Introduction
Thyroid cancer (THCA) is a disease with high
incidence, low malignancy and slow tumor growth (1). Papillary thyroid cancer (PTHCA)
accounts for ~85% of THCAs and is highly differentiated and with
good therapeutic effects (2). While
early PTCHA does not have any obvious symptoms, the disease
exhibits high invasion and metastasis at advanced stages such that
surgical resection and chemoradiotherapy are not fully effective
(3). Targeted therapy is an
effective method to combat PTCHA and novel therapeutic targets are
required.
Centromere protein F (CENPF) is a transient
kinetochore protein that regulates multiple cellular processes,
including chromosome segregation during mitosis (4,5). The
absence of CENPF causes various physical and developmental defects,
including loss of CENF in mouse embryos, resulting in developmental
failure (6-9).
CENPF is upregulated during the G2/M phase, inducing microtubule
attachment and regulating cellular functions (6,10).
Additionally, CENPF interacts with several key cell cycle
checkpoint proteins and late telophase proteins, including syntaxin
4 and synaptosomal-associated protein 25, to further affect
cellular processes (11,12).
CENPF upregulation has been observed in multiple
types of cancer, including hepatocellular carcinoma (HCC), breast
cancer, nasopharyngeal cancer, prostate cancer and gastric cancer
(11-15).
The involvement of CENPF in cancer progression and metastasis, and
its effects on prognosis, have been widely reported (16-19).
CENPF was associated with the prognosis of non-small cell lung
cancer (NSCLC) and prostate cancer (15-18).
Additionally, CENPF overexpression was associated with poor
prognosis and bone metastasis of breast cancer (19). Furthermore, a bioinformation
analysis revealed an association between CENPF expression and the
progression of non-muscle invasive bladder cancer (10). However, the potential role of CENPF
in PTHCA progression remains unclear.
The present study aimed to clarify the role of CENPF
in PTHCA progression. The current study demonstrated that CENPF
upregulation in human PTHCA tissues and CENPF expression was
correlated with the prognosis and clinical features in patients
PTHCA. The effects of CENPF on PTHCA cell proliferation and
apoptosis were further investigated. CENPF may be a promising
molecular target for PTHCA treatment.
Materials and methods
Bioinformatic analysis
Bioinformatic analysis was performed using Gene
Expression Profiling Interactive Analysis (http://gepia.cancer-pku.cn/detail.php?gene=CENPF/) to
analyze The Cancer Genome Atlas (TCGA; https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga)
data with a threshold of P<0.05 and LogFC >1 or <-1 for
differential genes. The median of survival rates was used as the
basis for dividing patients into two groups for Kaplan-Meier
survival analysis.
Antibodies, primers and short hairpin
(sh)RNA plasmids
Anti-CENPF [for immunohistochemical (IHC) assays,
1:500; for immunoblot assays, 1:2,000; cat. no. ab84697],
anti-β-actin (1:2,000; cat. no. ab8226), horseradish peroxidase
(HRP)-conjugated goat anti-rabbit IgG (1:5,000; cat. no. ab6721)
and HRP-conjugated rabbit anti-mouse IgG (1:5,000; cat. no. ab6728)
were purchased from Abcam.
Quantitative PCR (qPCR) primer sequences were as
follows: CENF forward, 5'-TCTGCTCGGGTTCAAACTGG-3' and reverse,
5'-TGTGAGTCCGTGACCGAGTA-3'; and GAPDH forward,
5'-CGACCACTTTGTCAAGCTCA-3' and reverse,
5'-GGTTGAGCACAGGGTACTTTATT-3'. CENPF shRNA plasmids (cat. no.
sc-37563-V) were purchased from Santa Cruz Biotechnology Co.,
Ltd.
Human tissue samples and analysis
A total of 87 human PTHCA tissues and corresponding
normal tissues were collected from patients with PTHCA at Binzhou
Medical University Hospital (Binzhou, China). Patients were divided
into CENPF low expression and high expression groups according to
CENPF expression in tumor tissues. Clinicopathological
characteristics, including patient age, sex, tumor (T) stage
(15), lymph node metastasis and
intraglandular dissemination, are presented in Table I. All procedures performed in the
current study were approved by the Ethics Committee of Binzhou
Medical University Hospital (Binzhou, China). Written informed
consent was obtained from all patients or their families.
 | Table ICorrelation between CENPF expression
and clinicopathological features of 87 patients with papillary
thyroid cancer. |
Table I
Correlation between CENPF expression
and clinicopathological features of 87 patients with papillary
thyroid cancer.
| | | CENPF expression | | |
|---|
| Clinicopathological
feature | No. of patients
(n=87) | Low (n=22) | High (n=65) | χ2 | P-value |
|---|
| Age (years) | | | | 2.213 | 0.137 |
|
<45 | 32 | 11 | 21 | | |
|
≥45 | 55 | 11 | 44 | | |
| Sex | | | | 3.804 | 0.051 |
|
Male | 22 | 9 | 13 | | |
|
Female | 65 | 13 | 52 | | |
| T stage | | | | 5.368 | 0.021a |
|
T1-T2 | 37 | 14 | 23 | | |
|
T3-T4 | 50 | 8 | 42 | | |
| Lymph node
metastasis | | | | 0.035 | 0.851 |
|
Yes | 42 | 11 | 31 | | |
|
No | 45 | 11 | 34 | | |
| Intraglandular
dissemination | | | | 4.148 | 0.042a |
|
Yes | 40 | 6 | 34 | | |
|
No | 47 | 16 | 31 | | |
IHC assays
To explore the possible association between CENPF
expression and PTHCA progression, IHC assays were performed. Tissue
sections (5-µm thickness) were fixed with 4% paraformaldehyde at
room temperature for 20 min and blocked with 2% BSA (Sigma-Aldrich;
Merck KGaA) for 20 min at room temperature. Slides were incubated
with CENPF antibodies for 2 h at room temperature. Sections were
then incubated with mice or rabbit biotinylated secondary
antibodies (1:5,000; cat. nos. ab6721 and ab6728; Abcam) for 1.5 h
at room temperature. Diaminobenzidine (Sigma-Aldrich; Merck KGaA)
was used as a chromogen substrate. A light microscope (IX71; Zeiss
AG) was used for imaging at x100 and x200 magnification.
IHC assays showed that CENPF was located in the
cytoplasm of cells in PTHCA tissues. The scoring method used to
analyze the proportion of positive stained cells was as follows: 0
(0% stained cells), 1 (1-20% stained cells), 2 (21-60% stained
cells) and 3 (61-100% stained cells).
Staining intensity was evaluated on a score of 0 (no
staining or low-level staining), 1 (modest staining) and 2 (high
staining). CENPF expression levels were divided according to the
staining index: Score of staining intensity + score of staining
cells percentage. A staining index <2 was considered as low
CENPF expression and ≥2 was considered as high CENPF
expression.
Cell cultures and transfections
Human PTHCA cell lines TPC-1 and KTC-1 were
purchased from the American Type Culture Collection. TPC-1 and
KTC-1 cells were incubated in DMEM supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) at 37˚C in a 5%
CO2 incubator for 48 h. CENPF shRNA plasmids (1 µg/µl;
5'-GTTTCAGCTTGACAGTCTCG-3') were transfected into TPC-1 and KTC-1
cells using Lipofectamine® 2000 (cat. no. 11668019;
Invitrogen, Thermo Fisher Scientific, Inc.) for 6 h. In 6-well
plates, 5 µl transfection reagent and 1 µg plasmids were mixed in
250 µl of serum-free DMEM, left to stand for 5 min and then mixed.
Following incubation at room temperature for 20 min, the mix was
added to serum-starved cells and incubated at 37˚C for 4 h. For the
control group, the shRNA targeting sequence was nonsense and did
not target intracellular RNAs. Only TPC-1 cells were used in the
animal assays, and stable CENPF-depleted cells were used for the
in vivo assays. Stable CENPF-depleted TPC-1 cells were
screened through CENPF shRNA lentivirus infection to stably deplete
CENPF expression and used for in vivo tumor growth
assays.
qPCR assays
Trizol® (cat. no. 15596026; Invitrogen,
Thermo Fisher Scientific, Inc.) was used to extract total RNA from
human PTHCA cells. RNA was reverse-transcribed using M-MLV reverse
transcriptase (cat. no. M1701; Promega Corporation). Total mRNA was
reverse transcribed into cDNA by using a cDNA synthesis system
(cat. no. 6110A; Takara Bio, Inc.) at 42˚C for 1 h. qPCR was
performed using a SYBR Ex Taq kit (cat. no. 638319; Takara Bio,
Inc.). The following thermocycling conditions were used for the
qPCR: Initial denaturation at 95˚C for 3 min; followed by 30 cycles
of denaturation at 95˚C for 30 sec, annealing at 58˚C for 30 sec
and extension a 72˚C for 30 sec. The
2-ΔΔCq method was used to quantify
results (20). CENPF expression
levels were normalized to GAPDH.
Western blotting
PTHCA cells or tissues were lysed using RIPA buffer
(cat. no. 9800; Cell Signaling Technology, Inc.). The BCA method
was used for protein determination. Proteins (20 mg/lane) were
separated via 8% SDS-PAGE. Proteins were then transferred onto PVDF
membranes. Membranes were blocked with 5% milk in TBS-T at 37˚C for
2 h and incubated with the aforementioned primary antibodies for
the detection of CENPF and β-actin at room temperature for 2 h.
Membranes were subsequently incubated with HRP-conjugate secondary
antibodies for 1 h at room temperature. Signals were detected using
an ECL kit (Novex™ Chemiluminescent Substrate Reagent kit; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
Signal intensity was measured using ImageJ (version 1.8.0; National
Institutes of Health).
Colony formation assays
PTHCA cells were plated into 6-well culture plates
and transfected with control or CENPF shRNA plasmids as
aforementioned. DMEM was replaced with fresh medium every 3 days.
After 14 days, cells were fixed with 4% paraformaldehyde for 30 min
at room temperature, stained with 0.1% crystal violet at room
temperature for 30 min and washed with PBS. Numbers of colonies
were counted manually. A light microscope (IX71; Zeiss AG) was used
for imaging at x50 magnification.
MTT assays
PTHCA cells were plated into 96-well plates (seeding
density, 1x103/well), transfected with control or CENPF
shRNA plasmids and incubated for 24 h. Cells were then incubated
with MTT for 4 h at room temperature and DMEM was removed.
Following this, 150 µl DMSO was added into each well to extract
stained cells. Optical density (OD) values were measured using a
microplate reader at 570 nm.
Cell cycle and apoptosis assays
An Annexin V-FITC Apoptosis Detection kit was
purchased from Beijing Solarbio Science & Technology Co., Ltd.
(cat. no. CA1020). For cell apoptosis assays, PTHCA cells were
fixed with 70% ethyl alcohol for 24 h at -20˚C and treated with
Annexin V-FITC and propidium iodide (PI) for 20 min at room
temperature. Cells were then analyzed using a FACS Calibur flow
cytometer (FACSAria III; BD Biosciences).
For cell cycle assays, PTHCA cells were fixed with
70% ethyl alcohol for 24 h at -20˚C and incubated with 50 µg/ml PI
at 37˚C for 30 min. The percentage of cells in different phases was
analyzed using a flow cytometer (FACSAria III; BD Biosciences).
Tumor growth assays
All animal experiments were approved by the
Institutional Animal Use Committee of Binzhou Medical University
Hospital, Tianjin, China. Female BALB/c nude mice (8-week-old; ~20
g) were supplied by Beijing Vital River Experimental Animal
Technology Co., Ltd.). Mice were fed with food and water ad
libitum and were fed at specific pathogen-free conditions at
20˚C, 60% humidity and alternating 12-h light/dark cycles. A total
of 12 athymic nude mice were included in control (n=6) and shRNA
(n=6) groups. TPC-1 cells were stably infected with control or
CENPF shRNA lentivirus as aforementioned. Subsequently,
~1x106 TPC-1 cells infected with the indicated
lentivirus were subcutaneously injected into the abdomen of athymic
nude mice. No mice died during the experiment. Mice were sacrificed
via cervical dislocation prior to the removal of tumor tissue.
Heartbeats were assessed to determine death and adequate
humanitarian care was given.
At 15 days post-injection, tumors began to form, and
tumor volumes were measured every 3 days. After 30 days, tumors
were excised and photographed. Tumor growth curves were calculated
and compared. Tumors were isolated and photographed, and tumor
growth curves were calculated and compared. The tumor volume was
calculated using the following formula: Tumor volume
(mm3) = tumor length (mm) x tumor width
(mm)2/2. In terms of weight, mice gained no more than 4%
and lost no more than 10% of their body weight. The weights of
different groups of mice were not significantly different between
groups during the whole feeding period. The maximum diameter of
tumors was 10 mm and the maximum tumor weight was 5% of the total
body weight.
Statistical analysis
GraphPad software (version 6.0; GraphPad Software,
Inc.) was used for statistical analysis. Data are presented as the
mean ± standard deviation. Associations between clinical features
of patients with PTHCA and CENPF expression levels were analyzed by
Pearson's χ2 test. Student's t-test was used for
statistical comparison between two groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
CENPF expression is enhanced in human
PTHCA tissues and is associated with the prognosis and clinical
features of patients
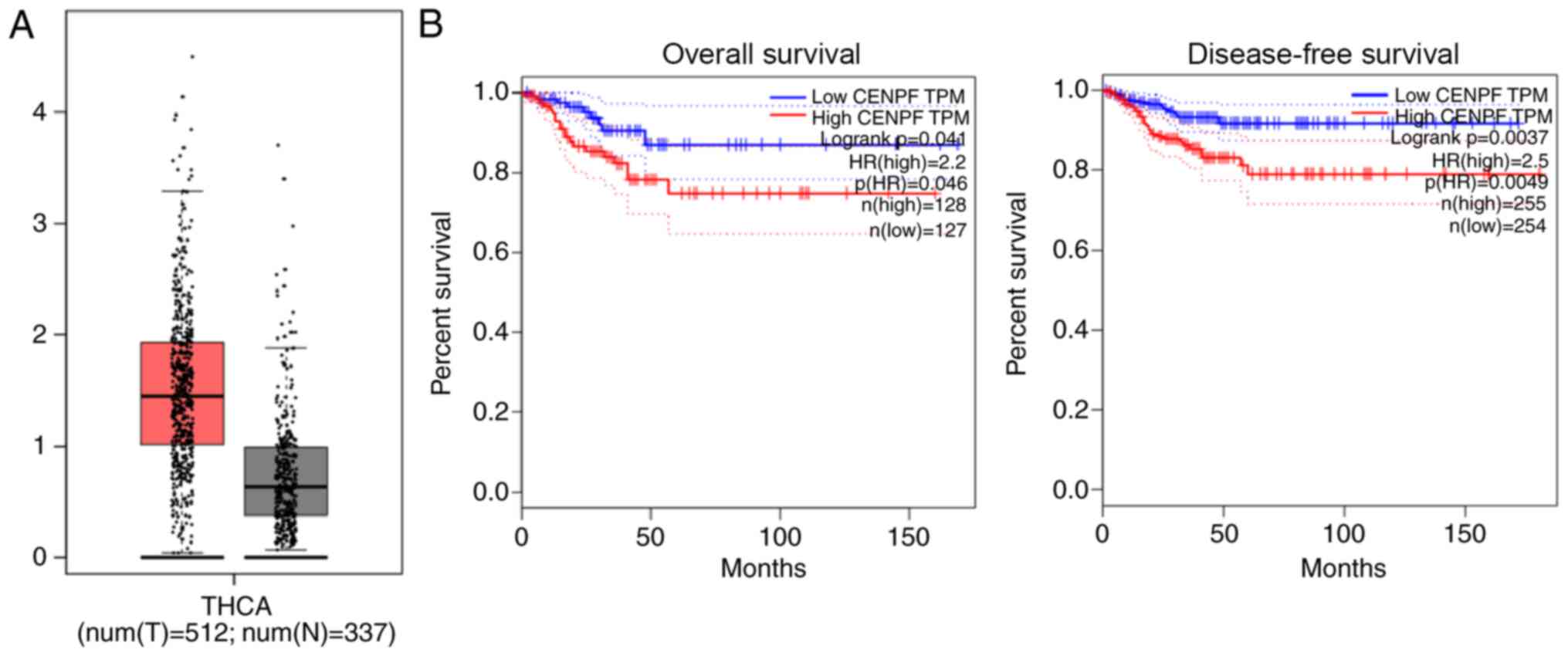
Relative mRNA levels of CENPF in human PTHCA tissues
were compared with normal tissues using the TCGA database.
According to the comparison between 512 tumor tissues and 337
normal tissues, high mRNA levels of CENPF were observed in PTHCA
tissues (Fig. 1A). Furthermore,
survival analysis of the database revealed that mRNA levels of
CENPF were associated with overall survival and disease-free
survival rates in patients with PTHCA (Fig. 1B), indicating an association between
CENPF expression and patient prognosis.
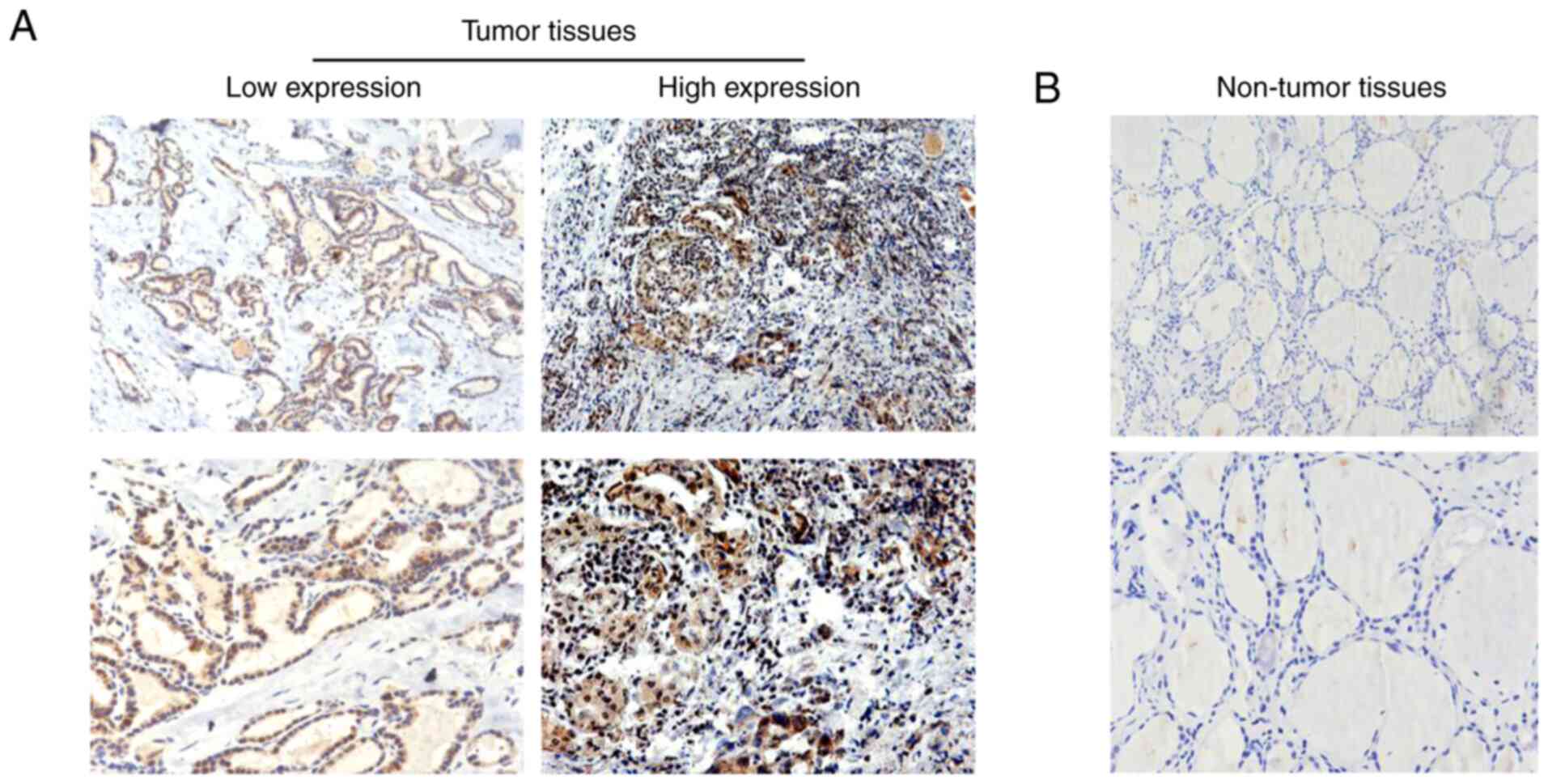
Human PTHCA tissues exhibit high CENF
expression in human PTHCA tissues
IHC assays were performed to investigate CENPF
protein levels in PTHCA tissues and corresponding normal tissues.
The results demonstrated that the expression of CENPF was markedly
higher in PTHCA tissues (Fig. 2A).
Furthermore, low CENPF expression was observed in corresponding
normal tissues (Fig. 2B). These
results confirmed high CENPF expression in human PTHCA tissues.
CENPF expression is associated with
the clinicopathological features of patients with PTHCA
The clinicopathological features of 87 patients with
PTHCA were analyzed. Patients were divided into CENPF low
expression and high expression groups according to CENPF expression
in tumor tissues. The results demonstrated that 22 (25.3%) patients
exhibited CENPF low expression and the remaining 65 (74.7%)
exhibited high expression (Table
I). Clinicopathological features, including age, sex, T stage,
lymph node metastasis and intraglandular dissemination, were
analyzed. There was no association between CENPF expression and age
(P=0.137), sex (P=0.051) and lymph node metastasis (P=0.851) in
patients with PTHCA. However, CENPF expression was associated with
T stage (P=0.021) and intraglandular dissemination (P=0.042) in
patients with PTHCA. The results revealed that CENPF expression was
associated with certain clinicopathological features in patients
with PTHCA.
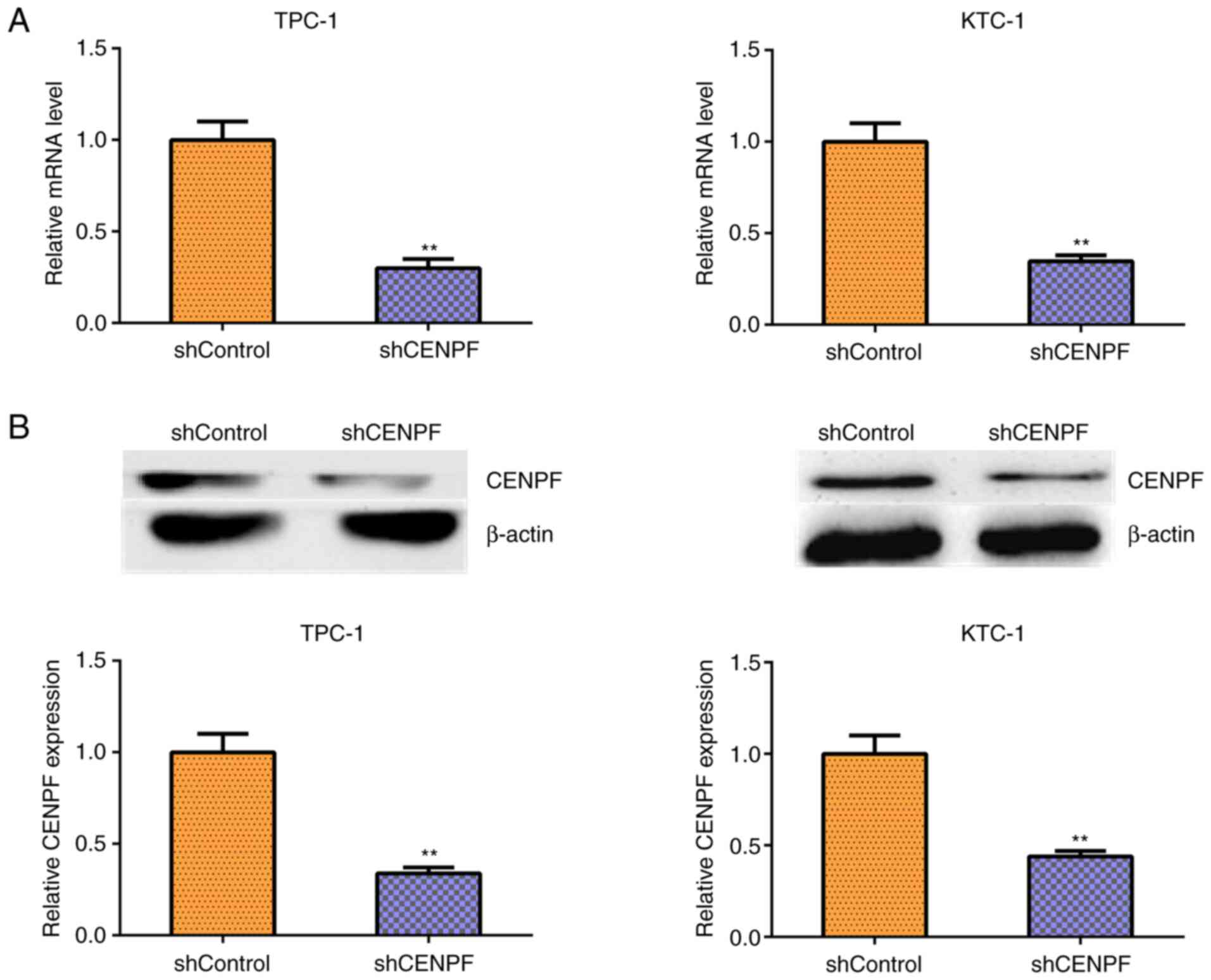
CENPF depletion suppresses
proliferation, stimulates apoptosis and causes cell cycle arrest in
PTHCA cells
To further assess the involvement of CENPF in PTHCA
progression, shRNA plasmids targeting CENPF were used to deplete
CENPF expression in human PTHCA cell lines TPC-1 and KTC-1.
Silencing efficiency was detected using qPCR assays (Fig. 3A) and western blotting (Fig. 3B). CENPF mRNA and protein levels
were significantly decreased following CENPF depletion.
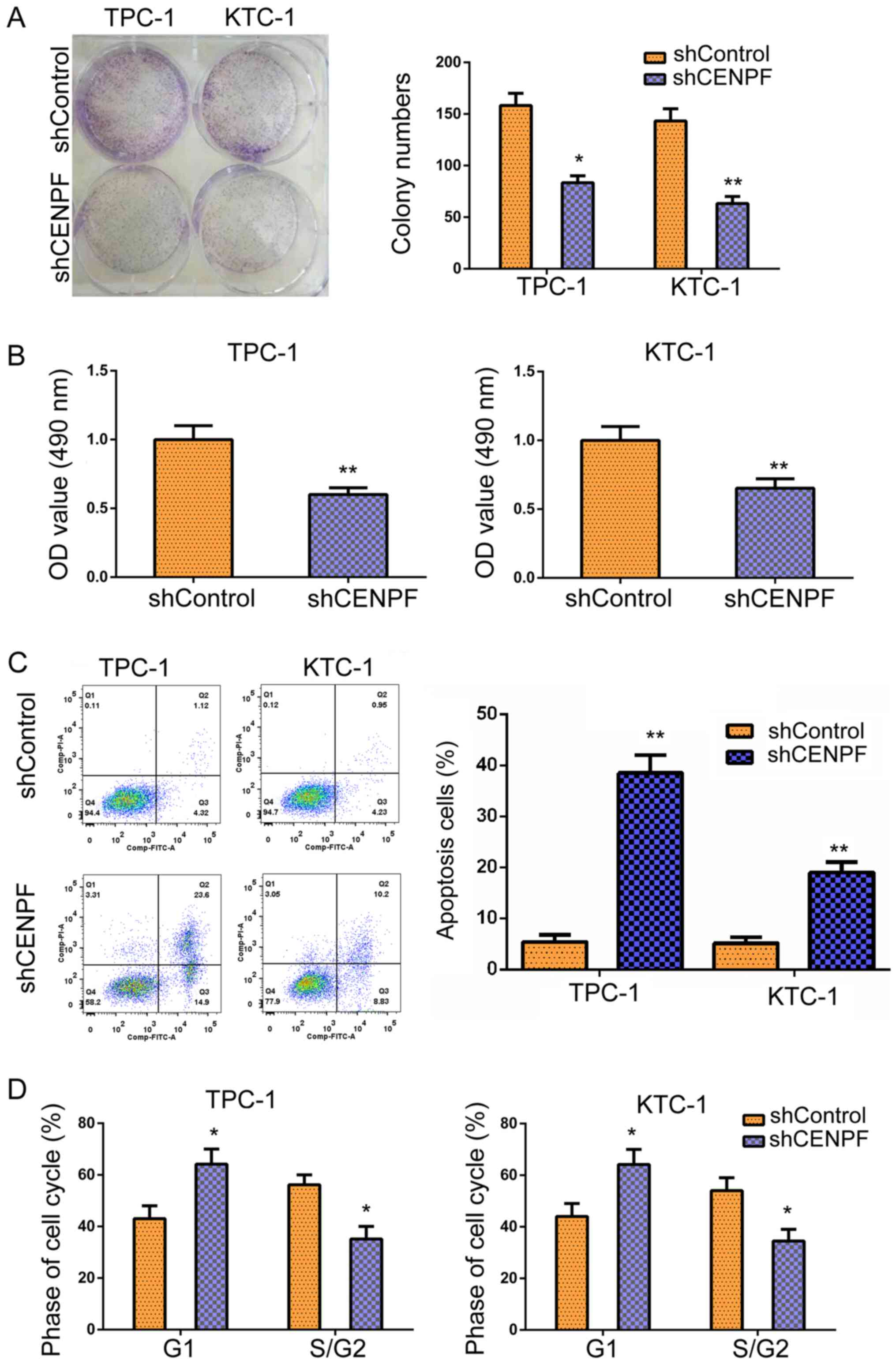
CENPF depletion results in
proliferation defects, cell cycle arrest and apoptosis
MTT assays and colony formation assays were
performed to assess the effects of CENPF on cytotoxicity in TPC-1
and KTC-1 cells. CENPF depletion resulted in significantly
decreased colony numbers in TPC-1 and KTC-1 cells, as evidenced by
colony formation assays (Fig. 4A).
Furthermore, MTT assays demonstrated that relative OD values were
significantly decreased in TPC-1 and KTC-1 cells following CENPF
depletion, indicating defects in PTHCA cell cytotoxicity (Fig. 4B). The effects of CENPF on apoptosis
and the cell cycle of TPC-1 and KTC-1 cells transfected with
control or CENPF shRNA plasmids were detected using flow cytometry
(FCM). The results demonstrated that CENPF depletion increased the
percentage of apoptotic cells in TPC-1 and KTC-1 cells (Fig. 4C). Additionally, CENPF depletion led
to the arrest of cell cycles in PTHCA cells, with the increase
proportion of cells at the G1 phase (Fig. 4D). In summary, the results
demonstrated the CENPF depletion resulted in proliferation defects
and cell cycle arrest and stimulated the apoptosis of thyroid
cancer cells in vitro.
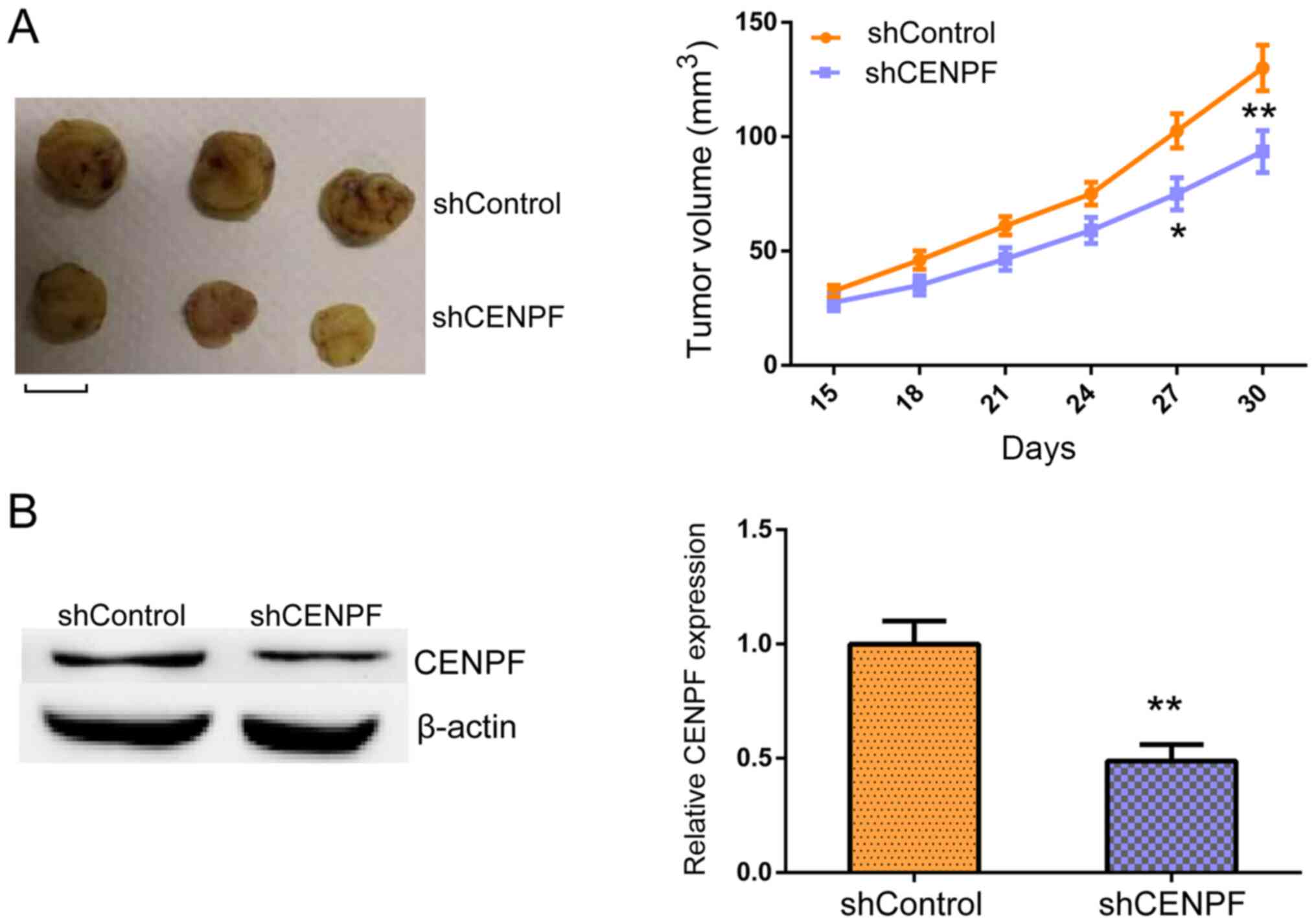
CENPF promotes the tumor growth of
PTHCA cells in vivo
Whether CENPF promoted tumor growth in PTHCA cells
was assessed using an animal model to further investigate the
effects of CENPF on PTHCA progression. TPC-1 cells infected with
control or CENPF shRNA lentivirus were subcutaneously injected into
nude mice. At 15 days post-injection, tumors began to form, and
tumor volumes were measured every 3 days. After 30 days, all tumors
were isolated and photographed. Representative images of tumors
were presented on the left and tumor growth curves on the right in
Fig. 5A. Tumor volumes in the
CENPF-depleted groups were significantly decreased compared with
control groups. Furthermore, western blotting was performed to
examine CENPF expression in tumor tissues in control or
CENPF-depleted groups. The results demonstrated that CENPF
expression levels were decreased in CENPF-depleted tumors (Fig. 5B). These results indicated that
CENPF promoted tumor growth of PTHCA cells in vivo.
Discussion
PTHCA is one of the most common endocrine
malignancies and worldwide incidence of PTHCA has markedly
increased since 2010(21). PTHCA is
the most common subtype of THCA and accounts for ~85% of PTHCA
cases and usually has a favorable prognosis (21). However, certain patients exhibit
high risk for tumor recurrence or death (22). Due to the high metastatic rate of
cancer, advances in targeted therapy are urgently required
(23,24). Therefore, novel treatments methods,
such as targeted therapy, need to be developed to improve the
survival rates of PTHCA (25). The
results of the current study demonstrated high CENPF expression in
human PTHCA tissues compared with corresponding normal tissues.
Furthermore, CENP expression was associated with patient prognosis
and clinicopathological features. The results indicated the
involvement of CENPF in PTHCA progression and suggested that CENPF
acted as a promising molecular target for PTHCA treatment.
Colony formation and MTT assays further confirmed
that CENPF contributed to the proliferation and cytotoxicity of
PTHCA cells in vitro. Furthermore, FCM assays demonstrated
that CENPF altered PTHCA cell apoptosis and regulated the cell
cycles of PTHCA cells. These results indicated that the depletion
of CENPF induced various anti-tumor effects on PTHCA and developing
inhibitors targeting CENPF may be an effective therapy to treat
this disease. Previous studies have demonstrated that CENPF was
abnormally expressed and altered the progression of various types
of tumors (13,17,26-29).
For instance, CENPF was associated with the prognosis of NSCLC and
was associated with tumor bone metastasis of breast cancer
(19). Additionally, CENPF was
regulated by cycling B1 and contributed to the proliferation and
metastasis of gastric cancer (13).
Furthermore, lymphoid-specific helicase may also promote the growth
and invasion of HCC by targeting CENPF (13). These previous studies, together with
the results of the current study, confirmed the critical role of
CENPF in cancer progression. Future studies should be conducted to
determine whether CENPF affects the migration and invasion of
gastric cancer cells in vitro and tumor metastasis in
vivo in mice models.
Through a series of assays, the current study
demonstrated that CENPF affected the proliferation and apoptosis of
PTHCA cells in vitro. Consistently, in vivo data
further confirmed that CENPF affected tumor growth in mice. As the
results of the current study confirmed this conclusion, the present
study hypothesized that CENPF altered the progression of PTHCA by
regulating cell proliferation and apoptosis.
Several previous studies have indicated that CENPF
may affect cancer progression through metabolism regulation
(16,18). Furthermore, CENPF depletion
remodeled prostate cancer cells by altering cellular metabolism
(16). Another previous study
reported that CENPF modulated cancer metabolism by mediating the
pyruvate kinase M2 phosphorylation pathway (18). Future studies should clarify whether
CENPF promotes PTHCA progression via this signaling pathway.
CENPF is also known as a microtubule binding protein
and regulates several microtubule-dependent cellular processes,
including mitosis (9,28). A previous study demonstrated that
CENPF promoted chromosome segregation and contributed to cell cycle
progression (28). Consistent with
these previous findings, the current study demonstrated that CENPF
depletion induced cell cycle arrest in PTHCA cells; this may be by
altering microtubule dynamics and mitosis. The present study also
showed that CENPF depletion stimulated PTHCA cell apoptosis.
However, whether CENPF exerts its effects through the regulation of
microtubules requires further study.
In conclusion, the current study reported enhanced
expression of CENPF in human PTHCA tissues. CENPF expression was
associated with prognosis and certain clinicopathological features
in patients with PTHCA, including T stage and intraglandular
dissemination. Further assays confirmed the effect of CENPF on
PTHCA cell proliferation and apoptosis in vitro and in
vivo tumor growth in mice. Therefore, CENPF may be a promising
therapeutic target for PTHCA treatment.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH, SX and YB performed the molecular biology
experiments and drafted the manuscript. KC, CD, SL and WZ
participated in study design and performed statistical analysis.
YB, YH, SX, KC, CD, SL and WZ conceived and designed the study and
aided in drafting the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All procedures performed in the current study were
approved by the Ethics Committee of Binzhou Medical University
Hospital, Binzhou, China. Written informed consent was obtained
from all patients or their families.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Su A, Zhao W, Wu W, Wei T, Ruan M, Li Z
and Zhu J: The association of preoperative thyroid-stimulating
hormone level and the risk of differentiated thyroid cancer in
patients with thyroid nodules: A systematic review and
meta-analysis. Am J Surg. 220:634–641. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shao W, Kuhn C, Mayr D, Ditsch N,
Kailuweit M, Wolf V, Harbeck N, Mahner S, Jeschke U, Cavaillès V
and Sixou S: Cytoplasmic and nuclear forms of thyroid hormone
receptor β1 are inversely associated with survival in primary
breast cancer. Int J Mol Sci. 21(330)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Qiu K, Xie Q, Jiang S and Lin T: Silencing
of DJ-1 reduces proliferation, invasion, and migration of papillary
thyroid cancer cells in vitro, probably by increase of PTEN
expression. Int J Clin Exp Pathol. 12:2046–2055. 2019.PubMed/NCBI
|
|
4
|
Alghamdi M, Alkhamis WH, Jamjoom D,
Al-Nafisah G, Tahir A and Abdouelhoda M: Expanding the phenotype
and the genotype of Stromme syndrome: A novel variant of the CENPF
gene and literature review. Eur J Med Genet.
63(103844)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fowler KJ, Saffery R, Irvine DV, Trowell
HE and Choo KH: Mouse centromere protein F (Cenpf) gene maps to the
distal region of chromosome 1 by interspecific backcross analysis.
Cytogenet Cell Genet. 82:180–181. 1998.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ozkinay F, Atik T, Isik E, Gormez Z,
Sagiroglu M, Sahin OA, Corduk N and Onay H: A further family of
Stromme syndrome carrying CENPF mutation. Am J Med Genet A.
173:1668–1672. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Waters AM, Asfahani R, Carroll P, Bicknell
L, Lescai F, Bright A, Chanudet E, Brooks A, Christou-Savina S,
Osman G, et al: The kinetochore protein, CENPF, is mutated in human
ciliopathy and microcephaly phenotypes. J Med Genet. 52:147–156.
2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhou CJ, Wang XY, Han Z, Wang DH, Ma YZ
and Liang CG: Loss of CENPF leads to developmental failure in mouse
embryos. Cell Cycle. 18:2784–2799. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Toralova T, Susor A, Nemcova L, Kepkova K
and Kanka J: Silencing CENPF in bovine preimplantation embryo
induces arrest at 8-cell stage. Reproduction. 138:783–791.
2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shi J, Zhang P, Liu L, Min X and Xiao Y:
Weighted gene coexpression network analysis identifies a new
biomarker of CENPF for prediction disease prognosis and progression
in nonmuscle invasive bladder cancer. Mol Genet Genomic Med.
7(e982)2019.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Kim HE, Kim DG, Lee KJ, Son JG, Song MY,
Park YM, Kim JJ, Cho SW, Chi SG, Cheong HS, et al: Frequent
amplification of CENPF, GMNN and CDK13 genes in hepatocellular
carcinomas. PLoS One. 7(e43223)2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pooley RD, Moynihan KL, Soukoulis V, Reddy
S, Francis R, Lo C, Ma LJ and Bader DM: Murine CENPF interacts with
syntaxin 4 in the regulation of vesicular transport. J Cell Sci.
121:3413–3421. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen EB, Qin X, Peng K, Li Q, Tang C, Wei
YC, Yu S, Gan L and Liu TS: HnRNPR-CCNB1/CENPF axis contributes to
gastric cancer proliferation and metastasis. Aging (Albany NY).
11:7473–7491. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Göbel C, Özden C, Schroeder C, Hube-Magg
C, Kluth M, Möller-Koop C, Neubauer E, Hinsch A, Jacobsen F, Simon
R, et al: Upregulation of centromere protein F is linked to
aggressive prostate cancers. Cancer Manag Res. 10:5491–5504.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li R, Wang X, Zhao X, Zhang X, Chen H, Ma
Y and Liu Y: Centromere protein F and Forkhead box M1 correlation
with prognosis of non-small cell lung cancer. Oncol Lett.
19:1368–1374. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shahid M, Kim M, Lee MY, Yeon A, You S,
Kim HL and Kim J: Downregulation of CENPF remodels prostate cancer
cells and alters cellular metabolism. Proteomics.
19(e1900038)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Aytes A, Mitrofanova A, Lefebvre C,
Alvarez MJ, Castillo-Martin M, Zheng T, Eastham JA, Gopalan A,
Pienta KJ, Shen MM, et al: Cross-species regulatory network
analysis identifies a synergistic interaction between FOXM1 and
CENPF that drives prostate cancer malignancy. Cancer Cell.
25:638–651. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shahid M, Lee MY, Piplani H, Andres AM,
Zhou B, Yeon A, Kim M, Kim HL and Kim J: Centromere protein F
(CENPF), a microtubule binding protein, modulates cancer metabolism
by regulating pyruvate kinase M2 phosphorylation signaling. Cell
Cycle. 17:2802–2818. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sun J, Huang J, Lan J, Zhou K, Gao Y, Song
Z, Deng Y, Liu L, Dong Y and Liu X: Overexpression of CENPF
correlates with poor prognosis and tumor bone metastasis in breast
cancer. Cancer Cell Int. 19(264)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mariniello RM, Orlandella FM, Stefano AE,
Iervolino PLC, Smaldone G, Luciano N, Cervone N, Munciguerra F,
Esposito S, Mirabelli P and Salvatore G: The TUSC2 tumour
suppressor inhibits the malignant phenotype of human thyroid cancer
cells via SMAC/DIABLO Protein. Int J Mol Sci.
21(702)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Moreno I, Hirsch D, Duskin-Bitan H,
Diker-Cohen T, Shimon I and Robenshtok E: Response to therapy
assessment in intermediate-risk thyroid cancer patients-is rhTSH
stimulation required? Thyroid. 30:863–870. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yang Y, Zhao Z, Xie CW and Zhao Y:
Dual-targeting liposome modified by glutamic hexapeptide and folic
acid for bone metastatic breast cancer. Chem Phys Lipids.
228(104882)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhao Z, Zhao Y, Xie CW, Chen CQ, Lin D,
Wang S, Lin D, Cui X, Guo Z and Zhou J: Dual-active targeting
liposomes drug delivery system for bone metastatic breast cancer:
Synthesis and biological evaluation. Chem Phys Lipids.
223(104785)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Qiu ZL, Shen CT, Sun ZK, Song HJ, Zhang GQ
and Luo QY: Lung metastases from papillary thyroid cancer with
persistently negative thyroglobulin and elevated thyroglobulin
Antibody levels during radioactive iodine treatment and follow-up:
Long-term outcomes and prognostic indicators. Front Endocrinol
(Lausanne). 10(903)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lin SC, Kao CY, Lee HJ, Creighton CJ,
Ittmann MM, Tsai SJ, Tsai SY and Tsai MJ: Dysregulation of
miRNAs-COUP-TFII-FOXM1-CENPF axis contributes to the metastasis of
prostate cancer. Nat Commun. 7(11418)2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lokody I: Signalling: FOXM1 and CENPF:
Co-pilots driving prostate cancer. Nat Rev Cancer. 14:450–451.
2014.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Mahmoud AD, Ballantyne MD, Miscianinov V,
Pinel K, Hung J, Scanlon JP, Iyinikkel J, Kaczynski J, Tavares AS,
Bradshaw AC, et al: The human-specific and smooth muscle
cell-enriched LncRNA SMILR promotes proliferation by regulating
mitotic CENPF mRNA and drives cell-cycle progression which can be
targeted to limit vascular remodeling. Circ Res. 125:535–551.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Dai Y, Liu L, Zeng T, Zhu YH, Li J, Chen
L, Li Y, Yuan YF, Ma S and Guan XY: Characterization of the
oncogenic function of centromere protein F in hepatocellular
carcinoma. Biochem Biophys Res Commun. 436:711–718. 2013.PubMed/NCBI View Article : Google Scholar
|