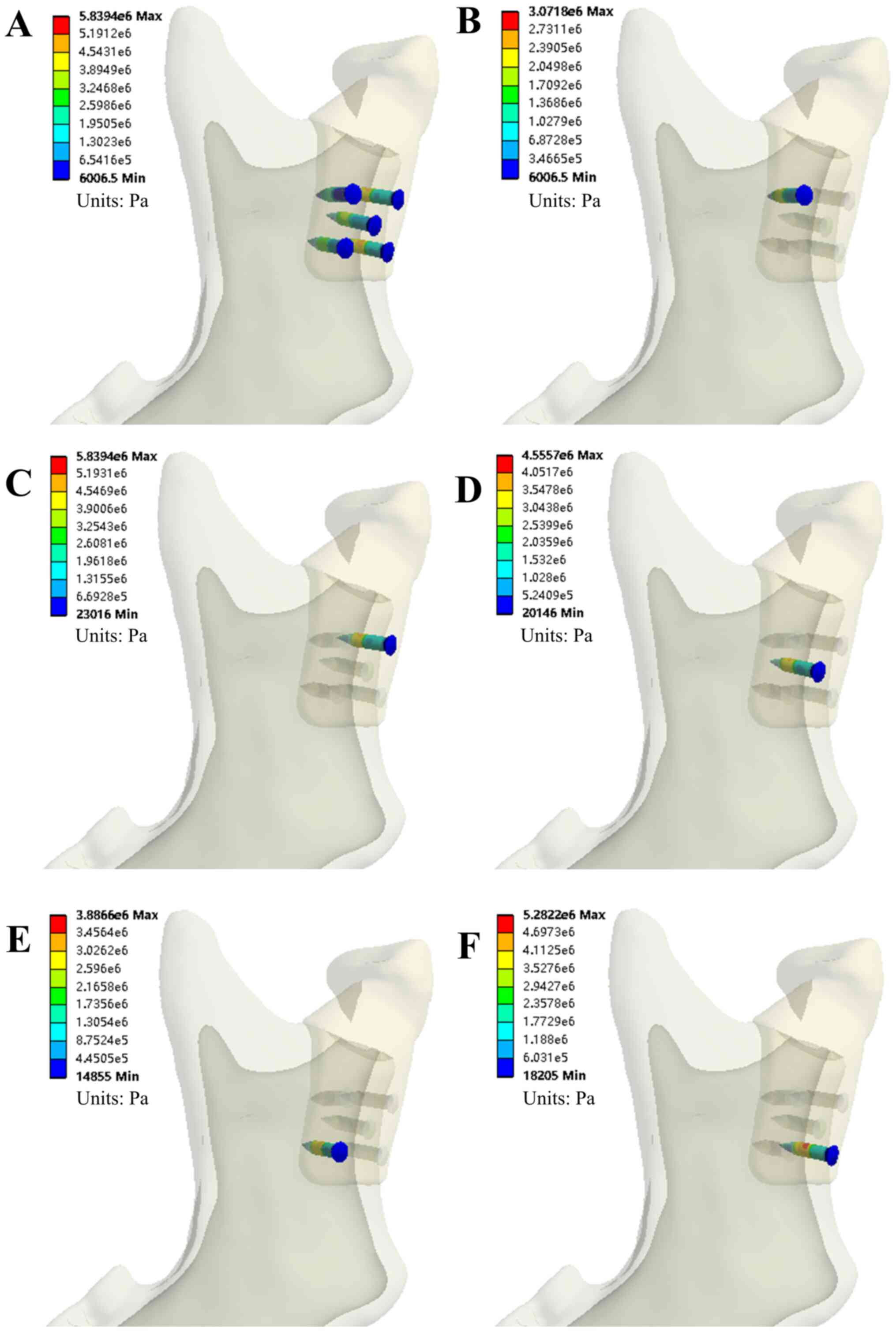
Figs. 4 and 5 provide the simulation results of the INC
task for model A. Tables II and
III present the evaluation
parameters for all four occlusion tasks for the two models.
 | Table IISimulation results of the four
clenching tasks (Model A). |
Table II
Simulation results of the four
clenching tasks (Model A).
| | Maximum value |
|---|
| Component/evaluation
parameter | INC | ICP | LMOL | RMOL |
|---|
| Condylar
prosthesis | | | | |
|
von Mises
stress (MPa) | 8.3723 | 10.733 | 4.8598 | 10.567 |
|
von Mises
strain (µ) | 3,263.7 | 4,261.5 | 1,889.3 | 4,027.2 |
| Cortical bone | | | | |
|
von Mises
stress (MPa) | 20.669 | 42.688 | 34.23 | 31.315 |
|
von Mises
strain (µ) | 1,781.5 | 4,897.2 | 3,388.1 | 2913.8 |
| Cancellous bone | | | | |
|
von Mises
stress (MPa) | 5.5876 | 10.826 | 3.7015 | 6.792 |
|
von Mises
strain (µ) | 4,825.7 | 7,772.4 | 2,920.5 | 5,857.4 |
| All screws | | | | |
|
von Mises
stress (MPa) | 5.8394 | 9.7075 | 5.3554 | 9.4276 |
|
von Mises
strain (µ) | 53.115 | 88.269 | 54.225 | 85.726 |
| Screw #1 | | | | |
|
von Mises
stress (MPa) | 3.0718 | 7.7425 | 4.7278 | 7.6845 |
|
von Mises
strain (µ) | 27.937 | 87.112 | 54.225 | 78.165 |
| Screw #2 | | | | |
|
von Mises
stress (MPa) | 5.8394 | 9.5725 | 5.3554 | 9.4276 |
|
von Mises
strain (µ) | 53.115 | 87.043 | 48.812 | 85.726 |
| Screw #3 | | | | |
|
von Mises
stress (MPa) | 4.5557 | 8.445 | 4.5214 | 7.6839 |
|
von Mises
strain (µ) | 41.441 | 76.817 | 41.128 | 69.894 |
| Screw #4 | | | | |
|
von Mises
stress (MPa) | 3.8866 | 7.7507 | 4.2435 | 6.7267 |
|
von Mises
strain (µ) | 35.34 | 70.501 | 38.600 | 61.188 |
| Screw #5 | | | | |
|
von Mises
stress (MPa) | 5.2822 | 9.7075 | 5.2122 | 9.2533 |
|
von Mises
strain (µ) | 48.042 | 88.269 | 47.394 | 84.139 |
 | Table IIISimulation results of the four
clenching tasks (Model B). |
Table III
Simulation results of the four
clenching tasks (Model B).
| | Maximum value |
|---|
|
Component/evaluation parameter | INC | ICP | LMOL | RMOL |
|---|
| Condylar
prosthesis | | | | |
|
von Mises
stress (MPa) | 5.9419 | 6.7679 | 1.9178 | 8.5229 |
|
von Mises
strain (µ) | 2,382.8 | 2,710.4 | 769.92 | 3,551.8 |
| Cortical bone | | | | |
|
von Mises
stress (MPa) | 13.93 | 34.535 | 43.038 | 24.148 |
|
von Mises
strain (µ) | 1,240.4 | 3,498.2 | 4,066.1 | 2,548.2 |
| Cancellous
bone | | | | |
|
von Mises
stress (MPa) | 2.5734 | 3.2374 | 2.4105 | 3.4237 |
|
von Mises
strain (µ) | 1,651.3 | 2,059.7 | 1,507.8 | 2,277.5 |
| All screws | | | | |
|
von Mises
stress (MPa) | 1.8837 | 1.9063 | 0.65778 | 2.7697 |
|
von Mises
strain (µ) | 17.267 | 17.441 | 6.041 | 25.332 |
| Screw #1 | | | | |
|
von Mises
stress (MPa) | 1.4413 | 1.3578 | 0.44294 | 1.6534 |
|
von Mises
strain (µ) | 13.455 | 12.69 | 4.1499 | 15.338 |
| Screw #2 | | | | |
|
von Mises
stress (MPa) | 1.8837 | 1.9063 | 0.65778 | 2.7697 |
|
von Mises
strain (µ) | 17.267 | 17.441 | 6.041 | 25.332 |
| Screw #3 | | | | |
|
von Mises
stress (MPa) | 1.3577 | 1.3148 | 0.44833 | 1.5328 |
|
von Mises
strain (µ) | 12.48 | 12.089 | 4.0937 | 14.087 |
| Screw #4 | | | | |
|
von Mises
stress (MPa) | 0.9578 | 0.86554 | 0.2715 | 0.90107 |
|
von Mises
strain (µ) | 8.8955 | 7.9824 | 2.5222 | 8.389 |
| Screw #5 | | | | |
|
von Mises
stress (MPa) | 1.3605 | 1.338 | 0.44003 | 1.7646 |
|
von Mises
strain (µ) | 12.528 | 12.311 | 4.2029 | 16.225 |
Evaluation of von Mises stress of the
TMJ condylar prosthesis and five bone screws
Mechanical analysis indicated that the stress values
of prosthesis Model A were <11 MPa in all four clenching tasks.
The von Mises stress on the five bone screws ranged from 3.07 MPa
(INC, screw #1) to 9.71 MPa (ICP, screw #5) for the same tasks.
Scre #5 was under the greatest von Mises stress during all tasks,
as shown in Table II. For Model B,
stress values of prosthesis were <9 MPa in all four occlusion
tasks. The von Mises stress on the five bone screws ranged from
0.27 MPa (LMOL, screw #4) to 2.77 MPa (RMOL, screw #2) and screw #2
was under the greatest stress during all tasks, as shown in
Table III.
Evaluation of the von Mises stress and
strain of the cortical and cancellous bones
The mechanical analysis of the entire mandible
cortical bone in Model A suggested that the maximum and minimum von
Mises stress occurred during the ICP (42.688 MPa) and INC (20.669
MPa) tests. The maximum and minimum von Mises strain also occurred
during the ICP (4,897.2 μ) and INC (1,781.5 μ) tests. However, in
the cancellous bone, the maximum and minimum von Mises stress and
strain occurred during the ICP (10.826 MPa and 7,772.4 μ,
respectively) and LMOL (3.7015 MPa and 2,920.5 μ, respectively)
tests, as shown in Table II.
Conversely, for the entire mandible cortical bone in Model B, the
maximum and minimum von Mises stress occurred during the LMOL
(43.038 MPa) and INC (13.93 MPa) tests, as did the maximum and
minimum von Mises strain (4,066.1 and 1,240.4 μ, respectively). In
the cancellous bone, the maximum and minimum von Mises stress and
strain occurred during the RMOL (3.4237 MPa and 2,277.5 μ,
respectively) and LMOL (2.4105 MPa and 1,507.8 μ, respectively)
tests, as shown in Table III.
Experimental results of the
prosthesis
The weights of the condyle prostheses printed by FDM
were 3.671 g (Model A) and 4.382 g (Model B). The compression test
results indicated that the maximum forces that the prostheses were
able to withstand were 3,814.7±442.6 N (Model A) and 4,245.7±348.3
N (Model B). The compressive force-displacement curves are
presented in Fig. 6.
Discussion
The present study introduced a novel TMJ prosthesis
that differs from the existing prostheses from Biomet and TMJ
Concepts. The custom condylar prostheses were designed using a
reverse engineering approach and were prepared by 3D printing using
PEEK as the printed material. PEEK is a high-strength
semi-crystalline thermoplastic that was first introduced to the
medical field in the early 1990s (18). PEEK has excellent biocompatibility
and its yield strength and Young's modulus are closer to cortical
bone than metal prostheses (13),
which facilitates the design of implants that reduce stress
shielding. Furthermore, high radiographic penetration may
effectively avoid artifacts during post-operative examination
(13). These advantages make PEEK a
better candidate for loadbearing orthopedic devices, particularly
in the spine. However, reports on the use of PEEK in TMJ
reconstruction are limited. In the present study, two PEEK condylar
prosthesis models were designed to reflect the different degrees of
surgical osteotomy. The FEM was used to analyze the biomechanical
behavior of the condylar prostheses, which was validated through
mechanical experiments.
To ensure the stability of the TMJ condylar
prostheses, the stress of each component of the prosthesis should
be less than the yield strength of the material. In the present
study, consistent with the study of Kashi et al (19) from 2010, the stress in the condylar
prostheses was predominantly concentrated in the anterior and
posterior areas of the condylar neck. The maximum stress in this
region was 10.733 MPa and 8.5229 in Models A and B, respectively,
which is far less than the yield strength of PEEK. Concerning the
five bone screws used to fix the PEEK condylar prostheses, the
maximum von Mises stresses in models A and B were 9.7075 MPa (screw
#5) and 2.7697 MPa (screw #2), far less than the yield strength of
titanium alloy. The peak stresses of the personalized PEEK condyle
prosthesis designed in this study and its retention screws are far
less than the yield strength of their respective materials.
Therefore, theoretically, the prostheses and screws would not
fracture. Moreover, the stress values of these screws are
significantly lower than the stress generated by a screw fixing
metal condyle prosthesis (17,20),
it showed that the PEEK condyle prostheses reduced the risk of the
retention screw fracture compared to the metal prostheses. In
addition, consistent with the study of Kashi et al (19) from 2010, the peak von Mises stresses
were observed in the upper and posterior regions of the mandible,
demonstrating the importance of the number and location of screws
when designing PEEK condyle prostheses.
The results of von Mises stress and strain generated
by the four clenching tasks in the entire mandible have the
following implications. First, the relationship between the von
Mises strain and variations in bone mass were divided into the
following ranges: disuse (bone loss): <10 µm/m (21), <50 µm/m (22) or <200 µm/m (23); normal load: 100-2,000 µm/m; mild
overload (bone gain): 2,000-4,000 µm/m (24); pathologic overload (irreversible
bone damage): >4,000 µm/m (24);
and bone fractures: 15,700-20,000 µm/m (24). With the exclusion of the right
condyle neck (near the fixed and restrained position) and the
lingual edge of the osteotomy surface (sharp edges after
osteotomy), the maximum strain on cortical bone and cancellous bone
was usually between 100 and 4,000 µm/m. Furthermore, regarding the
relationship between the von Mises stress and bone mass changes, a
study by Sugiura et al (25)
from 2000 indicated that the critical threshold for bone resorption
should be approximately -50 MPa. In the simulations of the present
study, the von Mises stress values in the bone were all <50 MPa.
In the present simulation of the four occlusal tasks, the stress
and strain of the mandible after adding a PEEK condyle prosthesis
were typically within the normal load range. Theoretically, no
disuse and overload absorption of the mandible was expected. In
addition, temporalis and part of the masseter muscles were resected
in Model B; therefore, the maximum stress of the condyle and the
stress and strain of the mandible were smaller than those in Model
A.
Personalized 3D-printed PEEK condylar prostheses are
able to meet individual requirements more effectively due to the
simple preparation method, one-time molding, convenient processing
and high material utilization. Furthermore, the intraoperative
adaptability is expected to surpass that of already available TMJ
prostheses. Furthermore, the experimental validation of the
biomechanical properties of the developed prostheses suggested that
the prostheses are able to withstand a maximum force of
3,814.7±442.6 N (Model A) and 4,245.7±348.3 N (Model B), which is
much larger than the combined maximum muscle force in the Z-axis
direction in the ICP. Therefore, the 3D-printed PEEK condyle
prosthesis developed in the present study is able to meet the
clinical requirements of TMJ reconstruction in terms of its
mechanical properties. This experiment revealed the following: FDM
printing is accomplished by the accumulation of materials layer by
layer and the molecular chains between the layers are less crossed
and entangled, resulting in a small interlayer force. When
designing the printing direction of the prosthesis, it is necessary
to consider the entire force environment and characteristics of the
prosthesis, so that frequent and larger forces act in the vertical
plane direction rather than the interlayer direction.
As with any theoretical simulation study, there are
certain limitations. First, a comparative study has yet to be
performed between the custom PEEK prostheses and a standard
prosthesis. In a future study, the stress and strain distribution
between the PEEK condylar prosthesis and the Biomet stock
prosthesis will be compared to further demonstrate the
biomechanical advantages of the proposed device. In addition, a
compression experiment will be conducted to evaluate the
biomechanics of the prosthesis. In subsequent studies, fatigue and
wear tests will be performed on the personalized PEEK condyle
prosthesis using mechanical testing equipment and validation in
animals.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the National Natural Science
Foundation of China (grant no. 81801034), the High-level Introduced
Talent Fund Project of Xi'an Medical University (grant no.
2018RCYJ03), the Key R&D Program of the Department of Science
and Technology of Shaanxi Province (grant no. 2020SF-023) and the
Key R&D Program of Guangdong Province (grant no.
2018B090906001).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MH and CL designed and supervised the project. FG,
SH, CY and DL performed the experiments. FG, SH and CY analyzed the
data. FG and SH confirmed the authenticity of the raw data. FG
wrote the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The patient provided informed consent.
Patient consent for publication
The patient has signed an informed consent for the
use of their CT data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rodrigues YL, Mathew MT, Mercuri LG, da
Silva JSP, Henriques B and Souza JCM: Biomechanical simulation of
temporomandibular joint replacement (TMJR) devices: A scoping
review of the finite element method. Int J Oral Maxillofac Surg.
47:1032–1042. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jones R: The use of virtual planning and
navigation in the treatment of temporomandibular joint ankylosis.
Aust Dent J. 58:358–367. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wolford LM, Dingwerth DJ, Talwar RM and
Pitta MC: Comparison of 2 temporomandibular joint total joint
prosthesis systems. J Oral Maxillofac Surg. 61:685–690; discussion
690. 2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhao Y, Wong SM, Wong HM, Wu S, Hu T,
Yeung KW and Chu PK: Effects of carbon and nitrogen plasma
immersion ion implantation on in vitro and in vivo biocompatibility
of titanium alloy. ACS Appl Mater Interfaces. 5:1510–1516.
2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Niki Y, Matsumoto H, Otani T, Suda Y and
Toyama Y: Metal ion concentrations in the joint fluid immediately
after total knee arthroplasty. Mod Rheumatol. 11:192–196.
2001.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang L, He S, Wu X, Liang S, Mu Z, Wei J,
Deng F, Deng Y and Wei S:
Polyetheretherketone/nano-fluorohydroxyapatite composite with
antimicrobial activity and osseointegration properties.
Biomaterials. 35:6758–6775. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sarot JR, Contar CM, Cruz AC and de Souza
Magini R: Evaluation of the stress distribution in CFR-PEEK dental
implants by the three-dimensional finite element method. J Mater
Sci Mater Med. 21:2079–2085. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jonkergouw J, van de Vijfeijken SE, Nout
E, Theys T, Van de Casteele E, Folkersma H, Depauw PR and Becking
AG: Outcome in patient-specific PEEK cranioplasty: A two-center
cohort study of 40 implants. J Craniomaxillofac Surg. 44:1266–1272.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lethaus B, Safi Y, ter Laak-Poort M,
Kloss-Brandstätter A, Banki F, Robbenmenke C, Steinseifer U and
Kessler P: Cranioplasty with customized titanium and PEEK implants
in a mechanical stress model. J Neurotrauma. 29:1077–1083.
2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zegers T, Ter Laak-Poort M, Koper D,
Lethaus B and Kessler P: The therapeutic effect of patient-specific
implants in cranioplasty. J Craniomaxillofac Surg. 45:82–86.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hu B, Yang X, Hu Y, Lyu Q, Liu L, Zhu C,
Zhou C and Song Y: The n-HA/PA66 cage versus the PEEK cage in
anterior cervical fusion with single-level discectomy during 7
years of follow-up. World Neurosurg. 123:e678–e684. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang J, Pan A, Zhou L, Yu J and Zhang X:
Comparison of unilateral pedicle screw fixation and interbody
fusion with PEEK cage vs. standalone expandable fusion cage for the
treatment of unilateral lumbar disc herniation. Arch Med Sci.
14:1432–1438. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kurtz SM and Devine JN: PEEK biomaterials
in trauma, orthopedic, and spinal implants. Biomaterials.
28:4845–4869. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Han ML, Li MH, Ji X, Han RY, Zhang N, Cui
LH, Sun LF and Han CM: Establishment of TMJ defect models and
evaluation on repair effect of CFR-PEEK material artificial joint
in rabbits. J Jilin Univ Med Εd. 43:903–909. 2017.
|
|
15
|
Arabshahi Z, Kashani J, Rafiq M, Kadir A
and Azari A: Influence of the TMJ implant geometry on stress
distribution. Adv Mat Res. 488:991–995. 2012.
|
|
16
|
Hsu JT, Huang HL, Tu MG and Fuh LJ: Effect
of bone quality on the artificial temporomandibular joint condylar
prosthesis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
109:e1–e5. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Huang HL, Su KC, Fuh LJ, Chen MY, Wu J,
Tsai MT and Hsu JT: Biomechanical analysis of a temporomandibular
joint condylar prosthesis during various clenching tasks. J
Craniomaxillofac Surg. 43:1194–1201. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Torstrick FB, Lin ASP, Potter D, Safranski
DL, Sulchek TA, Gall K and Guldberg RE: Porous PEEK improves the
bone-implant interface compared to plasma-sprayed titanium coating
on PEEK. Biomaterials. 185:106–116. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kashi A, Chowdhury AR and Saha S: Finite
element analysis of a TMJ implant. J Dent Res. 89:241–245.
2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen X, Wang Y, Mao Y, Zhou Z, Zheng J,
Zhen J, Qiu Y, Zhang S, Qin H and Yang C: Biomechanical evaluation
of Chinese customized three-dimensionally printed total
temporomandibular joint prostheses: A finite element analysis. J
Craniomaxillofac Surg. 46:1561–1568. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rubin CT and Lanyon LE: Regulation of bone
mass by mechanical strain magnitude. Calcif Tissue Int. 37:411–417.
1985.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Jaworski ZF, Liskova-Kiar M and Uhthoff
HK: Effect of long-term immobilisation on the pattern of bone loss
in older dogs. J Bone Joint Surg Br. 62-B:104–110. 1980.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lovell NC: Structure function, and
adaptation of compact bone. By R. Bruce Martin and David B. Burr.
Am J Phys Anthropol. 82:116–117. 1990.
|
|
24
|
Mellal A, Wiskott HWA, Botsis J, Scherrer
SS and Belser UC: Stimulating effect of implant loading on
surrounding bone. Comparison of three numerical models and
validation by in vivo data. Clin Oral Implants Res. 15:239–248.
2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sugiura T, Horiuchi K, Sugimura M and
Tsutsumi S: Evaluation of threshold stress for bone resorption
around screws based on in vivo strain measurement of miniplate. J
Musculoskelet Neuronal Interact. 1:165–170. 2000.PubMed/NCBI
|