Introduction
Dermal fibroblasts are major skin components that
produce extracellular matrix (ECM) for the modulation of cellular
structure. ECM homeostasis is regulated by fatty acid oxidation and
glycolysis in dermal fibroblasts, thereby leading to skin ECM
accumulation and degradation (1).
As a major protein in the ECM, collagen is responsible for
supporting cellular structure and skin stability and elasticity
(2,3). Type I collagen is the most abundant
class present in humans (4) and
promotes cell proliferation, as well as tissue connections and
attachments through collagen-binding integrins and discoidin domain
receptor 2(5). Collagen synthesis
is regulated by several cytokines at both the transcriptional and
posttranscriptional levels (6). For
example, insulin-like growth factor-I (IGF-I) treatment increases
procollagen synthesis, whereas cycloheximide completely inhibits
IGF-1-induced collagen expression (7). Moreover, transforming growth factor-β
(TGF-β) signaling contributes to increased collagen expression by
suppressing microRNA-196a levels (8) or by activating the extracellular
signal-regulated kinase 1/2 (ERK1/2) pathway (9). Once procollagen is synthesized, it is
further modified by the addition of hydroxyl groups to proline and
lysine residues, followed by peptide cleavage by collagen peptidase
and fibril assembly by lysyl oxidase to form a mature collagen
fibril (10-12).
Collagen is negatively regulated by interferon-α (IFN-α), IFN-γ and
matrix metalloproteinase (MMP)-induced proteolysis (13,14).
Additionally, activator protein-1 (AP-1), a transcription factor in
fibroblasts, is activated by UV irradiation to decrease collagen
production and increase MMP gene transcription, thereby
increasing collagen degradation (15). Thus, skin aging is accompanied by
the accumulation of damaged ECM components, as well as reduction of
collagen in dermal cells, which consequently decreases the
fibroblast-ECM interaction and results in skin shrinkage and
undesirable wrinkle effects. By contrast, stimulation of type I
collagen expression significantly improves tissue recovery and
appearance (16). Interestingly,
several studies show that polyphenolic compounds, such as flavonoid
glycosides, ginsenosides, or catechins, stimulate type I collagen
biosynthesis (17-19).
Emodin (1,3,8-trihydroxy-6-methylanthraquinone) is a
natural anthraquinone derivative belonging to the polyphenol family
and found in various Chinese herbs, such as Rheum palmatum,
Polygonum cuspidatum and Polygonum multiflorum
(20,21). Numerous studies report that emodin
exerts various biological effects, including antiviral,
anti-allergic, anticancer, immunosuppressive, anti-inflammatory and
neuroprotective effects (22-26).
Interestingly, previous studies also suggest that natural
anthraquinones, such as emodin and parietin, are implicated in the
wound-healing process (27-29),
which is strongly associated with collagen metabolism (30). Although Lin et al (31) have reported that emodin improves
wound healing by increasing the expression of cytokines, such as
monocyte chemoattractant protein-1, interleukin (IL)-1β, and
vascular endothelial growth factor, in a burn-wound mouse model,
the association between emodin and type I collagen expression and
the cellular mechanism by which emodin exerts its effect in human
dermal fibroblast cells remain unclear. Therefore, the present
study investigated whether emodin induced increase in type I
collagen synthesis in Hs27 cells and further elucidated the
intracellular signaling pathway responsible for emodin-induced
collagen expression.
Materials and methods
Cell culture and material
treatment
Human dermal fibroblasts (Hs27) were obtained from
the American Type Culture Collection (cat. no. ATCC CRL-1634) and
cultured in the Dulbecco's modified essential medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine
serum and maintained in an incubator with a 5% CO2
humidified atmosphere at 37˚C. For all experiments, Hs27 cells were
used between passage numbers five and ten. For time- and
dose-dependent treatments, Hs27 cells were serum-starved for 24 h
before exposure to the YIGSR peptide and emodin at specific doses
and times. The synthetic YIGSR peptide was purchased from Anygen.
emodin (cat. no. E7881), TGF-β (cat. no. T7039), and the inhibitors
U0126 (cat. no. 662009) and compound c (cat. no. 171260) were
purchased from Sigma-Aldrich (Merck KGaA).
Reverse
transcription-quantitative-PCR
Total RNA was extracted using the TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), and cDNA was reverse
transcribed from 1 µg of total RNA using oligo (dT) primers and
murine leukemia virus reverse transcriptase. The temperature
protocol for reverse transcription was as follows: 25˚C for 10 min
and 37˚C for 120 min, followed by heat inactivation at 85˚C for 5
min. A total volume of 20 µl PCR amplification mixtures were
prepared by mixing 10 µl of 2X SYBR-Green I Premix ExTaq (Takara
Bio, Inc.), 2 µl primer mix (1 µM forward and 1 µM reverse
primers), and 8 µl diluted cDNA templates. Real-Time quantitative
PCR was performed on the CFX96 real-time PCR system (Bio-Rad
Laboratories, Inc.) using the following conditions: 95˚C for 1 min,
followed by 40 amplification cycles comprising 95˚C for 15 sec,
60˚C for 15 sec (annealing), and 72˚C for 30 sec. After
amplification, melting curve analysis was performed according to
manufacturer's instructions (Bio-Rad Laboratories, Inc.). Primer
sequences for collagen were as follows: COL1A1 forward,
5'-gaacgcgtgtcatcccttgt-3'; COL1A1 reverse,
5'-gaacgaggtagtctttcagcaaca-3'; COL1A2 forward,
5'-cctggtgctaaaggagaaagagg-3'; COL1A2 reverse
5'-atcaccacgacttccagcagga-3'; COL2A1 forward,
5'-cctggcaaagatggtgagacag-3'; COL2A1 reverse,
5'-cctggttttccaccttcacctg-3'; COL3A1 forward,
5'-tggtctgcaaggaatgcctgga-3'; COL3A1 reverse,
5'-tctttccctgggacaccatcag-3'; COL4A3 forward,
5'-ggacaaaggagaaccaggtctc-3'; COL4A3 reverse,
5'-agtgctgcccaaatctcctctg-3'; COL5A1 forward,
5'-cacaacttgcctgatggaataaca-3', and COL5A1 reverse,
5'-gcagggtacagctgcttggt-3'. Collagen expression was measured using
the 2-ΔΔCq assay (32)
and normalized against GAPDH.
MTT assay
For the MTT assay, Hs27 cells were seeded in 96-well
culture plates (1x104 cells/well) and cultured for 24 h.
After serum starvation for 24 h, cells were exposed to emodin with
the given dose (0.05, 0.1, 0.5, 1, 3 and 5 µM) and time conditions.
After discarding the media, cells were treated with 0.5 mg/ml MTT
(Sigma-Aldrich; Merck KGaA) dissolved in serum-free media for 3 h
in a 37˚C CO2 incubator. After discarding the solution,
100 µl DMSO was added to each well, and the plate was vortexed for
10 min. The absorbance of the MTT solution was measured at a
wavelength of 540 nm.
Fluorescence-activated cell sorting
(FACS) analysis
For apoptosis analysis, Hs27 cells were seeded in
6-well culture plates and exposed to a given dose of emodin (1 and
5 µM) for 24 h. After trypsinization, the cells were detached and
harvested by centrifugation (400 x g for 5 min at 4˚C). Collected
cells were stained with FITC-Annexin V (cat. no. 556419; BD
Biosciences) and 7-ADD (cat. no. 559925; BD Biosciences) in the
dark for 20 min. Living and apoptotic populations were detected
using flow cytometry (FACS Aria; BD Biosciences).
Immunoblotting
The following antibodies were used for
immunoblotting: COL1 (Rockland Immunochemicals; cat. no.
600-401-103-0.5), phospho-5' AMP-activated protein kinase (AMPK;
Tyr172; Cell Signaling Technology, Inc.; cat. no. 2535),
phospho-Smad2 (Ser465/467; Cell Signaling Technology, Inc.; cat.
no. 3108), phospho-ERK (Thr202/Tyr204; Abcam;
cat. no. ab214362), MMP-1 (Cell Signaling Technology, Inc.; cat.
no. 54376) and β-actin (MP Biomedicals; cat. no. 08691331).
Immunoblotting was performed by harvesting the treated cells and by
isolating the total proteins. To prepare total cell lysates, the
cells were washed with cold phosphate-buffered saline and lysed in
cold lysis buffer [in mM: 40 HEPES (pH 7.5), 120 NaCl, 1 EDTA, 10
pyrophosphate, 10 glycerophosphate, 50 NaF, 1.5
Na3VO4, 1 PMSF, 5 MgCl2, 0.5%
Triton X-100 and protease-inhibitor mixture). After determining
protein concentration using a Bradford assay, protein lysates were
subsequently denatured by boiling in Lammeli sample buffer for 10
min at 95˚C. A total of 15 µg protein was loaded and separated by
8% SDS-PAGE gel, and transferred onto nitrocellulose membranes. The
membranes were subsequently blocked for 30 min at room temperature
with 5% non-fat milk in Tris-buffered saline containing 0.05%
Tween-20 (TBS-T; pH 7.6), followed by incubation with primary
antibodies (1:1,000). After washing the membranes three times with
TBS-T, blots were incubated with HRP-conjugated secondary antibody
(SeraCare KPL; goat anti-rabbit; cat. no. 5220-0336; anti-mouse;
cat. no. 5220-0342; each, 1:5,000) for 1 h at room temperature,
washed three times with TBS-T and detected by enhanced
chemiluminescence (ECL system; GE Healthcare Bio-Sciences).
Densitometric analysis was performed using the ImageJ software
(v.1.51; http://rsbweb.nih.gov/ij/index.html) and Multigauge
software (Fujifilm). Data were obtained from three independent
experiments.
Statistical analysis
All data were evaluated using the GraphPad software
5 (GraphPad Software, Inc.) and are expressed as mean ± SEM. The
significant differences among groups were determined by one-way
ANOVA with Dunnett's multiple comparison analysis. Comparison
between two groups was analyzed by unpaired 2-tailed Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Emodin induces collagen synthesis
To investigate the effects of emodin on type I
collagen expression in dermal fibroblasts, Hs27 human dermal
fibroblasts were initially exposed to emodin for 12 h (Fig. 1A). Hs27 cells were also treated with
YIGSR, a peptide that induces collagen synthesis in Hs27 cells
(33). As shown in Fig. 1A, YIGSR and emodin significantly
increased protein levels of type I collagen, and these levels
positively associate with emodin concentration (Fig. 1B). MMP-1 is a well-known protease
that degrades collagen proteins. To investigate whether emodin
affected MMP-1 expression, we analyzed MMP-1 levels following
dose-dependent emodin treatment. As shown in Fig. 1B, no significant changes in MMP-1
levels were observed, indicating that the increase in type I
collagen by emodin treatment was not caused by MMP-1
downregulation. Subsequently, the effect of emodin on mRNA
expression was determined by qPCR analysis. Similar to protein
levels, exposure to emodin increased transcript levels of type I
collagen without any effects on types II, III and V collagen
(Fig. 1C). Type IV collagen genes
were not detectable in Hs27 cells (data not shown). This suggests
that emodin specifically induces type I collagen expression in
vitro.
Emodin does not affect cell
viability
To verify the effect of emodin on cell viability an
MTT assay was performed. In particular, relatively high doses of
emodin were used for the viability assay to exclude potential
cytotoxicity with increased concentration. As a result, there was
no significant changes in cell viability at varying concentrations
of emodin (Fig. 2A). Light
microscopy images further demonstrated that 24-h emodin treatment
did not induce any changes in fibroblast cell morphology (Fig. 2B). Additionally, the number of
apoptotic cells following emodin treatment was evaluated by flow
cytometry, with Fig. 2C showing
that the percentage of apoptotic cells was similar between vehicle-
and emodin-treated groups. These results indicate that emodin
increases type I collagen without affecting cell viability.
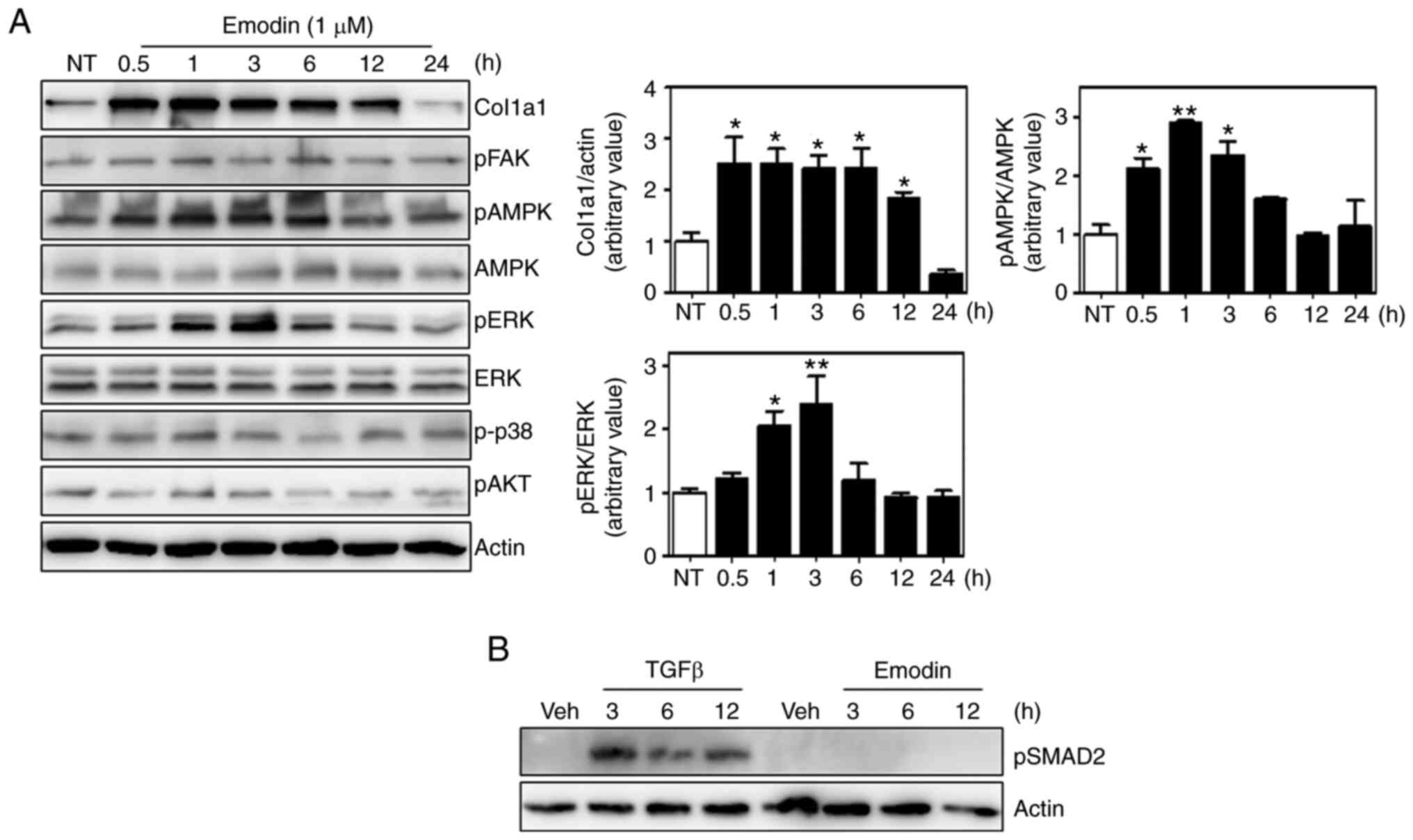
Emodin activates the ERK and AMPK
pathways in Hs27 fibroblasts
Several signaling pathways are involved in collagen
synthesis, including the focal adhesion kinase (FAK), ERK, p38
mitogen-activated protein kinase and AKT pathways (34-37).
Moreover, 5' AMP-activated protein kinase (AMPK) also appears to
affect collagen expression (38,39).
To investigate whether these pathways were responsible for
emodin-induced type I collagen synthesis, the phosphorylation of
each component was analyzed, and found that ERK and AMPK
phosphorylation was increased by ~2.5-fold at 3 h after emodin
treatment relative to untreated cells and without any effect on
total protein levels (Fig. 3A).
Phosphorylated p38, AKT and FAK were normalized with housekeeping
protein, actin. In contrast to AMPK and ERK, no significant
differences were observed in the phosphorylation of these proteins
between control and emodin treatment at each time point. Total
protein levels of p38, AKT and FAK were not examined, because there
were no changes in the phosphorylation status. Since TGF-β
signaling is an important regulatory pathway of collagen expression
(40), the phosphorylation status
of downstream molecules were also measured, which demonstrated that
Smad2 phosphorylation was also unaffected by emodin treatment
(Fig. 3B). Thus, the TGF-β pathway
is not involved in emodin-induced collagen synthesis.
Inhibition of the AMPK pathway
prevents emodin-induced type I collagen synthesis
To determine which of the two pathways (ERK and
AMPK) affected emodin-induced collagen expression, Hs27 cells were
treated with the chemical inhibitor U0126 to suppress the ERK
pathway. Although U0126 sufficiently decreased ERK phosphorylation,
emodin-induced type I collagen expression remained similar to that
in the vehicle-treated group (Fig.
4A). In marked contrast, collagen levels were largely inhibited
by the AMPK inhibitor, compound c (Fig.
4B), suggesting AMPK as a crucial regulatory enzyme for
emodin-induced type I collagen expression.
Discussion
The present study demonstrated that emodin increases
type I collagen transcripts and protein levels without affecting
the collagen-degradation pathway in Hs27 cells. Furthermore, the
varying concentrations of emodin used in the present study induced
type I collagen expression without any effect on cellular
viability. The present study clearly reveals a new function for
emodin through its effect on collagen type I synthesis in human
dermal fibroblasts.
The results of the present study indicate that
emodin increases the phosphorylation of ERK1/2 and AMPK within 3 h.
Although a previous study has reported that activation of ERK
induces the expression of type I collagen in human fibroblasts
(34), contrasting results have
also been published, showing that activation of ERK1/2 by IL-18 or
ceramide inhibits type I collagen expression (41,42).
Additionally, the AMPK pathway exerts inconsistent effects on
collagen synthesis. For example,
5-aminoimidazole-4-carboxamide-ribonucleoside (AICAR), an AMPK
activator, promotes collagen deposition and improves scar formation
by promoting myofibroblast maturation in the scar of aged ischemic
hearts (38). In rat kidney
fibroblasts and human embryonic kidney cells, activation of AMPKα1
induces fibroblast activation and increases ECM deposition,
including fibronectin and type I collagen (43). However, other studies report that
collagen production and secretion are decreased by AMPK
phosphorylation in renal and kidney fibroblasts (44,45).
These studies suggest that both the ERK1/2 and AMPK pathways have
varying effects on collagen metabolism depending on the cell type,
culture conditions, and presence of other cytokines. To elucidate
the role of each pathway and collagen expression in human dermal
fibroblasts, the conventional chemical inhibitors U0126 or compound
c were used. Pretreatment with compound c, a specific AMPK pathway
inhibitor, largely suppressed emodin-induced collagen levels,
thereby indicating that AMPK activation by emodin was responsible
for collagen synthesis. By contrast, the ERK1/2 pathway inhibition
by U0126 did not suppress emodin-induced collagen expression.
Wound healing is a complicated process controlled by
cell-ECM interactions and numerous growth factors. Among these,
collagen types I and III play a critical role during wound repair
by recruiting fibroblasts and increasing deposition of new collagen
in skin, bone and blood vessels (46). A previous report has shown that
emodin inhibits fibronectin and type III collagen synthesis by
suppressing the p38 and ERK pathways in the kidney (47). Additionally, Liu et al
(48) have shown that emodin
inhibits collagen synthesis by inhibiting the TGF-β1/ADAMTS-1
signaling pathway in pulmonary fibroblasts. However, inhibition of
these pathways were not observed following emodin treatment.
Although the reason for this difference remains unknown, one
possibility is that these studies used long-term treatment with
relatively high dose of emodin >100 µM, which can affect cell
viability or MMP1 expression compared with the low-concentration
condition. Moreover, emodin increases or suppresses ERK
phosphorylation according to the cell type (49-51).
Thus, it is speculated that emodin targets a different signaling
pathway for collagen synthesis between skin and other fibroblasts.
Importantly, the TGF-β signaling pathway regulates type I collagen
synthesis (40), and a previous
study reported that emodin treatment for 7 days significantly
increased TGF-β1 expression and collagen levels in
excisional wounds (28). Although
Wei et al (52) showed that
emodin treatment decreased collagen deposition during intestinal
surgery-induced abdominal adhesion, they suggested that
emodin-mediated anti-inflammatory effects might contribute to lower
collagen levels in the adhesion tissues, indicating the possibility
of an indirect effect. Interestingly, Xiao et al (53) showed that the AMPK pathway inhibited
inflammation or angiotensin-induced TGF-β signaling in cardiac
fibroblasts, whereas another study reported that AMPK activation
increased rather than suppressed TGF-β responsiveness, by
stimulating the non-canonical TGF-β pathway in myofibroblasts
(38). In the present study, Smad2
phosphorylation remained similar between vehicle and emodin
treatments, indicating that emodin did not increase TGF-β signaling
in dermal fibroblasts. Given these findings, further studies are
necessary to elucidate the detailed mechanisms by which emodin
mediates different responses to collagen expression in each cell
type.
In summary, the present study demonstrated that
emodin directly increases type I collagen levels in Hs27 cells, and
that AMPK activation is an important factor for collagen
expression. A limitation of the study is that newborn foreskin
fibroblasts (Hs27) were the only cells for confirming the overall
results. Previous studies report functional differences between
neonatal and adult dermal fibroblasts in terms of ECM components
and migration capacity (54,55);
therefore, determining whether emodin can increase type I collagen
levels in adult dermal fibroblasts is worthwhile. As collagen
synthesis is decreased in aged skin (56), and collagen-induction therapy is
considered a safe treatment strategy for wrinkles and scars, emodin
represents a potential therapeutic agent for alleviating skin
aging. Nevertheless, further in vivo investigations are
warranted to support these results.
Acknowledgements
Not applicable.
Funding
This research was supported by the KBRI Basic Research Program
(grant no. 20-BR-02-012) funded by the Ministry of Science and ICT
and by the Basic Science Research Program through the National
Research Foundation of Korea funded by the Ministry of Education
(grant no. 2018R1D1A1B07043929). This research was also supported
by grants from the National Cancer Center, Republic of Korea (grant
no. NCC 1810861-1) and by a National Research Foundation of Korea
grant funded by the Korean government (grant no.
2020R1C1C1005500).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PS wrote the manuscript and performed the
experiments. HSJ, YWK and SB contributed to the conceptualization
and design of the experiments. YK, BA and WSS performed the
experiments. JH and DL interpreted the data and reviewed the
manuscript. SHR helped analyze the data and revise the manuscript.
PS and YK confirm the authenticity of all the raw data. PS and JHY
supervised the entire project, conceived the study and wrote the
original draft. All authors read and approved this manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhao X, Psarianos P, Ghoraie LS, Yip K,
Goldstein D, Gilbert R, Witterick I, Pang H, Hussain A, Lee JH, et
al: Metabolic regulation of dermal fibroblasts contributes to skin
extracellular matrix homeostasis and fibrosis. Nat Metab.
1:147–157. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Avila Rodriguez MI, Rodriguez Barroso LG
and Sánchez ML: Collagen: A review on its sources and potential
cosmetic applications. J Cosmet Dermatol. 17:20–26. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
de Araújo R, Lôbo M, Trindade K, Silva DF
and Pereira N: Fibroblast growth factors: A controlling mechanism
of skin aging. Skin Pharmacol Physiol. 32:275–282. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mäkitie RE, Costantini A, Kämpe A, Alm JJ
and Mäkitie O: New insights into monogenic causes of osteoporosis.
Front Endocrinol (Lausanne). 10(70)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Irawan V, Sung TC, Higuchi A and Ikoma T:
Collagen scaffolds in cartilage tissue engineering and relevant
approaches for future development. Tissue Eng Regen Med.
15:673–697. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Armendariz-Borunda J, Katayama K and Seyer
JM: Transcriptional mechanisms of type I collagen gene expression
are differentially regulated by interleukin-1 beta, tumor necrosis
factor alpha, and transforming growth factor beta in Ito cells. J
Biol Chem. 267:14316–14321. 1992.PubMed/NCBI
|
|
7
|
Gillery P, Leperre A, Maquart FX and Borel
JP: Insulin-like growth factor-I (IGF-I) stimulates protein
synthesis and collagen gene expression in monolayer and lattice
cultures of fibroblasts. J Cell Physiol. 152:389–396.
1992.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Honda N, Jinnin M, Kajihara I, Makino T,
Makino K, Masuguchi S, Fukushima S, Okamoto Y, Hasegawa M, Fujimoto
M and Ihn H: TGF-β-mediated downregulation of microRNA-196a
contributes to the constitutive upregulated type I collagen
expression in scleroderma dermal fibroblasts. J Immunol.
188:3323–3331. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mishra R, Zhu L, Eckert RL and Simonson
MS: TGF-beta-regulated collagen type I accumulation: Role of
Src-based signals. Am J Physiol Cell Physiol. 292:C1361–C1369.
2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Murad S, Sivarajah A and Pinnell SR:
Prolyl and lysyl hydroxylase activities of human skin fibroblasts:
Effect of donor age and ascorbate. J Invest Dermatol. 75:404–407.
1980.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tsuji-Naito K, Ishikura S, Akagawa M and
Saeki H: α-Lipoic acid induces collagen biosynthesis involving
prolyl hydroxylase expression via activation of TGF-β-Smad
signaling in human dermal fibroblasts. Connect Tissue Res.
51:378–387. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yamauchi M and Sricholpech M: Lysine
post-translational modifications of collagen. Essays Biochem.
52:113–133. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tsutsumi Y, Kakumu S, Yoshioka K, Arao M,
Inoue M and Wakita T: Effects of various cytokines on collagen
synthesis by normal rat hepatocytes in primary cultures and
fibroblasts. Digestion. 44:191–199. 1989.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nakamuta M, Kotoh K, Enjoji M and Nawata
H: Effects of fibril- or fixed-collagen on matrix
metalloproteinase-1 and tissue inhibitor of matrix
metalloproteinase-1 production in the human hepatocyte cell line
HLE. World J Gastroenterol. 11:2264–2268. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Quan T, Qin Z, Xu Y, He T, Kang S,
Voorhees JJ and Fisher GJ: Ultraviolet irradiation induces
CYR61/CCN1, a mediator of collagen homeostasis, through activation
of transcription factor AP-1 in human skin fibroblasts. J Invest
Dermatol. 130:1697–1706. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ganceviciene R, Liakou AI, Theodoridis A,
Makrantonaki E and Zouboulis CC: Skin anti-aging strategies.
Dermatoendocrinol. 4:308–319. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Galicka A and Nazaruk J: Stimulation of
collagen biosynthesis by flavonoid glycosides in skin fibroblasts
of osteogenesis imperfecta type I and the potential mechanism of
their action. Int J Mol Med. 20:889–895. 2007.PubMed/NCBI
|
|
18
|
Kwok HH, Yue PY, Mak NK and Wong RN:
Ginsenoside Rb(1) induces type I collagen expression through
peroxisome proliferator-activated receptor-delta. Biochem
Pharmacol. 84:532–539. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lucarini M, Sciubba F, Capitani D, Di
Cocco ME, D'Evoli L, Durazzo A, Delfini M and Lombardi Boccia G:
Role of catechin on collagen type I stability upon oxidation: A NMR
approach. Nat Prod Res. 34:53–62. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dong X, Fu J, Yin X, Cao S, Li X, Lin L
and Huyiligeqi; Ni J: Emodin: A review of its pharmacology,
toxicity and pharmacokinetics. Phytother Res. 30:1207–1218.
2016.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Wang M, Zhao R, Wang W, Mao X and Yu J:
Lipid regulation effects of Polygoni Multiflori Radix, its
processed products and its major substances on steatosis human
liver cell line L02. J Ethnopharmacol. 139:287–293. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang W, Zhou Q, Liu L and Zou K:
Anti-allergic activity of emodin on IgE-mediated activation in
RBL-2H3 cells. Pharmacol Rep. 64:1216–1222. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Andersen DO, Weber ND, Wood SG, Hughes BG,
Murray BK and North JA: In vitro virucidal activity of selected
anthraquinones and anthraquinone derivatives. Antiviral Res.
16:185–196. 1991.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hwang JK, Noh EM, Moon SJ, Kim JM, Kwon
KB, Park BH, You YO, Hwang BM, Kim HJ, Kim BS, et al: Emodin
suppresses inflammatory responses and joint destruction in
collagen-induced arthritic mice. Rheumatology (Oxford).
52:1583–1591. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Srinivas G, Babykutty S, Sathiadevan PP
and Srinivas P: Molecular mechanism of emodin action: Transition
from laxative ingredient to an antitumor agent. Med Res Rev.
27:591–608. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ahn SM, Kim HN, Kim YR, Choi YW, Kim CM,
Shin HK and Choi BT: Emodin from Polygonum multiflorum
ameliorates oxidative toxicity in HT22 cells and deficits in
photothrombotic ischemia. J Ethnopharmacol. 188:13–20.
2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gundogdu G, Gundogdu K, Nalci KA,
Demirkaya AK, Yılmaz Tascı S, Demirkaya Miloglu F, Senol O and
Hacimuftuoglu A: The effect of parietin isolated from rheum ribes L
on in vitro wound model using human dermal fibroblast cells. Int J
Low Extrem Wounds. 18:56–64. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tang T, Yin L, Yang J and Shan G: Emodin,
an anthraquinone derivative from Rheum officinale Baill,
enhances cutaneous wound healing in rats. Eur J Pharmacol.
567:177–185. 2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Xiao D, Zhang Y, Wang R, Fu Y, Zhou T,
Diao H, Wang Z, Lin Y, Li Z, Wen L, et al: Emodin alleviates
cardiac fibrosis by suppressing activation of cardiac fibroblasts
via upregulating metastasis associated protein 3. Acta Pharm Sin B.
9:724–733. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kapoor M, Howard R, Hall I and Appleton I:
Effects of epicatechin gallate on wound healing and scar formation
in a full thickness incisional wound healing model in rats. Am J
Pathol. 165:299–307. 2004.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lin LX, Wang P, Wang YT, Huang Y, Jiang L
and Wang XM: Aloe vera and Vitis vinifera improve wound healing in
an in vivo rat burn wound model. Mol Med Rep. 13:1070–1076.
2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yoon JH, Kim J, Lee H, Kim SY, Jang HH,
Ryu SH, Kim BJ and Lee TG: Laminin peptide YIGSR induces collagen
synthesis in Hs27 human dermal fibroblasts. Biochem Biophys Res
Commun. 428:416–421. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bhogal RK and Bona CA: Regulatory effect
of extracellular signal-regulated kinases (ERK) on type I collagen
synthesis in human dermal fibroblasts stimulated by IL-4 and IL-13.
Int Rev Immunol. 27:472–496. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kimoto K, Nakatsuka K, Matsuo N and
Yoshioka H: p38 MAPK mediates the expression of type I collagen
induced by TGF-beta 2 in human retinal pigment epithelial cells
ARPE-19. Invest Ophthalmol Vis Sci. 45:2431–2437. 2004.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Rajshankar D, Wang Y and McCulloch CA:
Osteogenesis requires FAK-dependent collagen synthesis by
fibroblasts and osteoblasts. FASEB J. 31:937–953. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yokoyama K, Kimoto K, Itoh Y, Nakatsuka K,
Matsuo N, Yoshioka H and Kubota T: The PI3K/Akt pathway mediates
the expression of type I collagen induced by TGF-β2 in human
retinal pigment epithelial cells. Graefes Arch Clin Exp Ophthalmol.
250:15–23. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cieslik KA, Taffet GE, Crawford JR, Trial
J, Mejia Osuna P and Entman ML: AICAR-dependent AMPK activation
improves scar formation in the aged heart in a murine model of
reperfused myocardial infarction. J Mol Cell Cardiol. 63:26–36.
2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Crane JD, MacNeil LG, Lally JS, Ford RJ,
Bujak AL, Brar IK, Kemp BE, Raha S, Steinberg GR and Tarnopolsky
MA: Exercise-stimulated interleukin-15 is controlled by AMPK and
regulates skin metabolism and aging. Aging Cell. 14:625–634.
2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Walton KL, Johnson KE and Harrison CA:
Targeting TGF-beta mediated SMAD signaling for the prevention of
fibrosis. Front Pharmacol. 8(461)2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kim HJ, Song SB, Choi JM, Kim KM, Cho BK,
Cho DH and Park HJ: IL-18 downregulates collagen production in
human dermal fibroblasts via the ERK pathway. J Invest Dermatol.
130:706–715. 2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Reunanen N, Foschi M, Han J and Kahari VM:
Activation of extracellular signal-regulated kinase 1/2 inhibits
type I collagen expression by human skin fibroblasts. J Biol Chem.
275:34634–34639. 2000.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wang Y, Jia L, Hu Z, Entman ML, Mitch WE
and Wang Y: AMP-activated protein kinase/myocardin-related
transcription factor-A signaling regulates fibroblast activation
and renal fibrosis. Kidney Int. 93:81–94. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Chen KH, Hsu HH, Lee CC, Yen TH, Ko YC,
Yang CW and Hung CC: The AMPK agonist AICAR inhibits TGF-β1 induced
activation of kidney myofibroblasts. PLoS One.
9(e106554)2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lu J, Shi J, Li M, Gui B, Fu R, Yao G,
Duan Z, Lv Z, Yang Y, Chen Z, et al: Activation of AMPK by
metformin inhibits TGF-β-induced collagen production in mouse renal
fibroblasts. Life Sci. 127:59–65. 2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Chattopadhyay S and Raines RT: Review
collagen-based biomaterials for wound healing. Biopolymers.
101:821–833. 2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zhu B, Lin Y, Zhu CF, Zhu XL, Huang CZ, Lu
Y, Cheng XX and Wang YJ: Emodin inhibits extracellular matrix
synthesis by suppressing p38 and ERK1/2 pathways in
TGF-β1-stimulated NRK-49F cells. Mol Med Rep. 4:505–509.
2011.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Liu L, Yin H, He J, Xie M, Wang Z and Xiao
H: Emodin inhibits the proliferation, transdifferentiation and
collagen synthesis of pulmonary fibroblasts. Xi Bao Yu Fen Zi Mian
Yi Xue Za Zhi. 32:921–925. 2016.PubMed/NCBI(In Chinese).
|
|
49
|
Leung SW, Lai JH, Wu JC, Tsai YR, Chen YH,
Kang SJ, Chiang YH, Chang CF and Chen KY: Neuroprotective effects
of emodin against ischemia/reperfusion injury through activating
ERK-1/2 signaling pathway. Int J Mol Sci. 21(2899)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Lin W, Zhong M, Yin H, Chen Y, Cao Q, Wang
C and Ling C: Emodin induces hepatocellular carcinoma cell
apoptosis through MAPK and PI3K/AKT signaling pathways in
vitro and in vivo. Oncol Rep. 36:961–967.
2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zhou X, Song B, Jin L, Hu D, Diao C, Xu G,
Zou Z and Yang S: Isolation and inhibitory activity against ERK
phosphorylation of hydroxyanthraquinones from rhubarb. Bioorg Med
Chem Lett. 16:563–568. 2006.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Wei G, Wu Y, Gao Q, Zhou C, Wang K, Shen
C, Wang G, Wang K, Sun X and Li X: Effect of emodin on preventing
postoperative intra-abdominal adhesion formation. Oxid Med Cell
Longev. 2017(1740317)2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Xiao Y, Ye J, Zhou Y, Huang J, Liu X,
Huang B, Zhu L, Wu B, Zhang G and Cai Y: Baicalin inhibits pressure
overload-induced cardiac fibrosis through regulating
AMPK/TGF-β/Smads signaling pathway. Arch Biochem Biophys.
640:37–46. 2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Krejčí E, Kodet O, Szabo P, Borský J,
Smetana K Jr, Grim M and Dvořánková B: In vitro differences of
neonatal and later postnatal keratinocytes and dermal fibroblasts.
Physiol Res. 64:561–569. 2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Mateu R, Živicová V, Krejčí ED, Grim M,
Strnad H, Vlček Č, Kolář M, Lacina L, Gál P, Borský J, et al:
Functional differences between neonatal and adult fibroblasts and
keratinocytes: Donor age affects epithelial-mesenchymal crosstalk
in vitro. Int J Mol Med. 38:1063–1074. 2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Varani J, Dame MK, Rittie L, Fligiel SE,
Kang S, Fisher GJ and Voorhees JJ: Decreased collagen production in
chronologically aged skin: Roles of age-dependent alteration in
fibroblast function and defective mechanical stimulation. Am J
Pathol. 168:1861–1868. 2006.PubMed/NCBI View Article : Google Scholar
|