Introduction
The spinal cord, which functions as a transporter to
relay messages from the brain to the body and vice versa, transmits
messages from the autonomic nervous system, which regulates
numerous physiological functions, including heartbeat, blood
pressure and temperature regulation. However, damage to the spinal
cord can perturb all these functions. Spinal cord injury (SCI) is a
severe central nervous system disorder that has been increasingly
observed in the clinic over the past few years in China (1). Due to lack of successful treatment for
SCI, 50-80% of patients with SCI suffer with long-term moderate to
severe traumatic pain, which significantly impacts the families of
the patients both financially and in providing care (2).
Since significant damage is produced by secondary
injury alone, the inhibition of processes involved in secondary
injury may be one of the most effective approaches to prevent the
deterioration of SCI and promote functional recovery (3,4). The
secretion of stimulating cytokines, which can facilitate neuronal
damage and cell apoptosis, triggers the stimulus response and
secondary injury. Previous studies have demonstrated that the
levels of inflammatory factors, including IL-1β, TNF-α and IL-6,
were elevated at the early SCI stage (5-7).
Interestingly, inhibiting the secretion of these inflammatory
factors was reported to serve a central role in protecting nerve
cells (6). Thus, investigating the
molecular mechanism by which inflammatory cytokines could be
inhibited may be a novel and effective strategy for the treatment
of SCI.
Long non-coding RNAs (lncRNAs) are non-coding RNAs
of >200 nucleotides in length that lack protein-coding ability
(8). LncRNAs were reported to be
associated with the initiation and progression of numerous types of
diseases, including Parkinson's disease, leukemia and periodontitis
(9-13).
Knockdown of small nucleolar RNA host gene 14 (SNHG14) protected
nerve cells from damage by alleviating inflammation (14). In addition, lncRNA SNHG14 was
revealed to promote neuronal impairment and inflammatory response
induced by cerebral ischemia/reperfusion injury by regulating the
microRNA (miRNA/miR)-136-5p/Rho-associated protein kinase 1 (ROCK1)
signaling pathway, while SNHG14 knockdown could improve
neurological function in vivo (15). SNHG14 knockdown was also discovered
to alleviate lipopolysaccharide (LPS)-induced acute lung injury
through the miR-34c-3p-dependent inhibition of Wnt inducible
signaling pathway protein 1(16).
miRNAs are a class of non-coding RNAs that serve as
important gene expression regulators (17). miRNAs negatively regulate target
gene expression at the post-transcriptional level, which in turn
regulates numerous cellular processes, such as cell
differentiation, aging and apoptosis (17,18).
Previous studies have reported that miR-181a/b-5p, which was found
to modulate vascular inflammation and endotoxin tolerance,
alleviated inflammation in monocrotaline-induced pulmonary arterial
hypertension by targeting endocan (19,20).
To the best of our knowledge, no previous studies
have reported the role of SNHG14 in SCI. Therefore, the present
study aimed to investigate whether SNHG14 could participate in the
neuronal protection and alleviate inflammation by regulating
miR-181a/b-5p in SCI, in addition to determining the underlying
mechanism.
Materials and methods
Cell culture and treatment
The PC-12 cell line was purchased from The Cell Bank
of Type Culture Collection of the Chinese Academy of Sciences. The
cells were cultured at a density of 1x104 cells/ml in
DMEM-high glucose (Sigma-Aldrich; Merck KGaA) supplemented with 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.) and 100 U/ml
penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.).
The cells were maintained in a humidified incubator with 5%
CO2 at 37˚C and the medium was replaced every 2-3
days.
To stimulate inflammatory cell injury following SCI,
PC-12 cells were treated with 1, 2, 5 or 10 µg/ml LPS
(Sigma-Aldrich; Merck KGA) for 12 h. In addition, the 12-h LPS
treatment of PC-12 cells was conducted after transfection.
Cell transfection
Short hairpin RNA (shRNA) targeting SNHG14, vector,
miR-181b-5p mimic, miR-181b-5p inhibitor and their corresponding
negative controls (NCs) were purchased from Shanghai GenePharma
Co., Ltd. miR-181b-5p mimic, 5'-AACAUUCAUUGCUGUCGGUGGGCA
CCGACAGCAAUGAAUGUUUU-3'; mimic NC,
5'-UUCUCCGAACGUGUCACGUTTACGUGACACGUUCGGAGAATT-3'; miR-181b-5p
inhibitor, 5'-CCCACCGACAGCAAUGAAUGUU-3' and Inhibitor NC:
5'-UCACAACCUCCUAGAAAGAGUAGA-3'. A total of 80 nM
shRNA-SNHG14/shRNA-NC, 50 nM miR-181b-5p mimic/mimic-NC and 50 nM
miR-181b-5p inhibitor/inhibitor-NC were transfected into PC12 cells
at 80% confluence using Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Following 48 h of
transfection, the cells were collected for subsequent
experiments.
Cell Counting Kit-8 (CCK-8) assay
The cells were seeded into 96-well plates at a
density of 1x104 cells/well and stimulated with LPS at
37˚C for 12 h. Following stimulation, 10 µl CCK-8 reagent (Dojindo
Molecular Technologies, Inc.) was added into the medium and
incubated at 37˚C for a further 2 h. Finally, the absorbance of the
cells was determined using a spectrophotometer at the wavelength of
450 nm.
TUNEL assay
A TUNEL detection kit (OriGene Technologies, Inc.)
was used to analyze the apoptosis of PC12 cells following LPS
induction. A six-well plate was added with a small amount of
culture medium (500 µl) in order for the slides to be in close
contact with the plate. Coverslips were then carefully placed in
the plate. Following indicated treatments, PC-12 cell suspension
was added to the coverslip at a density of 1x105
cells/well, which was incubated at 37˚C in a 5% CO2
chamber. The coverslips were washed in 37˚C PBS three times for 3-5
sec each time, fixed in 4% paraformaldehyde for 15 min at room
temperature and then cleaned with 37˚C deionized water. The
coverslips were dried on filter paper and glued to the slides with
neutral balsam mounting medium. The sample slide was digested with
protease K and then treated with terminal deoxynucleotidyl
transferase and biotin-dUTP at 37˚C for 60 min. The slides were
then sealed with sealing liquid and incubated with
streptavidin-horseradish peroxidase (HRP) working liquid (cat. no.
AR100017; OriGene Technologies, Inc.) and 3,3-diaminobenzidine
color reagent (OriGene Technologies, Inc.). Cell nucleus were
stained with hematoxylin at room temperature for 5 min and washed
by PBS for three times. Finally, the color was observed and
apoptotic cells were counted using a light microscope
(magnification, x200) from five fields of view.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from PC-12 cells was extracted and
purified using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). Total RNA was reverse transcribed into
cDNA using a PrimeScript™ II 1st Strand cDNA Synthesis kit (Takara
Bio, Inc.) using the following protocol: 65˚C for 10 min, 25˚C for
10 min, 37˚C for 60 min and 70˚C for 10 min. qPCR was subsequently
performed on an ABI Prism 7500 Sequence Detection system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) with a SYBR Green
Real-Time PCR kit (Takara Bio, Inc.). The PCR reaction mixture (20
µl) consisted of 10 µl Real Time PCR Master mix, 2 µl primer mix
(0.2 µM) and 20 ng cDNA diluted in RNase-free water. The primer
sequences were as follows: SNHG14 forward,
5'-CGTTGTCGAAAGCTAAAAGGA-3' and reverse,
5'-TGTTTCCATCTCACCAAATGC-3'; GAPDH forward,
5'-GCACCGTCAAGGCTGAGAAC-3' and reverse, 5'-TGGTGAAGACGCCAGTGGA-3';
miR-181b-5p forward, 5'-ACACTCCAGCTGGGACTTGGGCACTGA AACA-3' and
reverse, 5'-TGGTGTCGTGGAGTCG-3' and U6 forward,
5'-CTCGCTTCGGCAGCACA-3' and reverse, 5'-AACGCTTCACGAATTTGCGT-3'.
The thermocycling conditions were as follows: Initial denaturation
at 95˚C for 5 min, then 95˚C for 30 sec, 60˚C for 30 sec and 72˚C
for 30 sec, for a total of 40 cycles. GAPDH and U6 served as the
internal controls. Expression levels were quantified using the
2-ΔΔCq method (21).
Western blotting
Following treatment, whole cell lysates were
acquired using RIPA lysis buffer (Beyotime Institute of
Biotechnology). Protein concentration was quantified using a
bicinchoninic acid Protein Assay kit (Pierce; Thermo Fisher
Scientific, Inc.) and 20 µg protein was separated via 10% SDS-PAGE.
The separated proteins were subsequently transferred onto
nitrocellulose membranes and blocked in 5% non-fat milk for 2 h at
room temperature. The membranes were then incubated with the
following primary antibodies at 4˚C overnight: Anti-Bcl-2 (cat. no.
ab32124; 1:1,000; Abcam), anti-Bax (cat. no. ab32503; 1:1,000;
Abcam), anti-cleaved caspase-9 (cat. no. ab2324; 1:1,000; Abcam),
anti-cleaved caspase-3 (cat. no. ab32042; 1:1,000; Abcam),
anti-caspase 3 (cat. no. ab13847; 1:1,000; Abcam), anti-caspase-9
(cat. no. ab32539; 1:1,000; Abcam) and anti-GAPDH (cat. no. ab8245;
1:1,000; Abcam). Following primary antibody incubation, the
membranes were incubated with horseradish peroxidase-conjugated
secondary antibody (cat. no. 7074; 1:1,000; Cell Signaling
Technology, Inc.) at room temperature for 2 h in the dark. Protein
bands were visualized using an ECL Western Blotting Detection
reagent (GE Healthcare) and densitometric analysis was performed
using Image J software (v.1.52; National Institutes of Health).
ELISA
The cell supernatant was collected by centrifugation
at 12,000 x g for 10 min at 4˚C and the concentrations of
inflammatory cytokines were measured using interleukin (IL)-1β
ELISA kit (cat. no. PI305; Beyotime Institute of Biotechnology),
IL-6 ELISA kit (cat. no. PI330; Beyotime Institute of
Biotechnology) and tumor necrosis factor (TNF)-α kit (cat. no.
PT518; Beyotime Institute of Biotechnology) according to the
manufacturers' protocols. All experiments were repeated in
triplicate.
Dual luciferase reporter assay
The combination between SNHG14 and miR-181b-5p were
predicted using StarBase 3.0 (https://starbase.sysu.edu.cn/index.php). Cells were
seeded into 24-well plates at a density of 5x104
cells/well and cultured at 37˚C for 24 h. The fragment of SNHG14
containing the predicted wild-type (WT) or mutant (MUT)
miR-181b-5p-binding sequences were amplified by Shanghai GenePharma
Co., Ltd., and inserted into the pmirGLO luciferase reporter gene
vector (Promega Corporation) to produce the reporter plasmids
SNHG14-WT and SNHG14-MUT, respectively. Subsequently, the cells
were co-transfected with SNHG14-WT/SNHG14-MUT and miR-181b-5p
mimic/mimic-NC using Lipofectamine 2000 reagent for 24 h. The
culture medium was then removed, and the cells were rinsed twice
with PBS. The cells were added to the cell lysates, which were
swirled for 10 min and centrifuged at 12,000 x g for 10 min at 4˚C,
and the supernatant was subsequently transferred to a new Eppendorf
tube. A dual luciferase kit (cat. no. D0010; Beijing Solarbio
Science & Technology Co., Ltd.) was used to measure luciferase
activity according to the manufacturer's protocol. Firefly
luminescence and Renilla luminescence were detected by
enzyme markers, and Renilla luminescence was used as
internal reference.
Statistical analysis
Each experiment was performed three times and data
are presented as the mean ± SD. Statistical analysis was performed
using GraphPad Prism 5 software (GraphPad Software, Inc.).
Statistical differences between groups were analyzed using one-way
ANOVA with Tukey's test or Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
LPS induces PC-12 cell inflammatory
injury
CCK-8 assay was performed to determine the dose of
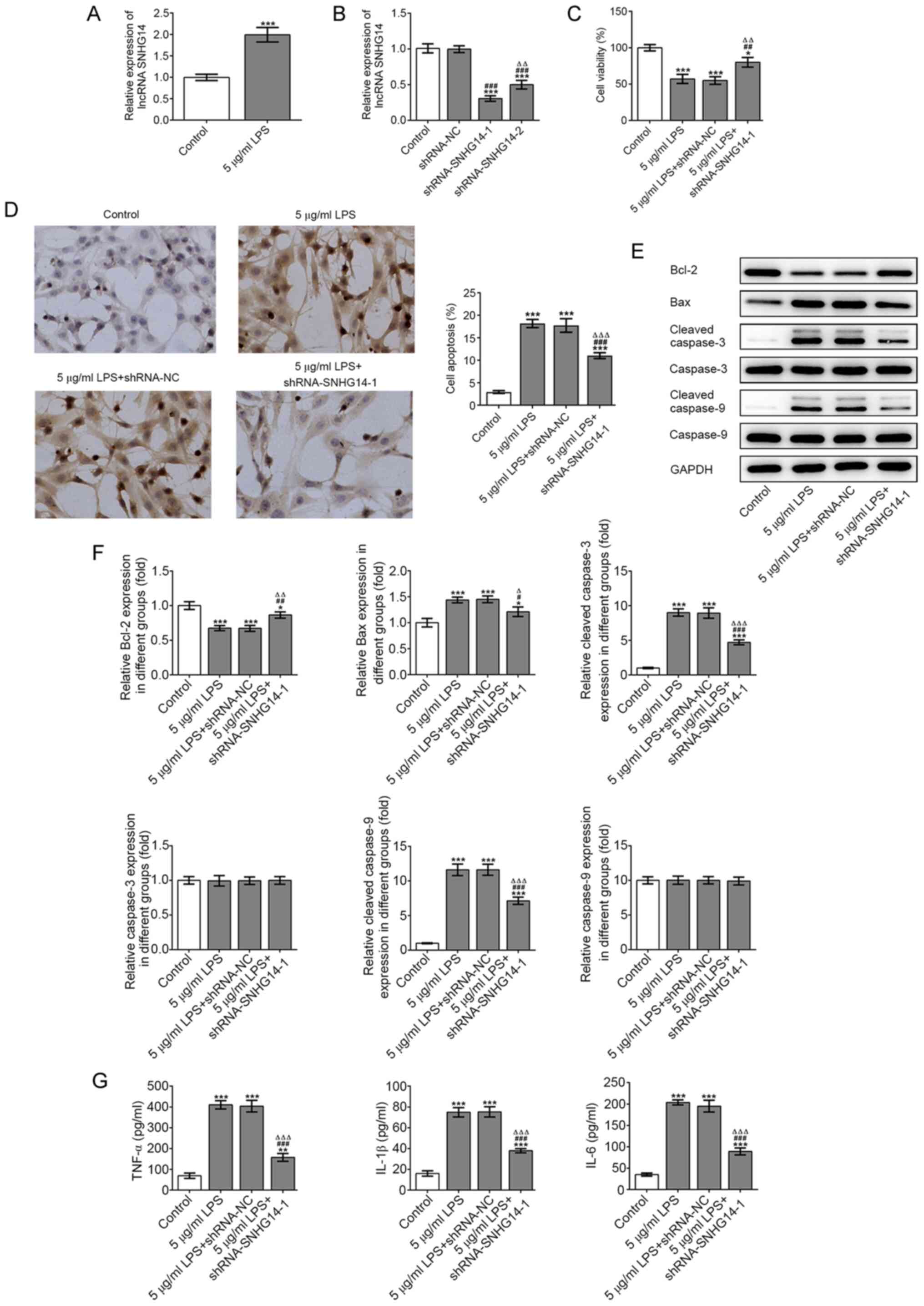
LPS to use for further experiments. As presented in Fig. 1A, PC-12 cell viability was barely
affected following treatment with 1 µg/ml LPS, whilst treatment
with 5 µg/ml LPS significantly decreased the cell viability
compared with the control group. Therefore, 5 µg/ml LPS was used
for subsequent experiments. TUNEL assay results revealed a
significant increase in cell apoptosis in LPS-treated cells
compared with controls (Fig. 1B).
These findings were validated using western blotting, which
revealed that compared with controls, the expression levels of
Bcl-2 were significantly downregulated, while the expression levels
of Bax, cleaved caspase-9 and cleaved caspase-3 were significantly
upregulated following treatment with 5 µg/ml LPS (Fig. 1C and D). As presented in Fig. 1E, the secretory levels of
pro-inflammatory factors, including IL-1β, IL-6 and TNF-α, were
significantly increased in the 5 µg/ml LPS group compared with
controls. These results suggested that LPS could induce PC-12 cell
inflammatory injury.
Knockdown of SNHG14 alleviates
LPS-induced inflammation and apoptosis of PC-12 cells
RT-qPCR analysis revealed that the expression levels
of SNHG14 in PC-12 cells were significantly upregulated following
treatment with 5 µg/ml LPS compared with controls (Fig. 2A). After constructing the
interference plasmids and transfecting them into PC-12 cells,
shRNA-SNHG14-1 demonstrated greater transfection efficiency
compared with shRNA-SNHG14-2. Therefore, shRNA-SNHG14-1 was
selected for use in subsequent experiments (Fig. 2B). PC-12 cells treated with LPS and
transfected with shRNA-SNHG14 had significantly higher cell
viability (Fig. 2C) and
significantly lower cell apoptosis levels (Fig. 2D) compared with those treated with
LPS alone. In addition, western blotting results revealed that the
expression levels of Bcl-2 were significantly upregulated, while
the expression levels of Bax, cleaved caspase-9 and cleaved
caspase-3 were significantly downregulated in cells treated with 5
µg/ml LPS and transfected with shRNA-SNHG14 compared with the 5
µg/ml LPS group (Fig. 2E and
F). ELISA results illustrated that
the secretory levels of IL-1β, IL-6 and TNF-α in PC-12 cells
following LPS treatment were significantly downregulated when the
cells were also transfected with shRNA-SNHG14 (Fig. 2G). These results suggested SNHG14
knockdown may alleviate LPS-induced inflammation and apoptosis of
PC-12 cells.
SNHG14 negatively regulates
miR-181b-5p expression levels in PC-12 cells
To investigate the regulatory mechanism of SNHG14 in
PC-12 cells, RT-qPCR analysis was conducted to determine the
interaction between SNHG14 and miR-181b-5p. As presented in
Fig. 3A, compared with the control,
the mRNA expression levels of miR-181b-5p were significantly
downregulated following treatment with 5 µg/ml LPS. However, the
expression levels of miR-181b-5p in PC-12 cells were significantly
upregulated following SNHG14 knockdown compared with the control
group (Fig. 3B). Subsequently, a
miR-181b-5p mimic was constructed and successfully transfected into
PC-12 cells to overexpress miR-181b-5p, as presented in Fig. 3C. The expression levels of SNHG14
were significantly downregulated in PC-12 cells transfected with
miR-181b-5p mimic compared with the control (Fig. 3D). The binding sites between SNHG14
and miR-181b-5p were subsequently predicted (Fig. 3E). Dual luciferase reporter assay
results revealed that the relative luciferase activities of PC-12
cells did not change following co-transfection with mimic-NC +
SNHG14-MUT or miR-181b-5p mimic + SNHG14-MUT; however, the relative
luciferase activity was significantly decreased following
co-transfection with miR-181b-5p mimic + SNHG14-WT (Fig. 3F). These findings indicated that
SNHG14 may negatively regulate miR-181b-5p in PC-12 cells.
 | Figure 3SNHG14 negatively regulates
miR-181b-5p in PC-12 cells. (A) miR-181b-5p expression in PC12
cells after LPS induction was detected by RT-qPCR.
***P<0.001 vs. Control group. (B) miR-181b-5p
expression in PC12 cells transfected with shRNA-SNHG14-1 was
detected by RT-qPCR. ***P<0.001 vs. Control group.
###P<0.001 vs. shRNA-NC group. (C) The transfection
efficiency of miR-181b-5p mimic was verified by RT-qPCR.
***P<0.001 vs. Control group.
###P<0.001 vs. mimic-NC group. (D) SNHG14 expression
in PC-12 cells transfected with miR-181b-5p mimic was detected by
RT-qPCR. ***P<0.001 vs. Control group.
###P<0.001 vs. mimic-NC group. (E) Binding sites
between SNHG14 and miR-181b-5p. (F) Luciferase reporter assay
verified the direct binding relationship between SNHG14 and
miR-181b-5p. **P<0.01 vs. mimic-NC group. SNHG14,
small nucleolar host gene 14; LPS, lipopolysaccharide; shRNA, short
hairpin RNA; NC, negative control; RT-qPCR, reverse
transcription-quantitative PCR; miR, microRNA; WT, wild-type; MUT,
mutant; luc, luciferase; R-Luc, Renilla luciferase; lncRNA,
long non-coding RNA. |
Knockdown of SNHG14 alleviates
LPS-induced inflammation and apoptosis of PC-12 cells via
upregulating miR-181b-5p expression levels
To confirm whether SNHG14 could reverse inflammation
and apoptosis in PC-12 cells through the newly identified
regulatory mechanism, a CCK-8 assay was performed. The results
revealed that the decreased cell viability induced by LPS
stimulation was rescued following transfection with shRNA-SNHG14,
while the addition of the miR-181b-5p inhibitor significantly
decreased cell viability (Fig. 4A).
In addition, as presented in Fig.
4B, cell apoptosis was significantly decreased following
stimulation with LPS and transfection with shRNA-SNHG14, while
co-transfection with shRNA-SNHG14 and miR-181b-5p inhibitor into
LPS-treated cells attenuated cell apoptosis. The downregulated
expression levels of Bcl-2 and upregulated expression levels of
Bax, cleaved caspase-9 and cleaved caspase-3 induced by 5 µg/ml LPS
were reversed by SNHG14 knockdown (Fig.
4C and D). However, miR-181b-5p
inhibition suppressed the effects of SNHG14 knockdown in
LPS-induced cells. As presented in Fig.
4E, the secretory levels of proinflammatory factors IL-1β, IL-6
and TNF-α were downregulated following stimulation with LPS and
transfection with shRNA-SNHG14. However, following LPS stimulation,
the secretory levels of these inflammatory factors were increased
when the cells were co-transfected with shRNA-SNHG14 and
miR-181b-5p inhibitor compared with those transfected with
shRNA-SNHG14 alone. These results suggested that SNHG14 knockdown
may upregulate the expression levels of miR-181b-5p and
subsequently attenuate LPS-induced inflammation and apoptosis of
PC-12 cells.
 | Figure 4SNHG14 knockdown alleviates
inflammation and apoptosis of PC-12 cells induced by LPS via
upregulating miR-181b-5p. (A) LPS-induced PC12 cell viability after
transfection was detected by Cell Counting Kit-8 assay.
***P<0.001 vs. Control group.
###P<0.001 vs. 5 µg/ml LPS group.
∆∆∆P<0.001 vs. 5 µg/ml LPS + shRNA-NC + inhibitor-NC
group. $$P<0.01 vs. 5 µg/ml LPS + shRNA-SNHG14-1 +
inhibitor-NC group. (B) LPS-induced PC12 cell apoptosis after
transfection was detected by TUNEL assay. Magnification, x200.
***P<0.001 vs. Control group. #P<0.05
and ###P<0.001 vs. 5 µg/ml LPS group.
∆∆∆P<0.001 vs. 5 µg/ml LPS + shRNA-NC + inhibitor-NC
group. $$P<0.01 vs. 5 µg/ml LPS + shRNA-SNHG14-1 +
inhibitor-NC group. (C) The expression of apoptosis-related
proteins was analyzed by western blot analysis and (D) quantified.
**P<0.01 and ***P<0.001 vs. Control
group. #P<0.05, ##P<0.01 and
###P<0.001 vs. 5 µg/ml LPS group.
∆∆P<0.01 and ∆∆∆P<0.001 vs. 5 µg/ml LPS
+ shRNA-NC + inhibitor-NC group. $P<0.05 and
$$$P<0.001 vs. 5 µg/ml LPS + shRNA-SNHG14-1 +
inhibitor-NC group. (E) The levels of TNF-α, IL-1β and IL-6 in the
supernatant of LPS-induced PC12 cells after transfection were
detected by ELISA. **P<0.01 and
***P<0.001 vs. Control group. #P<0.05,
##P<0.01 and ###P<0.001 vs. 5 µg/ml LPS
group. ∆∆∆P<0.001 vs. 5 µg/ml LPS + shRNA-NC +
inhibitor-NC group. $$$P<0.001 vs. 5 µg/ml LPS +
shRNA-SNHG14-1 + inhibitor-NC group. SNHG14, small nucleolar host
gene 14; LPS, lipopolysaccharide; shRNA, short hairpin RNA; NC,
negative control; miR, microRNA; WT, wild-type; MUT, mutant; luc,
luciferase; lncRNA, long non-coding RNA. |
Discussion
Despite progress being made in understanding the
basic neurobiology of SCI, strategies available for the treatment
of SCI remain limited (22).
Current clinical treatments, including surgical procedures and
high-dose methylprednisolone, can improve the overall survival rate
of patients, but they cannot repair nerve function that has been
damaged (23). In the present
study, the role of lncRNA SNHG14 in LPS-induced PC-12 cells was
investigated, in addition to its potential mechanism, with the aim
to provide a novel strategy for the treatment of SCI.
lncRNAs serve a role in various fundamental
biochemical and cellular processes and have emerged as pivotal
regulators that can enhance neural regeneration in the development
of SCI (24). Moreover, the
expression levels of certain lncRNAs, such as SNHG5 and tectonic
family member 2, have been discovered to be upregulated in SCI
(25,26). Therefore, therapies that target
lncRNAs may hold promise for the treatment of SCI (27). SNHG14 is located within the
Prader-Willi critical region and extends into the region of the
ubiquitin protein ligase E3A gene, whose deficiency in brain cells
in children was revealed to contribute to neurogenetic disorders
(28). A previous study has
verified that SNHG14 may aggravate inflammation in cerebral
ischemia/reperfusion injury by damaging the miR-136-5p-dependent
inhibition of ROCK1(15). It is
well known that inflammation is one of the most common features in
the occurrence of the SCI (29).
Therefore, it was hypothesized that SNHG14 may also induce the
development of SCI. In the present study, inflammatory injury
following SCI was stimulated in cells by LPS treatment, and the
results revealed that the expression levels of SNHG14 were
upregulated following LPS stimulation compared with the control
group. Following the subsequent construction of interference
plasmids targeting SNHG14 and transfection into PC-12 cells, it was
observed that SNHG14 knockdown could rescue cell viability under an
LPS-induced inflammatory environment. Since the sub-acute phase of
secondary SCI is involved in apoptosis, the expression levels of
apoptosis-related proteins in PC-12 cells were analyzed (30). The results revealed that the genetic
silencing of SNHG14 could reduce cell apoptosis and alleviate
inflammation. These findings suggested that SNHG14 may facilitate
the progression of SCI, which was consistent with the findings of
Jiao et al (31), who
reported that SNHG14 participated in the deterioration of non-small
cell lung cancer.
Using advanced high-throughput sequencing
technologies, a large number of differentially expressed genes
(lncRNA LINRIS, lncRNA HOXA-AS2 and lncRNA ZNF667-AS1), which
participated in the development of various types of disease, have
been identified (32-34).
Previous research has also noted the role of miRNAs following SCI
and suggested that they could be novel biomarkers for the
diagnosis, treatment and prognosis of such injuries (35). Accumulating evidence has verified
that miRNAs can regulate their expression and subsequent biological
functions by interacting with lncRNAs (36). LncRNAs have been found to exert
their effects by targeting or sponging miRNAs in various types of
cancer (37-40).
However, whether lncRNAs could sponge or target a certain miRNA in
SCI remain to be determined. The present study used StarBase 3.0
software to predict that miR-181b-5p, a member of the miR-181
family, was a target of SNHG14. Indeed, the genetic knockdown of
SNHG14 upregulated miR-181b-5p expression levels, while SNHG14
expression levels in PC-12 cells transfected with a miR-181b-5p
mimic were downregulated, indicating the negative regulatory role
of SNHG14 on miR-181b-5p expression levels. Furthermore,
miR-181b-5p was discovered to promote cell viability and inhibit
the apoptosis of PC-12 cells.
In conclusion, the results of the present study
suggested that SNHG14 may serve as a pathogenic lncRNA in PC-12
cells, as the genetic knockdown of SNHG14 alleviated LPS-induced
inflammation and apoptosis of PC-12 cells by negatively regulating
miR-181b-5p expression levels. These results may provide a novel
perspective for future therapeutic strategies for SCI. However, the
present study is that only performed in vitro experiments.
Future studies will involve in vivo experiments and assess
other underlying mechanisms.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YX conceived and designed the experiments. HJ and JN
performed all the experiments and YZ helped HJ and JN to collect
experimental data. HJ and JN authenticated the raw data in this
study. YZ performed the statistical analysis. HJ and JN wrote the
paper together which was revised and polished by YX. All authors
reviewed and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lu Y, Yang J, Wang X, Ma Z, Li S, Liu Z
and Fan X: Research progress in use of traditional Chinese medicine
for treatment of spinal cord injury. Biomed Pharmacother.
127(110136)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Watanabe S, Uchida K, Nakajima H, Matsuo
H, Sugita D, Yoshida A, Honjoh K, Johnson WE and Baba H: Early
transplantation of mesenchymal stem cells after spinal cord injury
relieves pain hypersensitivity through suppression of pain-related
signaling cascades and reduced inflammatory cell recruitment. Stem
Cells. 33:1902–1914. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hamann K, Nehrt G, Ouyang H, Duerstock B
and Shi R: Hydralazine inhibits compression and acrolein-mediated
injuries in ex vivo spinal cord. J Neurochem. 104:708–718.
2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hamann K and Shi R: Acrolein scavenging: A
potential novel mechanism of attenuating oxidative stress following
spinal cord injury. J Neurochem. 111:1348–1356. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Saxena T, Loomis KH, Pai SB, Karumbaiah L,
Gaupp E, Patil K, Patkar R and Bellamkonda RV: Nanocarrier-mediated
inhibition of macrophage migration inhibitory factor attenuates
secondary injury after spinal cord injury. ACS Nano. 9:1492–1505.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lv ZC, Cao XY, Guo YX, Zhang XD, Ding J,
Geng J, Feng K and Niu H: MiR-137-5p alleviates inflammation by
upregulating IL-10R1 expression in rats with spinal cord injury.
Eur Rev Med Pharmacol Sci. 23:4551–4557. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhu Y, Zhu H, Wang Z, Gao F, Wang J and
Zhang W: Wogonoside alleviates inflammation induced by traumatic
spinal cord injury by suppressing NF-κB and NLRP3 inflammasome
activation. Exp Ther Med. 14:3304–3308. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Iyer MK, Niknafs YS, Malik R, Singhal U,
Sahu A, Hosono Y, Barrette TR, Prensner JR, Evans JR, Zhao S, et
al: The landscape of long noncoding RNAs in the human
transcriptome. Nat Genet. 47:199–208. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Zhang Y, Xia Q and Lin J: LncRNA H19
Attenuates apoptosis in MPTP-induced Parkinson's disease through
regulating miR-585-3p/PIK3R3. Neurochem Res. 45:1700–1710.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gao J, Wang F, Wu P, Chen Y and Jia Y:
Aberrant lncRNA expression in leukemia. J Cancer. 11:4284–4296.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhu W, Liu H, Wang X, Lu J and Yang W:
Long noncoding RNAs in bladder cancer prognosis: A meta-analysis.
Pathol Res Pract. 215(152429)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Loewen G, Jayawickramarajah J, Zhuo Y and
Shan B: Functions of lncRNA HOTAIR in lung cancer. J Hematol Oncol.
7(90)2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu Y, Liu C, Zhang A, Yin S, Wang T, Wang
Y, Wang M, Liu Y, Ying Q, Sun J, et al: Down-regulation of long
non-coding RNA MEG3 suppresses osteogenic differentiation of
periodontal ligament stem cells (PDLSCs) through miR-27a-3p/IGF1
axis in periodontitis. Aging (Albany NY). 11:5334–5350.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li Z, Ho IHT, Li X, Xu D, Wu WKK, Chan
MTV, Li S and Liu X: Long non-coding RNAs in the spinal cord
injury: Novel spotlight. J Cell Mol Med. 23:4883–4890.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhong Y, Yu C and Qin W: LncRNA SNHG14
promotes inflammatory response induced by cerebral
ischemia/reperfusion injury through regulating miR-136-5p/ROCK1.
Cancer Gene Ther. 26:234–247. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhu J, Bai J, Wang S and Dong H:
Down-regulation of long non-coding RNA SNHG14 protects against
acute lung injury induced by lipopolysaccharide through
microRNA-34c-3p-dependent inhibition of WISP1. Respir Res.
20(233)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297.
2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang X, Sun H, Liu H, Ma L, Jiang C, Liao
H, Xu S, Xiang J and Cao Z: MicroRNA-181b-5p modulates tumor
necrosis factor-α-induced inflammatory responses by targeting
interleukin-6 in cementoblasts. J Cell Physiol. 234:22719–22730.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhao H, Guo Y, Sun Y, Zhang N and Wang X:
miR-181a/b-5p ameliorates inflammatory response in
monocrotaline-induced pulmonary arterial hypertension by targeting
endocan. J Cell Physiol. 235:4422–4433. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Demirel A, Li J, Morrow C, Barnes S,
Jansen J, Gower B, Kirksey K, Redden D and Yarar-Fisher C:
Evaluation of a ketogenic diet for improvement of neurological
recovery in individuals with acute spinal cord injury: Study
protocol for a randomized controlled trial. Trials.
21(372)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Haldrup M, Schwartz OS, Kasch H and
Rasmussen MM: Early decompressive surgery in patients with
traumatic spinal cord injury improves neurological outcome. Acta
Neurochir (Wien). 161:2223–2228. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhu S, Zhou Z, Li Z, Shao J, Jiao G, Huang
YE and Lin Y: Suppression of LINC00707 alleviates
lipopolysaccharide-induced inflammation and apoptosis in PC-12
cells by regulated miR-30a-5p/Neurod 1. Biosci Biotechnol Biochem.
83:2049–2056. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang F, Liu J, Wang X, Chen J, Kong Q, Ye
B and Li Z: The emerging role of lncRNAs in spinal cord injury.
Biomed Res Int. 2019(3467121)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ren XD, Wan CX and Niu YL: Overexpression
of lncRNA TCTN2 protects neurons from apoptosis by enhancing cell
autophagy in spinal cord injury. FEBS Open Bio. 9:1223–1231.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sun X, Shen H, Liu S, Gao J and Zhang S:
Long noncoding RNA SNHG14 promotes the aggressiveness of
retinoblastoma by sponging microRNA124 and thereby upregulating
STAT3. Int J Mol Med. 45:1685–1696. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Qi X, Shao M, Sun H, Shen Y, Meng D and
Huo W: Long non-coding RNA SNHG14 promotes microglia activation by
regulating miR-145-5p/PLA2G4A in cerebral infarction. Neuroscience.
348:98–106. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Venkatesh K, Ghosh SK, Mullick M,
Manivasagam G and Sen D: Spinal cord injury: Pathophysiology,
treatment strategies, associated challenges, and future
implications. Cell Tissue Res. 377:125–151. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Alizadeh A, Dyck SM and Karimi-Abdolrezaee
S: Traumatic spinal cord injury: An overview of pathophysiology,
models and acute injury mechanisms. Front Neurol.
10(282)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jiao P, Hou J, Yao M, Wu J and Ren G:
SNHG14 silencing suppresses the progression and promotes cisplatin
sensitivity in non-small cell lung cancer. Biomed Pharmacother.
117(109164)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang Y, Lu JH, Wu QN, Jin Y, Wang DS, Chen
YX, Liu J, Luo XJ, Meng Q, Pu HY, et al: LncRNA LINRIS stabilizes
IGF2BP2 and promotes the aerobic glycolysis in colorectal cancer.
Mol Cancer. 18(174)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang J, Su Z, Lu S, Fu W, Liu Z, Jiang X
and Tai S: LncRNA HOXA-AS2 and its molecular mechanisms in human
cancer. Clin Chim Acta. 485:229–233. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li JW, Kuang Y, Chen L and Wang JF: LncRNA
ZNF667-AS1 inhibits inflammatory response and promotes recovery of
spinal cord injury via suppressing JAK-STAT pathway. Eur Rev Med
Pharmacol Sci. 22:7614–7620. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Li F and Zhou MW: MicroRNAs in contusion
spinal cord injury: Pathophysiology and clinical utility. Acta
Neurol Belg. 119:21–27. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang Q, Teng Y, Wang R, Deng D, You Y,
Peng Y, Shao N and Zhi F: The long non-coding RNA SNHG14 inhibits
cell proliferation and invasion and promotes apoptosis by sponging
miR-92a-3p in glioma. Oncotarget. 9:12112–12124. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liang Y, Song X, Li Y, Chen B, Zhao W,
Wang L, Zhang H, Liu Y, Han D, Zhang N, et al: LncRNA BCRT1
promotes breast cancer progression by targeting miR-1303/PTBP3
axis. Mol Cancer. 19(85)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zhao W, Geng D, Li S, Chen Z and Sun M:
LncRNA HOTAIR influences cell growth, migration, invasion, and
apoptosis via the miR-20a-5p/HMGA2 axis in breast cancer. Cancer
Med. 7:842–855. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Luan X and Wang Y: LncRNA XLOC_006390
facilitates cervical cancer tumorigenesis and metastasis as a ceRNA
against miR-331-3p and miR-338-3p. J Gynecol Oncol.
29(e95)2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hu YP, Jin YP, Wu XS, Yang Y, Li YS, Li
HF, Xiang SS, Song XL, Jiang L, Zhang YJ, et al: LncRNA-HGBC
stabilized by HuR promotes gallbladder cancer progression by
regulating miR-502-3p/SET/AKT axis. Mol Cancer.
18(167)2019.PubMed/NCBI View Article : Google Scholar
|