Introduction
As the most common musculoskeletal disease in the
elderly population, osteoarthritis (OA) is characterised by joint
pain, tenderness, stiffness, crepitus and limitation of movement
(1). Histologically, the major
characteristics of OA are early fragmentation of the cartilage
surface, vertical clefts of the cartilage, cloning of chondrocytes,
variable crystal deposition and violation of the tidemark by blood
vessels (2,3). Worldwide disease estimates have
demonstrated that 18% of women and 9.6% of men aged 60 and older
have symptomatic OA (4). Although
there are numerous treatments for OA, including acupuncture
therapy, drug treatment and surgical therapy (5,6), OA
remains difficult to cure. Therefore, it is imperative to assess
the molecular mechanisms underlying OA in order to identify novel
therapeutic targets.
Long non-coding RNAs (lncRNAs) are a class of
nucleotide sequences >200 nucleotides in length that lack
protein-coding potential (7). A
previous study reported that lncRNAs may function as important
participants in regulating the progression of OA (8). Liu et al (9) reported that the silencing of lncRNA
XIST may restrain the development of OA by inhibiting cell
apoptosis and extracellular matrix (ECM) degradation (9). Chen et al (10) demonstrated that the lncRNA HOTAIR
participates in OA progression by stimulating ECM degradation and
chondrocyte apoptosis. Zhang et al (11) revealed that lncRNA SNHG15 suppresses
OA progression by acting as a sponge of microRNA
(miRNA/miR)-141-3p. Notably, a recent study indicated that lncRNA
KCNQ1 opposite strand/antisense transcript 1 (KCNQ1OT1) may serve a
critical role in OA progression by sponging hsa-miR-1202/ETS1
interactions (12). However,
research on the underlying mechanism of KCNQ1OT1 in OA remains in
the exploratory stage.
miRNAs are highly conserved non-coding RNAs with a
length of 19-25 nucleotides, which have been reported to
participate in the process of OA (13,14).
Luo et al (15) reported
that miR-34a induces OA synovial cell apoptosis by regulating
transforming growth factor-β-induced factor homeobox 2. Zhong et
al (16) confirmed that
miR-335-5p may markedly alleviate inflammation in OA chondrocytes.
Huang et al (17) revealed
the essential role of miR-211 in maintaining joint homeostasis and
counteracting the progression of OA. Notably, previous studies on
the expression and function of miR-211-5p in OA have received
significant attention (18,19). It has been reported that miR-211-5p
manifests a downregulation in sclerotic bone compared with
non-sclerotic OA bone samples, and miR-211-5p has been confirmed to
decrease the mineralisation capacity of ossified osteoblasts in OA
(18). Furthermore, miR-211-5p has
been reported to be markedly diminished in the articular cartilage
tissues and functions to restrain ECM degradation and the
production of inflammatory cytokines in OA (19). Despite these findings, the
interaction between miR-211-5p and KCNQ1OT1 in OA has not been
fully understood.
The aim of the present study was to investigate the
role and molecular mechanism of KCNQ1OT1 in OA, and to identify the
associations between KCNQ1OT1, miR-211-5p and transcription factor
4 (TCF4). Through this work, the present study aimed to develop a
solid foundation for targeted OA therapy.
Materials and methods
Cartilage tissue samples
From January 2018 to December 2019, OA cartilage
tissues were obtained from the knee joints of 25 patients (12 males
and 13 females; age range, 52-70 years; mean age, 60.5±6.4) who
underwent artificial total knee replacement surgery. Normal
cartilage tissues were derived from the knee joints of 25 subjects
(12 males and 13 females; age range, 48-66 years; mean age,
56.7±6.4) who had femoral neck fractures without OA or rheumatoid
arthritis. The diagnostic criteria for OA were based on the
American College of Rheumatology standards (20). The diagnostic criteria included: i)
Pain in the knee, hip and interphalangeal joints, ii) presence of
osteophytes detected through X-ray examination, iii) synovial fluid
laboratory examination consistent with OA, iv) morning stiffness
for <30 min, v) age of at least 40 years, and vi) presence of
crepitus. Patients who met i and ii; i, iii, v and vi; or i, iv, v
and vi were diagnosed with OA. The clinicopathological data of
these patients were as follows: Age range, 53-75 years; mean age,
63.3±5.9 years; sex, 9 men and 16 women; and OA grade I, 2; grade
II, 10; and grade III, 13. The present study was approved by the
Ethics Committee of The Second Xiangya Hospital of Central South
University. Written informed consent was obtained from each
participant.
Cell culture
The normal human cartilage cell line (C28/I2) was
purchased from the American Type Culture Collection. C28/I2 cells
were cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc.)-Nutrient Mixture F12 supplemented with 10%
foetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.). All
cells were maintained in an incubator with 5% CO2 at
37˚C. An OA model was established in vitro in accordance
with previous studies (21,22). In brief, C28/I2 cells were treated
with lipopolysaccharide (LPS; 5 µg/ml; Sigma-Aldrich; Merck KGaA)
for 12 h at 37˚C, and untreated C28/I2 cells were used as
controls.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells and tissues using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), and cDNA was synthesised using a high-capacity RT kit at
42˚C for 45 min (Applied Biosystems; Thermo Fisher Scientific,
Inc.). qPCR was performed using SYBR Green PCR Master mix (Takara
Biotechnology Co., Ltd.). The thermocycling conditions were as
follows: 95˚C for 5 min, 40 cycles of denaturation at 95˚C for 10
sec, annealing at 50˚C for 1 min and extension at 72˚C for 30 sec.
All primers were purchased from Invitrogen (Thermo Fisher
Scientific, Inc.), and their sequences are presented in Table I. Experimental results were
calculated using the 2-ΔΔCq method (23). KCNQ1OT1 and TCF4 expression levels
were normalised to GAPDH, and miR-211-5p expression levels were
normalised to U6.
 | Table IPrimers for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Primers for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward | Reverse |
|---|
| KCNQ1OT1 |
5'-TTGGTAGGATTTTGTTGAGG-3' |
5'-CAACCTTCCCCTACTACC-3' |
| miR-211-5p |
5'-TCGGCAGGTCCCTTTGTCATCC-3' |
5'-TGCAGGTCAACTGGTGTCGT-3' |
| TCF4 |
5'-CCTGGCTATGCAGGAATGTT-3' |
5'-CAGGAGGCGTACAGGAAGAG-3' |
| GAPDH |
5'-CCAGGTGGTCTCCTCTGA-3' |
5'-GCTGTAGCCAAATCGTTGT-3' |
| U6 |
5'-CTCGCTTCGGCAGCACA-3' |
5'-AACGCTTCACGAATTTGCGT-3' |
Cell transfection
When C28/I2 cells reached 80% confluence, they were
seeded onto 6-well cell culture plates (5x103
cells/well). The short hairpin (sh) RNA targeting KCNQ1OT1
(sh-KCNQ1OT1; 5'-GGUUCAGAUUCAUAAACUAGA-3'), sh-negative control
(sh-NC; 5'-CAUAGUCGAAUUUCGCUAGUGAGUU-3'), miR-NC
(5'-ACCGCUAAUCAUACGAAUACAC-3'), miR-211-5p mimics
(5'-UUCCCUUUGUCAUCCUUCGCCU-3'), miR-211-5p inhibitor
(5'-AGGCAAAGGAUGACAAGGGAA-3'), inhibitor NC
(5'-CAGUACUUUUGUAGUACAAA-3'), overexpression plasmids
(pcDNA-KCNQ1OT1 and pcDNA-TCF4) and empty vector (pcDNA-NC) were
purchased from Guangzhou RiboBio Co., Ltd. C28/I2 cells
(6x105 cells/well) were transfected with these factors
(all, 50 nM) for 48 h at 37˚C with Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocols.
Dual-luciferase reporter (DLR)
assay
The targeting relationship between KCNQ1OT1 and
miR-211-5p was analysed using the lncBase Predicted database
(version 2.0; http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php?r=lncbasev2/index-predicted).
Additionally, the targeting relationship between miR-211-5p and
TCF4 was predicted using TargetScan (release 7.2; http://www.targetscan.org/vert_72/) and miRDB
(version 4.0; http://mirdb.org/). The 3'-untranslated
region (UTR) of KCNQ1OT1 or TCF4 harbouring the predicted binding
sites for miR-211-5p was introduced into the pGL3 vector (Promega
Corporation) to construct the KCNQ1OT1 wild-type (wt)/mutant type
(mut) or TCF4 wt/mut. Next, C28/I2 cells (1x105
cells/well) were seeded onto a 24-well plate and co-transfected
with one of the aforementioned plasmids (80 ng) and miR-211-5p
mimics or miR-NC (50 nM) using Lipofectamine 3000. After 48 h at
37˚C, the relative luciferase activity was determined using the DLR
Assay kit (Promega Corporation). The activity of firefly luciferase
was normalized to that of Renilla luciferase.
RNA immunoprecipitation (RIP)
assay
The RIP assay was performed using the Magna RIP
RNA-Binding Protein Immunoprecipitation kit (EMD Millipore) in
accordance with the manufacturer's protocol. In brief, C28/I2 cells
were transfected with miR-211-5p mimics or miR-NC. After 48 h, the
cells were collected and lysed using RIP lysis buffer (Beyotime
Institute of Biotechnology). An anti-argonaute2 (Ago2) antibody
(1:5,000; cat. no. 03-110; EMD Millipore) was conjugated to
magnetic beads and incubated at 55˚C for 30 min with the whole cell
extract. Samples were then centrifuged (at 450 x g for 20 min at
20˚C) and washed three times with Hank's Balanced Salt Solution
(Sigma-Aldrich; Merck KGaA). The immunoprecipitated RNAs were
isolated, and an aforementioned RT-qPCR assay was used to detect
the expression levels of KCNQ1OT1 and TCF4.
MTT assay
Transfected cells were seeded onto 96-well plates
(5x103 cells/well). After 48 h of culture, the cells
were incubated with 10 µl MTT reagent (Beyotime Institute of
Biotechnology) for another 4 h. Thereafter, 100 µl dimethyl
sulfoxide was added to dissolve the formazan. The optical density
was measured at 490 nm using a spectrophotometer (Thermo Fisher
Scientific, Inc.).
ELISA
The levels of IL-6 (cat. no. PD6050; R&D
Systems, Inc.), IL-1β (cat. no. PDLB50; R&D Systems, Inc.) and
TNF-α (cat. no. PDTA00D; R&D Systems, Inc.) in culture media
were measured using Quantikine ELISA kits according to the
manufacturer's protocols. The optical density was determined at 450
nm using a microplate reader (Molecular Devices, LLC).
Western blotting
Total proteins were extracted using
radioimmunoprecipitation lysis buffer (Beyotime Institute of
Biotechnology). Total protein was quantified using a BCA Protein
assay kit (Invitrogen; Thermo Fisher Scientific, Inc.). Protein
samples (20 µg per lane) were fractionated by 10% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis and then transferred
onto polyvinylidene difluoride membranes. After blocking with 5%
skimmed milk-Tris-buffered saline with TBST (Tween-20, 0.05%) for 2
h at 25˚C, the membranes were incubated with primary antibodies
(Abcam) against matrix metalloproteinase (MMP)-3 (dilution,
1:1,000; cat. no. ab53015), MMP-13 (dilution, 1:1,000; cat. no.
ab51072), collagen II (dilution, 1:2,000; cat. no. ab34712),
collagen X (dilution, 1:1,000; cat. no. ab182563), TCF4 (dilution,
1:1,000; cat. no. ab130014) and β-actin (dilution, 1:5,000; cat.
no. ab6276) at 4˚C overnight. After the membranes were washed with
TBST three times, the horseradish peroxidase-conjugated secondary
antibody (dilution, 1:5,000; cat. no. 14709; Cell Signaling
Technology, Inc.) was added and incubated at 37˚C for 1 h. β-actin
was selected as the internal reference. The immunoreactive bands
were visualised using an enhanced chemiluminescent detection kit
(Thermo Fisher Scientific, Inc.), and the relative protein
expression levels were semiquantified using the ChemiDoc XRS system
(Bio-Rad Laboratories, Inc.).
Statistical analysis
All data derived from independent experiments
performed in triplicate are presented as the mean ± standard
deviation. A paired Student's t-test was employed for comparisons
between two groups. Differences between multiple groups were
compared using a one-way analysis of variance followed by Tukey's
multiple comparisons test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Silencing of lncRNA KCNQ1OT1 alleviates
LPS-induced chondrocyte injury in C28/I2 cells. To assess the
function of lncRNA KCNQ1OT1 in OA, the expression levels of
KCNQ1OT1 were detected through RT-qPCR. KCNQ1OT1 was significantly
upregulated in OA cartilage tissues compared with that in normal
cartilage tissues (P<0.01; Fig.
1A). KCNQ1OT1 expression levels were also increased in C28/I2
cells treated with LPS (P<0.01; Fig.
1B). KCNQ1OT1 was then silenced or overexpressed to investigate
its role in OA. As shown in Fig.
1C, KCNQ1OT1 expression was markedly diminished
post-transfection with sh-KCNQ1OT1, whereas it was increased
following transfection with pcDNA-KCNQ1OT1 in C28/I2 cells
(P<0.01). The MTT assay demonstrated that cell viability was
suppressed by LPS in C28/I2 cells, and sh-KCNQ1OT1 enhanced cell
viability in LPS-induced C28/I2 cells (all P<0.01; Fig. 1D). In addition, the ELISA results
indicated that IL-6, IL-1β and TNF-α levels in the culture medium
were enhanced by LPS, whereas the levels of IL-6, IL-1β and TNF-α
were decreased by sh-KCNQ1OT1 in C28/I2 cells treated with LPS (all
P<0.01; Fig. 1E-G). Furthermore,
the expression levels of ECM degradation-related proteins
(MMP-3/-13 and collagen II) and the hypertrophy marker collagen X
were determined. Western blot analysis revealed that the expression
levels of MMP-3/-13 and collagen X were enhanced by LPS in C28/I2
cells, whereas collagen II expression levels were decreased by LPS
in C28/I2 cells (all P<0.01; Fig.
1H). MMP-3/-13 and collagen X were diminished, whereas collagen
II was increased following transfection of LPS-treated C28/I2 cells
with sh-KCNQ1OT1 (all P<0.01; Fig.
1H).
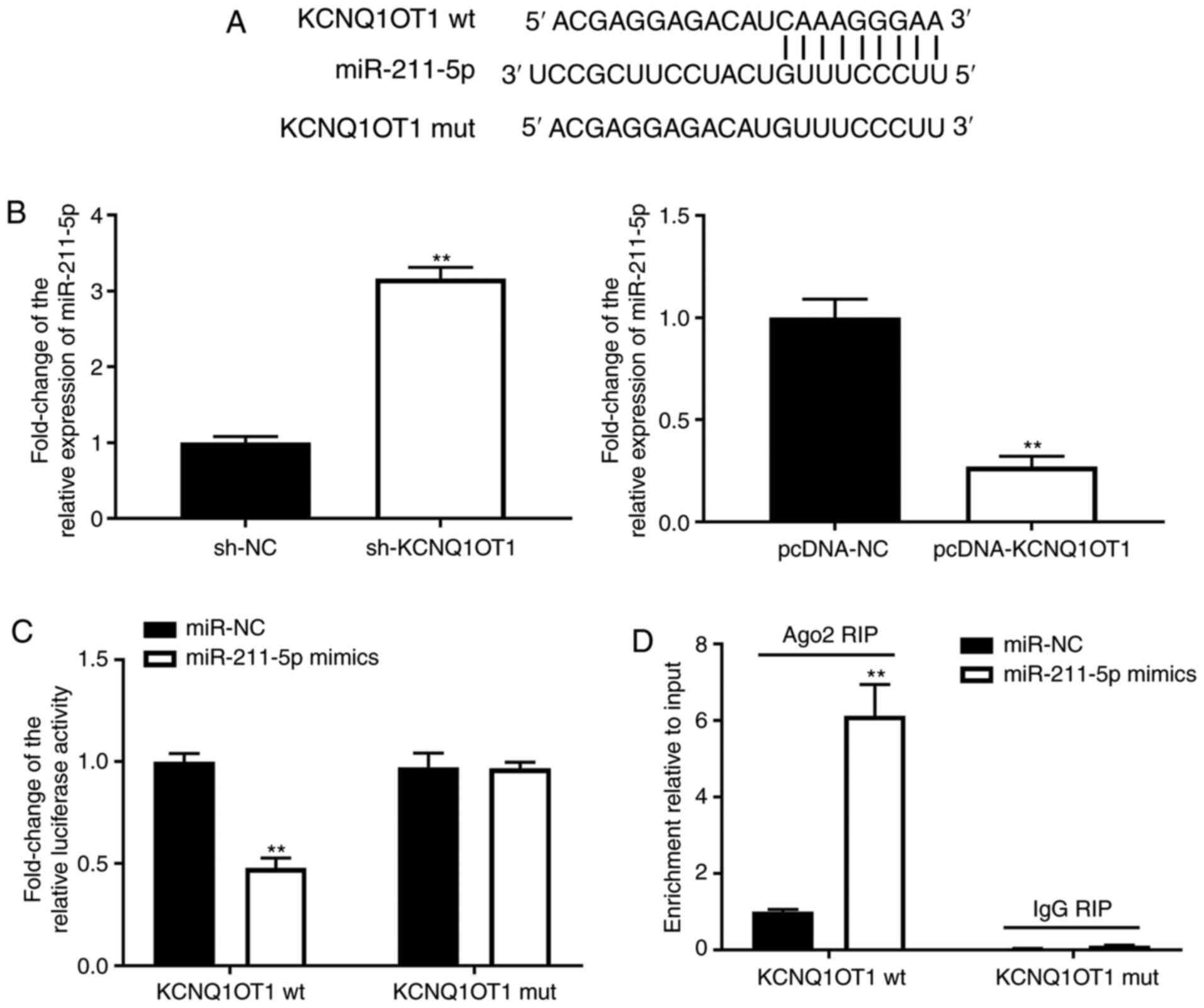
KCNQ1OT1 may serve as a competitive
endogenous RNA of miR-211-5p
To further investigate the mechanism of KCNQ1OT1 in
OA, the binding sites of KCNQ1OT1 were predicted using lncBase
Predicted v.2. miR-211-5p was predicted to be the target of
KCNQ1OT1 (Fig. 2A). The expression
levels of miR-211-5p were increased by sh-KCNQ1OT1 but inhibited by
pcDNA-KCNQ1OT1 in C28/I2 cells (P<0.01; Fig. 2B-C). Furthermore, the DLR and RIP
assays were utilised to verify the interaction between KCNQ1OT1 and
miR-211-5p. As shown in Fig. 2D,
the relative luciferase activity of C28/I2 cells was decreased
post-transfection with the KCNQ1OT1 wt vector and miR-211-5p,
compared with C28/I2 cells transfected with the KCNQ1OT1 wt vector
and miR-NC (P<0.01). As shown in Fig. 2E, KCNQ1OT1 immunoprecipitates
obtained from C28/I2 cells were notably enriched in the miR-211-5p
mimics group, compared with the miR-NC group (P<0.01).
Overexpression of miR-211-5p reduces
LPS-induced chondrocyte injury in C28/I2 cells
Through investigating the role of miR-211-5p in OA,
it was revealed that the expression levels of miR-211-5p in OA
cartilage tissues were downregulated compared with those in normal
cartilage tissues (P<0.01; Fig.
3A). miR-211-5p expression levels were also decreased in
LPS-induced C28/I2 cells compared with those in C28/I2 cells not
treated with LPS (P<0.01; Fig.
3B). Following transfection with miR-211-5p mimics, miR-211-5p
expression levels exhibited a significant increase in C28/I2 cells
(P<0.01; Fig. 3C), indicating
that overexpression of miR-211-5p was successful in subsequent
functional studies. As shown in Fig.
3D, cell viability was increased by transfection of LPS-treated
C28/I2 cells with miR-211-5p mimics (P<0.01). Overexpression of
miR-211-5p also suppressed the inflammatory response by decreasing
the levels of IL-6, IL-1β and TNF-α in C28/I2 cells treated with
LPS (all P<0.01; Fig. 3E-G). In
addition, the upregulation of collagen II and downregulation of
MMP-3/-13/collagen X implied that overexpression of miR-211-5p may
suppress ECM degradation and chondrocyte hypertrophy (all
P<0.01; Fig. 3H).
TCF4 is a target gene of
miR-211-5p
TargetScan and miRDB software were employed to
search for the target gene of miR-211-5p. It was discovered that
miR-211-5p had certain complementary bases with the sequence of
TCF4 3'-UTR, indicating that TCF4 may be the target gene of
miR-211-5p (Fig. 4A). Western blot
analysis revealed that overexpression of miR-211-5p markedly
decreased the protein expression levels of TCF4 (P<0.01;
Fig. 4B), whereas KCNQ1OT1
overexpression increased the protein expression levels of TCF4
(P<0.01; Fig. 4C). The results
of DLR and RIP assays verified the interaction between miR-211-5p
and TCF4. It was revealed that the relative luciferase activity was
markedly decreased by the introduction of miR-211-5p mimics in
C28/I2 cells transfected with the TCF4 wt vector (P<0.01;
Fig. 4D) but did not differ
following the introduction of miR-211-5p mimics in C28/I2 cells
transfected with the TCF4 mut vector (Fig. 4D). The RIP assay demonstrated that
endogenous TCF4 was significantly enriched in the miR-211-5p mimic
group compared with that in the miR-NC group (P<0.01; Fig. 4E), revealing the direct binding
between miR-211-5p and TCF4.
sh-KCNQ1OT1 may regulate the
miR-211-5p/TCF4 axis to inhibit OA progression
To confirm the associations between KCNQ1OT1,
miR-211-5p and TCF4 in OA, the expression levels of TCF4 in OA were
determined. It was revealed that the mRNA expression levels of TCF4
were upregulated in the cartilage tissues of patients with OA, and
the protein expression levels of TCF4 were also upregulated in
LPS-induced C28/I2 cells, compared with their respective controls
(all P<0.01; Fig. 5A and
B). In addition, the expression
levels of miR-211-5p were decreased by transfection with miR-211-5p
inhibitor, and TCF4 expression levels were increased
post-transfection with pcDNA-TCF4 (P<0.01; Fig. 5C). Subsequently, rescue experiments
were implemented, and it was revealed that sh-KCNQ1OT1 led to a
significant decrease in TCF4 in LPS-treated C28/I2 cells
(P<0.01; Fig. 5D). The
suppressive effect of sh-KCNQ1OT1 on TCF4 was reversed by
pcDNA-TCF4 or the miR-211-5p inhibitor in LPS-treated C28/I2 cells
(all P<0.01; Fig. 5D). Cell
viability was promoted by sh-KCNQ1OT1 in LPS-treated C28/I2 cells
(P<0.01; Fig. 5E), whereas the
effect of sh-KCNQ1OT1 on cell viability was reversed by pcDNA-TCF4
or the miR-211-5p inhibitor (all P<0.01; Fig. 5E). Furthermore, inflammation was
suppressed by sh-KCNQ1OT1 in LPS-treated C28/I2 cells (all
P<0.01; Fig. 5F-H). The
suppressive effects of sh-KCNQ1OT1 on IL-6, IL-1β and TNF-α were
reversed by pcDNA-TCF4 or the miR-211-5p inhibitor in LPS-induced
C28/I2 cells (all P<0.01; Fig.
5F-H). As illustrated in Fig.
5I, the decrease in MMP-3/-13/collagen X and increase in
collagen II induced by transfection of LPS-induced C28/I2 cells
with sh-KCNQ1OT1 (all P<0.01) was reversed by transfection with
pcDNA-TCF4 or the miR-211-5p inhibitor (P<0.05).
 | Figure 5KCNQ1OT1 indirectly regulates
expression of TCF4 by competitively binding to miR-211-5p. (A)
Relative expression level of TCF4 in OA cartilage tissues and
normal cartilage tissues was determined by RT-qPCR.
**P<0.01 vs. normal. (B) Relative protein expression
level of TCF4 in C28/I2 cells was determined by western blotting.
**P<0.01 vs. control. (C) Expression levels of
miR-211-5p and TCF4 were detected by RT-qPCR.
**P<0.01 vs. inhibitor NC or pcDNA-NC. (D) Relative
protein expression level of TCF4 in transfected C28/I2 cells were
determined by western blotting. **P<0.01 vs. LPS +
sh-NC; ##P<0.01 vs. LPS + sh-KCNQ1OT1. (E) Cell
viability was assessed by MTT assay. **P<0.01 vs. LPS
+ sh-NC; ##P<0.01 vs. LPS + sh-KCNQ1OT1. Levels of
(F) IL-6, (G) IL-1β and (H) TNF-α in culture medium were measured
by ELISA. **P<0.01 vs. LPS + sh-NC;
##P<0.01 vs. LPS + sh-KCNQ1OT1. (I) Protein
expression levels of MMP-3, MMP-13, collagen X and collagen II were
detected by western blotting. **P<0.01 vs. LPS +
sh-NC; ##P<0.01 vs. LPS + sh-KCNQ1OT1. miR, microRNA;
OA, osteoarthritis; LPS, lipopolysaccharide; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; sh, short
hairpin RNA; NC, negative control; KCNQ1OT1, KCNQ1 opposite
strand/antisense transcript 1; TCF4, transcription factor 4; MMP,
matrix metalloproteinase. |
Discussion
OA is characterised as a degenerative joint disease
that poses a serious threat to public health (24). Previous studies on lncRNA profiles
have demonstrated upregulation of various lncRNAs is involved in
the pathogenesis of OA, including lncRNA ANRIL (25), lncRNA CTBP1-AS2(26) and lncRNA FAS-AS1(27). Consistent with these findings, the
present study demonstrated that lncRNA KCNQ1OT1 was markedly
upregulated in OA cartilage tissues and LPS-induced C28/I2 cells.
Therefore, it was hypothesized that KCNQ1OT1 may be a therapeutic
target for OA in the clinic. Furthermore, an increasing number of
studies have reported that KCNQ1OT1 may serve vital roles in cell
proliferation and inflammation (28-30).
Li et al (29) revealed that
silencing KCNQ1OT1 promoted cell viability, and decreased the
production of TNF-α, IL-6 and IL-1β in H9c2 cells induced by oxygen
and glucose deprivation/reoxygenation (29). Ye et al (30) revealed that KCNQ1OT1 suppressed the
proliferation of vascular smooth muscle cells and secretion of
inflammatory cytokines in intimal hyperplasia (30). Furthermore, KCNQ1OT1 has been
demonstrated to have a crucial impact on OA progression by sponging
the hsa-miR-1202/ETS1 axis (12).
The present study reported that knockdown of KCNQ1OT1 promoted cell
viability, suppressed inflammation and decreased the degradation of
ECM in OA cells. These results validated the crucial impact of
KCNQ1OT1 on the progression of OA in vitro. Given these
results, measurements of cell viability, inflammatory factor levels
and ECM degradation in OA may determine whether KCNQ1OT1 is
involved in OA, thereby highlighting a potential targeted treatment
for OA in the clinic.
Several studies have reported that miR-211-5p is
decreased in OA tissues and serves a regulatory role in OA
(18,19). Prasadam et al (18) reported that miR-211-5p was
downregulated in the OA meniscectomy model and was confirmed to
participate in decreasing the mineralisation capacity of ossified
osteoblasts in OA. Liu and Luo (19) determined that miR-211-5p was
markedly diminished in OA articular cartilage tissues, and was able
to restrain ECM degradation and the production of inflammatory
cytokines in OA. Similar to the aforementioned outcomes, miR-211-5p
was downregulated in OA tissues and cells in the present study, and
overexpression of miR-211-5p was shown to facilitate cell
viability, suppress inflammation and decrease ECM degradation,
implying that miR-211-5p may alleviate OA in vitro.
Furthermore, previous studies have reported that lncRNAs exert
their regulatory functions by interacting with miRNAs in OA
(31,32). More importantly, the crucial role of
PVT1/miR-211-3p axis (33) and
XIST/miR-211(34) axis in OA have
been verified. In the present study, miR-211-5p was identified as a
target of KCNQ1OT1 and was inversely modulated by it. Furthermore,
inhibition of miR-211-5p reversed the suppressive effects of
sh-KCNQ1OT1 on inflammation and ECM degradation, as well as the
promotive effect of sh-KCNQ1OT1 on viability in OA cells. Given
these outcomes, it was deduced that knockdown of KCNQ1OT1
attenuated OA by regulating miR-211-5p in vitro.
TCF4 is a crucial risk gene on human chromosome 18
that has been reported to be associated with OA (35,36).
Previous studies have confirmed the high expression of TCF4 in OA
(22,36), and revealed that TCF4 may induce
chondrocyte apoptosis and cartilage degradation to facilitate OA
development (37). In agreement
with previous studies, the present study reported that TCF4
expression was increased in OA tissues and cells, implying that
TCF4 contributed toward the development of OA. Furthermore, a
previous study revealed that TCF4 may serve as the target gene of
numerous miRNAs in OA, including miR-137(38), miR-130a-3p (22) and miR-93-5p (37). In the present study, TCF4 was
targeted and inversely regulated by miR-211-5p. Based on the
aforementioned results, it was concluded that miR-211-5p exerted an
inhibitory effect on the progression of OA by targeting TCF4.
Furthermore, it was revealed that TCF4 was positively regulated by
KCNQ1OT1, and overexpression of TCF4 reversed the suppressive
effects of sh-KCNQ1OT1 on inflammation and ECM degradation, and
reversed the promotive effect of sh-KCNQ1OT1 on proliferation in OA
in vitro. Therefore, it was concluded that sh-KCNQ1OT1
suppressed OA progression by modulating the miR-211-5p/TCF4 axis
in vitro.
In conclusion, KCNQ1OT1 and TCF4 expression was
increased, whereas miR-211-5p was decreased in OA tissues and
cells. It was suggested that KCNQ1OT1 acted as a sponge for
miR-211-5p and TCF4 was targeted by miR-211-5p. Knockdown of
KCNQ1OT1 facilitated cell viability, but inhibited inflammation and
ECM degradation by mediating the miR-211-5p/TCF4 axis in
vitro. However, the present study has certain limitations.
There may be other possible mediators aside from TCF4. In addition,
in vivo experiments were not conducted to elucidate the
regulatory mechanism of the KCNQ1OT1/miR-211-5p/TCF4 axis. These
limitations should be addressed in future studies. Nonetheless, the
present study may provide a solid foundation for treating OA in
clinical settings.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DA made substantial contributions to the conception
and design of the study. TW, YG, ZC and WW made substantial
contributions to the acquisition, analysis and interpretation of
data, as well as the drafting and revision of the manuscript. All
authors agreed to be accountable for the study, and read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Second Xiangya Hospital of Central South
University. Written informed consent was obtained from each
participant.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ashford S and Williard J: Osteoarthritis:
A review. Nurse Pract. 39:1–8. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Madry H, Luyten FP and Facchini A:
Biological aspects of early osteoarthritis. Knee Surg Sports
Traumatol Arthrosc. 20:407–422. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Xia B, Di C, Zhang J, Hu S, Jin H and Tong
P: Osteoarthritis pathogenesis: A review of molecular mechanisms.
Calcif Tissue Int. 95:495–505. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Woolf AD and Pfleger B: Burden of major
musculoskeletal conditions. Bull World Health Organ. 81:646–656.
2003.PubMed/NCBI
|
|
5
|
Migliore A, Paoletta M, Moretti A, Liguori
S and Iolascon G: The perspectives of intra-articular therapy in
the management of osteoarthritis. Expert Opin Drug Deliv.
17:1213–1226. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Taruc-Uy RL and Lynch SA: Diagnosis and
treatment of osteoarthritis. Prim Care. 40:821–836, vii.
2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fritah S, Niclou SP and Azuaje F:
Databases for lncRNAs: A comparative evaluation of emerging tools.
RNA. 20:1655–1665. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chen WK, Yu XH, Yang W, Wang C, He WS, Yan
YG, Zhang J and Wang WJ: lncRNAs: Novel players in intervertebral
disc degeneration and osteoarthritis. Cell Prolif.
50(e12313)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu Y, Liu K, Tang C, Shi Z, Jing K and
Zheng J: Long non-coding RNA XIST contributes to osteoarthritis
progression via miR-149-5p/DNMT3A axis. Biomed Pharmacother.
128(110349)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen Y, Zhang L, Li E, Zhang G, Hou Y,
Yuan W, Qu W and Ding L: Long-chain non-coding RNA HOTAIR promotes
the progression of osteoarthritis via sponging miR-20b/PTEN axis.
Life Sci. 253(117685)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang X, Huang CR, Pan S, Pang Y, Chen YS,
Zha GC, Guo KJ and Zheng X: Long non-coding RNA SNHG15 is a
competing endogenous RNA of miR-141-3p that prevents osteoarthritis
progression by upregulating BCL2L13 expression. Int
Immunopharmacol. 83(106425)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liu C, Gao J, Su G, Xiang Y and Wan L:
MicroRNA-1202 plays a vital role in osteoarthritis via KCNQ1OT1
has-miR-1202-ETS1 regulatory pathway. J Orthop Surg Res.
15(130)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Swingler TE, Niu L, Smith P, Paddy P, Le
L, Barter MJ, Young DA and Clark IM: The function of microRNAs in
cartilage and osteoarthritis. Clin Exp Rheumatol. 37 (Suppl
120):S40–S47. 2019.PubMed/NCBI
|
|
14
|
Iliopoulos D, Malizos KN, Oikonomou P and
Tsezou A: Integrative microRNA and proteomic approaches identify
novel osteoarthritis genes and their collaborative metabolic and
inflammatory networks. PLoS One. 3(e3740)2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Luo C, Liang JS, Gong J, Zhang HL, Feng
ZJ, Yang HT, Zhang HB and Kong QH: The function of microRNA-34a in
osteoarthritis. Bratisl Lek Listy. 120:386–391. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhong G, Long H, Ma S, Shunhan Y, Li J and
Yao J: Corrigendum to ‘miRNA-335-5p relieves chondrocyte
inflammation by activating autophagy in osteoarthritis’ [Life Sci
226 (2019) 164-172]. Life Sci. 240(117135)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Huang J, Zhao L, Fan Y, Liao L, Ma PX,
Xiao G and Chen D: The microRNAs miR-204 and miR-211 maintain joint
homeostasis and protect against osteoarthritis progression. Nat
Commun. 10(2876)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Prasadam I, Batra J, Perry S, Gu W,
Crawford R and Xiao Y: Systematic identification, characterization
and target gene analysis of microRNAs involved in osteoarthritis
subchondral bone pathogenesis. Calcif Tissue Int. 99:43–55.
2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu H and Luo J: miR-211-5p contributes to
chondrocyte differentiation by suppressing Fibulin-4 expression to
play a role in osteoarthritis. J Biochem. 166:495–502.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Altman R, Asch E, Bloch D, Bole G,
Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg
M, et al: Development of criteria for the classification and
reporting of osteoarthritis. Classification of osteoarthritis of
the knee. Diagnostic and Therapeutic Criteria Committee of the
American Rheumatism Association. Arthritis Rheum. 29:1039–1049.
1986.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hu Y, Li S and Zou Y: Knockdown of lncRNA
H19 relieves LPS-induced damage by modulating miR-130a in
osteoarthritis. Yonsei Med J. 60:381–388. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Luo X, Wang J, Wei X, Wang S and Wang A:
Knockdown of lncRNA MFI2-AS1 inhibits lipopolysaccharide-induced
osteoarthritis progression by miR-130a-3p/TCF4. Life Sci.
240(117019)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Endo Y, Nwawka OK, Smith S and Burket JC:
Tarsometatarsal joint communication during fluoroscopy-guided
therapeutic joint injections and relationship with patient age and
degree of osteoarthritis. Skeletal Radiol. 47:271–277.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li X, Huang TL, Zhang GD, Jiang JT and Guo
PY: lncRNA ANRIL impacts the progress of osteoarthritis via
regulating proliferation and apoptosis of osteoarthritis
synoviocytes. Eur Rev Med Pharmacol Sci. 23:9729–9737.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang H, Li J, Shao W and Shen N: lncRNA
CTBP1-AS2 is upregulated in osteoarthritis and increases the
methylation of miR-130a gene to inhibit chondrocyte proliferation.
Clin Rheumatol. 39:3473–3478. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhu JK, He TD, Wei ZX and Wang YM: lncRNA
FAS-AS1 promotes the degradation of extracellular matrix of
cartilage in osteoarthritis. Eur Rev Med Pharmacol Sci.
22:2966–2972. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen B, Ma J, Li C and Wang Y: Long
noncoding RNA KCNQ1OT1 promotes proliferation and
epithelialmesenchymal transition by regulation of SMAD4 expression
in lens epithelial cells. Mol Med Rep. 18:16–24. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li X, Dai Y, Yan S, Shi Y, Han B, Li J,
Cha L and Mu J: Down-regulation of lncRNA KCNQ1OT1 protects against
myocardial ischemia/reperfusion injury following acute myocardial
infarction. Biochem Biophys Res Commun. 491:1026–1033.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ye B, Wu ZH, Tsui TY, Zhang BF, Su X, Qiu
YH and Zheng XT: lncRNA KCNQ1OT1 suppresses the inflammation and
proliferation of vascular smooth muscle cells through IkappaBa in
intimal hyperplasia. Mol Ther Nucleic Acids. 20:62–72.
2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu Y, Lin L, Zou R, Wen C, Wang Z and Lin
F: MSC-derived exosomes promote proliferation and inhibit apoptosis
of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in
osteoarthritis. Cell Cycle. 17:2411–2422. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cao L, Wang Y, Wang Q and Huang J: lncRNA
FOXD2-AS1 regulates chondrocyte proliferation in osteoarthritis by
acting as a sponge of miR-206 to modulate CCND1 expression. Biomed
Pharmacother. 106:1220–1226. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xu K, Meng Z, Xian XM, Deng MH, Meng QG,
Fang W, Zhang D and Long X: lncRNA PVT1 induces chondrocyte
apoptosis through upregulation of TNF-alpha in synoviocytes by
sponging miR-211-3p. Mol Cell Probes: 101560, 2020.
|
|
34
|
Li L, Lv G, Wang B and Kuang L: The role
of lncRNA XIST/miR-211 axis in modulating the proliferation and
apoptosis of osteoarthritis chondrocytes through CXCR4 and MAPK
signaling. Biochem Biophys Res Commun. 503:2555–2562.
2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wu L, Guo H, Sun K, Zhao X, Ma T and Jin
Q: Sclerostin expression in the subchondral bone of patients with
knee osteoarthritis. Int J Mol Med. 38:1395–1402. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ma B, Zhong L, van Blitterswijk CA, Post
JN and Karperien M: T cell factor 4 is a pro-catabolic and
apoptotic factor in human articular chondrocytes by potentiating
nuclear factor kappaB signaling. J Biol Chem. 288:17552–17558.
2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Xue H, Tu Y, Ma T, Wen T, Yang T, Xue L,
Cai M, Wang F and Guan M: miR-93-5p attenuates IL-1β-induced
chondrocyte apoptosis and cartilage degradation in osteoarthritis
partially by targeting TCF4. Bone. 123:129–136. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wang J, Fang L, Ye L, Ma S, Huang H, Lan X
and Ma J: miR-137 targets the inhibition of TCF4 to reverse the
progression of osteoarthritis through the AMPK/NF-κB signaling
pathway. Biosci Rep. 40(BSR20200466)2020.PubMed/NCBI View Article : Google Scholar
|