Introduction
Osteoporotic fractures are the most severe
complications related to osteoporosis, which is characterized by
skeletal fragility and microarchitectural deterioration of bone
tissue (1). Previous studies have
shown that elderly female patients are at a higher risk of delayed
union and nonunion fractures (2);
thus, alternative techniques and treatments for osteoporotic
fractures have gained increasing research interest over recent
years.
Antiresorptive pharmacological agents, such as
bisphosphonates and estrogen, can improve bone health and reduce
the risk of fractures (3).
Nevertheless, their effect on fracture healing remains unclear. For
example, bisphosphonates cannot stimulate new bone formation, and
long-term use of bisphosphonates can delay fracture healing and
lead to a prolonged presence of callus and delayed callus
remodeling (4,5). Therefore, the delayed administration
of bolus-dose bisphosphonates may be optimal for the treatment of
fragility fractures (6,7). Due to an increased risk of breast
carcinoma, endometrial cancer and cardiovascular disease, estrogen
is no longer recommended as first-line therapy for osteoporosis
according to new guidelines (8,9).
Additionally, due to the adverse effects related to synthetic
drugs, there is an increased demand for alternative or natural
medicines for the treatment of osteoporosis and associated
complications. Previous studies have shown that phytoestrogens,
which can be found in certain traditional Chinese medicines, have
anti-osteoporotic potential (10-12).
Psoralen is an active component extracted from
Psoraleacorylifolia that belongs to the phytoestrogen group
of the furanocoumarin class of compounds (13). Previous in vitro studies have
revealed that psoralen may induce the proliferation of osteoblasts,
increase the expression of osteoprotegerin (OPG) and decrease the
expression levels of receptor activator of nuclear factor-κB ligand
(RANKL) to suppress the differentiation and maturation of
osteoclasts (14,15). In sex hormone deficiency-induced
osteoporosis animal models, the administration of psoralen at
different doses (10 and 20 mg/kg) significantly increased the
values of alkaline phosphatase/tartrate-resistant acid phosphatase
(TRACP) and bone volume (BV)/tissue volume (TV) in intact bone,
exhibiting anti-osteoporotic effects in female and male mice after
8 weeks (16). Furthermore, in a
previous study by the authors of the current study, it was found
that the administration of 20 mg/kg psoralen for 6 weeks could
increase the levels of serum alkaline phosphatase and improve bone
biomechanics in ovariectomized (OVX) mice (17). Due to these properties, psoralen
could be considered as an alternative option for the management of
osteoporosis; nevertheless, to the best of our knowledge, its
effect on fracture healing has not yet been examined.
In the present study, a standardized femoral
osteotomy model and bone fixation with an intramedullary pin was
used in the OVX mouse model to mimic the condition in
post-menopausal women. The effects of psoralen on the osteoporotic
fracture healing process and the underlying mechanisms were
examined.
Materials and methods
Establishment of an osteoporosis model
in mice
A total of 7-10-week-old, 20 g, female C57BL/6 mice
were obtained from the Hubei Research Center of Laboratory Animals.
All animals were housed in an environment with a temperature of
22±1˚C, a relative humidity of 50±1% and a light/dark cycle of
12/12 h. Animals were allowed free access to water and food
pellets. In addition, all animal studies, including the euthanasia
procedure, were approved and performed in compliance with the
regulations and guidelines of the Medical Ethical Committee of the
First Affiliated Hospital of Yangtze University (approval no.
2017-060; Jingzhou, China) and conducted according to The
Association for Assessment and Accreditation of Laboratory Animal
Care International and the Institutional Animal Care and Use
Committee guidelines (18).
When the mice reached 12 weeks of age, 10 mice were
used initially to check whether the OVX model could be successfully
established and subsequently, 60 mice were used for further
experiments. A total of 10 mice were randomly divided into two
groups (n=5/per group): Sham-operated group and OVX group. Under
anesthesia, the sham-operated group mice were subjected to a
bilateral laparotomy, without affecting the ovaries. OVX group was
subject to bilateral OVX using the dorsal approach. After surgery,
mice were left untreated for 6 weeks, following which all the mice
in each group were sacrificed. The successful establishment of the
OVX model was confirmed via atrophy of gonads and femoral
metaphysis determined via histological evaluation.
Experimental design
Once the successful establishment of the OVX model
was confirmed, 60 OVX mice were divided into two subgroups: i) OVX
group, treated with physiological saline solution; and ii)
psoralen+OVX group treated with 20 mg/kg psoralen (≥98% purity;
Yongjian Pharmaceutical Co., Ltd.). These drug doses (20 mg/kg
psoralen) have been shown to promote bone metabolism and
biomechanics in ovariectomized mice (14). Under anesthesia, a standardized
fracture was created on the mid diaphysis of the left femur in the
OVX and psoralen+OVX groups, and stabilized with an intramedullary
pin, as described below. After osteotomy, saline and psoralen
treatments were given via an oral gavage route for a total of 21
days.
Surgical protocol
Mice were anesthetized by intraperitoneal injection
of ketamine hydrochloride (100 mg/kg body weight) and xylazine (20
mg/kg body weight). After successful anesthesia and sterilization,
the skin and thigh muscle were incised to expose the midshaft of
the femur. Transverse-shaped osteotomy was performed on the middle
femoral shaft with a 0.3-mm diameter micro-high-speed milling
cutter. At the same time, saline solutions were used for local
washing and cooling. Then, the fracture was reduced, following
which a retrograde intramedullary pin fixation (0.55 mm in
diameter) was performed. The muscular layer and skin were sutured.
All of the animals received an intramuscular antibiotic (10 mg/kg
penicillin) and analgesic (1 mg/kg meloxicam) injection for three
days after the operation.
Psoralen
Psoralen powder was ground and suspended in
physiological saline to achieve a final concentration of 2 mg/ml.
The psoralen+OVX group was treated with psoralen at a dose of 20
mg/kg/day from the first post operative day.
Specimen collection and
preparation
Mice were sacrificed at 10 days (n=15/per group) or
21 days (n=15/per group) post-surgery to assess the endochondral
ossification phase and the bone remodeling stage of fracture
healing. The osteotomized femurs were evaluated using X-ray,
micro-CT, histology and immunohistochemistry. Fracture callus
tissues were harvested for reverse transcription-quantitative
(RT-q) PCR and western blot analysis at 10 and 21 days.
X-ray and micro-CT analysis of
fracture healing
Radiographs were taken 21 days after fracture to
evaluate the fracture healing, using an X-ray mammography system
(Siemens AG) with an exposure of 39 kV for 32 sec. On day 21,
micro-CT was performed using a Scanco Micro-CT 60 system (Scanco
Medical AG) and a previously described method (19). Briefly, the range covered 10 mm of
the callus; the fracture in the center was scanned and images were
reconstructed to nominal 12-mm voxel size. Only the newly formed
callus was used to define the tissue perimeters as the volume of
interest. Two fixed, global thresholds with the lower limit
corresponding to a mineral density of 421 mg
hydroxyapatite/cm3 and the upper limit corresponding to
3,000 mg hydroxyapatite/cm3 were used to distinguish
mineralized from non-mineralized tissues. Quantitative parameters
used for evaluation included TV, BV, BV/TV and tissue mineral
density (TMD).
Histology and immunohistochemical
examination
The fractured femurs were harvested. Soft tissue was
removed without disturbing the callus, and the fractured femurs
were fixed in 10% buffered formalin for 48 h in 4˚C. The
formalin-fixed samples were decalcified in 10% EDTA and then
embedded in paraffin. Sections of 5 µm were cut in the sagittal
plane, and analyzed using the following approach. Samples were
stained with hematoxylin and eosin (H&E) for basic morphology
or with tartrate-resistant acid phosphatase (TRAP) to analyze the
osteoclast cells. In the same mice, a total of six slides were
analyzed. Three specimens were used for HE staining and TRAP
staining, respectively. Osteoclasts were identified as TRACP+cells
with ≥three nuclei. Finally, three fields of view (magnification,
x200) were randomly selected for each slice. The number of stained
cells in each field was calculated using Image-Pro Plus 6.0
analysis software (Media Cybernetics, Inc.). Immunohistochemical
staining included a standard immunohistochemical procedure for bone
morphogenetic protein-2 (BMP-2), estrogen receptor (ER)-α and ER-β
protein expression that was performed on the paraffin sections
obtained on day 21. In brief, Paraffin sections were dewaxed by
successively placing the slices into xylene I, II and III (each for
15 min), and then water ethanol I, absolute ethanol II, 85% alcohol
and 75% alcohol (each for 5 min). Samples were then washed with
distilled water. Tissue sections were treated with citric acid
antigen repair buffer (pH 6.0) in a microwave oven for antigen
retrieval. Samples were subjected to moderate heating in the
microwave for 8 mins to boil, left to rest for 8 min and then
turned to medium low heating for 7 min. This process should prevent
the buffer excessive evaporation, do not dry tablets. After natural
cooling, the slides were placed in PBS (pH 7.4) and shaken on the
decolorizing shaker. The samples were washed in PBS (pH 7.4) in
triplicate, with each wash lasting for 5 min. Endogenous peroxidase
was quenched using 3% hydrogen peroxide for 10 min, and nonspecific
binding of epitopes was blocked using 3% BSA for 30 min at room
temperature. Slides were incubated at 4˚C overnight with the
primary antibodies for BMP-2 (1:500; cat. no. GB11252), ER-α
(1:200; cat. no. GB13205) and ER-β (1:500; cat. no. GB13003), all
purchased from Wuhan Servicebio Technology Co., Ltd. Tissues were
incubated at room temperature for 50 min with goat anti-mouse IgG
secondary antibodies (cat. no. G1214; Wuhan Servicebio Technology
Co., Ltd.; 1:200;) and Morphometric analysis was performed by
measuring the average relative optical staining areas using
Image-Pro Plus 6.0 analysis software.
All stained sections were analyzed under a light
microscope (Leica RM2156; Leica Microsystems GmbH; magnification,
x200). The region of interest was selected as the center of the
fracture, encompassing an entire cross-sectional area of a
callus.
Callus mRNA quantification by
RT-qPCR
Total RNA was extracted from the fracture callus and
1 mm of normal bone margin from each femur, and purified using RNA
extracting solution (cat. no. G3013; Wuhan Servicebio Technology
Co., Ltd.). The total RNA concentration was determined using a
NanoDrop™ 2000 (NanoDrop Technologies; Thermo Fisher Scientific,
Inc.), then stored at -80˚C until further processing. Reverse
transcription was performed with a commercial RevertAid First
Strand cDNA Synthesis kit (cat. no. K1622; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Relative quantification of mRNA was performed using FastStart
Universal SYBR-Green Master (Rox) (cat. no. 04913914001; Roche
Diagnostics GmbH) and ABI real-time PCR System (StepOnePlus™;
Applied Biosystems; Thermo Fisher Scientific, Inc.). RT-qPCR assays
for BMP-2, RANKL and OPG were performed using the primer sequences
listed in Table I. All gene
expression levels were normalized to β-actin. The thermocycling
conditions for qPCR were as follows: Initial denaturation at 95˚C
for 2 min; followed by 40 cycles of denaturation at 95˚C for 10
sec, annealing and elongation at 60˚C for 30 sec; with a final
extension step at 72˚C for 30 sec. For qPCR, cDNA was used for
amplification of the target genes in triplicate. Relative levels of
BMP-2, RANKL and OPG were measured using β-actin as a reference
gene (20). Expression levels were
determined using the 2-ΔΔCq formula (21).
 | Table IOligonucleotide primer sequences for
reverse transcription-quantitative PCR analysis of gene
expression. |
Table I
Oligonucleotide primer sequences for
reverse transcription-quantitative PCR analysis of gene
expression.
| Gene | NCBI accession
number | Sequence
(5'"3') |
|---|
| β-actin | NM_007393.3 | F:
GTGACGTTGACATCCGTAAAGA |
| | | R:
GTAACAGTCCGCCTAGAAGCAC |
| BMP-2 | NM_007553.3 | F:
CGAATTTGAGTTGAGGCTGCTC |
| | | R:
GCCGTTTTCCCACTCATCTCT |
| RANKL | NM_011613.3 | F:
CCATCGGGTTCCCATAAAGTCA |
| | | R:
CAGTTTTTCGTGCTCCCTCCTT |
| OPG | NM_008764.3 | F:
CCAGATGGGTTCTTCTCAGGTG |
| | | R:
GTCCACCAAAACACTCAGCCAA |
Western blotting
The callus tissue was lysed in RIPA buffer (cat. no.
G2002; Wuhan Servicebio Technology Co., Ltd.) and protease
inhibitor cocktail (cat. no. G2006; Wuhan Servicebio Technology
Co., Ltd.). Protein concentrations were determined using the BCA
protein quantitative detection kit according to the manufacturer's
instructions (cat. no. G2026; Wuhan Servicebio Technology Co.,
Ltd.). Equivalent amounts of protein (25 µg) were size-fractionated
using 10% SDS-PAGE (cat. no. G2003; Wuhan Servicebio Technology
Co., Ltd.) and electrotransferred onto Protran®
nitrocellulose membranes (cat. no. IPVH00010; EMD Millipore). The
membranes were then blocked with 5% non-fat milk in Tris-buffered
saline for 10 h at room temperature. Consequently, the membranes
were incubated at 4˚C overnight with anti-mouse ER-α (cat. no.
MS-354-P0; Thermo Fisher Scientific, Inc.; 1:1,000), rabbit
anti-mouse ER-β (cat. no. 14007-1-AP; ProteinTech Group, Inc.;
1:1,000) and anti-mouse β-actin (cat. no. GB12001; Wuhan Servicebio
Technology Co., Ltd.; 1:3,000) antibodies, and subsequently with
certain horseradish peroxidase-conjugated secondary antibodies,
including ER-α (cat. no. GB11205; Wuhan Servicebio Technology Co.,
Ltd. 1:3,000), β-actin (cat. no. GB12001, Wuhan Servicebio
Technology Co., Ltd. 1:3,000) and ER-β (cat. no. 14007-1-AP; Wuhan
Sanying Biotechnology. 1:3,000) at room temperature for 30 min.
Proteins were visualized using the enhanced chemiluminescence
detection system solution (EMD Millipore). The intensity of the
specific bands was semi-quantified by densitometry using Alpha
Innotech software (Version 4.0.0; FluorChem HD2; PoteinSimple).
Statistical analysis
Values are presented as the mean ± SD of three
independent repeats. All statistical comparisons were performed
using SPSS 17.0 software for Windows (SPSS, Inc.). For normally
distributed data, the statistical analysis was performed with a
Student's t-test. Data that were not normally distributed were
analyzed using the Mann-Whitney U test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Confirmation of osteoporotic
model
The successful establishment of the osteoporotic
model was evaluated by uterine atrophy and femoral metaphysis
histology. After six weeks, bilateral OVX led to marked gonadal
hypertrophy in OVX mice compared with the sham mice (Fig. 1A). In addition, H&E staining
images of femoral metaphysis in OVX mice showed that the bone
trabeculae had notably higher separation and was sparsely arranged
compared to the sham group (Fig. 1B
and C), which further confirmed
that the osteoporotic model was successfully established.
Radiographic healing and callus
histology
Twenty-one days after fracture, the callus of the
psoralen+OVX group mice appeared radiopaque, compared with OVX
group mice (Fig. 2A). Furthermore,
representative 3D views of the callus structure demonstrated that
an intact periosteal callus appeared to bridge the fracture in
psoralen+OVX group mice, compared with the OVX group in which bony
callus development and cortical bridging were notably disrupted in
the mice (Fig. 2B and C). Data for quantitative parameters are
shown in Fig. 2D-G. The TV was
similar between the psoralen+OVX and OVX groups, which suggested
that psoralen treatment did not affect fracture callus size.
However, BV and BV/TV were significantly increased in the calluses
of the psoralen+OVX group compared with the OVX group
(P<0.05).
Compared with the OVX group, histological data
showed more rapid endochondral ossification and bone formation in
the psoralen+OVX group. On day 10, the psoralen+OVX group showed
increased mineralized callus tissue and new bone formation in the
callus (Fig. 3A and B). There was also an apparent difference
in bone and cartilage composition between psoralen+OVX and OVX
groups on day 21. The psoralen+OVX group showed an increased
maturation and mineralization of callus at the fracture gap while
abundant cartilage persisted in the OVX group (Fig. 3C and D).
Effect of psoralen treatment on BMP-2
expression
Results of RT-qPCR analyses for gene expression
showed that there was increased BMP-2 expression at days 10 and 21
in the psoralen+OVX group compared with the OVX group (Fig. 4D and E). These data were further confirmed by
immunohistochemistry findings at the protein level; after 21 days,
immunohistochemical staining showed higher signal intensities for
BMP-2 in the psoralen+OVX group compared with the OVX group
(Fig. 4A-C).
Effect of psoralen treatment on
osteoclasts
The callus stained with TRAP at 21 days showed that
TRAP-positive multinucleated giant cells were easily observed in
the OVX group along with the newly formed woven bone tissue
(Fig. 5A and B). By contrast, the number of osteoclasts
was significantly reduced in the psoralen+OVX group (Fig. 5C).
The mRNA expression levels of OPG and RANKL in
fracture calluses were determined to evaluate the potential
mechanisms of osteoclast activity. On days 10 and 21, increased OPG
gene expression levels and decreased expression levels of RANKL
were observed in the psoralen+OVX group compared with the OVX group
(Fig. 4D and E).
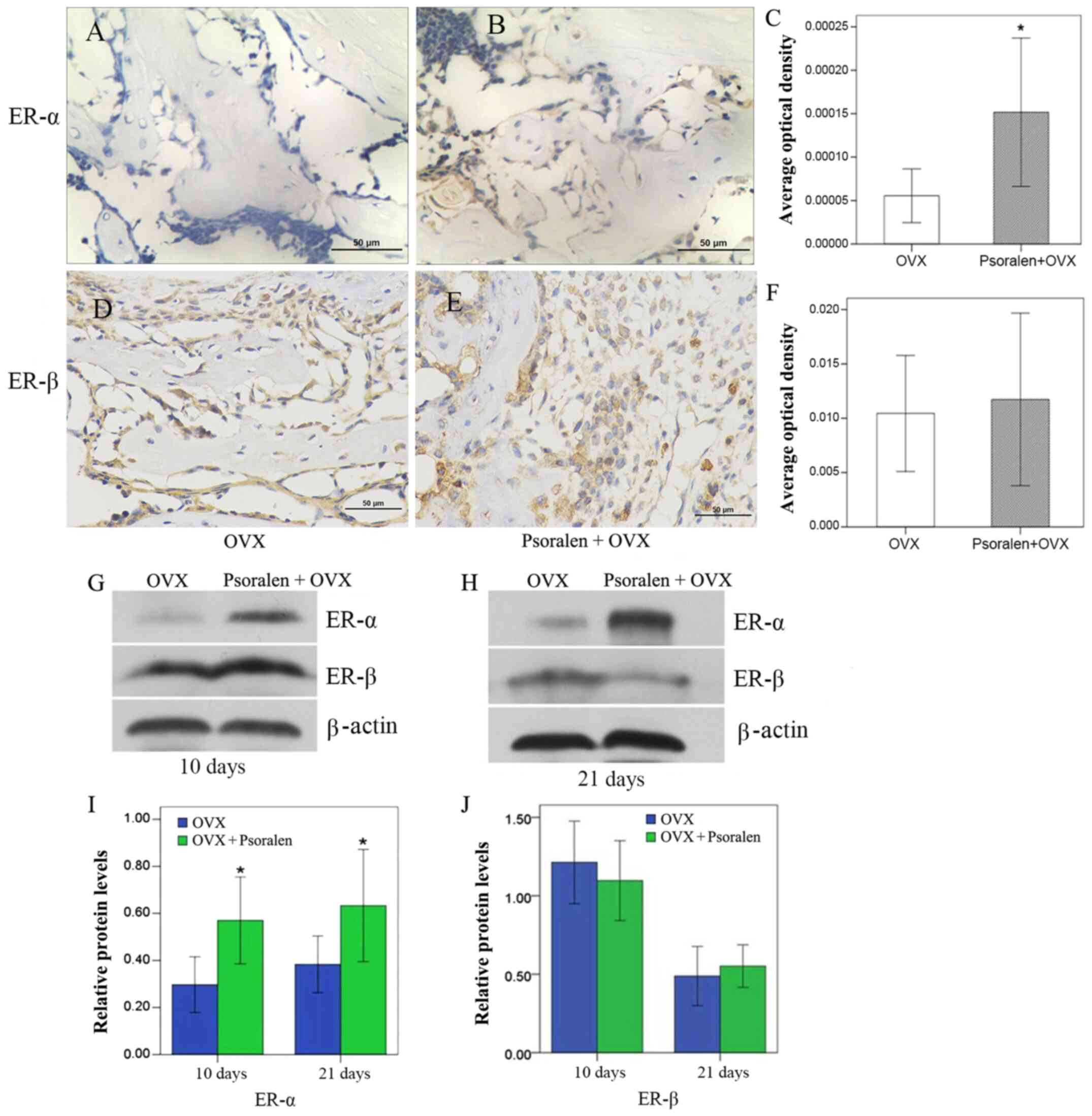
Effect of psoralen treatment on
ER
Western blot analysis of ER-α and ER-β expression
levels in the callus showed that psoralen was able to significantly
increase ER-α, but had no effect on ER-β expression (Fig. 6G-J). These results were further
confirmed by immunohistochemistry. ER-α was increased in the
psoralen+OVX group compared with the OVX group, while no difference
was observed for ER-β (Fig.
6A-F).
Discussion
Over previous years, several natural herbal drugs
and plant-based bioactive molecules with anabolic potential have
been examined as an alternative to traditional anti-osteoporotic
medications (22). In the present
study, an OVX-induced osteoporotic mouse model was established to
investigate the effect of psoralen on osteoporotic fracture
healing. Briefly, X-ray and 3D micro-CT imaging revealed progressed
callus formation in the psoralen+OVX group compared with the OVX
group. Furthermore, quantitative morphometry of callus structure
analysis revealed that psoralen significantly increased BV and
BV/TV in osteoporotic fractures in OVX mice. Moreover, a faster
endochondral ossification process was observed in mice treated with
psoralen compared with the control group. These findings suggest
that psoralen treatment can improve fracture healing in OVX mice.
In a previous study, Wong and Rabie (23) showed that psoralen treatment could
increase the number of bone-forming osteoblasts and new bone
formation following a bone defect in rats. Similarly, Yang et
al (15) revealed that psoralen
may significantly improve bone mass by stimulating osteoblastic
differentiation in OVX animal models.
At the molecular level, BMP signaling is involved in
osteoporotic delayed fracture healing (23,24).
Our previous study indicated that the combination of locally
delivered BMP-2 and systemically administered psoralen may improve
bone healing in ovariectomized mice, compared with BMP-2 alone
(25). Furthermore, the current
study determined that psoralen may significantly increase BMP-2
expression in callus tissue. Psoralen upregulated the expression of
BMP-2 and BMP-4 genes in primary osteoblasts (14). Taken together, the above results
suggest that psoralen accelerates fracture healing in OVX mice,
presumably by increasing bone formation and stimulating BMP-2
expression in osteoblasts.
Estrogen treatment has a direct effect on
osteocytes, osteoclasts and osteoblasts, which in turn leads to the
inhibition of bone resorption and maintenance of bone formation
(26,27). A previous study of OVX-induced
osteoporotic fracture reported a moderate correlation between ER-α
and BMP-2, thus suggesting that a high ER-α/ER-β ratio favors
callus formation (28). It has been
reported that phytoestrogens possess estrogenic activity and may
act as a potential alternative for estrogen deficiency to prevent
and treat postmenopausal osteoporosis (29,30).
Psoralen is a type of phytoestrogen that binds to both ER-α and
ER-β (31), in turn stimulating the
proliferation of human breast cancer T47D cells (32). Yang et al (15) demonstrated that psoralen may reduce
the gene expression and serum levels of the cytokines, tumor
necrosis factor-α and interleukin-17, in ovariectomized rats by
increasing the levels of estradiol and ER-β. In the present study,
high expression of ER-α was observed during fracture healing in the
psoralen+OVX group compared with the OVX group. This was consistent
with that of a previous study (17), which indicated that psoralen as an
activator, could promote the expression of ER-α.
The ratio of RANKL/OPG has a role in regulating the
differentiation and activation of osteoclasts, and is associated
with bone loss (33). The present
results showed that psoralen increased OPG mRNA expression and
decreased RANKL expression to inhibit osteoclastogenesis. The
histological assessment of local osteoclast activity within callus
tissues showed smaller numbers of TRACP-positive multinucleated
cells in the calluses of psoralen-treated mice compared with the
OVX group. Gerstenfeld et al (34) showed that denosumab (10 mg/kg),
which is a fully human monoclonal antibody (IgG2) that inhibits
bone resorption by binding to RANKL, can almost completely
eliminate TRACP-positive cells from callus tissues within 21 days,
thus leading to a considerable delay in the resorption of the
cartilage in a mouse fracture model.
In conclusion, these data indicated that psoralen
could promote callus formation and inhibit osteoclast genesis by
increasing BMP-2 and ER-α, and increasing the OPG/RANKL ratio,
respectively. Thus, it may be a useful treatment approach for
osteoporotic fracture-healing complications.
Acknowledgements
Not applicable.
Funding
Funding: This work was financially supported by the Hubei
Province Health and Family Planning Scientific Research Project
(grant no. WJ2019M086) and the Science and Technology Development
Plan Project of Jingzhou City (grant no. 2017041).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KBL designed the experiment, analyzed the results
and revised the article. KH completed the experiment, statistical
analysis and the writing of the first draft of the article. YQS
assisted in the completion and design of the experiment, and
assisted in the writing and modification of the article. XFC
completed the sample detection and data analysis. FT helped produce
the tissue sections and analyzed immunohistochemical staining data.
FC assisted in the detection of gene expression and data analysis.
QLG assisted in raising and collecting animals and analysis and
interpretation of data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal studies, including the euthanasia
procedure, were approved and performed in compliance with the
regulations and guidelines of the Medical Ethical Committee of the
First Affiliated Hospital of Yangtze University (approval no.
2017-060; Jingzhou, China) and conducted according to The
Association for Assessment and Accreditation of Laboratory Animal
Care International and the Institutional Animal Care and Use
Committee guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Compston JE, McClung MR and Leslie WD:
Osteoporosis. Lancet. 393:364–376. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cheung WH, Miclau T, Chow SK, Yang FF and
Alt V: Fracture healing in osteoporotic bone. Injury. 47 (Suppl
2):S21–S26. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Khosla S and Hofbauer LC: Osteoporosis
treatment: Recent developments and ongoing challenges. Lancet
Diabetes Endocrinol. 5:898–907. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Larsson S and Fazzalari NL:
Anti-osteoporosis therapy and fracture healing. Arch Orthop Trauma
Surg. 134:291–297. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hegde V, Jo JE, Andreopoulou P and Lane
JM: Effect of osteoporosis medications on fracture healing.
Osteoporosis Int. 27:861–871. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ha KY, Park KS, Kim SI and Kim YH: Does
bisphosphonate-based anti-osteoporosis medication affect
osteoporotic spinal fracture healing? Osteoporosis Int. 27:483–488.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Silverman SL, Kupperman ES and Bukata SV:
Members of IOFFWG. Fracture healing: A consensus report from the
International Osteoporosis Foundation Fracture Working Group.
Osteoporosis Int. 27:2197–2206. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cosman F, de Beur SJ, LeBoff MS, Lewiecki
EM, Tanner B, Randall S and Lindsay R: National Osteoporosis
Foundation. Clinician's guide to prevention and treatment of
osteoporosis. Osteoporosis Int. 25:2359–2381. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Compston J, Cooper A, Cooper C, Francis R,
Kanis JA, Marsh D, McCloskey EV, Reid DM, Selby P and Wilkins M:
National Osteoporosis Guideline Group (NOGG). Guidelines for the
diagnosis and management of osteoporosis in postmenopausal women
and men from the age of 50 years in the UK. Maturitas. 62:105–108.
2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lin J, Zhu J, Wang Y, Zhang N, Gober HJ,
Qiu X, Li D and Wang L: Chinese single herbs and active ingredients
for postmenopausal osteoporosis: From preclinical evidence to
action mechanism. Biosci Trends. 11:496–506. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Leung PC and Siu WS: Herbal treatment for
osteoporosis: A current review. J Tradit Complement Med. 3:82–87.
2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Salari Sharif P, Nikfar S and Abdollahi M:
Prevention of bone resorption by intake of phytoestrogens in
postmenopausal women: A meta-analysis. Age (Dordr). 33:421–431.
2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lim SH, Ha TY, Ahn J and Kim S: Estrogenic
activities of Psoralea corylifolia L. seed extracts and main
constituents. Phytomedicine. 18:425–430. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tang DZ, Yang F, Yang Z, Huang J, Shi Q,
Chen D and Wang YJ: Psoralen stimulates osteoblast differentiation
through activation of BMP signaling. Biochem Biophys Res Commun.
405:256–261. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yang Z, Huang JH, Liu SF, Zhao YJ, Shen
ZY, Wang YJ and Bian Q: The osteoprotective effect of psoralen in
ovariectomy-induced osteoporotic rats via stimulating the
osteoblastic differentiation from bone mesenchymal stem cells.
Menopause. 19:1156–1164. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yuan X, Bi Y, Yan Z, Pu W, Li Y and Zhou
K: Psoralen and isopsoralen ameliorate sex hormone
deficiency-induced osteoporosis in female and male mice. BioMed Res
Int. 2016(6869452)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xin D, Wang H, Yang J, Su YF, Fan GW, Wang
YF, Zhu Y and Gao XM: Phytoestrogens from Psoralea
corylifolia reveal estrogen receptor-subtype selectivity.
Phytomedicine. 17:126–131. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Thulin JD, Bradfield JF, Bergdall VK,
Conour LA, Grady AW, Hickman DL, Norton JN and Wallace JM: The cost
of self-imposed regulatory burden in animal research. FASEB J.
28:3297–3300. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bouxsein ML, Boyd SK, Christiansen BA,
Guldberg RE, Jepsen KJ and Muller R: Guidelines for assessment of
bone microstructure in rodents using micro-computed tomography. J
Bone Miner Res. 25:1468–1486. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Naik AA, Xie C, Zuscik MJ, Kingsley P,
Schwarz EM, Awad H, Guldberg R, Drissi H, Puzas JD, Boyce B, et al:
Reduced COX-2 expression in aged mice is associated with impaired
fracture healing. J Bone Miner Res. 24:251–264. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ng JJ, Wei Y, Zhou B, Bernhard J, Robinson
S, Burapachaisri A, Guo XE and Vunjak-Novakovic G: Recapitulation
of physiological spatiotemporal signals promotes in vitro formation
of phenotypically stable human articular cartilage. Proc Natl Acad
Sci USA. 114:2556–2561. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Jia M, Nie Y, Cao DP, Xue YY, Wang JS,
Zhao L, Rahman K, Zhang QY and Qin LP: Potential antiosteoporotic
agents from plants: A comprehensive review. Evid Based Complement
Alternat Med. 2012(364604)2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wong RW and Rabie AB: Effect of psoralen
on bone formation. J Orthop Res. 29:158–164. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chen L, Jiang W, Huang J, He BC, Zuo GW,
Zhang W, Luo Q, Shi Q, Zhang BQ, Wagner ER, et al: Insulin-like
growth factor 2 (IGF-2) potentiates BMP-9-induced osteogenic
differentiation and bone formation. J Bone Miner Res. 25:2447–2459.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Huang K, Wu G, Zou J and Peng S:
Combination therapy with BMP-2 and psoralen enhances fracture
healing in ovariectomized mice. Exp Ther Med. 16:1655–1662.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Khosla S, Oursler MJ and Monroe DG:
Estrogen and the skeleton. Trends Endocrinol Metab. 23:576–581.
2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wilkinson HN and Hardman MJ: The role of
estrogen in cutaneous ageing and repair. Maturitas. 103:60–64.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chow SK, Leung KS, Qin L, Wei F and Cheung
WH: Callus formation is related to the expression ratios of
estrogen receptors-alpha and -beta in ovariectomy-induced
osteoporotic fracture healing. Arch Orthop Trauma Surg.
134:1405–1416. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sirotkin AV and Harrath AH: Phytoestrogens
and their effects. Eur J Pharmacol. 741:230–236. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lagari VS and Levis S: Phytoestrogens in
the prevention of postmenopausal bone loss. J Clin Densitom.
16:445–449. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhao PW, Niu JZ, Wang JF and Wang LQ:
Research on phytoestrogenic effects and its mechanism of psoralen.
Zhongguo Zhong Yao Za Zhi. 33:59–63. 2008.PubMed/NCBI(In Chinese).
|
|
32
|
Soto AM, Murai JT, Siiteri PK and
Sonnenschein C: Control of cell proliferation: Evidence for
negative control on estrogen-sensitive T47D human breast cancer
cells. Cancer Res. 46:2271–2275. 1986.PubMed/NCBI
|
|
33
|
Catalano A, Loddo S, Bellone F, Pecora C,
Lasco A and Morabito N: Pulsed electromagnetic fields modulate bone
metabolism via RANKL/OPG and Wnt/β-catenin pathways in women with
postmenopausal osteoporosis: A pilot study. Bone. 116:42–46.
2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gerstenfeld LC, Sacks DJ, Pelis M, Mason
ZD, Graves DT, Barrero M, Ominsky MS, Kostenuik PJ, Morgan EF and
Einhorn TA: Comparison of effects of the bisphosphonate alendronate
versus the RANKL inhibitor denosumab on murine fracture healing. J
Bone Miner Res. 24:196–208. 2009.PubMed/NCBI View Article : Google Scholar
|