Introduction
Mesonephric carcinoma of the female genital tract is
a rare variant of adenocarcinoma that originates from the remnants
of the mesonephric ducts (Wolffian ducts). Although the majority of
cases arise in the wall of the cervix uteri, they may occur
throughout the whole female genital tract (1,2).
Morphologically, they can be composed of a purely epithelial
components (carcinoma) or include biphasic elements consisting of
epithelial carcinomatous and mesenchymal sarcomatous components
(1). This latter pathological
entity, known as malignant mixed mesonephric tumour (MMMsT) or
carcinosarcoma, is even less common than pure mesonephric carcinoma
and is associated with worse prognosis than monophasic mesonephric
carcinoma (1). Among mesonephric
neoplasms of the female genital tract, cervical MMMsTs are
exceedingly rare (3) and there is
no report of the molecular characteristics of this condition. The
present study reports a case of cervical MMMsT with full molecular
characterisation by next-generation sequencing (NGS) analysis.
Case report
Clinical history and methods. In February 2019 a
58-year-old woman presented at San Carlo di Nancy Hospital (Rome,
Italy) with a history of vaginal bleeding over several months.
Gynaecological examination revealed a nodular mass in the posterior
aspect of the vagina. Ultrasonography showed a solid mass with
irregular borders measuring 25 mm in the maximum dimension. Her
past medical history showed that, in July 2013 the patient had
undergone hysterosalpingo-oophorectomy for a cervical
carcinosarcoma of probable mesonephric origin and International
Federation of Gynaecology and Obstetrics stage IB at San Giovanni
Hospital (Rome, Italy). There was no history of other neoplasms.
Chemotherapy and/or radiotherapy were not performed. A biopsy of
the vaginal mass was taken. Slides of the original tumour were
requested and only haematoxylin and eosin (H&E) sections were
made available for review. Resection of the vaginal recurrence,
with clear margins, was performed.
Immunohistochemistry (IHC) was performed on formalin
fixed paraffin embedded (FFPE) tissue blocks. Tissues were fixed in
10% neutral buffered formalin for 24 h at a room temperature.
Sections of 3 µm thickness were cut and stained on a BOND III
Automated Immunohistochemistry System (Leica Biosystems) using a
Leica Bond Polymer Refine Detection (Leica Biosystems; cat no.
DS9800) and the antigen-unmasking solutions Epitope Retrieval
Solution 1 (ER1) at pH 6.0 (Leica Biosystems; cat. no. AR9961) and
Epitope Retrieval Solution 2 (ER2) at pH 9.0 (Leica Biosystems;
cat. no. AR9640) for 20 min with the following antibodies: EMA
(ready to use in ER1; mouse mAb clone GP1.4; Leica Biosystems; cat.
no. PA0035), MNF116 (1:50 in ER2; mouse mAb clone MNF116; Dako;
Agilent Technologies, Inc.; cat. no. M082101-2), cytokeratin 7
(1:50 in ER1; mouse mAb clone RN7; Leica Biosystems; cat. no.
CK7-560-L-CE), cytokeratin 20 (1:400 in ER2; mouse mAb clone PW31;
Leica Biosystems; cat. no. CK20-561-L-CE), PAX-8 (1:50 in ER2;
mouse mAb clone MRQ-50; Cell Marque™; Sigma-Aldrich; Merck KGaA;
cat. no. 363M-16), β-catenin (1:400 in ER1; rabbit pAb; Cell
Signaling Technology, Inc.; cat. no. 9562S), cyclin D1 (1:40 in
ER2; rabbit mAb clone SP4; Cell Marque™; Sigma-Aldrich; Merck KGaA;
cat. no. 241R-16), GATA 3 (1:100 in ER2; mouse mAb clone L50-823;
Cell Marque™; Sigma-Aldrich; Merck KGaA; cat. no. 390M-16), CD10
(1:50 in ER2; mouse mAb clone 55C6; Leica Biosystems; cat. no.
CD10-270-L-CE), androgen receptor (1:200 in ER2; rabbit mAb clone
EP120; Cell Marque™; Sigma-Aldrich; Merck KGaA; cat. no. AC-0071),
p16 (1:10 in ER1; mouse mAb clone G175-405; BD Biosciences; cat.
no. 550834), CEA (ready to use in ER2; mouse mAb clone COL-1; Leica
Biosystems; cat. no. PA0364), ER (1:50 in ER1; mouse mAb clone
6F11; Leica Biosystems; cat. no. ER-6F11-L-F), PR (1:100; ER1;
mouse mAb clone 16; Leica Biosystems; cat. no. PGR-312-L-F), TTF1
(ready to use in ER1; mouse mAb clone SPT24; Leica Biosystems; cat.
no. PA0848), WT1 (ready to use in ER2; mouse mAb clone WT49; Leica
Biosystems; cat. no. PA0562), calretinin (ready to use in ER2;
mouse mAb clone CAL6; Leica Biosystems; cat. no. PA0346); p53
(ready to use in ER2; mouse mAb clone DO-7; Leica Biosystems; cat.
no. PA0057) and Ki67 (ready to use in ER2; mouse mAb clone MM1;
Leica Biosystems; cat. no. PA0230). A total of 20 slides were
examined for H&E and IHC. An upright light microscope (Olympus
Corporation; model BX43) was used for examination of the stained
slides.
Molecular analysis on both the primary and recurrent
tumours was performed using a targeted semiconductor sequencing
approach on an Ion Torrent Personal Genome Machine (PGM; Thermo
Fisher Scientific, Inc.) (4).
Tumour cellularity of the recurrent tumour (part 1, mainly
sarcomatous) was estimated at 51-75%, whereas the primary tumour
(part 2, epithelial and sarcomatous) was at 5-20%. Both samples
were within assay acceptance criteria. FFPE histological sections
were microdissected to include mainly tumour cells. Nucleic acid
isolation was performed using a Gene Read FFPE DNA kit (Qiagen
GmbH; cat. no. 180134). Extracted DNA was quantified using an
Agilent 4200 TapeStation (Agilent Technologies, Inc.); thereafter,
DNA samples were diluted to the desired concentration of 5 ng/µl.
The Ion Library Equalizer™ Kit (Life Technologies; Thermo Fisher
Scientific, Inc.; cat. no. 4482298) was used for library
normalization at 100 pM. Tru-Q DNA Reference Standards (Horizon
Discovery Group, plc; cat. nos. HD728, 729, 730 and 731) were
included in the run.
The Ion AmpliSeq™ Cancer Hotspot Panel v2 (Life
Technologies; Thermo Fisher Scientific, Inc.; cat. no. 4475346) and
The Ion AmpliSeq™ Library Kit 2.0 (Life Technologies; Thermo Fisher
Scientific, Inc.; cat. no. 4475345) were used to perform multiplex
PCR for preparation of amplicon libraries (~250 bp in length with
3' P1 adapter incorporated by PCR) from genomic ‘hot spot’ regions.
A panel covering ~2,800 Catalogue Of Somatic Mutations In Cancer
(COSMIC) mutations from 50 oncogenes and tumour suppressors, with
the following genes, was used: ABL1, AKT1,
ALK, APC, ATM, BRAF, CDH1,
CDKN2A, CSF1R, CTNNB1, EGFR,
ERBB2, ERBB4, EZH2, FBXW7,
FGFR1, FGFR2, FGFR3, FLT3,
GNA11, GNAQ, GNAS, HNF1A, HRAS,
IDH1, IDH2, JAK2, JAK3, KDR,
KIT, KRAS, MET, MLH1, MPL,
NOTCH1, NPM1, NRAS, PDGFRA,
PIK3CA, PTEN, PTPN11, RB1, RET,
SMAD4, SMARCB1, SMO, SRC, STK11,
TP53 and VHL.
Sequencing was performed on the PGM instrument using
an Ion PGM™ Hi-Q™ View sequencing kit (Life Technologies; Thermo
Fisher Scientific, Inc.; cat. no. A30044) with the Ion PGM™ Hi-Q™
View OT2 kit (Life Technologies; Thermo Fisher Scientific, Inc.;
cat. no. A29900) and run on an Ion 318™ Chip Kit v2 BC (Life
Technologies; Thermo Fisher Scientific, Inc.; cat. no. 4488150).
For mutation analysis, Torrent Suite v5.0.5 and Variant Caller
v5.0.4.0 (Life Technologies; Thermo Fisher Scientific, Inc.) were
used, followed by variant annotation in Ion Reporter v5.10 software
(Life Technologies; Thermo Fisher Scientific, Inc.) with custom
filters and functional characterization via MutationTaster
(http://www.mutationtaster.org) and
Varsome (https://varsome.com) to group the
Variant Caller output into the reported categories
(single-nucleotide variant and small indels). Variant descriptors
were reported according to Human Genome Variation Society
recommended nomenclature with the corresponding COSMIC reference
number. The patient did not consent for matched normal sample
and/or blood molecular analysis, due to the family implications of
germline mutation testing.
Results
Histological examination of the original tumour and
recurrent mass demonstrated a biphasic neoplasm composed of
malignant epithelial adenocarcinomatous and mesenchymal sarcomatous
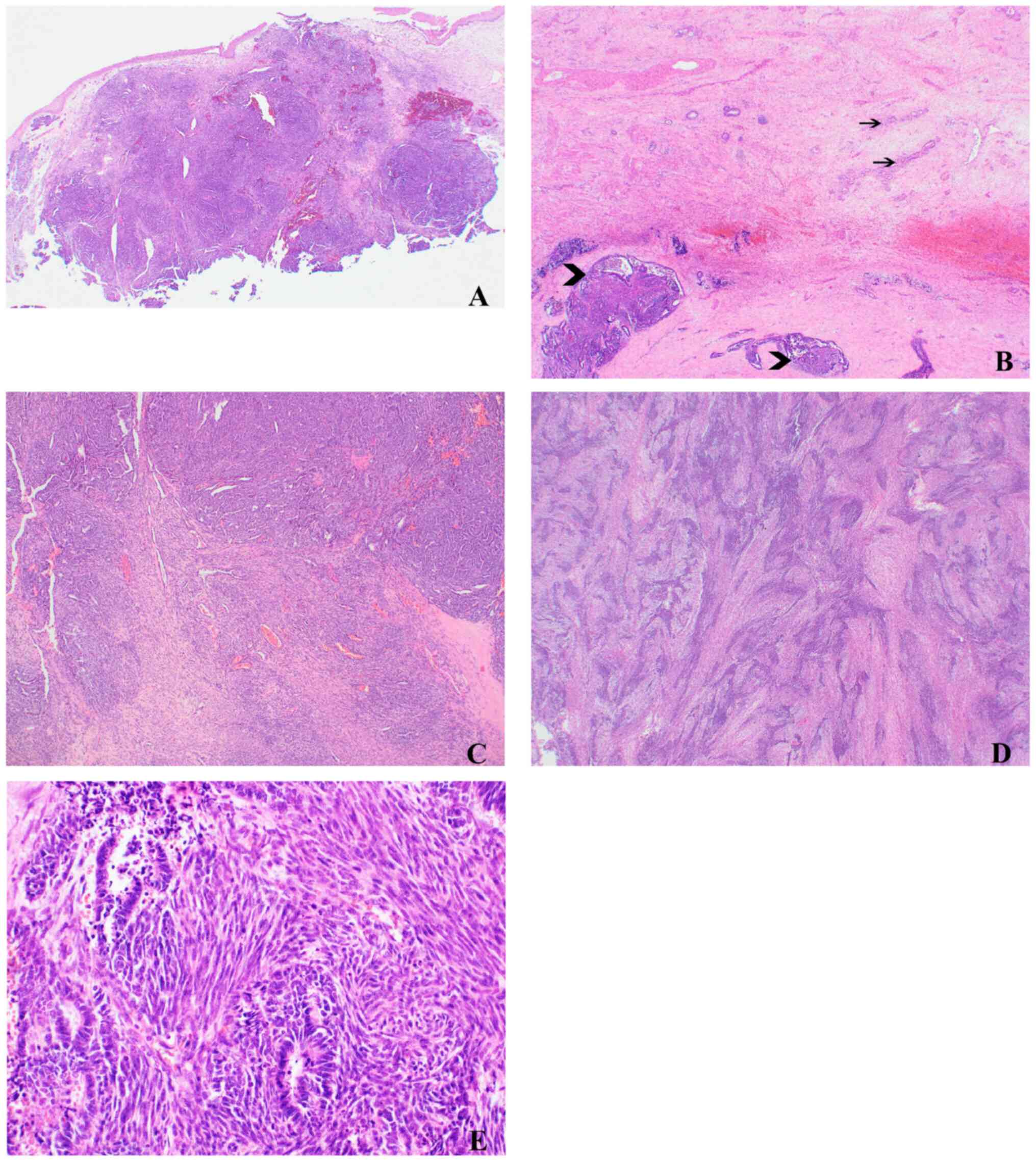
components (Fig. 1A). The original
neoplasm presented infiltrative margins and had arisen in the
background of mesonephric hyperplasia (Fig. 1B) that consisted of clusters of
small/medium-sized tubules lined by bland cuboidal cells, with no
nuclear atypia and/or pleomorphism. Most of the tubule lumens
contained a densely eosinophilic secretion. The recurrent tumour
did not contain foci of benign mesonephric remnants.
The primary and recurrent tumour showed similar
morphological features. However, the original tumour contained a
higher proportion of carcinomatous components while the recurrent
tumour contained a higher proportion of sarcomatous components. The
epithelial components displayed a variety of architectural
patterns, including tubular, solid, papillary, retiform and ductal
(glandular) elements (Fig. 1C),
which were lined by a single row of cuboidal cells, with moderate
atypia. Occasional pseudo-papillae and slit-like spaces,
reminiscent of serous carcinoma, were present. Some tubules
contained eosinophilic secretions. In the primary tumour, the
tubules infiltrated and dissected the cervical wall in a random,
hazardous manner. The mesenchymal sarcomatous component displayed
alternating hypocellular areas with dense, hypercellular areas
(Fig. 1D) and was composed of cells
with spindle-cell nuclei and scanty basophilic cytoplasm, arranged
in short intersecting fascicles. There was moderate nuclear atypia
and frequent mitoses. Myxoid changes were present and no
heterologous elements were identified. The transition between
epithelial and mesenchymal components was abrupt (Fig. 1E). Mitotic figures, in both
components, were frequent, ~12 per 10 high power fields (HFP). No
necrosis was identified. There was no lymphovascular invasion.
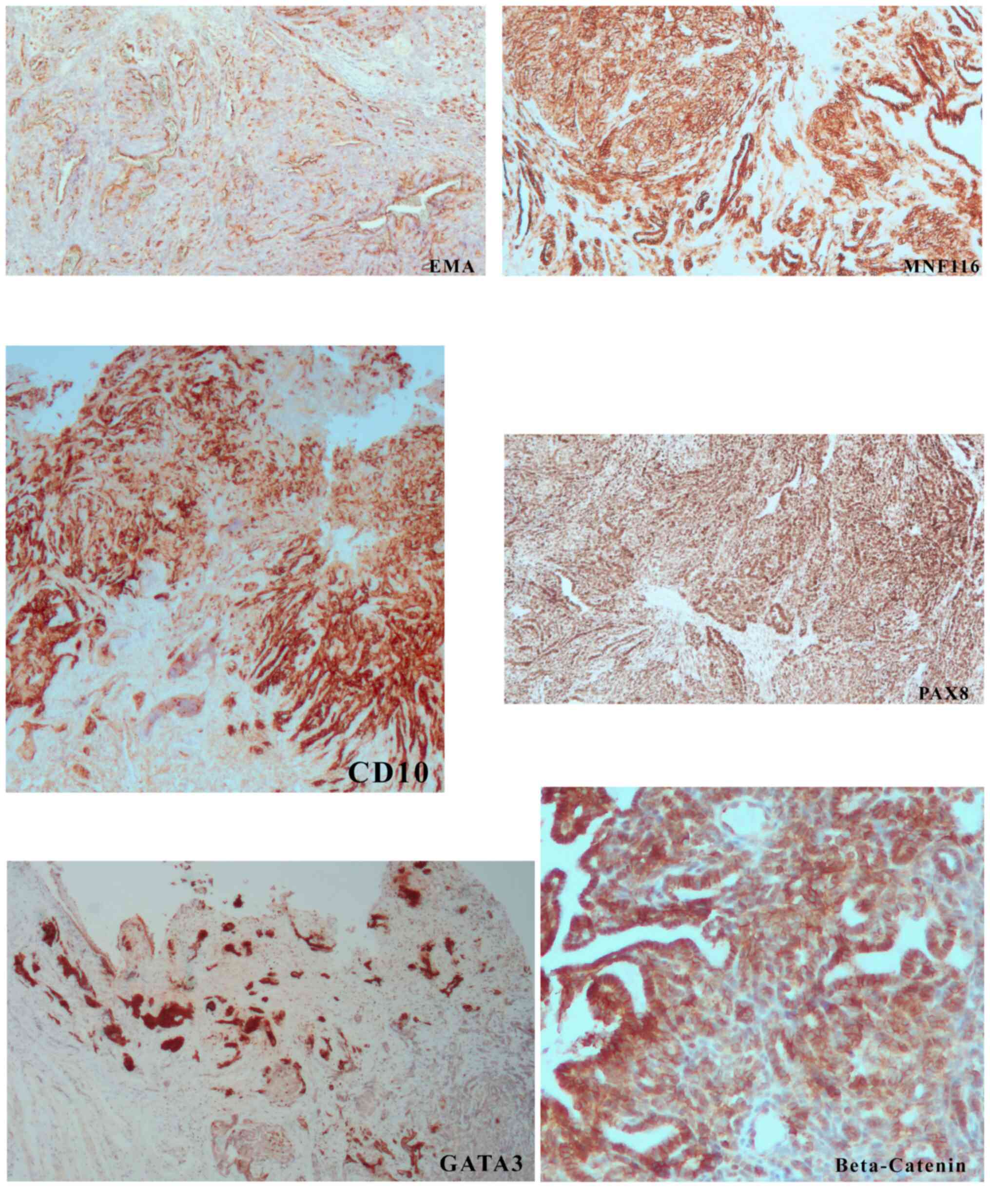
Immunohistochemistry demonstrated diffuse positive
staining of EMA, MNF116, PAX-8 (Fig.
2) and β-catenin (membranous).
Cytokeratin 7 and cyclin D1 were present in ~15 and
40% of lesional cells, respectively. GATA 3 and CD10
(apical/luminal) were positive in ~60% of cells (Fig. 2). Androgen receptor positivity was
detected in ~5% of cells. p16 showed a patchy positivity with no
‘block-type’ pattern. Cytokeratin 20, monoclonal CEA, ER, PR, TTF1,
WT1 and calretinin were negative. p53 showed normal levels of
expression. Ki67 staining, a marker of proliferation, showed
positive nuclear staining in ~70% of the lesional cells. Positive
CD10 and GATA3 and negative p16 immunostaining were consistent with
a mesonephric origin.
No mutations with currently established therapeutic
implications were detected either in primary or recurrent samples.
In particular, there were no mutations in TP53, BRAF,
EGFR, KRAS, NRAS and CTNNB1. However, a
missense variant of ATM (p.Phe858Leu, c.2572 T>C,
COSM21826)-[Sequence Ontology: SO:0001583], was observed in 65% of
the sequencing reads in the original tumour and in 96% of the
sequencing reads in the recurrent tumour.
The patient is alive and well without recurrence one
year after surgery. No additional treatment was administered.
Discussion
Cervical malignant mesonephric tumours are rare, and
the majority are adenocarcinomas (1). MMMsTs are extremely rare and,
therefore, understudied. To the best of our knowledge, only 11
cases of MMMsTs have been reported (3). MMMsTs are also called carcinosarcomas;
however, this may be an improper term, as it may evoke a Mullerian
tract derived neoplasm. Mesonephric tumours arise from mesonephric
remnants that are usually found in the lateral walls of the cervix
and less frequently adjacent to the ovarian hilum, in the broad
ligament and in the vagina (1). The
prevalence of mesonephric remnants varies from 1-22% in adults and
up to 40% in children (5,6).
The morphological and immunohistochemical data of
the present case are consistent with those of previous reports
(3). The diagnosis of cervical
MMMsTs from resection specimens should not be arduous, as the
neoplasm arises in the context of mesonephric hyperplasia and often
the background of benign mesonephric remnants is present. Diagnosis
from biopsy samples, however, may be problematic. If only the
epithelial component is present, differential diagnosis with florid
mesonephric hyperplasia can be difficult. Morphological features
such as atypia, brisk mitotic activity and the aforementioned
architectural pattern are helpful criteria. The rate of
proliferation, assessed by Ki67, is also a useful diagnostic clue
(7). Cervical mixed Mullerian
malignant tumour (carcinosarcoma) is also included in the
differential diagnosis. However, this neoplasm may show squamous
differentiation that is not present in MMMsTs. Endometrioid
endometrial adenocarcinoma, that extends into the cervix, may show
mesonephric morphological features (8). The CD10+, ER-
and R- immunoprofile of MMMsTs distinguishes them from
an endometrioid carcinoma. When a sarcomatous component is present,
the differential diagnosis is with endometrial stromal sarcoma
(ESS). MMMsTs do not exhibit the typical vasculature pattern of
ESS, as low-grade ESS is ER+ and PR+. In
high-grade MMMsTs, molecular analysis can rule out ESS (9).
Data on the molecular features of mesonephric
malignancies of the female genital tract, and MMMsT in particular,
are very limited. Mesonephric carcinomas with no sarcomatous
component have been characterised at the molecular level using NGS
(10). A total of 81% of cases had
a KRAS or NRAS mutation, while mutations in
ARID1A, ARID1B or SMARCA4 (chromatin
remodelling genes) were identified in 62% of cases. In 75% of
cases, a copy number alteration (1q gain) was identified. No
mutations in PIK3CA and PTEN were present. Based on
these findings the authors concluded that the molecular aberrations
in mesonephric carcinomas differed from cervical and endometrial
adenocarcinoma, which displayed KRAS and NRAS
mutations in 7 and 25% of cases, respectively.
The ATM protein belongs to the phosphatidylinositol
3-kinase (PIK3) family of proteins that participate in DNA repair
and encodes a protein kinase that phosphorylates multiple targets
following double stranded breaks; it plays a role in cell cycle
checkpoint arrest, DNA repair and apoptosis (11). Germline mutations in ATM
result in ataxia telangiectasia syndrome (ATS), and patients with
ATS show increased cancer predisposition, primarily development of
lymphomas or leukaemia (12,13).
Sporadic ATM mutations are relatively common in
haematological malignancies, as well as in a wide spectrum of
tumours, including breast, pancreatic and lung cancers (12,14).
To the best of the authors' knowledge, ATM
mutations in the female genital tract mesonephric neoplasms
(adenocarcinoma and MMMsT) have not been reported. The results of
NGS analysis in the case reported herein are interesting; however,
they should be interpreted with great caution. In the primary
tumour the missense mutation was seen in 65% of the sequencing
reads, and may be the result of either germline or somatic
mutation. The same ATM variant was found in 96% of the
recurrent tumour DNA. In a scenario with a single clone of cells,
we would expect possibilities of finding 0% mutation, 50% (one
allele inactivated) or 100% (both alleles inactivated), unless
there was a gene amplification. Our results of between 50 and 100%
suggest that the cell population is not monoclonal and includes
stromal and inflammatory cells or consists of a different
proportion of heterozygous and homozygous mutated cells, either
germline or somatic. However, considering the difference in the
proportion of the tumour epithelial and sarcomatous components, and
the tumour cellularity in both samples, with the sarcomatous
component being predominant in the recurrent tumour, the higher
value of 96% may be associated with the expansion of one clone.
Nevertheless, 96% is very close to the assumed homozygous germline
mutation rate of 100%. Therefore, an artefactual effect due to
excessive formalin fixation of the specimen cannot be excluded. The
possibility that the same presumed somatic ATM variant
detected in the primary neoplasm was also identified in the
recurrent tumour cannot be ruled out. As the patient did not
consent for normal tissue and blood molecular analysis, a germline
mutation cannot be excluded.
There is no clear evidence that this missense
variant (p.Phe858Leu) results in loss of function of the ATM
gene. Both germline and somatic mutations span the entire
ATM gene and occur in each functional domain (11). This heterogeneity makes it
challenging to make firm predictions of the clinical consequences.
Therefore, this variant would be considered of uncertain clinical
significance with respect to the pathogenesis of MMMsTs. In terms
of clinical consequences, analysis of breast cancer and control
cases by Stredrick et al (15) concluded that the p.Phe858Leu,
c.2572T>C missense variant was seen at ~2% frequency and was
associated with a significantly increased risk of breast cancer in
the USA, but not in Poland (15).
Estiar and Mehdipour (16) reported
in their comprehensive review on breast and brain tumour ATM
mutational studies, that the c.2572T>C mutation was mainly
detected in breast, but not in brain tumours. Additionally, the
same mutation was found in two endometrial carcinoma samples
reported in the COSMIC database by Rosa-Rosa et al (17) but was not considered pathogenic.
The ATM mutation p.(Phe858Leu), c.2572 T>C
(http://www.ncbi.nlm.nih.gov/clinvar/variation/132736/),
has been most commonly reported as benign, likely-benign or
conflicting-interpretations-of-pathogenicity (18,19).
However, in view of the results of the case reported herein, the
involvement of this variant in the pathogenesis of MMMsT cannot be
ruled out. Therefore, investigation into mutations in the ATM gene
should be considered in further suspected cases of MMMTs.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All the authors were involved in conceiving and
designing the present study. CM designed, drafted and wrote the
manuscript. IA, CCY and EW were responsible for collecting and
analysing patient data. SR designed, drafted the manuscript and
revised it critically for important intellectual content. All
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Signed informed consent was obtained from the
patient.
Patient consent for publication
The patient gave signed consent for publication;
however, the authors also made efforts to remove any identifying
information to protect the privacy of the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Howitt BE and Nucci MR: Mesonephric
proliferations of the female genital tract. Pathology. 50:141–150.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yano M, Shintani D, Katoh T, Hamada M, Ito
K, Kozawa E, Hasegawa K and Yasuda M: Coexistence of endometrial
mesonephric-like adenocarcinoma and endometrioid carcinoma suggests
a Müllerian duct lineage: A case report. Diagn Pathol.
14(54)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ribeiro B, Silva R, Dias R and Patrício V:
Carcinosarcoma of the uterine cervix: A rare pathological finding
originating from mesonephric remnants. BMJ Case Rep.
12(e227050)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Exome Sequencing by Ion Torrent
Next-Generation Sequencing. Thermo Fisher Scientific, UK, 2020;
Accessed July 19, 2020; Available from: https://www.thermofisher.com/uk/en/home/life-science/sequencing/dna-sequencing/exome-sequencing/exome-sequencing-ion-torrent-next-generation-sequencing.html.
|
|
5
|
Cavalcanti MS, Schultheis AM, Ho C, Wang
L, DeLair DF, Weigelt B, Gardner G, Lichtman SM, Hameed M and Park
KJ: Mixed mesonephric adenocarcinoma and high-grade neuroendocrine
carcinoma of the uterine cervix: Case description of a previously
unreported entity with insights into its molecular. Int J Gynecol
Pathol. 36:76–89. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ferry JA and Scully RE: Mesonephric
remnants, hyperplasia, and neoplasia in the uterine cervix. A study
of 49 cases. Am J Surg Pathol. 14:1100–1111. 1990.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jimenez C, Nucci M and Zaloudek C:
Mesonephric adenocarcinomas of the uterus and cervix - A
clinicopathologic study of 10 cases. Mod Pathol.
25(278A)2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tambouret R, Clement PB and Young RH:
Endometrial endometrioid adenocarcinoma with a deceptive pattern of
spread to the uterine cervix: A manifestation of stage IIb
endometrial carcinoma liable to be misinterpreted as an independent
carcinoma or a benign lesion. Am J Surg Pathol. 27:1080–1088.
2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee CH and Nucci MR: Endometrial stromal
sarcoma - the new genetic paradigm. Histopathology. 67:1–19.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mirkovic J, Sholl LM, Garcia E, Lindeman
N, MacConaill L, Hirsch M, Dal Cin P, Gorman M, Barletta JA, Nucci
MR, et al: Targeted genomic profiling reveals recurrent KRAS
mutations and gain of chromosome 1q in mesonephric carcinomas of
the female genital tract. Mod Pathol. 28:1504–1514. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shiloh Y: ATM: Expanding roles as a chief
guardian of genome stability. Exp Cell Res. 329:154–161.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Choi M, Kipps T and Kurzrock R: ATM
mutations in cancer: Therapeutic implications. Mol Cancer Ther.
15:1781–1791. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Abdolrahimzadeh S, Plateroti AM, Recupero
SM and Lambiase A: An update on the ophthalmologic features in the
phakomatoses. J Ophthalmol. 2016(3043026)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Forbes SA, Beare D, Gunasekaran P, Leung
K, Bindal N, Boutselakis H, Ding M, Bamford S, Cole C, Ward S, et
al: COSMIC: Exploring the world's knowledge of somatic mutations in
human cancer. Nucleic Acids Res. 43(D805-D811)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Stredrick DL, Garcia-Closas M, Pineda MA,
Bhatti P, Alexander BH, Doody MM, Lissowska J, Peplonska B, Brinton
LA, Chanock SJ, et al: The ATM missense mutation p.Ser49Cys
(c.146C>G) and the risk of breast cancer. Hum Mutat. 27:538–544.
2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Estiar MA and Mehdipour P: ATM in breast
and brain tumors: A comprehensive review. Cancer Biol Med.
15:210–227. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rosa-Rosa JM, Leskelä S, Cristóbal-Lana E,
Santón A, López-García MA, Muñoz G, Pérez-Mies B, Biscuola M, Prat
J, Esther O, et al: Molecular genetic heterogeneity in
undifferentiated endometrial carcinomas. Mod Pathol. 29:1390–1398.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
11-108137983-108138023 | gnomAD. Retrieved
July 20, 2020; Available from: https://gnomad.broadinstitute.org/region/11-108137983-108138023?dataset=gnomad_r2_1.
|
|
19
|
VCV000132736:10 - ClinVar - NCBI.
Retrieved July 22, 2020; Available from: https://www.ncbi.nlm.nih.gov/clinvar/variation/132736/.
|