Introduction
Ischemic heart disease (IHD) is a leading cause of
premature mortality worldwide (1,2). IHD
affects ~126 million individuals globally (1,655 per 100,000),
which is estimated to be 1.72% of the world's population (1). Restoration of coronary artery
perfusion is currently one of the main treatment options but it can
lead to myocardial ischemia-reperfusion injury (MIRI), which refers
to a more serious myocardial injury caused by blood recanalization
after a period of myocardial ischemia compared with that induced by
vascular occlusion (3,4). MIRI is reported to increase the risk
of cardiovascular disease, which imposes health, social and human
burdens on societies worldwide, and thus is a public health issue
(5-8).
Therefore, treatment of MIRI is important for the prevention of
cardiovascular disease, the end stage of cardiac dysfunction and
mortality.
Previous studies have reported that the spinal cord
is closely associated with the occurrence and development of MIRI
(9-11).
Following cardiac ischemia and reperfusion, chemical mediators such
as dynorphin and suppresses substance P within the myocardium
activate cardiac afferent fibres projecting from the dorsal root
ganglion to the upper thoracic dorsal horn of the spinal cord. This
results in the release of inflammatory factors and neuropeptides
within the spinal cord (12-15),
which triggers abnormal changes in gene transcription and
translation within the spinal cord (16-18).
Despite these recognized spinal responses, the genomic mechanisms
that enable differences during these spinal processes of MIRI
remain largely elusive.
It has been revealed that long non-coding (lnc)RNAs
serve a key role in the processing of gene expression signals via
the regulation of cell cycle distribution, stem cell
differentiation and transcriptional and post-transcriptional
control (19,20). Moreover, there are aberrant
expression levels of spinal lncRNAs in peripheral diseases, such as
pruritus (21,22), acute kidney injury (23), neuropathic pain and
inflammation-related pain (24).
In the present study, the altered expression of
mRNAs and lncRNAs in the thoracic (T)1-T4 spinal cord were analyzed
using high-throughput RNA-sequencing (RNA-seq) between a group of
rats established with MIRI and a sham group. Using microarrays, the
current study identified 470 mRNAs and 126 lncRNAs that were
significantly differentially expressed in rats with MIRI, and
further analyzed the biologic roles of these mRNAs and lncRNAs by
performing Gene Ontology (GO) and pathway analyses. Subsequently,
the differentially expressed mRNAs and lncRNAs were validated using
reverse transcription-quantitative (RT-q) PCR. The present results
may facilitate the development of molecular-targeting therapies for
MIRI.
Materials and methods
Animals
In total, 26 male Sprague Dawley rats (specific
pathogen free grade; weight, 250-300 g; age, 8-10 weeks) from Hunan
Slack Jingda Experimental Animal Company were used in the present
study. The animals were housed at a controlled room temperature
(25±0.5˚C) and relative humidity (40-60%) with a 12-h light/dark
cycle and had unlimited access to food and water. The number of
animals in each group was three, and each animal was used only
once. All experiments were performed with the approval of the
Institutional Animal Care and Use Committee of Tongji Hospital,
Huazhong University of Science and Technology (IRB ID, TJ-A0804)
and the National Institutes of Health Guide for the Care and Use of
Laboratory Animals (25).
MIRI
Rats were randomly divided into MIRI (model, n=13)
and sham (control, n=13) groups. MIRI experiments were performed
according to previously published protocols (18,26-29).
The rats were maintained in deep anesthetic state on a heating pad
using sodium pentobarbital (50 mg/kg; intraperitoneal), which was
indicated by disappearance of the foot withdrawal reflex.
In the model group, the left anterior descending
coronary artery (LAD) was ligated 2-mm below the left atrial
appendage for 30 min and then reperfused for 2 h, while sutures
were placed without LAD ligation in control operations. The
reperfused rats were considered as the animal model of MIRI.
Sham-operated age-matched rats served as controls. During the
experiment, the electrocardiogram (ECG) of animals was monitored
using a electrocardiogram machine (model no. ECG-2303B; Guangzhou
Sanrui Electronic Technology Co., Ltd.) at the following time
points: Before the operation, ischemia for 0, 15 and 30 min,
reperfusion for 0 min, 30 min and 2 h. After reperfusion for 2 h,
150 mg/kg sodium pentobarbital via intraperitoneal injection was
used for euthanasia and the confirmation of euthanasia was
decapitation. Then, the hearts were collected for
2,3,5-triphenyltetrazolium chloride (TTC) staining, hematoxylin and
eosin (H&E) staining and Masson staining. At the end of
reperfusion, the rats were deeply anesthetized, blood (~1 ml)
samples were collected from the aorta for cardiac troponin I (cTnI)
detection before euthanasia.
In the present experiment, ~20% of the animals died
due to hemorrhage and arrhythmia caused by ligation of coronary
artery at the early stage of reperfusion. When ventricular
fibrillation and bleeding were uncontrollable, euthanasia was
performed on animals as humane endpoints. Time points of 30 min for
ischemia and 2 h for reperfusion were selected for this animal
model is sufficient to result in significant histopathological and
functional myocardial injury, which can be confirmed using the
results of ECG, cTnI, TTC, H&E and Masson staining (30).
cTnI detection and myocardial tissue
staining
cTnI level was determined using an Automated Blood
or Urine Chemical Analyzer (Vitro 350; Ortho Clinical Diagnostics).
H&E staining and Masson trichrome staining were conducted in
the myocardial tissue samples in order to observe the myocardial
pathology. Heart tissues were fixed in 10% formaldehyde for 24 h at
room temperature and embedded with paraffin, before 4-µm thick
sections were cut. After deparaffinization by washing with xylene
followed by rehydration in a descending ethanol gradient, sections
were immersed in hematoxylin for 5-7 min, differentiated in 1% acid
alcohol for 2-5 sec and stained with 0.5% eosin for 2 min. After
rinsing with distilled water for 30 sec, sections were dehydrated
with an ascending ethanol gradient and were cleared in xylene. All
procedures were carried out at room temperature. Using a light
microscope (magnification x200; Leica DM 4000B; Leica Microsystems,
Inc.), pathological changes of myocardial tissues were observed.
The procedure of Masson's trichrome staining was similar to H&E
staining, which was described previously (31).
The infarct size was assessed using TTC staining 2 h
after reperfusion. After surgery, the hearts were removed and
frozen for 20 min at -20˚C, then transversally cut into sections
with a thickness of 1-2 mm. Tissue sections were incubated in 2%
TTC for 10 min at 37˚C in dark conditions and then fixed in 10%
formaldehyde at 4˚C overnight. Under light microscopic observation,
the infarct area was a white color, and the healthy tissues had a
red color. The degree of myocardial infarction was represented by
the percentage of infarcted area accounting for the left
ventricle.
Tissue extraction and RNA
isolation
In total, three rats in the MIRI group and three
from the sham group were anesthetized, and the T1-T4 spinal cord
segments were quickly extracted. Total RNAs were collected from the
dorsal horn of the spinal cord using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) based on the
manufacturer's instructions. The RNA concentration and purity were
examined using a spectrophotometer (Invitrogen; Thermo Fisher
Scientific, Inc.) (32,33). Equal amounts of mRNA (1 µg) from one
rat with the same treatment was mixed as one sample after these
tests. A total of six samples were selected for microarray
analysis.
Gene expression analysis
High-throughput lncRNA-seq was used to identify
novel genes in the spinal cord that regulate cardiac function in
the rat MIRI model. Briefly, total RNA was isolated from the T1-T4
of spinal cord tissues from the three MIRI- and sham-group rats,
and the concentration, purity and integrity of the RNA were
assessed on an Agilent 2100 Bioanalyzer (Agilent Technologies,
Inc.) and checked using RNase-free 1% agarose gel electrophoresis
containing GelRed® (cat. no. 41003; Biotium, Inc.).
Ribosomal RNA was then removed from the total RNA. The mRNA and
ncRNA were mostly retained and the strand-specific library was then
constructed using NEBNext® Ultra™ RNA Library Prep Kit
for Illumina® (cat. no. E7530L; New England BioLabs,
Inc.) according to the manufacturer's protocols. Briefly, the mRNA
was fragmented and used as a template to synthesize cDNA with
random primers. The first strand cDNA was synthesized using the
following progress: 25˚C for 10 min, 42˚C for 15 min, 70˚C for 15
min and final holding at 4˚C. A Second Strand Marking Master Mix
was used to synthesize second strand cDNA at 16˚C for 60 min. After
using a ligate adapter, uracil-N-glycosylase treatment and PCR
amplification were used to construct a sequencing library. The
loading concentration of DNA was 12.182 nM. The PCR thermocycling
parameters were set as follows: Initial denaturation at 98˚C for 30
sec; followed by 15 cycles of 98˚C for 10 sec, 60˚C for 30 sec and
72˚C for 30 sec; 1 cycle at 72˚C for 5 min and final holding at
10˚C. The library was then sequenced by Illumina HiSeqÔ 4000 with
pair ends reads of 150 bp. The data were analyzed by Gene Denovo
Biotechnology Co. (https://www.genedenovo.com; Guangzhou, China). The
differentially expressed mRNAs and lncRNAs were identified with
|log2 fold change (FC)| and P-values as calculated with
a t-test. |log2FC| >1 and P<0.05 were set as the
up- and downregulated gene thresholds. Hierarchical clustering and
volcano plots determined the different expression patterns of mRNAs
and lncRNAs among the samples.
Bioinformatics analysis
The differentially expressed lncRNAs and mRNAs with
statistical significance were identified via volcano plot
filtering. The threshold used to screen RNAs with
|log2FC| >1 was P<0.05. |log2FC| >1
is to screen genes with multiples of difference >2 times
(34,35). Gene Ontology (GO) analysis
(http://www.geneontology.org) described
the molecular function, cellular component and biological process
of differentially expressed transcripts. GO analysis and a Kyoto
Encyclopedia of Genes and Genomes (KEGG) analysis were applied to
determine the biologic roles of these differentially expressed
lncRNAs and mRNAs based on the latest KEGG data (http://www.genome.jp/kegg/).
RT-qPCR analysis for upper thoracic
spinal cord
The total RNA, extracted from the upper T1-T4
segments of the spinal cord using the TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions, was used for the generation of cDNA
(21,22,36-40).
The primers were designed using the Primer Express 3.0 software
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and the
specific forward and reverse primer sequences are listed in
Table I. RNA samples were
quantified using a spectrophotometer (BioPhotometer; Eppendorf AG)
and then synthesized to cDNA by reverse transcription using the
PrimeScript™ RT reagent kit (Takara Bio, Inc.). The temperature
protocol was conducted using the following protocol: 15 min at
37˚C, 5 sec at 85˚C and holding at 4˚C cDNA was quantitated via
RT-qPCR using a TB green® Premix Ex Taq (cat. no.
RR420A; Takara Bio, Inc.). The thermocycling conditions for PCR
were as follows: Initial denaturation for 30 sec at 95˚C, followed
by 40 cycles of 15 sec at 95˚C, 15 sec at 60˚C and 45 sec at 72˚C.
The threshold cycle (Cq) was used to estimate the amount of target
mRNA. The comparative Cq method with a formula for relative FC
(2-ΔΔCq) was used to quantify the amplified transcripts
(32,33,41).
The relative gene expression was determined via normalization to
GAPDH. Experiments were evaluated in triplicate and repeated ≥3
times.
 | Table IPrimer sequences for reverse
transcription-quantitative PCR. |
Table I
Primer sequences for reverse
transcription-quantitative PCR.
| A, mRNA |
|---|
| Primer name | Primer sequences
(5'→3') |
|---|
| Frs3 | F:
GAGCCAGTCATCATCACACGGAAC |
| | R:
GAAGCCATTGGAGAAGCTGGAGAC |
| Zfp523 | F:
CGCTGACTTCCTGTGAGTGTGAC |
| | R:
ACCTTGGCTCTGGCTCTCGTC |
| Dnajc6 | F:
GACAAGCCTCATGGAGCCAAGAAG |
| | R:
GGAGAAGTTCGTGCTCACATCGG |
| Nedd4l | F:
TACGCAGTGGCACCGACCTAG |
| | R:
GTGTGCGGCCTCCTGATTGATC |
| Tep1 | F:
GGATGGATACGGAGCTGCTGAATG |
| | R:
GGACACTGACGAAGAGGCACTTG |
| Myef2 | F:
CCAGGTGGACAGCCAATTAGTGC |
| | R:
GCCTGCCATGCTATTCATTGCTTC |
| Tgfbr1 | F:
CATTGCTGGTCCAGTCTGCTTCG |
| | R:
TGGTGAATGACAGTGCGGTTATGG |
| Fgf12 | F:
TGCTGTGCGACTGTGAGATTGC |
| | R:
CGTGCGGCTCGTTGTACTCG |
| Mef2c | F:
TGCTGTGCGACTGTGAGATTGC |
| | R:
CGTGCGGCTCGTTGTACTCG |
| Tfdp1 | F:
AAGAAGATGCCGCCGAGTTGTG |
| | R:
CGCTGGCTTCACGACACATCC |
| GAPDH | F:
CGCTAACATCAAATGGGGTG |
| | R:
TTGCTGACAATCTTGAGGGAG |
| B, Long non-coding
RNA |
| Primer name | Primer sequences
(5'→3') |
|
ENSRNOT00000080713 | F:
CACTTCCGCCCACATCACA |
| | R:
AAACCCCTCAGTCTCAAAACCT |
|
ENSRNOT00000090564 | F:
GACCACGCATCTACA |
| | R:
GCGAACTGAGGTGAGAAGGGA |
|
ENSRNOT00000082588 | F:
TGCTTCACTTCTCCCACTCTTC |
| | R:
AGGCCTTCCATCAAACAAATC |
|
ENSRNOT00000091080 | F:
GGTGGTGGACAAGGATGAG |
| | R:
TGGACGGAGGTTGGGGAGAT |
|
ENSRNOT00000091570 | F:
CAAGAAATCCAATACGACCC |
| | R:
TCCCCGTGGAAATAGGTGTAA |
|
ENSRNOT00000087777 | F:
GGAAGAGGGACGTTGGGTA |
| | R:
AGCCAGGTCCAAGTCACAGG |
|
ENSRNOT00000082061 | F:
CATTTCTTCCTCCCTTCCCTT |
| | R:
ACGGTTCTCCAATCGCACA |
|
ENSRNOT00000091108 | F:
CCGTGGAATATGGGAAGGAC |
| | R:
CAAGATGGAAAGGCAACGAG |
|
ENSRNOT00000087028 | F:
ACGGGGTGAAAGGGAAGA |
| | R:
TGCTGGTTTGTTAAGGGGATG |
|
ENSRNOT00000086475 | F:
CAGTTTGGTGTCTGTTTCCC |
| | R:
TGCTTCTTCGAGCCGGTATT |
Statistical analysis
Data are presented as the mean ± SEM, and were
analyzed using GraphPad Prism software v5.0 (GraphPad Software,
Inc.). RT-qPCR parameters were analyzed using unpaired t-test for
repeated measures. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of ischemic myocardial
tissues
Serum cTnI levels in the model group were
significantly increased compared with the control group (Fig. 1B). In addition, the results of TTC
staining (Fig. 1A), H&E
staining and Masson staining (Fig.
1C) all confirmed the successful establishment of the rat MIRI
model. In the control group, the myocardium had a physiological
myocardial architecture, and the myocardial fibres and myocardial
cells were complete and arranged in an orderly manner. In the model
group, structural disorder of the cardiac tissue was observed, with
different degrees of vacuolar degeneration and necrosis, as well as
loose stroma. Moreover, the number of cardiomyocyte fibres were
markedly increased after MIRI.
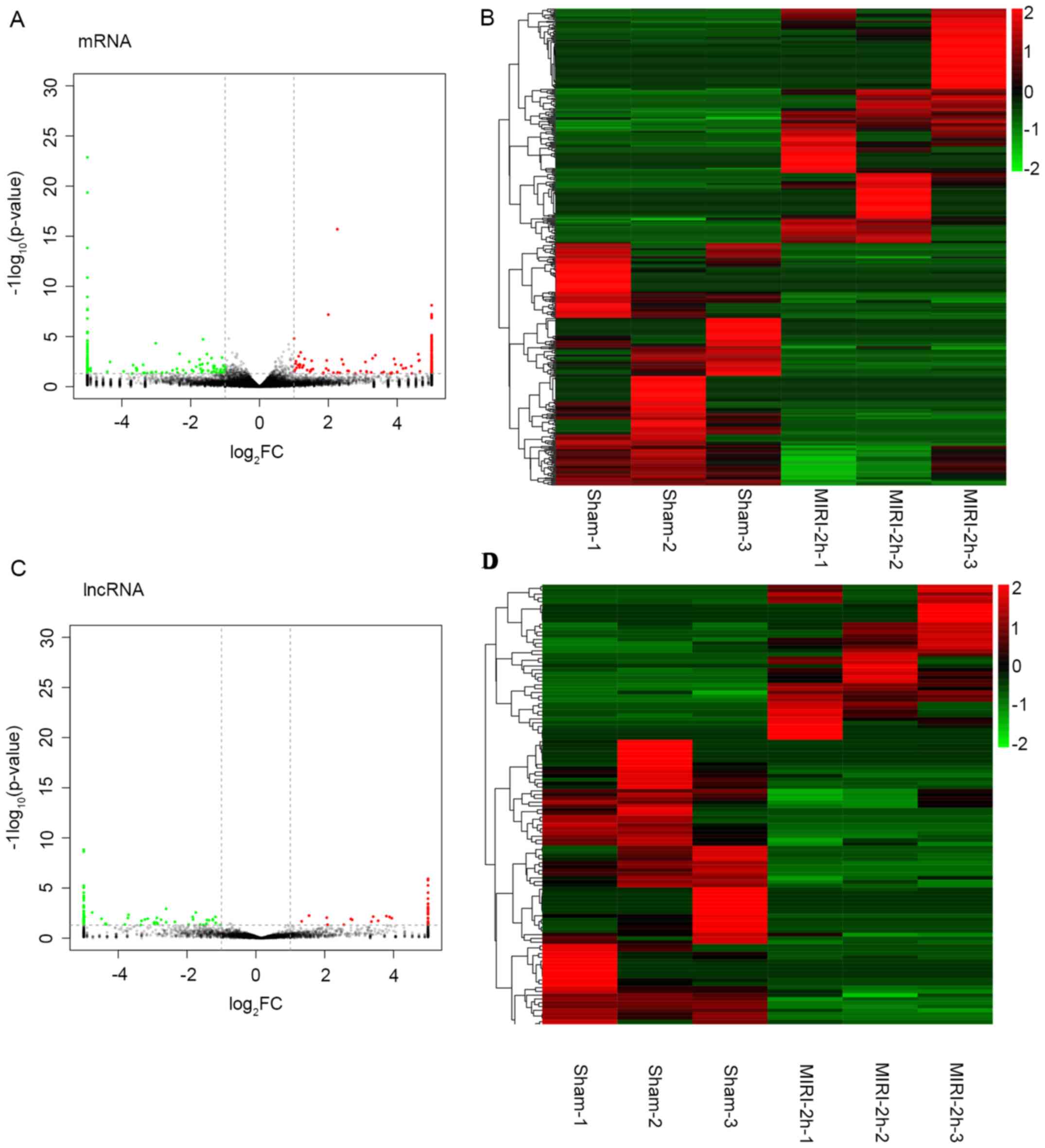
mRNAs and lncRNAs expression profiles
in MIRI
The patterns of the mRNA and lncRNA expressions in
the T1-T4 spinal cord at 2 h after MIRI or sham operations were
then examined using microarray. From the volcano map and
hierarchical clustering analysis results, a landscape of the
expression characteristics of the mRNAs and lncRNAs was obtained.
The volcano plots demonstrated that large numbers of the mRNAs and
lncRNAs were differentially expressed between the two groups
(Fig. 2A and C). The hierarchical cluster analysis of
the lncRNAs and mRNAs indicated that the samples of two groups were
clustered together and the signal intensity was consistent in the
two groups (Fig. 2B and D). Furthermore, these differential
alterations of the mRNAs and lncRNAs in the T1-T4 spinal cord were
associated with MIRI.
Differentially expressed mRNAs and
lncRNAs
To further analyze the differentially expressed
mRNAs and lncRNAs, a significance analysis of the microarrays
method with the criteria P<0.05 and FC >1 was performed.
In the differentially expressed mRNAs, there were
470 genes that exhibited an FC that was >1. The number of
downregulated mRNAs was 239, whereas the number of upregulated
mRNAs was 231. The most upregulated mRNAs were Tfdp1, Map2, Cep19,
Aurkaip1, Tpbg, Myef2, Ap2m1, Tgfbr1, Fgf12 and Mef2c. The most
downregulated mRNAs were Frs3, Zfp523, Tpm4, Apold1, Ctnnb1, Lss,
Psen2, Dnajc6, Nedd4l and Tep1. Detailed information regarding the
differentially expressed mRNAs is listed in Table II.
 | Table IIDetails of the top 10 downregulated
and upregulated mRNAs in the T1-T4 spinal cord between MIRI and
sham groups. |
Table II
Details of the top 10 downregulated
and upregulated mRNAs in the T1-T4 spinal cord between MIRI and
sham groups.
| A,
Downregulation |
|---|
| ID | Log2FC
(MIRI/sham) | Symbol | P-value | Description |
|---|
|
ENSRNOT00000019404 | -12.0846 | Frs3 |
4.50x10-20 | Fibroblast growth
factor receptor substrate 3 |
|
ENSRNOT00000000595 | -11.2761 | Zfp523 |
1.51x10-14 | Zinc finger protein
523 |
|
ENSRNOT00000089056 | -10.8893 | Tpm4 | 0.001717 | Tropomyosin 4 |
|
ENSRNOT00000010289 | -10.5635 | Apold1 |
1.1610-9 | Apolipoprotein L
domain containing 1 |
|
ENSRNOT00000079085 | -10.1716 | Ctnnb1 |
8.32x10-5 | Catenin beta 1 |
|
ENSRNOT00000081903 | -9.81911 | Lss | 0.013918 | Lanosterol synthase
(2,3-oxido squalene-lanos terol cyclase) |
|
ENSRNOT00000091715 | -9.75933 | Psen2 | 0.000372 | Presenilin 2 |
|
ENSRNOT00000086539 | -9.30682 | Dnajc6 |
5.25x10-5 | DnaJ heat shock
protein family (Hsp40) member C6 |
|
ENSRNOT00000083363 | -9.15482 | Nedd4l | 0.005334 | Neural precursor
cell expressed, developmentally down-regulated 4-like, E3 ubiquitin
protein ligase |
|
ENSRNOT00000088981 | -8.70275 | Tep1 | 0.001598 | Telomerase
associated protein 1 |
| B,
Upregulation |
| ID | Log2FC
(MIRI/sham) | Symbol | P-value | Description |
|
ENSRNOT00000087192 | 12.22179 | Tfdp1 |
6.42x10-8 | Transcription
factor Dp-1 |
|
ENSRNOT00000045766 | 11.86754 | Map2 |
5.07x10-5 |
Microtubule-associated protein 2 |
|
ENSRNOT00000037795 | 11.22882 | Cep19 |
2.22x10-5 | Centrosomal protein
19 |
|
ENSRNOT00000025531 | 11.16113 | Aurkaip1 | 0.000724 | Aurora kinase A
interacting protein 1 |
|
ENSRNOT00000014326 | 10.76763 | Tpbg | 0.000962 | Trophoblast
glycoprotein |
|
ENSRNOT00000081533 | 10.69407 | Myef2 |
7.98x10-9 | Myelin expression
factor 2 |
|
ENSRNOT00000088821 | 10.64386 | Ap2m1 | 0.004070 | Adaptor-related
protein complex 2, mu 1 subunit |
|
ENSRNOT00000081090 | 9.813781 | Tgfbr1 | 0.005577 | Transforming growth
factor, beta receptor 1 |
|
ENSRNOT00000071586 | 9.781360 | Fgf12 | 0.004286 | Fibroblast growth
factor 12 |
|
ENSRNOT00000076230 | 8.748193 | Mef2c |
1.98x10-5 | Myocyte enhancer
factor 2C |
The results identified that 126 lncRNAs, which
included 41 upregulated and 85 downregulated lncRNAs, were
significantly altered in the MIRI group compared with the sham
group. The most upregulated lncRNAs were ENSRNOT00000086475,
ENSRNOT00000087028, ENSRNOT00000091108, ENSRNOT00000031815,
ENSRNOT00000082061 and ENSRNOT00000087777. The most downregulated
lncRNAs were ENSRNOT00000080713, ENSRNOT00000090564,
ENSRNOT00000082588, ENSRNOT00000091080, ENSRNOT00000091570,
ENSRNOT00000077319, ENSRNOT00000077509, ENSRNOT00000089288,
ENSRNOT00000083187 and ENSRNOT00000091435. Additional information
regarding the differentially expressed lncRNAs is presented in
Table III.
 | Table IIIDetails of the downregulated and
upregulated long non-coding RNAs in the T1-T4 spinal cord between
MIRI and sham group. |
Table III
Details of the downregulated and
upregulated long non-coding RNAs in the T1-T4 spinal cord between
MIRI and sham group.
| A,
Downregulation |
|---|
| ID | Log2FC
(MIRI/sham) | P-value | Symbol |
|---|
|
ENSRNOT00000080713 | -9.98726 | 0.000442 | LOC102550026 |
|
ENSRNOT00000090564 | -8.10329 | 0.040576 | AABR07050135.1 |
|
ENSRNOT00000082588 | -7.49185 |
4.01x10-5 | AABR07000385.2 |
|
ENSRNOT00000091080 | -6.9542 |
1.14x10-6 | AABR07044980.1 |
|
ENSRNOT00000091570 | -6.71425 | 0.025341 | AABR07071198.1 |
|
ENSRNOT00000077319 | -6.22882 | 0.005841 | AABR07071383.1 |
|
ENSRNOT00000077509 | -5.32193 | 0.048185 | AABR07027371.1 |
|
ENSRNOT00000089288 | -4.16993 | 0.01174 | AABR07044001.4 |
|
ENSRNOT00000083187 | -3.52356 | 0.024012 | AABR07060560.4 |
|
ENSRNOT00000091435 | -3.38082 | 0.016282 | AABR07042668.1 |
| B,
Upregulation |
| ID | Log2FC
(MIRI/sham) | P-value | Symbol |
|
ENSRNOT00000086475 | 8.797662 | 0.013366 | AABR07070801.2 |
|
ENSRNOT00000087028 | 6.129283 | 0.027308 | LOC103692514 |
|
ENSRNOT00000091108 | 4.058894 | 0.020796 | AABR07006724.1 |
|
ENSRNOT00000031815 | 3.297681 | 0.041965 | Rn50_X_0744.6 |
|
ENSRNOT00000082061 | 2.966833 | 0.034871 | AABR07071395.1 |
|
ENSRNOT00000087777 | 2.917538 | 0.024331 | AABR07016845.1 |
Functional prediction of
differentially expressed mRNAs in MIRI
To investigate the spinal molecular mechanisms in
MIRI, GO and KEGG pathway analyses of the differentially expressed
mRNAs in MIRI vs. sham were performed.
To identify major biochemical metabolic pathways and
signal transduction pathways for differential gene enrichment, KEGG
pathway analysis (Fig. 3) was
conducted. Using the KEGG pathway analysis, it was found that
differential genes were mainly enriched in the ‘PI3K/Akt signaling
pathway’, ‘protein digestion and uptake’, ‘p53 signaling pathway’
and ‘neuroactive ligand-dependent body interaction’ (Fig. 3; Table
IV).
 | Table IVDifferential gene enriched in Kyoto
Encyclopedia of Genes and Genomes pathway. |
Table IV
Differential gene enriched in Kyoto
Encyclopedia of Genes and Genomes pathway.
| Pathway ID | Pathway | Gene number | P-value |
|---|
| ko04151 | PI3K/Akt signaling
pathway | 20 | 0.00039 |
| ko04974 | Protein digestion
and absorption | 8 | 0.001448 |
| ko04115 | p53 signaling
pathway | 7 | 0.001795 |
| ko04510 | Focal adhesion | 13 | 0.005058 |
| ko04080 | Neuroactive
ligand-receptor interaction | 16 | 0.005635 |
| ko04512 | ECM-receptor
interaction | 7 | 0.008425 |
| ko00534 | Glycosaminoglycan
biosynthesis-heparan sulfate/heparin | 3 | 0.019966 |
| ko04960 |
Aldosterone-regulated sodium
reabsorption | 4 | 0.023467 |
| ko04015 | Rap1 signaling
pathway | 12 | 0.027612 |
| ko04721 | Synaptic vesicle
cycle | 5 | 0.030579 |
| ko00600 | Sphingolipid
metabolism | 4 | 0.036733 |
| ko04020 | Calcium signaling
pathway | 11 | 0.03857 |
The differential genes associated with cellular
components were mainly enriched in the ‘extracellular fraction’,
‘cytoplasm’, ‘microtubule cytoskeleton’, ‘cytoskeleton’ and
‘intracellular’, whereas the genes participating in biological
processes were mainly enriched in ‘cell signal transduction’
(Fig. 4; Table V).
 | Table VFunctional classification of
differential genes via GO analysis. |
Table V
Functional classification of
differential genes via GO analysis.
| A, Cellular
component |
|---|
| GO ID | Description | Gene number | P-value |
|---|
| GO:0044424 | Intracellular
part | 48 | 0.013126 |
| GO:0005737 | Cytoplasm | 30 | 0.025862 |
| GO:0015630 | Microtubule
cytoskeleton | 3 | 0.026874 |
| GO:0005856 | Cytoskeleton | 7 | 0.041896 |
| GO:0005622 | Intracellular | 63 | 0.044992 |
| B, Biological
process |
| GO ID | Description | Gene number | P-value |
| GO:0030198 | Extracellular
matrix organization | 3 | 0.010728 |
| GO:0043062 | Extracellular
structure organization | 3 | 0.010729 |
| GO:0007267 | Cell-cell
signaling | 4 | 0.028507 |
| GO:0032196 | Transposition | 1 | 0.035381 |
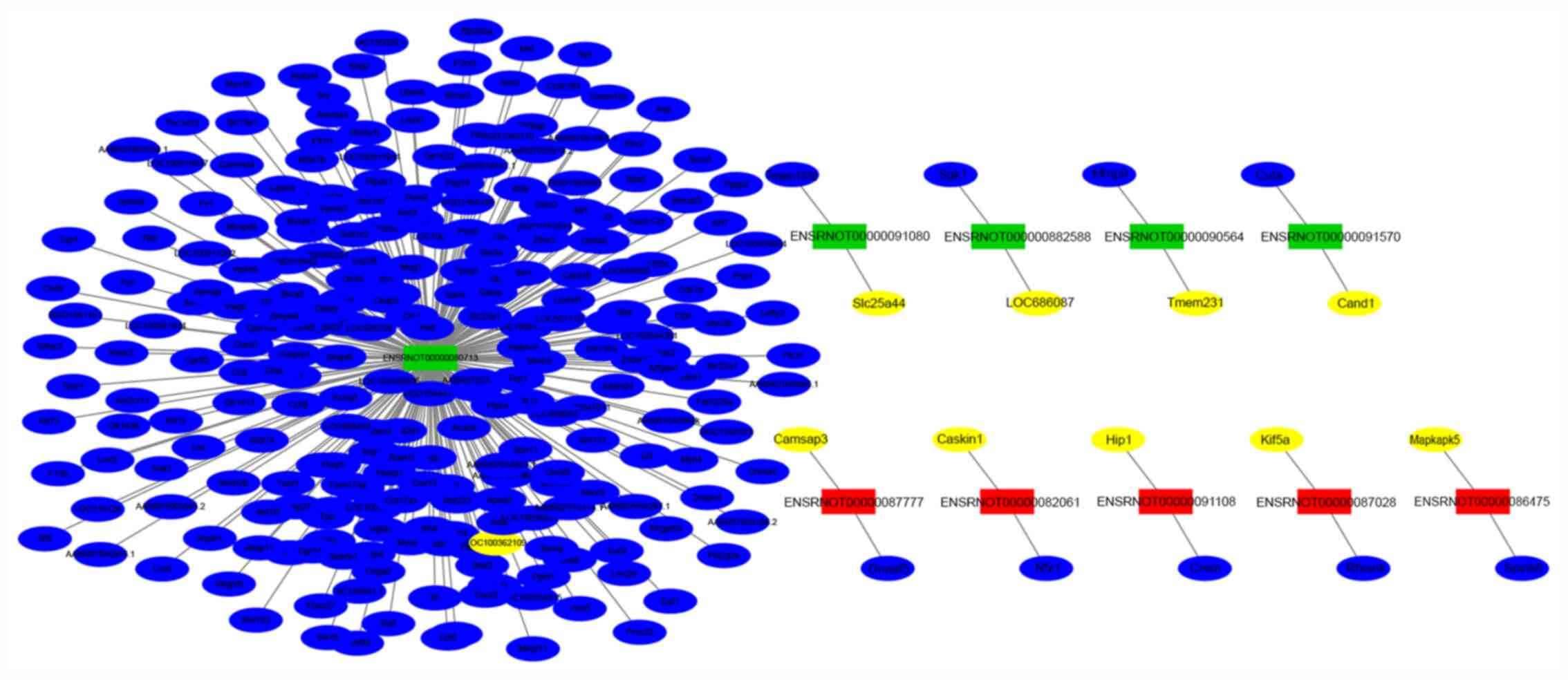
Gene co-expression networks were presented to
identify interactions between lncRNAs and their co-expression genes
(Fig. 5). The co-expressed network
showed that 10 highly-dysregulated lncRNAs were highly co-expressed
with 277 target genes, of which 10 were positively correlated
(colored with yellow) and 267 were negatively correlated (colored
with blue). ENSRNOT00000080713 was at the core of the lncRNA-mRNA
co-expression network and had a co-expression relationship with
many mRNAs.
RT-qPCR confirmation of mRNA and
lncRNA expression from microarray
To confirm the reliability of the microarray data
and analyze the temporal alterations in mRNA and lncRNA expression
after MIRI, the lncRNAs, ENSRNOT00000080713, ENSRNOT00000090564,
ENSRNOT00000082588, ENSRNOT00000091080, ENSRNOT00000091570,
ENSRNOT00000087777, ENSRNOT00000082061, ENSRNOT00000091108,
ENSRNOT00000087028 and ENSRNOT00000086475, and the mRNAs,
fibroblast growth factor receptor substrate 3 (Frs3), zinc finger
protein 76 (Zfp523), DnaJ heat shock protein family (Hsp40) member
C6 (Dnajc6), NEDD4 like E3 ubiquitin protein ligase (Nedd4l),
telomerase associated protein 1 (Tep1), myelin expression factor 2
(Myef2), transforming growth factor β receptor 1 (Tgfbr1),
fibroblast growth factor 12 (Fgf12), myocyte enhancer factor 2C
(Mef2c) and transcription factor Dp-1 (Tfdp1), were randomly
selected and detected via RT-qPCR.
The results demonstrated that expression of lncRNAs
ENSRNOT00000080713, ENSRNOT00000090564, ENSRNOT00000082588,
ENSRNOT00000091080 and ENSRNOT00000091570 were markedly decreased
in MIRI group compared with those in the control group, whilst
ENSRNOT00000087777, ENSRNOT00000082061, ENSRNOT00000091108,
ENSRNOT00000087028 and ENSRNOT00000086475 were markedly increased.
In addition, the mRNAs Frs3, Zfp523, Dnajc6, Nedd4l and Tep1 were
markedly decreased in the MIRI group compared with those in the
control group, but Myef2, Tgfbr1, Fgf12, Mef2c and Tfdp1 were
markedly increased. These RT-qPCR results were consistent with the
results from the transcriptome sequencing (Fig. 6), which further supported the
reliability of the microarray data.
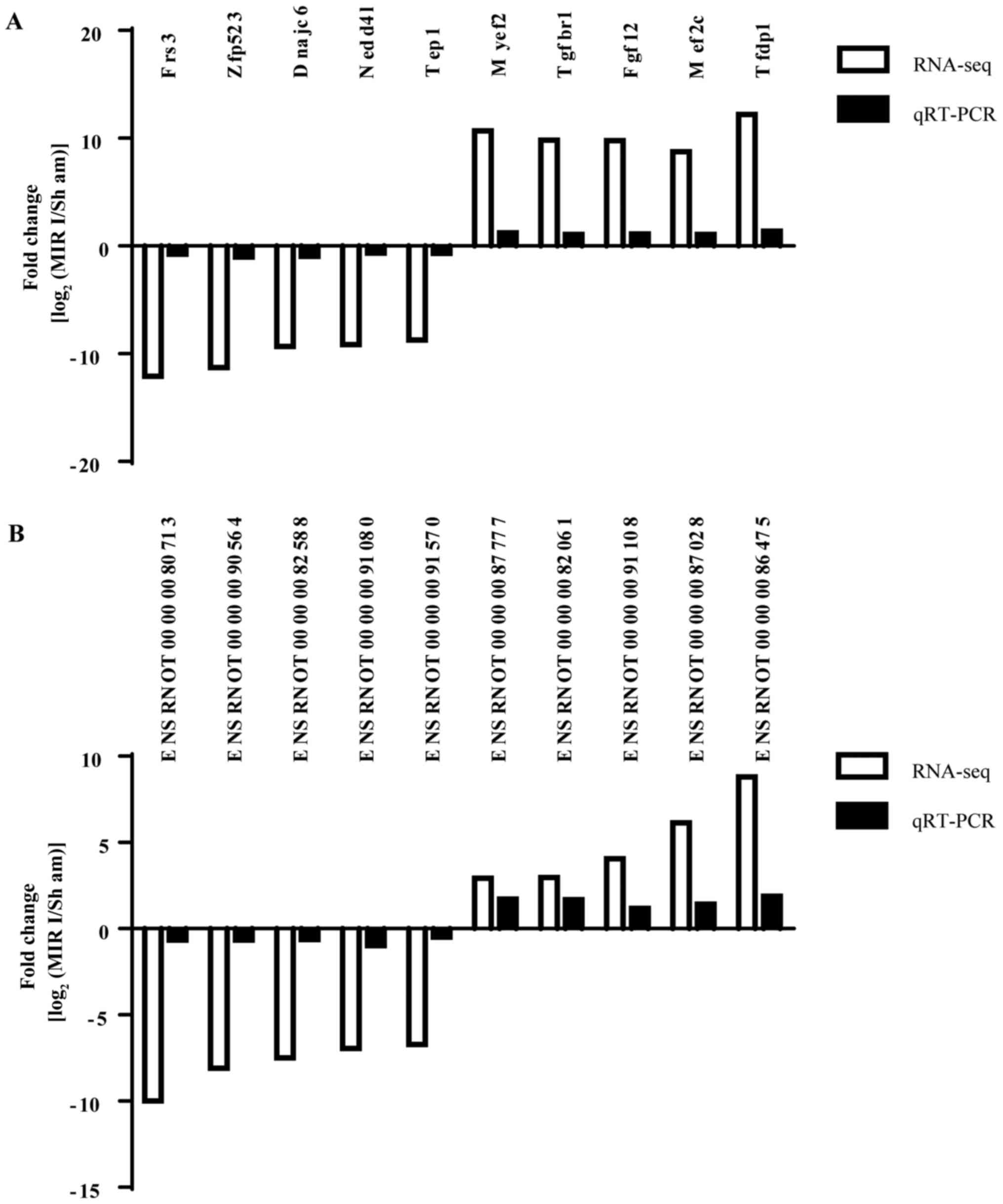 | Figure 6RT-qPCR confirmation of mRNA and
lncRNA expression from the microarray. (A) mRNA expression levels
in spinal cord tissue of MIRI rats. (B) lncRNA expression levels in
spinal cord tissue of MIRI rats. The expression of mRNAs and
lncRNAs in the spinal cord of rats with MIRI was consistent with
the results of transcriptome sequencing, indicating the reliability
of the microarray data. lncRNA, long non-coding RNA; Frs3,
fibroblast growth factor receptor substrate 3; Zfp523, zinc finger
protein 76; Dnajc6, DnaJ heat shock protein family (Hsp40) member
C6; Nedd4l, NEDD4 like E3 ubiquitin protein ligase; Tep1,
telomerase associated protein 1; Myef2, myelin expression factor 2;
Tgfbr1, transforming growth factor β receptor 1; Fgf12, fibroblast
growth factor 12; Mef2c, myocyte enhancer factor 2C; Tfdp1,
transcription factor Dp-1; RT-qPCR, reverse
transcription-quantitative PCR; seq, sequencing; MIRI, myocardial
ischemia-reperfusion injury. |
RT-qPCR validation of mRNAs and
lncRNAs in T1-T4 spinal cord
The results of RT-qPCR demonstrated that the mRNA
expression levels of Frs3, Nedd4l and Tep1 were significantly
downregulated in the model group, while the expression of mRNA
Myef2 was significantly upregulated in the model group. The
expression levels of mRNA Zfp523, Dnajc6 and Myef2 were not
significantly different between the two groups (Fig. 7). The expression levels of lncRNA
ENSRNOT00000080713, ENSRNOT00000090564, ENSRNOT00000082588 and
ENSRNOT00000091570 were significantly downregulated in the model
group, but the expression levels of lncRNA ENSRNOT00000087777,
ENSRNOT00000082061, ENSRNOT00000087028 and ENSRNOT00000086475 were
significantly upregulated in the model group. However, the
expression levels of lncRNA ENSRNOT00000091080 and
ENSRNOT00000091108 were not significantly different between the two
groups (Fig. 8).
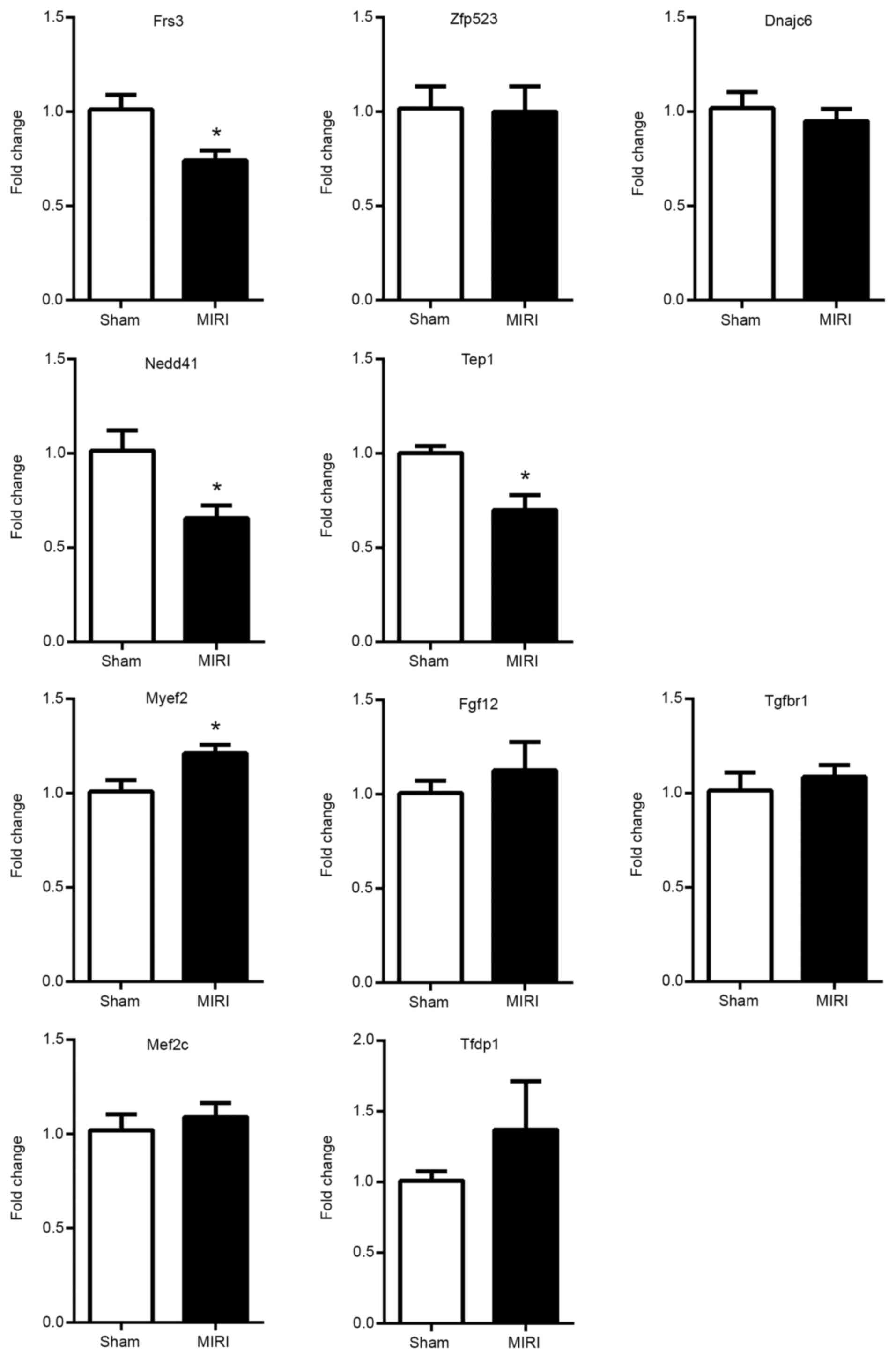 | Figure 7Reverse transcription-quantitative
PCR validation of the mRNAs in the T1-T4 spinal cord. The
expression levels of mRNA Frs3, Nedd4l and Tep1 were significantly
downregulated in model group, whereas the expression of mRNA Myef2
was significantly upregulated. The expression levels of mRNA
Zfp523, Dnajc6 and Myef2 were not statistically different between
the two groups. Data are presented as the mean ± SEM (n=6).
*P<0.05 vs. control. Frs3, fibroblast growth factor
receptor substrate 3; Zfp523, zinc finger protein 76; Dnajc6, DnaJ
heat shock protein family (Hsp40) member C6; Nedd4l, NEDD4 like E3
ubiquitin protein ligase; Tep1, telomerase associated protein 1;
Myef2, myelin expression factor 2; Tgfbr1, transforming growth
factor β receptor 1; Fgf12, fibroblast growth factor 12; Mef2c,
myocyte enhancer factor 2C; Tfdp1, transcription factor Dp-1; MIRI,
myocardial ischemia-reperfusion injury. |
Discussion
MIRI causes nociceptive chemical and electrical
signals that affect dorsal root ganglia and the upper thoracic
spinal cord (42). Previous studies
have reported that spinal gene expression is associated with in the
development of MIRI (9,27) and have identified the molecular
mechanisms during MIRI (26,27,42);
however, the impact of the spinal process during MIRI requires
further investigation.
Emerging evidence indicates that the inflammatory
and immune responses that occur in the spinal cord are vital
contributors to the progression of MIRI (9,13,14,16).
Moreover, autonomic dysregulation following MIRI is an important
pathogenic event (43-47).
Interactions between the heart and spinal autonomic nervous system
serve a role in both the development and maintenance of MIRI, and
are also crucial in myocardial ischemia-induced angina pectoris
(48-52).
Based on the importance of the spinal autonomic nervous system in
the pathophysiology of MIRI, there has been a notable effort in
this field to study changes in the genomics, transcriptomics,
proteomics and metabolomics in the spinal cord after MIRI, which
could facilitate both existing interventions and novel therapies
directed at cardiac-autonomic targets (53).
In the present study, the differentially expressed
lncRNAs in the T1-T4 spinal cord during MIRI, along with their
characteristics and possible relationships with mRNAs, were
identified. A total of 126 lncRNAs were observed in the T1-T4
spinal cord of the MIRI model rats. Among these lncRNAs, 41 lncRNAs
were upregulated, and 85 lncRNAs were downregulated at 2 h after
MIRI. Moreover, RT-qPCR was used to validate the microarray
analysis results, for which the conclusion was consistent. Based on
these findings, it was suggested that the aforementioned lncRNAs
may serve a role in MIRI pathology. Furthermore, the present
research contributes to understanding the molecular mechanism of
MIRI.
To further investigate the roles of these
differentially expressed lncRNAs in the development of MIRI, GO and
KEGG pathway analyses were performed. Based on the GO and KEGG
enrichment analyses of these lncRNAs, the results suggested that
the significantly enriched biologic processes and molecular
functions of the upregulated genes after MIRI were associated with
gene sets termed as follows: ‘PI3K/Akt signaling pathway’, ‘protein
digestion and absorption’, ‘p53 signaling pathway’ and ‘neuroactive
ligand-dependent body interaction’.
The PI3K/Akt signaling pathway is associated with
MIRI (54). p53 is a tumor
suppressor, which exerts an important role in the process of
apoptosis and is associated with the occurrence and development of
cardiovascular disease (55-57).
Previous studies have shown that apoptosis is a typical
manifestation and primary pathological mechanism of MIRI (58). The loss of cardiomyocytes caused by
apoptosis is an important reason for the development of various
heart diseases (59). Preventing
the process of apoptosis can prevent myocardial damage caused by
reperfusion, thus slowing or preventing the occurrence of heart
failure and even mortality (60).
The present study has several limitations. For
example, the reperfusion time for tissue collection lasted only 2
h. Therefore, it remains unknown if the expression of lncRNA has
the same regulation for a longer period after ischemia. Moreover, a
functional analysis of differentially expressed genes was performed
without further verification. LncRNAs are poorly conserved and only
part of the sequence is conserved by multiple species (61,62).
Previous studies have reported that there are 481 segments longer
>200 bp (range, 200-779 bp) that are conserved between
orthologous regions of the mouse, rat and human genomes (63), and these conserved lncRNAs can
provide an insight into their key functional roles (64,65).
As a result, further research is required to examine the functions
of lncRNAs and the conservation of differentially expressed lncRNAs
in rats and humans, which will identify their predicted targets and
provide a detailed expression profile of lncRNA.
In conclusion, the present study demonstrated that
lncRNA transcripts of the upper thoracic spinal cord were
differentially expressed after MIRI. These spinal lncRNAs may
regulate the expression of MIRI-related proteins and genes, and
contribute to the pathogenesis of MIRI. Future investigations of
these spinal lncRNAs found in the present study will mainly focus
on their functions and their relationship with MIRI. Nonetheless,
the present study may provide important evidence for further basic
research and clinical investigations on improved therapeutic
interventions of MIRI.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by grants from National Natural
Science Foundation of P.R. China (grant nos. 82070302, 81873467 and
81770283), the Clinical Medical Research Center of Peritoneal
Cancer of Wuhan (grant no. 2015060911020462), Research Foundation
of Health and Family Planning Commission of Hubei Province (grant
nos. WJ2015MA010 and WJ2017M249), Natural Science Foundation of
Hubei Province (grant no. 2015CFA027) and the National Natural
Science Foundation of Hubei Province (grant no. 2016CFB625).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZXL and YJL performed the surgical procedures and
PCR experiments. QW and ZGH participated in the experimental
design. MHF and ZGH analyzed the data. HBX and MHF contributed to
the study concept and design and supervised the whole project. All
authors contributed to the final manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Ethical Committee of Tongji Hospital, Tongji Medical College,
Huazhong University of Science and Technology (approval no.
TJ-A20150804).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Khan MA, Hashim MJ, Mustafa H, Baniyas MY,
Al Suwaidi SK, AlKatheeri R, Alblooshi FM, Almatrooshi ME, Alzaabi
ME, Al Darmaki RS and Lootah SN: Global epidemiology of ischemic
heart disease: Results from the global burden of disease study.
Cureus. 12(e9349)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Roth GA, Johnson C, Abajobir A, Abd-Allah
F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, et al:
Global, regional, and national burden of cardiovascular diseases
for 10 causes, 1990 to 2015. J Am Coll Cardiol. 70:1–25.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yellon DM and Hausenloy DJ: Myocardial
reperfusion injury. N Engl J Med. 357:1121–1135. 2007.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Grace PA: Ischemia-reperfusion injury. Br
J Surg. 81:637–647. 1994.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cheng YF, Chang YT, Chen WH, Shih HC, Chen
YH, Shyu BC and Chen CC: Cardioprotection induced in a mouse model
of neuropathic pain via anterior nucleus of paraventricular
thalamus. Nat Commun. 8(826)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Xu A, Song Z, Peng Y and Xiang H: Change
of mitochondrial function in the early stage after cardiac
ischemia-reperfusion injury in mice. Int J Clin Exp Med.
9:2549–2554. 2016.
|
|
7
|
De Hert S and Moerman A: Myocardial injury
and protection related to cardiopulmonary bypass. Best Pract Res
Clin Anaesthesiol. 29:137–149. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Murphy E and Steenbergen C: Mechanisms
underlying acute protection from cardiac ischemia-reperfusion
injury. Physiol Rev. 88:581–609. 2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Saddic LA, Howard-Quijano K, Kipke J, Kubo
Y, Dale EA, Hoover D, Shivkumar K, Eghbali M and Mahajan A:
Progression of myocardial ischemia leads to unique changes in
immediate-early gene expression in the spinal cord dorsal horn. Am
J Physiol Heart Circ Physiol. 315:H1592–H1601. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Waldron NH, Fudim M, Mathew JP and Piccini
JP: Neuromodulation for the treatment of heart rhythm disorders.
JACC Basic Transl Sci. 4:546–562. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shen MJ and Zipes DP: Role of the
autonomic nervous system in modulating cardiac arrhythmias. Circ
Res. 114:1004–1021. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hua F, Ardell JL and Williams CA: Left
vagal stimulation induces dynorphin release and suppresses
substance P release from the rat thoracic spinal cord during
cardiac ischemia. Am J Physiol Regul Integr Comp Physiol.
287:R1468–R1477. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Steagall RJ, Sipe AL, Williams CA, Joyner
WL and Singh K: Substance p release in response to cardiac ischemia
from rat thoracic spinal dorsal horn is mediated by trpv1.
Neuroscience. 214:106–119. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ding X, Ardell JL, Hua F, McAuley RJ,
Sutherly K, Daniel JJ and Williams CA: Modulation of cardiac
ischemia-sensitive afferent neuron signaling by preemptive C2
spinal cord stimulation: Effect on substance P release from rat
spinal cord. Am J Physiol Regul Integr Comp Physiol. 294:R93–R101.
2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gao C, Howard-Quijano K, Rau C, Takamiya
T, Song Y, Shivkumar K, Wang Y and Mahajan A: Inflammatory and
apoptotic remodeling in autonomic nervous system following
myocardial infarction. PLoS One. 12(e0177750)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Niu YL, Guo Z and Zhou RH: Up-Regulation
of TNF-alpha in neurons of dorsal root ganglia and spinal cord
during coronary artery occlusion in rats. Cytokine. 47:23–29.
2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Guo Z, Niu YL, Zhang JW and Yao TP:
Coronary artery occlusion alters expression of substance P and its
mRNA in spinal dorsal horn in rats. Neuroscience. 145:669–675.
2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang Q, He ZG, Li ZX, Li SY, Chen YL, Feng
MH, Hong QX and Xiang HB: Bioinformatics analysis of gene
expression profile data to screen key genes involved in cardiac
ischemia-reperfusion injury. Int J Clin Exp Med. 11:4955–4966.
2018.
|
|
19
|
Trapnell C, Roberts A, Goff L, Pertea G,
Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL and Pachter L:
Differential gene and transcript expression analysis of RNA-seq
experiments with TopHat and Cufflinks. Nat Protoc. 7:562–578.
2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Trapnell C, Williams BA, Pertea G,
Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ and Pachter
L: Transcript assembly and quantification by RNA-Seq reveals
unannotated transcripts and isoform switching during cell
differentiation. Nat Biotechnol. 28:U511–U174. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Chen M, Li ZX, Wang Q and Xiang HB:
Altered expression of differential genes in thoracic spinal cord
involved in experimental cholestatic itch mouse model. Curr Med
Sci. 38:679–683. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang Q, Li ZX, Liu BW, He ZG, Liu C, Chen
M, Liu SG, Wu WZ and Xiang HB: Altered expression of differential
gene and lncRNA in the lower thoracic spinal cord on different time
courses of experimental obstructive jaundice model accompanied with
altered peripheral nociception in rats. Oncotarget.
8:106098–106112. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu QQ, Liu H, He ZG, Zhang SJ, Liu BW,
Wang L, Qiu WH, Xu Q, Xiang HB and Lv YM: Differential gene and
lncRNA expression in the lower thoracic spinal cord following
ischemia/reperfusion-induced acute kidney injury in rats.
Oncotarget. 8:53465–53481. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang QL, Ai HZ, Liu JL, Xu M, Zhou Z, Qian
C, Xie Y and Yan J: Characterization of novel lnc RNAs in the
spinal cord of rats with lumbar disc herniation. J Pain Res.
12:501–512. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
National Research Council (US) Institute
for Laboratory Animal Research. In: Guide for the Care and Use of
Laboratory Animals. National Academies Press (US), Washington, DC,
1996.
|
|
26
|
Wang Q, Li ZX, Li YJ, Manyande A, Li SY,
Feng MH, Wu DZ and Xiang HB: Alterations in amino acid levels and
metabolite ratio of spinal cord in rat with myocardial
ischemia-reperfusion injury by proton magnetic resonance
spectroscopy. Am J Transl Res. 11:3101–3108. 2019.PubMed/NCBI
|
|
27
|
Wang Q, Li ZX, Li YJ, He ZG, Chen YL, Feng
MH, Li SY, Wu DZ and Xiang HB: Identification of lncRNA and mRNA
expression profiles in rat spinal cords at various timepoints
following cardiac ischemia/reperfusion. Int J Mol Med.
43:2361–2375. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Pan XC, Li ZX, Wu DZ, Li SY, Xiang HB and
Song YT: Mapping changes of whole brain blood flow in rats with
myocardial ischemia/reperfusion injury assessed by positron
emission tomography. Curr Med Sci. 39:653–657. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li SY, Li ZX, He ZG, Wang Q, Li YJ, Yang
Q, Wu DZ, Zeng HL and Xiang HB: Quantitative proteomics reveal the
alterations in the spinal cord after myocardial
ischemia-reperfusion injury in rats. Int J Mol Med. 43:1877–1887.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Xu Z, Alloush J, Beck E and Weisleder N: A
murine model of myocardial ischemia-reperfusion injury through
ligation of the left anterior descending artery. J Vis Exp.
10(51329)2014.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Liu S, Yang Y, Song YQ, Geng J and Chen
QL: Protective effects of N(2)LalanylLglutamine mediated by the
JAK2/STAT3 signaling pathway on myocardial ischemia reperfusion.
Mol Med Rep. 17:5102–5108. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liu BW, Li ZX, He ZG, Wang Q, Liu C, Zhang
XW, Yang H and Xiang HB: Altered expression of itch-related
mediators in the lower cervical spinal cord in mouse models of two
types of chronic itch. Int J Mol Med. 44:835–846. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liu QQ, Liu H, He ZG, Zhang SJ, Liu BW,
Wang L, Qiu WH, Xu Q, Xiang HB and Lv YM: Differential gene and
lncRNA expression in the lower thoracic spinal cord following
ischemia/reperfusion-induced acute kidney injury in rats.
Oncotarget. 8:53465–53481. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Pan N, Bhatti MZ, Zhang H, Ni B, Fan X and
Chen J: The encystment-related micrornas and its regulation
molecular mechanism in pseudourostyla cristata revealed by high
throughput small RNA sequencing. Int J Mol Sci.
21(2309)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sun H, Wang J, Que J, Peng Y, Yu Y, Wang
L, Ye H, Huang K, Xue Y, Zhou Y and Ji K: RNA sequencing revealing
the role of AMP-activated protein kinase signaling in mice
myocardial ischemia reperfusion injury. Gene. 703:91–101.
2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ke C, Gao F, Tian X, Li C, Shi D, He W and
Tian Y: Slit2/Robo1 mediation of synaptic plasticity contributes to
bone cancer pain. Mol Neurobiol. 54:295–307. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liu BW, Li ZX, He ZG, Liu C, Xiong J and
Xiang HB: Altered expression of target genes of spinal cord in
different itch models compared with capsaicin assessed by RT-qPCR
validation. Oncotarget. 8:74423–74433. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Fu Q, Shi D, Zhou Y, Zheng H, Xiang H,
Tian X, Gao F, Manyande A, Cao F, Tian Y and Ye D: MHC-I promotes
apoptosis of GABAergic interneurons in the spinal dorsal horn and
contributes to cancer induced bone pain. Exp Neurol. 286:12–20.
2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Guan XH, Fu QC, Shi D, Bu HL, Song ZP,
Xiong BR, Shu B, Xiang HB, Xu B, Manyande A, et al: Activation of
spinal chemokine receptor CXCR3 mediates bone cancer pain through
an Akt-ERK crosstalk pathway in rats. Exp Neurol. 263:39–49.
2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Xu B, Guan XH, Yu JX, Lv J, Zhang HX, Fu
QC, Xiang HB, Bu HL, Shi D, Shu B, et al: Activation of spinal
phosphatidylinositol 3-kinase/protein kinase B mediates pain
behavior induced by plantar incision in mice. Exp Neurol.
255:71–82. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Huang C, Wang J, Wang N, Du F, Xiong W,
Qian J, Zhong K, Cai A, Xu S, Huang J, et al: Effect of myocardial
ischemic preconditioning on ischemia-reperfusion
stimulation-induced activation in rat thoracic spinal cord with
functional MRI. Int J Cardiol. 285:59–64. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Rajendran PS, Nakamura K, Ajijola OA,
Vaseghi M, Armour JA, Ardell JL and Shivkumar K: Myocardial
infarction induces structural and functional remodelling of the
intrinsic cardiac nervous system. J Physiol. 594:321–341.
2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Huang WA, Boyle NG and Vaseghi M: Cardiac
innervation and the autonomic nervous system in sudden cardiac
death. Card Electrophysiol Clin. 9:665–679. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Dae MW, Lee RJ, Ursell PC, Chin MC,
Stillson CA and Moise NS: Heterogeneous sympathetic innervation in
German shepherd dogs with inherited ventricular arrhythmia and
sudden cardiac death. Circulation. 96:1337–1342. 1997.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Stramba-Badiale M, Lazzarotti M and
Schwartz PJ: Development of cardiac innervation, ventricular
fibrillation, and sudden infant death syndrome. Am J Physiol.
263:H1514–H1522. 1992.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Schwartz PJ: Cardiac sympathetic
innervation and the prevention of sudden death. Cardiologia.
35:51–54. 1990.PubMed/NCBI
|
|
48
|
Scalercio L, Vitter J and Elliott CE:
Placement of a continuous stellate ganglion block for treatment of
refractory ventricular fibrillation in the setting of known
prinzmetal angina during pregnancy: A case report. A A Pract.
12:106–108. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Imran TF, Malapero R, Qavi AH, Hasan Z, de
la Torre B, Patel YR, Yong RJ, Djousse L, Gaziano JM and
Gerhard-Herman MD: Efficacy of spinal cord stimulation as an
adjunct therapy for chronic refractory angina pectoris. Int J
Cardiol. 227:535–542. 2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Dobias M, Michalek P, Neuzil P, Stritesky
M and Johnston P: Interventional treatment of pain in refractory
angina. A review. Biomed Pap Med Fac Univ Palacky Olomouc Czech
Repub. 158:518–527. 2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Deer TR, Mekhail N, Provenzano D, Pope J,
Krames E, Thomson S, Raso L, Burton A, DeAndres J, Buchser E, et
al: The appropriate use of neurostimulation of the spinal cord and
peripheral nervous system for the treatment of chronic pain and
ischemic diseases: The neuromodulation appropriateness consensus
committee. Neuromodulation. 17:515–550. 2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Sylvén C: Neurophysiological aspects of
angina pectoris. Z Kardiol. 86:95–105. 1997.PubMed/NCBI
|
|
53
|
Pan X, Bao H, Si Y, Xu C, Chen H, Gao X,
Xie X, Xu Y, Sun F and Zeng L: Spinal cord stimulation for
refractory angina pectoris: A systematic review and meta-analysis.
Clin J Pain. 33:543–551. 2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Hombach V, Grebe O, Merkle N, Waldenmaier
S, Höher M, Kochs M, Wöhrle J and Kestler HA: Sequelae of acute
myocardial infarction regarding cardiac structure and function and
their prognostic significance as assessed by magnetic resonance
imaging. Eur Heart J. 26:549–557. 2005.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Bär FW, Tzivoni D, Dirksen MT,
Fernández-Ortiz A, Heyndrickx GR, Brachmann J, Reiber JH, Avasthy
N, Tatsuno J, Davies M, et al: Results of the first clinical study
of adjunctive CAldaret (MCC-135) in patients undergoing primary
percutaneous coronary intervention for ST-elevation myocardial
infarction: The randomized multicentre CASTEMI study. Eur Heart J.
27:2516–2523. 2006.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Hearse DJ, Humphrey SM and Chain EB:
Abrupt reoxygenation of the anoxic potassium-arrested perfused rat
heart: A study of myocardial enzyme release. J Mol Cell Cardiol.
5:395–407. 1973.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Herzog WR, Vogel RA, Schlossberg ML,
Edenbaum LR, Scott HJ and Serebruany VL: Short-Term low dose
intracoronary diltiazem administered at the onset of reperfusion
reduces myocardial infarct size. Int J Cardiol. 59:21–27.
1997.PubMed/NCBI View Article : Google Scholar
|
|
58
|
De Stefani D, Raffaello A, Teardo E, Szabò
I and Rizzuto R: A forty-kilodalton protein of the inner membrane
is the mitochondrial calcium uniporter. Nature. 476:336–340.
2011.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Hausenloy DJ and Yellon DM: The
mitochondrial permeability transition pore: Its fundamental role in
mediating cell death during ischaemia and reperfusion. J Mol Cell
Cardiol. 35:339–341. 2003.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Argaud L, Gateau-Roesch O, Muntean D,
Chalabreysse L, Loufouat J, Robert D and Ovize M: Specific
inhibition of the mitochondrial permeability transition prevents
lethal reperfusion injury. J Mol Cell Cardiol. 38:367–374.
2005.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Pang KC, Frith MC and Mattick JS: Rapid
evolution of noncoding RNAs: Lack of conservation does not mean
lack of function. Trends Genet. 22:1–5. 2006.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Wang P, Luo ML, Song E, Zhou Z, Ma T, Wang
J, Jia N, Wang G, Nie S, Liu Y and Hou F: Long noncoding RNA
lnc-TSI inhibits renal fibrogenesis by negatively regulating the
TGF-beta/smad3 pathway. Sci Transl Med. 10:2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Bejerano G, Pheasant M, Makunin I, Stephen
S, Kent WJ, Mattick JS and Haussler D: Ultraconserved elements in
the human genome. Science. 304:1321–1325. 2004.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Li D and Yang MQ: Identification and
characterization of conserved lncRNAs in human and rat brain. BMC
Bioinformatics. 18(489)2017.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Sun Y, Fan W, Xue R, Dong B, Liang Z, Chen
C, Li J, Wang Y, Zhao J, Huang H, et al: Transcribed ultraconserved
regions, Uc.323, ameliorates cardiac hypertrophy by regulating the
transcription of CPT1b (Carnitine Palmitoyl transferase 1b).
Hypertension. 75:79–90. 2020.PubMed/NCBI View Article : Google Scholar
|