Introduction
Insulin-like growth factors (IGFs) are an essential
growth factor system connected in both the development of an
organism and the sustainment of normal function of various cells of
the body (1). IGFs are also
reported to possess powerful anti-apoptotic effects (1). Similarly, IGFs and IGF-binding
proteins may play an important role in tumor proliferation
(2). One kind of IGF, IGF-2, is
widely applied for the regulation of various functions of many
cells (3,4). IGF-2 has been shown to play a critical
role in adult neurogenesis and cognitive function, which results in
acting as a memory enhancer (3).
IGF-2 enhanced functions of antigen-specific regulatory B cells,
which resulted in enhancing the inhibitory effects on allergic
inflammation (4).
Recently, IGF-2 has been reported to have modulatory
effects on stem cells. IGF-2 modulated hematopoietic stem cell
maintenance (5). IGF-2 promoted
stemness of neural stem cells, evidenced by increased expression of
Oct4, Sox1, and FABP7 mRNA levels (6). IGF-2 induced the differentiation of
hematopoietic stem cells (4). Human
mesenchymal stem cells derived from human bone marrow can
differentiate into epithelial-like cells using various growth
factors, including IGF-2, hepatocyte growth factor, keratinocyte
growth factor, and epidermal growth factor (7). IGF-2 facilitated the transformation of
fibroblasts to myofibroblasts, as well as enhanced the
transformation of stem cells into epithelial cells (8). Moreover, IGF-2 has been shown to
potentiate osteogenic differentiation (9). Three-dimensional in vitro
culturing methods including spheroid culture have been of great
interest because they represent in vivo cell biology better
(10). Spheroids is known to mimic
the solid tissues by simulating the cell-to-cell interaction and
secreting their own extracellular matrix along with displaying
differential nutrient availability (11). Moreover, spheroid culture is
reported to enhance osteogenic differentiation potential of
mesenchymal stem cells (12). The
hypothesis of this study is that the application of IGF-2 may have
beneficial effects on the osteogenic differentiation of stem cells
in spheroid formation without application of the scaffold. This
study was conducted to evaluate the effects of IGF-2 on the
maintenance of morphology, improvements of cellular viability, and
enhancement of osteogenic differentiation of stem cell
spheroids.
Materials and methods
Cell spheroids using bone marrow
mesenchymal stem cells
The present study protocol was reviewed and approved
by the Institutional Review Board of Seoul St Mary's Hospital,
College of Medicine, the Catholic University of Korea
(KC19SESI0234). Informed consent was obtained from the
participants. All experiments were performed based on the relevant
guidelines and regulations specified in the Declaration of
Helsinki.
Human bone marrow mesenchymal stem cells (BMSCs;
Catholic MASTER cells) were obtained from the Catholic Institute of
Cell Therapy (CIC, Seoul, South Korea). Isolation and
characterization of the BMSCs were performed following previously
reported methods (13). The CIC
verified that all samples showed >90% positive CD 73 and CD 90
expression. We plated the cells on a culture dish, and cells that
were attached to the dish were removed. We changed the culture
medium every 2 or 3 days. The cells were grown in an incubator at
37˚C with 95% air and 5% CO2.
Fig. 1 shows an
overview of the study's design. We used commercially available
concave microwells (H389600, StemFIT 3D; MicroFIT) to fabricate
stem cell spheroids. We loaded a total of 1x106 BMSCs in
each well and evaluated the cell response. We treated cell
spheroids made of BMSCs with IGF-2 at predetermined concentrations
of 0, 10, and 100 ng/ml. We evaluated the morphological
characteristics on Days 1, 3, 5, and 7.
Determination of cellular
viability
We cultured stem cell spheroids in osteogenic media.
We used the commercially available two-color assay based on plasma
membrane integrity and esterase activity (Live/Dead Kit assay,
Molecular Probes,) for qualitative analysis of the stem cell
spheroids on Days 1, 3, 5, and 7(14). We also performed a quantitative
cellular viability test using an assay kit based on water-soluble
tetrazolium salt (Cell Counting Kit-8, Dojindo) (15).
Level of alkaline phosphatase activity
and calcium deposition
The level of alkaline phosphatase activity and an
anthraquinone dye assay for calcium deposit evaluation were used to
assess osteogenic differentiation (16). We obtained cell spheroids grown on
culture plates with osteogenic media on Days 3, 7, 14, and 21. We
used a commercially available kit (K412-500, BioVision, Inc.) to
evaluate level of alkaline phosphatase activity.
We used an anthraquinone dye assay for calcium
deposit evaluation to assess osteogenic differentiation on Days 7,
14, and 21(17). We washed three
times with phosphate-buffered saline and then fixed with 4%
paraformaldehyde in phosphate-buffered saline at room temperature
for 15 min. After that, we carefully removed the fixative and
washed three times with deionized water. We added 2% Alizarin Red S
Staining solution, and then incubated for 20 min. We removed dye
and washed three times with deionized water. The quantification of
the bound dyes was performed afterwards by adding 10%
cetylpyridinium chloride for 15 min at ambient temperature.
Spectrophotometric quantification was performed at 560 nm.
Total RNA extraction and
quantification of Runx2 and Col1 by quantitative polymerase chain
reaction
We harvested the cells on Day 7(18). We isolated total RNA using
purification (Thermo Fisher Scientific, Inc.); RNA was used as a
template for reverse transcription using SuperiorScript II reverse
transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.), and we
determined quantities were within ratios of absorbance at 260 and
280 nm spectrophotometrically (ND-2000, Thermo Fisher Scientific,
Inc.).
We detected mRNA expression by quantitative
polymerase chain reaction (18,19).
We designed the sense and antisense primers based on GenBank. The
primer sequences were as follows: Runx2 forward 5':
AATGATGGTGTTGACGCTGA-3'; reverse 5': TTGATACGTGTGGGATGTGG-3'; Col1
forward 5': CCAGAAGAACTGGTACATCAGCAA-3', reverse 5':
CGCCATACTCGAACTGGAATC-3', β-actin forward 5':
TGGCACCCAGCACAATGAA-3', and reverse 5':
CTAAGTCATAGTCCGCCTAGAAGCA-3'. Normalization was performed by
applying β-actin as a housekeeping gene.
Statistical analysis
We presented the data as means ± standard deviations
of the experiments. Tests of normality and equality of variances
were conducted. We performed two-way analysis of variance to
evaluate the effects of concentration and time points with Tukey's
post hoc test. We tested the differences among groups by applying
one-way analysis of variance with Tukey's post hoc test (SPSS 12
for Windows, SPSS Inc.; P<0.05).
Results
Formation of cell spheroids with human
bone marrow-derived stem cells
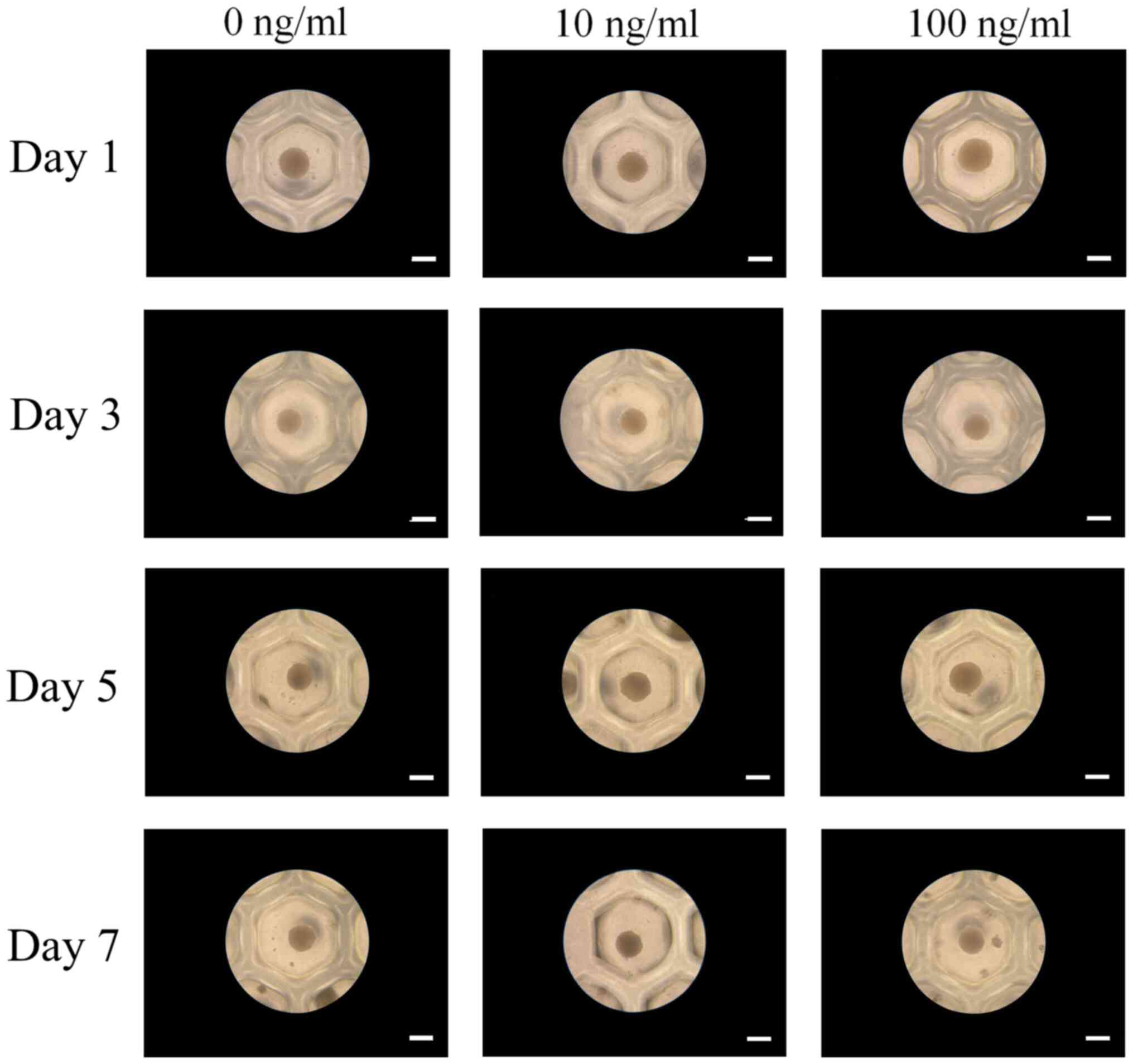
Spheroids were fabricated in each microwell on Day 1
(Fig. 2). The addition of IGF-2 at
10 and 100 ng/ml concentrations did not produce any morphological
changes. Extended incubation on Days 3, 5, and 7 did not show any
morphological changes.
Determination of cellular
viability
Qualitative results of the viability of cell
spheroids were analyzed using a Live/Dead kit assay on Days 1, 3,
5, and 7 (Fig. 3A-D). Most of the
cells on the surface produced green fluorescence, indicating live
cells. The quantitative values for cellular viability on Days 1, 3,
5, and 7 are shown in Fig. 3E. The
relative values for IGF-2 at 0, 10, and 100 ng/ml at Day 1 were
0.193±0.002, 0.191±0.002, and 0.201±0.006, respectively
(P>0.05).
 | Figure 3Live, dead and merged images of stem
cell spheroids. (A) Live, dead and merged images of stem cell
spheroids on day 1. Scale bar, 100 µm. (B) Live, dead and merged
images of stem cell spheroids on day 3. Scale bar, 100 µm. (C)
Live, dead and merged images of stem cell spheroids on day 5. Scale
bar, 100 µm. (D) Live, dead and merged images of stem cell
spheroids on day 7. Scale bar, 100 µm. (E) Cellular viability was
assessed using a Cell Counting Kit-8 assay on days 1, 3, 5 and 7.
*P<0.05 vs. IGF-2 at 0 ng/ml on day 5;
**P<0.05 vs. IGF-2 at 0 ng/ml on day 7. IGF-2,
insulin-like growth factor 2. |
Level of alkaline phosphatase activity
and calcium deposition
The levels of the alkaline phosphatase activity
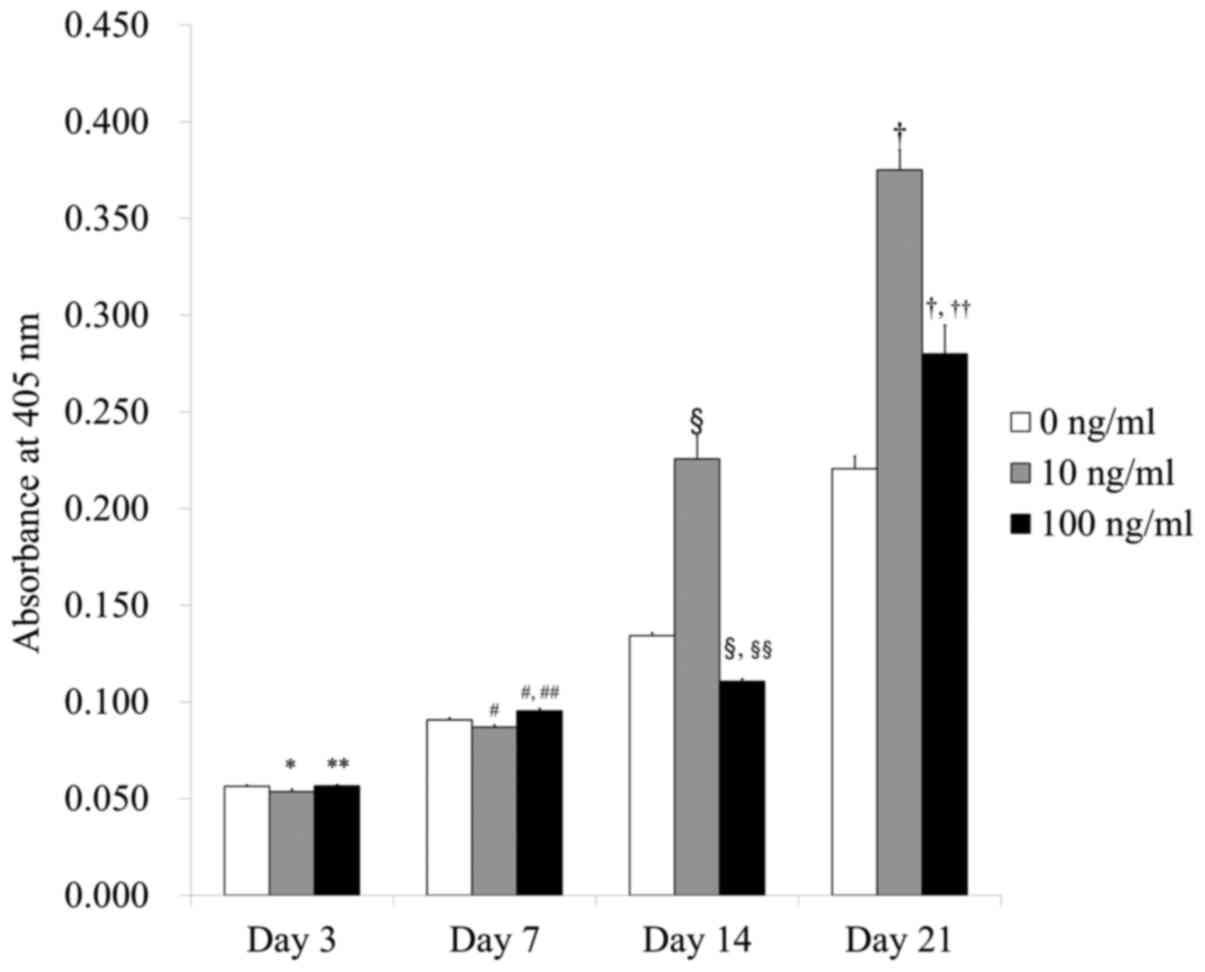
assays on Days 3, 7, 14, and 21 are shown in Fig. 4. The absorbance values at 405 nm on
Day 21 for IGF-2 at 0, 10, and 100 ng/ml were 0.221±0.006,
0.375±0.010, and 0.280±0.015, respectively. There were
significantly higher values for IGF-2 in the 10 and 100 ng/ml
groups when compared with the control (P<0.05).
Fig. 5A shows the
results for Alizarin Red S staining. Calcium deposits were clearly
noted in each group. The quantitative results of the anthraquinone
dye assay at Days 7, 14, and 21 are shown in Fig. 5B. The absorbance values at 560 nm on
Day 21 for IGF-2 at 0, 10, and 100 ng/ml were 0.057±0.002,
0.065±0.002 and 0.053±0.004, respectively. There were significantly
higher values for IGF-2 in the 10 ng/ml group when compared with
the unloaded control at Day 21 (P<0.05).
Evaluation of Runx2 and Col1 by
reverse transcription-quantitative polymerase chain reaction
Quantitative real-time polymerase chain reaction
revealed that mRNA levels of Runx2 were 1.0±0.2, 3.3±0.5, and
4.0±0.9 for IGF-2 at 0, 10, and 100 ng/ml, respectively. The
results showed that the addition of IGF-2 produced a significant
increase of Runx2 in 100 ng/ml group (P<0.05) (Fig. 6A).
Reverse transcription-quantitative polymerase chain
reaction revealed that mRNA levels of Col1 were 1.0±0.1, 0.5±0.1,
and 2.4±0.3 for IGF-2 at 0, 10, and 100 ng/ml, respectively. The
results showed that application of IGF-2 produced a significant
increase of Col1 in 100 ng/ml group (P<0.05) (Fig. 6B).
Discussion
This study evaluated the effects of IGF-2 on the
maintenance of morphology, improvements of cellular viability, and
enhancement of osteogenic differentiation of stem cell spheroids.
Collectively, this study shows that the addition of IGF-2 increased
the osteogenic differentiations and Runx2 and Col1 expression of
stem cell spheroids.
IGFs are reported to be key regulators for bone
growth, bone repair, and bone remodeling, and IGF-2 has been shown
to be a chemotactic factor for human mesenchymal progenitor cells
(20). IGF-2 rescued
microRNA-repressed osteogenic differentiation (21). An increase in IGF-2 expression can
aid in osteogenic differentiation of BMSCs (22). The enhanced differentiation of
osteoprogenitor cells by IGF-2 was obtained in a dose-dependent
pattern (23). In this report, the
IGF-2 significantly produced higher values for alkaline phosphatase
activity and anthraquinone dye assay. Moreover, IGF-2 is reported
to be involved in the promotion of cell proliferation and survival
(24,25). It was reported that the effects of
IGF-2 on cellular behavior are mediated by IGF type I receptor and
it transports survival signals to the cell through a complex
network of signaling mechanisms (26).
The effects of concentration of IGF-2 were tested in
previous reports. While subphysiologic doses of IGF-2 caused a
modest stimulation of erythropoiesis, addition of a physiologic
concentration (100 ng/ml) resulted in up to a 4-fold enhancement in
erythroid colony formation (27).
Stimulation of cell migration was noticed with the addition of
IGF-2 from 1 to 100 ng/ml in a dose-dependent way (20). In this study, significantly higher
values for alkaline phosphatase activity assays were seen at 10 and
100 ng/ml. Alizarin Red S staining data showed that higher values
for IGF-2 were seen at 10 ng/ml. The highest gene expressions of
Runx2 and Col1 were noted at 100 ng/ml. In general, the application
of IGF-2 increased alkaline phosphatase activity, Alizarin red
staining, and Runx2 and Col1 expression of stem cell spheroids. The
differences in the overall effects or maximal effective dose may be
due to the culturing condition and culturing period (28).
The effects of IGF-2 may be enhanced synergistically
by applying additional molecules. IGF-2 in combination with
platelet-derived growth factor and neurotrophin-3 resulted in
induction of trans differentiation of muscle-derived cells into
Schwann cell-like cells (29).
IGF-2 was delivered with bone morphogenetic proteins to evaluate
the synergistic effects on osteogenic differentiation (30).
IGF-2 has been proposed to act on via various
pathways (5,23,31-33).
IGF-2 appeared to exert effects on human marrow erythroid
progenitors via a direct mechanism involving the IGF-1 receptor
(31). IGF-2 augmented in
vitro hematopoiesis primarily through its interaction with
IGF-1 and possibly insulin receptors, rather than
IGF-2/cation-independent mannose 6-phosphate receptors (32). IGF-2 promoted stemness of stem cells
via the A isoform of the insulin receptor and not through
activation of either the IGF-1 or the IGF-2 receptors (33). IGF-2 regulates hematopoietic stem
cell cycle by upregulation of p57(5). Moreover, IGF-stimulated
osteoprogenitor differentiation is mediated through IGF-1 receptor
(23). The change in the Runx2
expression can be used as the evaluation tool for the osteogenic
differentiation potential for spheroids (19,34,35).
Further research including the mitogen-activated protein kinase
pathways and Smad signaling pathways may be warranted to evaluate
the underlying mechanism (26,36).
This study evaluated the effects of IGF-2 on the
maintenance of morphology, improvements of cellular viability, and
enhancement of osteogenic differentiation of stem cell spheroids.
Collectively, this study shows that the addition of IGF-2 increased
the osteogenic differentiation and Runx2 and Col1 expression of
stem cell spheroids. Future studies are warranted for the
application of IGF-2 along with stem cell spheroids to various
models, including in vivo studies using bony defects models
in calvaria, mandible and femur.
Acknowledgements
BMSCs (Catholic MASTER cells) were supplied by
Catholic Institute of Cell Therapy (CIC, Seoul, Korea). The cells
were derived from human bone marrow donated by healthy donors after
informed consent was obtained from the participants.
Funding
Funding: The present study was supported by the National
Research Foundation of Korea (NRF) grant funded by the Korea
government (MSIT) (grant no. 2020R1A2C4001624). The present study
was also supported by Research Fund of Seoul St. Mary's Hospital,
The Catholic University of Korea.
Availability of data and materials
All data generated or analyzed during this study are
included in the published article.
Authors' contributions
SKM, MK and JBP collaborated to design the study.
SKM, MK and JBP were responsible for data access and analysis. SKM,
MK and JBP performed the experiments. SKM, MK and JBP wrote the
manuscript. SKM, MK and JBP reviewed the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study protocol was reviewed and approved
by the Institutional Review Board of Seoul St Mary's Hospital,
College of Medicine, the Catholic University of Korea
(KC19SESI0234). Informed consent for participation was obtained
from the participants. All experiments were performed in accordance
with relevant guidelines and regulations specified in the
Declaration of Helsinki.
Patient consent for publication
Patient consent for publication was obtained from
the participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
LeRoith D and Roberts CT Jr: The
insulin-like growth factor system and cancer. Cancer Lett.
195:127–137. 2003.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dawczynski K, Kauf E and Zintl F: Changes
of serum growth factors (IGF-I,-II and IGFBP-2,-3) prior to and
after stem cell transplantation in children with acute leukemia.
Bone Marrow Transplant. 32:411–415. 2003.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Iwamoto T and Ouchi Y: Emerging evidence
of insulin-like growth factor 2 as a memory enhancer: A unique
animal model of cognitive dysfunction with impaired adult
neurogenesis. Rev Neurosci. 25:559–574. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Geng XR, Yang G, Li M, Song JP, Liu ZQ,
Qiu S, Liu Z and Yang PC: Insulin-like growth factor-2 enhances
functions of antigen (Ag)-specific regulatory B cells. J Biol Chem.
289:17941–17950. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Thomas DD, Sommer AG, Balazs AB, Beerman
I, Murphy GJ, Rossi D and Mostoslavsky G: Insulin-like growth
factor 2 modulates murine hematopoietic stem cell maintenance
through upregulation of p57. Exp Hematol. 44:422–433.e1.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ziegler AN, Schneider JS, Qin M, Tyler WA,
Pintar JE, Fraidenraich D, Wood TL and Levison SW: IGF-II promotes
stemness of neural restricted precursors. Stem Cells. 30:1265–1276.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Păunescu V, Deak E, Herman D, Siska IR,
Tănasie G, Bunu C, Anghel S, Tatu CA, Oprea TI, Henschler R, et al:
In vitro differentiation of human mesenchymal stem cells to
epithelial lineage. J Cell Mol Med. 11:502–508. 2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jiang Y, Ju Z, Zhang J, Liu X, Tian J and
Mu G: Effects of insulin-like growth factor 2 and its receptor
expressions on corneal repair. Int J Clin Exp Pathol.
8:10185–10191. 2015.PubMed/NCBI
|
|
9
|
Chen L, Jiang W, Huang J, He BC, Zuo GW,
Zhang W, Luo Q, Shi Q, Zhang BQ, Wagner ER, et al: Insulin-like
growth factor 2 (IGF-2) potentiates BMP-9-induced osteogenic
differentiation and bone formation. J Bone Miner Res. 25:2447–2459.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Verjans ET, Doijen J, Luyten W, Landuyt B
and Schoofs L: Three-dimensional cell culture models for anticancer
drug screening: Worth the effort? J Cell Physiol. 233:2993–3003.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Antoni D, Burckel H, Josset E and Noel G:
Three-dimensional cell culture: A breakthrough in vivo. Int J Mol
Sci. 16:5517–5527. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Moritani Y, Usui M, Sano K, Nakazawa K,
Hanatani T, Nakatomi M, Iwata T, Sato T, Ariyoshi W, Nishihara T
and Nakashima K: Spheroid culture enhances osteogenic potential of
periodontal ligament mesenchymal stem cells. J Periodontal Res.
53:870–882. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jeong CH, Kim SM, Lim JY, Ryu CH, Jun JA
and Jeun SS: Mesenchymal stem cells expressing brain-derived
neurotrophic factor enhance endogenous neurogenesis in an ischemic
stroke model. Biomed Res Int. 2014(129145)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kang SH, Park JB, Kim I, Lee W and Kim H:
Assessment of stem cell viability in the initial healing period in
rabbits with a cranial bone defect according to the type and form
of scaffold. J Periodontal Implant Sci. 49:258–267. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kim BB, Tae JY, Ko Y and Park JB:
Lovastatin increases the proliferation and osteoblastic
differentiation of human gingiva-derived stem cells in
three-dimensional cultures. Exp Ther Med. 18:3425–3430.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lee H, Son J, Min SK, Na CB, Yi G, Koo H
and Park JB: A study of the effects of doxorubicin-containing
liposomes on osteogenesis of 3D stem cell spheroids derived from
gingiva. Materials (Basel). 12(2693)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lee H and Park JB: Dimethyl sulfoxide
leads to decreased osteogenic differentiation of stem cells derived
from gingiva via Runx2 and collagen I expression. Eur J Dent.
13:131–136. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lee H, Lee H, Na CB and Park JB: The
effects of simvastatin on cellular viability, stemness and
osteogenic differentiation using 3-dimensional cultures of stem
cells and osteoblast-like cells. Adv Clin Exp Med. 28:699–706.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tae JY, Lee H, Lee H, Ko Y and Park JB:
Osteogenic potential of cell spheroids composed of varying ratios
of gingiva-derived and bone marrow stem cells using concave
microwells. Exp Ther Med. 16:2287–2294. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fiedler J, Brill C, Blum WF and Brenner
RE: IGF-I and IGF-II stimulate directed cell migration of
bone-marrow-derived human mesenchymal progenitor cells. Biochem
Biophys Res Commun. 345:1177–1183. 2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ding W, Li J, Singh J, Alif R,
Vazquez-Padron RI, Gomes SA, Hare JM and Shehadeh LA: miR-30e
targets IGF2-regulated osteogenesis in bone marrow-derived
mesenchymal stem cells, aortic smooth muscle cells, and
ApoE-/- mice. Cardiovasc Res. 106:131–142.
2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Han LC, Qi MC, Sun H, Hu J, Zou SJ and Li
JH: Response of bone marrow mesenchymal stem cells to mechanical
stretch and gene expression of transforming growth factor-beta and
insulin-like growth factor-II under mechanical strain. Hua Xi Kou
Qiang Yi Xue Za Zhi. 27:381–385. 2009.PubMed/NCBI(In Chinese).
|
|
23
|
Jia D and Heersche JN: Insulin-like growth
factor-1 and -2 stimulate osteoprogenitor proliferation and
differentiation and adipocyte formation in cell populations derived
from adult rat bone. Bone. 27:785–794. 2000.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Corcoran RB, Bachar Raveh T, Barakat MT,
Lee EY and Scott MP: Insulin-like growth factor 2 is required for
progression to advanced medulloblastoma in patched1 heterozygous
mice. Cancer Res. 68:8788–8795. 2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Guillaud-Bataille M, Ragazzon B, de
Reyniès A, Chevalier C, Francillard I, Barreau O, Steunou V,
Guillemot J, Tissier F, Rizk-Rabin M, et al: IGF2 promotes growth
of adrenocortical carcinoma cells, but its overexpression does not
modify phenotypic and molecular features of adrenocortical
carcinoma. PLoS One. 9(e103744)2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Franceschi RT, Xiao G, Jiang D,
Gopalakrishnan R, Yang S and Reith E: Multiple signaling pathways
converge on the Cbfa1/Runx2 transcription factor to regulate
osteoblast differentiation. Connect Tissue Res. 44 (Suppl
1):S109–S116. 2003.PubMed/NCBI
|
|
27
|
Sanders M, Sorba S and Dainiak N:
Insulin-like growth factors stimulate erythropoiesis in
serum-substituted umbilical cord blood cultures. Exp Hematol.
21:25–30. 1993.PubMed/NCBI
|
|
28
|
Lee H, Son J, Yi G, Koo H and Park JB:
Cellular viability and osteogenic differentiation potential of
human gingiva-derived stem cells in 2D culture following treatment
with anionic, cationic, and neutral liposomes containing
doxorubicin. Exp Ther Med. 16:4457–4462. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tang Y, He H, Cheng N, Song Y, Ding W,
Zhang Y, Zhang W, Zhang J, Peng H and Jiang H: PDGF, NT-3 and IGF-2
in combination induced transdifferentiation of muscle-derived stem
cells into Schwann cell-like cells. PLoS One.
9(e73402)2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Acil Y, Ghoniem AA, Wiltfang J and
Gierloff M: Optimizing the osteogenic differentiation of human
mesenchymal stromal cells by the synergistic action of growth
factors. J Craniomaxillofac Surg. 42:2002–2009. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Merchav S, Silvian-Drachsler I, Tatarsky
I, Lake M and Skottner A: Comparative studies of the
erythroid-potentiating effects of biosynthetic human insulin-like
growth factors-I and -II. J Clin Endocrinol Metab. 74:447–452.
1992.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Schwartz GN, Warren MK, Sakano K, Szabo
JM, Kessler SW, Pashapour A, Gress RE and Perdue JF: Comparative
effects of insulin-like growth factor II (IGF-II) and IGF-II
mutants specific for IGF-II/CIM6-P or IGF-I receptors on in vitro
hematopoiesis. Stem Cells. 14:337–350. 1996.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ziegler AN, Chidambaram S, Forbes BE, Wood
TL and Levison SW: Insulin-like growth factor-II (IGF-II) and
IGF-II analogs with enhanced insulin receptor-a binding affinity
promote neural stem cell expansion. J Biol Chem. 289:4626–4633.
2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Almalki SG and Agrawal DK: Key
transcription factors in the differentiation of mesenchymal stem
cells. Differentiation. 92:41–51. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
He S, Yang S, Zhang Y, Li X, Gao D, Zhong
Y, Cao L, Ma H, Liu Y, Li G, et al: LncRNA ODIR1 inhibits
osteogenic differentiation of hUC-MSCs through the
FBXO25/H2BK120ub/H3K4me3/OSX axis. Cell Death Dis.
10(947)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Javelaud D and Mauviel A: Crosstalk
mechanisms between the mitogen-activated protein kinase pathways
and Smad signaling downstream of TGF-beta: Implications for
carcinogenesis. Oncogene. 24:5742–5750. 2005.PubMed/NCBI View Article : Google Scholar
|