Introduction
Doxorubicin (DOX) is a double-edged sword as a
classic anthracycline anti-tumor drug (1). On one hand, it is favored by doctors
and patients because of its powerful anti-tumor effects (2). On the other hand, its dose
accumulation and time-dependent cardiotoxicity concerns doctors and
patients (3). The mechanism of
cardiac toxicity caused by DOX is mostly related to the increase of
oxidative stress, damage to mitochondrial function and finally
apoptosis (4). Cytochrome c serves
a key role in mitochondrial injury (5). The increased permeability of damaged
mitochondrial membrane leads to the leakage of cytochrome c
from mitochondria and finally leads to cardiomyocyte apoptosis
(6). Although some drugs have been
used in the prevention and treatment of DOX-induced cardiotoxicity
for a number of years, the overall effect remains poor (7,8).
At present, dexrazoxane is the only anthracycline
anti-tumor drug cardioprotective agent approved by the FDA (USA),
but it is rarely used clinically in China due to its high price and
lack of popularity (9,10). Therefore, it is urgent to find a
more effective protective drug to protect the heart from the damage
of anthracycline anti-tumor drugs.
Wogonin (WG; 5,7-dihydroxy-8-methoxyflavone), an
important flavonoid extracted from Scutellaria baicalensis
root, has been proved to possess anti-inflammatory, antioxidant,
anti-allergic and anti-apoptotic properties (11,12).
A number of studies have shown that it protects the heart from
diabetes, obesity and ischemia-reperfusion injury (13-15).
However, whether WG can protect heart from DOX damage remains to be
elucidated.
In the present study, DOX was used to induce cardiac
injury and WG was administered by gavage to observe its protective
effect. The aim of the present study was to investigate the
anti-apoptotic effect of WG on DOX-induced cardiotoxicity and its
key mechanism.
Materials and methods
Materials
Wogonin (WG) and Doxorubicin (DOX), purity ≥98%,
were purchased from MilliporeSigma. Malondialdehyde (MDA; cat. no.
A003-1-1) and superoxide dismutase (SOD; cat. no. A001-1-1) test
kits were obtained from Nanjing Jiancheng Bioengineering Institute.
Brain natriuretic peptide (BNP; cat. no. 1608B), creatine kinase MB
(CK-MB; cat. no. 6930B) and cardiac troponin T (cTnT; cat. no.
7278A) test kits were all purchased from Shanghai Meixuan
Biotechnology Co., Ltd. ROS detection kit and CCK-8 cell viability
and toxicity detection kit were purchased from Biosharp Life
Sciences. The antibody of cytochrome c (cat. no. 11940), COX
Ⅳ (cat. no. 4850), pro/cleaved-caspase-3 (cat. no. 14220S/cat. no.
9664S) and cleaved-caspase-9 (cat. no. 9507) were obtained from
Cell Signaling Technology, Inc. All chemicals and reagents were
analytical grade.
Animal model
The present study was approved by the Animal Ethics
Committee of Hubei Medical College (approval no. 42000900000093). A
total of 40 Male SD rats (weight, 150-180 g; age, 5-6 weeks) were
obtained from the Hubei Medical College experimental animal center.
Rats were randomly distributed equally into four groups: CON group
(normal, n=10), WG group (WG gavage, 100 mg kg-1, n=10),
DOX group (3 mg kg-1 DOX was injected via caudal vein
once a week, n=10) and WG+THP group (3 mg kg-1 THP was
injected via caudal vein once a week with WG gavage, 100 mg
kg-1, n=10). The rats were kept at a standard room
temperature of 22±3˚C, 45±10% humidity and 12-h light/dark cycle.
The animals were supplied with standard laboratory food and ad
libitum access to tap water before experiments. The dosages of
DOX and WG in animal experiments referred to relevant studies
(16-18).
Electrocardiography and
echocardiography
Following the experiments, rats were anesthetized by
ketamine (55 mg/kg) plus xylazine (15 mg/kg) (19). Following anesthesia, Doppler
echocardiography of rats were measured by a Vivid E95 ultrasonic
diagnostic instrument (General Electric Company) and ejection
fraction (EF) and fractional shortening (FS) values in
echocardiography were measured. electrocardiograms (ECG) of rats
were recorded by BL-420E biological function measurement system
(Chengdu Taimeng Technology Co., Ltd.) and the R-wave, T-wave and
QT interval in ECG were measured.
Sample collection and processing
Under anesthesia, the rats were sacrificed by
cervical dislocation. Blood samples were collected and centrifuged
at 3,000 x g for 30 min at 4˚C immediately. The supernatant was
collected and the levels of BNP, CK-MB, cTnT, SOD and MDA were
measured immediately according to the kit instructions. Part of the
left ventricular tissue was fixed in 10 times volume of 4%
paraformaldehyde solution for 72 h at 4˚C. Then, following
dehydration and infiltration, it was embedded in paraffin and
sectioned at 4-5 µm thickness and finally stained according to the
instructions of H&E and TUNEL staining kit (Beyotime Institute
of Biotechnology) for 1 h at room temperature. The remaining heart
tissue was cryopreserved at -80˚C.
Cell culture and treatment
The ventricular parts of neonatal SD rats were
quickly separated under sterile conditions, cleaned and then cut
into fragments with a diameter of less than 1 mm, digested by
trypsin + type II collagenase, then filtered, centrifuged at 1,000
x g for 5 min at room temperature, suspended and seeded
(~5x106 cells/well). Finally, primary rat cardiomyocytes
were isolated by the differential adhesion method (5). The primary cardiomyocytes were
divided into four groups: Normal group (CON), WG group (WG, 10 µM,
12 h), DOX group (DOX, 5 µM, 12 h), DOX and WG co-culture group
(DOX, 5 µM + WG 10 µM, 12 h).
Western blotting
The mitochondria and cytoplasm of rat ventricular
tissue and primary rat cardiomyocytes were separated according to
the instructions of tissue or cell mitochondrial extraction kit
(Beyotime Institute of Biotechnology). A BCA kit was used to
determine the protein concentration thereafter. Then, ~30 µg heart
tissue or cell lysate was loaded per lane onto 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis, after which proteins
were transferred to an FL membrane (MilliporeSigma) at 4˚C for 2 h.
After blocking with 5% blocking protein powder (non-fat milk
powder; Beyotime Institute of Biotechnology) at room temperature
for 2 h, the following primary antibodies were added and incubated
overnight at 4˚C: Cyt C (1:1,000), pro/cleaved caspase-3
(cat. no. 14220S/cat. no. 9664S; 1:1,000), cleaved caspase-9 (cat.
no. 9507; 1:1,000), COX IV (cat. no. 4850; 1:1,000) and GAPDH (cat.
no. 10494-1-AP; 1:5,000). Subsequently, HRP-conjugated goat
anti-rabbit IgG (H+L) secondary antibodies (1:10,000; Thermo Fisher
Scientific, Inc.; cat. no. #31460) were added and incubated at room
temperature for 1.5 h. The results of western blotting were
analyzed using BeyoECL Plus (Beyotime Institute of Biotechnology)
and Image Lab software (version 5.2.1; Bio-Rad Laboratories, Inc.).
The specific protein expression levels were normalized to GAPDH or
COX IV.
Reverse transcription-quantitative
(RT-q) PCR
TRIzol® (Thermo Fisher Scientific, Inc.)
was used to extract total RNA from frozen, crushed rat hearts and
5x106 cultured primary cardiac myocytes (n=3) in
accordance with the manufacturers protocol, and quantified at 260
nm using a NanoPhotometer (cat. no. P300; Implen GmbH). The total
RNA was transcribed in two steps by super script first strand
synthesis system used in accordance with the manufacturers protocol
(cat. no. K1073; Apexbio Technology LLC). The PCR products were
quantified by SYBR Green PCR Master Mix (Applied Biosystems; Thermo
Fisher Scientific, Inc.) and the results were standardized with
β-Actin Gene expression. The thermocycling conditions were as
follows: Initial denaturation at 95˚C for 30 sec, followed by 40
cycles of denaturation at 94˚C for 5 sec, annealing at 58˚C for 30
sec and extension at 70˚C for 5 sec. β-actin served as the internal
reference gene and results were analyzed using the
2-ΔΔCq method (20).
The primer sequences were: Voltage-dependent anion-selective
channel 1 (VDAC-1): Forward ATGTCTTCACCAAGGGCTAT, Reverse
TCTGGGTCACTCGGGATT; adenine nucleotide transporter 1 (ANT1):
Forward TGGGCGACTGTATCATCAAG, Reverse TCACACTCTGGGCAATCATC;
cyclophilin D (Cyp D): Forward TTCCATCTTATGCTCTTCACCG, Reverse
GGTTGAAGAAGTCCTTGTCTGC; β-actin: Forward CTCTTCCAGCCTTCCTTCCT,
Reverse AGCACTGTGTTGGCGTACAG.
Statistical analysis
Data were presented as mean ± SD. The significance
of differences between groups were analyzed statistically using one
way analysis of variance (ANOVA), followed by a Tukey's
multiple-comparison post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
WG improves the abnormalities of
echocardiography and ECG in DOX rats
At the end of the experiment, a certain number of
succumbed in DOX group and the mortality was ~30% (7 survived and 3
succumbed). There was no mortality the in other groups.
Following DOX administration, echocardiography and
ECG changes occurred in rats, suggesting that DOX caused cardiac
injury in rats. Echocardiography (Fig.
1A) showed a decrease in EF (Fig.
1C) and FS (Fig. 1D) in rats.
However, the changes of EF and FS were reversed following WG
treatment. Similar to echocardiography, DOX also caused ECG
(Fig. 1B) abnormalities in rats,
which were characterized by increased R wave (Fig. 1E) and T wave (Fig. 1F) and prolonged QT interval
(Fig. 1G). WG treatment also
reversed these changes.
 | Figure 1WG ameliorates DOX-induced myocardial
injury in rats. WG improved the damage induced by DOX in rats as
shown by of (A) echocardiography and (B) ECG. Specifically, WG
reversed the decrease of (C) EF and (D) FS, the increase of (E) R
wave and (F) T wave and the prolongation of (G) QT interval in DOX
rats. In addition, WG also improved the contents of serum (H) cTnT,
(I) CK-MB and (J) BNP in DOX rats. All values are the mean ± SD.
*P<0.05, **P<0.01 vs. CON;
#P>0.05, ##P>0.01 vs. DOX. WG, wogonin;
DOX, doxorubicin; ECG, electrocardiograms; EF, ejection fraction;
FS, fractional shortening; CON, control; cTnT, cardiac troponin T;
CK-MB, creatine kinase MB; BNP, brain natriuretic peptide. |
WG improves myocardial injury and
increase of oxidative stress in DOX rats
Myocardial injury is often accompanied by the
increase of serum myocardial injury markers and myocardial
oxidative stress (21). In the DOX
rat model, the contents of serum cTnT (Fig. 1H), CK-MB (Fig. 1I) and BNP (Fig. 1J) increased. However, the above
myocardial injury markers decreased following WG treatment.
Similarly, in myocardial tissue, SOD activity (Fig. 2A), glutathione (GSH) content
(Fig. 2C) and CAT content
(Fig. 2D) decreased and MDA
content (Fig. 2B) increased. WG
effectively alleviated the abnormal oxidative stress. These results
suggested that WG effectively alleviated the heart injury caused by
DOX and the abnormal increase of oxidative stress.
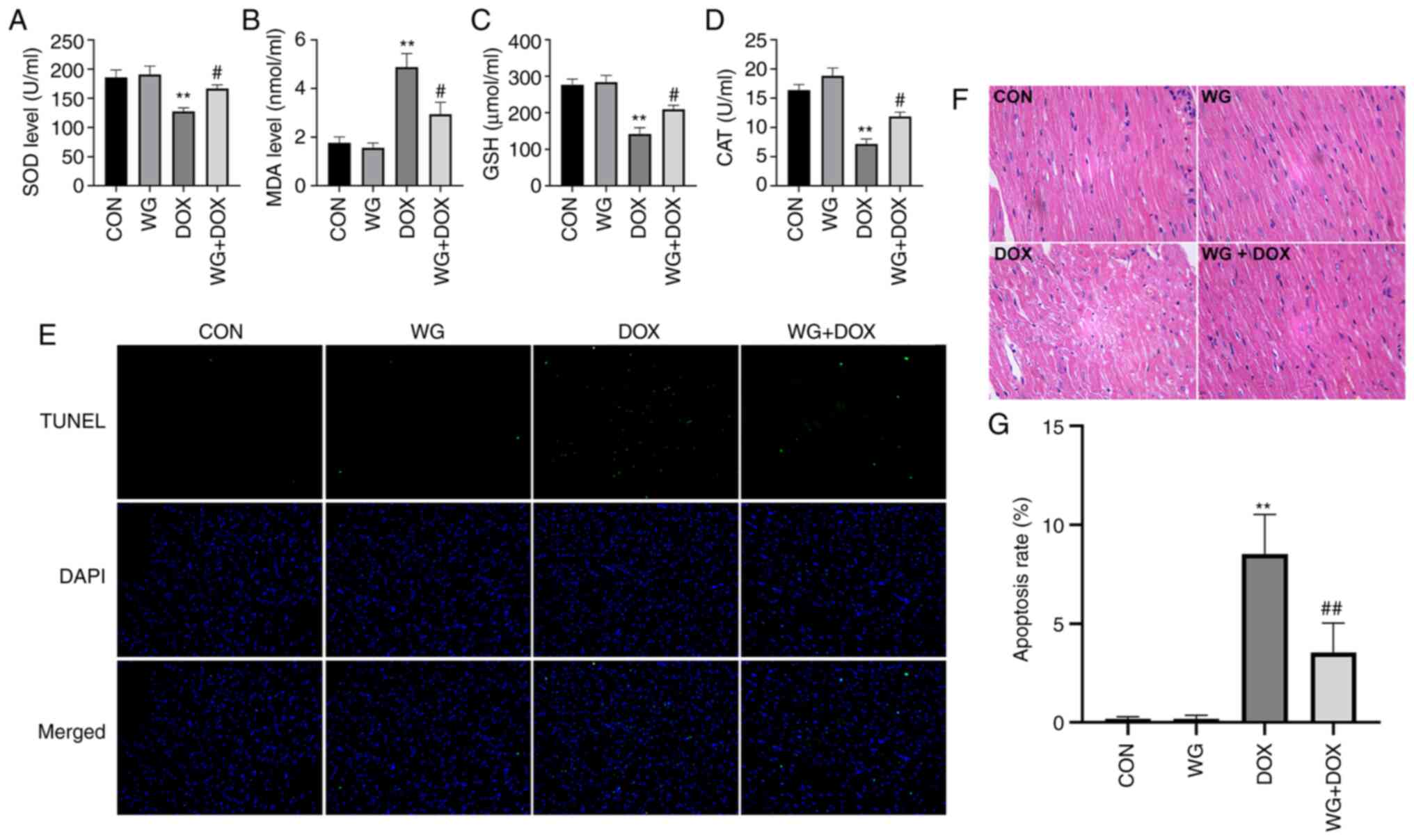 | Figure 2WG improves the DOX-induced oxidative
stress and apoptosis in rat hearts. WG reversed the decrease of (A)
SOD, (C) GSH and (D) CAT and the increase of (B) MDA in myocardial
tissue of DOX rats. (E) WG also improved cardiomyocyte apoptosis in
DOX rats (magnification, x200). (F) Hematoxylin and eosin staining
(magnification, x200). (G) Quantitative analysis. All values are
the mean ± SD. **P<0.01 vs. CON;
#P>0.05, ##P>0.01 vs. DOX. WG, wogonin;
DOX, doxorubicin; SOD, superoxide dismutase; GSH, glutathione; CAT,
catalase; MDA, malondialdehyde. |
WG improves DOX induced cardiac tissue
damage and cardiomyocyte apoptosis in rats
DOX caused the disorder of cardiac tissue
arrangement, the increase of cell gap and the abnormality of
nucleus in rats. The related changes were alleviated following WG
treatment (Fig. 2F). As shown in
Fig. 2E, TUNEL staining showed
that there was no cardiomyocyte apoptosis in CON and WG group, but
there was regional cardiomyocyte apoptosis in DOX injection group.
The treatment of WG effectively improved DOX-induced cardiomyocyte
apoptosis. The quantitative results were shown in Fig. 2G.
Effect of WG on DOX-induced
apoptosis-related signal pathway in rats
Mitochondrial membranes are composed of Cyp D, VDAC1
and ANT1. Abnormal increased permeability cause cytochrome c
to leak into cytoplasm from mitochondria and start caspase related
apoptosis signal pathway. The mRNA level of the components of
mitochondrial membranes was detected by RT-qPCR. The results showed
that the mRNA expressions of Cyp D, VDAC1 and ANT1 in DOX model
rats were significantly higher than those in the control group
(Fig. 3A). As shown in Fig. 3A, the mRNA levels of these genes in
the WG group were significantly lower than those in the DOX group,
which showed that under the action of WG, there were fewer
mitochondrial permeability conversion pores composed of the above
components than DOX group, which inhibited the release of
cytochrome c from mitochondria to cytoplasm.
 | Figure 3Effects of WG and DOX on apoptosis
related signaling pathways in rat heart. (A) WG effectively
improved the increased mRNA expression of Cyp D, VDAC1 and ANT1 in
DOX rats, (B) the decreased expression of mitochondrial cytochrome
c protein and (D) the increased the expression of
cytoplasmic cytochrome c protein, the increased expression
of cleaved-caspase-9/3 and the decreased expression of
pro-caspase-3. (C and E) Semi quantitative analysis. The expression
level of mitochondrial Cyt C specific protein was normalized to COX
IV. All values are the mean ± SD. *P<0.05,
**P<0.01 vs. CON; #P>0.05,
##P>0.01 vs. DOX. WG, wogonin; DOX, doxorubicin;
VDAC1, voltage-dependent anion-selective channel 1; Cyp D,
cyclophilin D; ANT1, adenine nucleotide transporter 1; Cyt C,
cytochrome c. |
By contrast, western blotting was used to detect the
changes of mitochondrial membrane permeability related apoptosis
signal pathway. The results showed that the expression of
mitochondrial cytochrome c protein in DOX rats was
significantly lower compared with the control group (Fig. 3B), while the expression of
cytoplasmic cytochrome c protein was significantly higher
compared with that in the control group (Fig. 3D). This indicated that cytochrome
c leaked from mitochondria into the cytoplasm. In addition,
the cytoplasmic cleaved-caspase-9/3 of DOX model rats was
significantly higher compared with of the control group, while
pro-caspase-3 was significantly lower compared with of the control
group (Fig. 3D). This suggests
that caspase dependent apoptosis was activated. However, compared
with DOX group, WG treatment group effectively reversed the above
changes (Fig. 3B and D). The semi-quantitative analysis results
were shown in Fig. 3C and E.
Effect of WG on DOX-induced
apoptosis-related signal pathway in cardiomyocytes
In order to verify whether WG has the same
protective effect on DOX induced-cardiomyocyte apoptosis in cells,
a DOX cell model we cultured and treated with WG. As with the
animal experiments, WG prevented the abnormal increase of
mitochondrial membrane permeability caused by DOX, the leakage of
cytochrome c from mitochondria into cytoplasm and the
activation of caspase dependent apoptosis pathway in DOX cell
group.
Specifically, WG effectively inhibited the
significant increase of Cyp D, VDAC1 and ANT1 mRNA expression
(Fig. 4A), the significant
decrease of mitochondrial cytochrome c (Fig. 4B) and cytoplasmic pro-caspase-3
protein expression (Fig. 4D) and
the significant increase of cytoplasmic cytochrome c and
cleaved-caspase-9/3 (Fig. 4D)
protein expression caused by DOX. The semi-quantitative analysis
results were shown in Fig. 4C and
E.
 | Figure 4Effects of WG and DOX on apoptosis
related signaling pathways in cardiomyocytes. (A) As in the animal
models, WG effectively improved the increased mRNA expression of
Cyp D, VDAC1 and ANT1 in DOX rats, (B) the decreased expression of
mitochondrial cytochrome c protein and (D) the increased the
expression of cytoplasmic cytochrome c protein and the
increased expression of cleaved-caspase-9/3 and the decreased
expression of pre-caspase-3. (C and E) semi-quantitative analysis.
All values are the mean ± SD. *P<0.05,
**P<0.01 vs. CON, #P>0.05,
##P>0.01 vs. DOX. WG, wogonin; DOX, doxorubicin;
VDAC1, voltage-dependent anion-selective channel 1; Cyp D,
cyclophilin D; ANT1, adenine nucleotide transporter 1. |
Discussion
Doxorubicin (DOX) is an anthracycline anti-tumor
antibiotic, which has a wide spectrum of anti-tumor effects
(2). It is commonly used in the
treatment of acute leukemia, breast cancer, malignant lymphoma and
bronchial lung cancer (22-24).
However, its clinical application is seriously limited due to its
dose-dependent and time-dependent cardiotoxicity (3,7,25).
The cardiotoxicity of DOX is often manifested as arrhythmia,
myocardial conduction disorder, myocarditis, cardiomyopathy and
heart failure (26). Traditional
wisdom suggests that the safe cumulative dose of DOX is 550
mg/m2 (27). However,
there are obvious individual differences in the cardiotoxicity of
DOX and there is no absolute safe dose (28). Previous studies have confirmed that
cardiomyocyte apoptosis serves a very important role in DOX-induced
cardiotoxicity (29,30). DOX leads to a large amount of ROS
accumulation and calcium overload in cells and destroys the
permeability of mitochondrial membrane (31,32).
A large number of pro-apoptotic factors such as cytochrome c
enter the cytoplasm from mitochondria, activate the caspase
pathway, initiate the endogenous apoptosis pathway and finally lead
to cardiomyocyte death (33).
Other studies also found that DOX can cause abnormal mitochondrial
morphology and structure, lipid deposition and irregular
arrangement of myofilaments, accompanied by myofilament breakage
and dissolution (4,10). This indicates that mitochondrial
function serves an important role in DOX induced heart injury.
WG, also known as 5,7-dihydroxy-8-methoxyflavone, is
a flavonoid extracted from the root of Scutellaria
baicalensis. It has significant pharmacological effects in a
variety of diseases, such as anti-inflammatory, anti-apoptosis,
anti-oxidation, cell cycle regulation and anti-cancer and leukemia
(13,15). In addition, WG also shows powerful
cardioprotective effect in animal models (14,15),
but its pharmacological effect in heart injury caused by DOX
remains to be elucidated. The present study showed that 100 mg/kg
WG improved the changes of echocardiography and ECG induced by DOX
and decreased the serum levels of BNP, CK-MB and cTnT (34). Elevated levels of cardiac markers
suggested that DOX caused myocardial injury, but WG effectively
alleviated the heart injury caused by DOX.
In the present study, it was also observed that the
serum SOD, GSH and CAT increased and the serum MDA level decreased
in DOX rats following WG treatment, indicating that WG exerts an
anti-oxidant effect. Relevant studies have pointed out that WG
significantly reduces the myocardial infarction area, serum cardiac
markers, lipid peroxidation products (MDA) and inflammatory markers
in myocardial infarction rats. Significantly upregulating the
expression of Nrf2 and HO-1 protein has also been demonstrated,
which has a powerful myocardial protective effect on myocardial
injury (11,13,15).
Compared with DOX group, WG decreased the levels of
cytosolic cytochrome c and cleaved-caspase-3/9 protein and
increased mitochondrial cytochrome c. At the same time, WG
also reduced the levels of VDAC1, ANT1 and Cyp D mRNA and the
reduction of mitochondrial cytochrome c release at the gene
level, thus reducing the occurrence of apoptosis; this is similar
to the results of Zhang et al (33) on the cardiotoxicity of another
anthracycline in rats. Mitochondrial function and its role in
maintaining cellular redox/oxidation balance are the basis of
regulating cellular homeostasis (35). ROS is the main by-product of normal
mitochondrial metabolism and stable internal environment (36). Under physiological conditions, the
balance between ROS generation and clearance is highly controlled
(37). DOX causes excessive
accumulation of ROS and upregulates oxidation, which may lead to
serious mitochondrial damage, cell damage and death and lead to the
failure of the whole organ and organism (38,39).
Mitochondrial permeability transition pore (mPTP) serves a central
role in regulating cell death (40). A number of factors, such as high
concentration of ROS, seem to lead to the opening of mPTP (41). The mPTP is hypothesized to be
regulated by VDAC in mitochondrial outer membrane, ANT in
mitochondrial inner membrane and Cyp D in mitochondrial matrix
(40,42). The abnormal increase of mPTP
permeability from various reasons leads to apoptosis through
mitochondrial mediated pathway (43). In short, DOX significantly
increases the level of cytochrome c in cytoplasm and the
expression of cleaved-caspase-3/9 protein and decreases the level
of cytochrome c in mitochondria. Cytochrome c is not only an
important factor for mitochondria to promote apoptosis, but also a
key factor for the initiation of apoptosis signal pathway (44,45).
Once released, cytochrome c forms a complex with Apaf1 and
pre-caspase-9, which activates cleaved-caspase-9/3, resulting in
programmed cell death (45,46).
The opening of mPTP leads to mitochondrial depolarization and
mitochondrial membrane potential dissipation, followed by
progressive mitochondrial swelling and the loss of soluble
components of respiratory chain, which eventually leads to the
rupture of mitochondrial outer membrane and the leakage of proteins
from mitochondria to cytoplasm, thus accelerating the occurrence of
apoptosis (47,48). The present study showed that WG
reversed the opening of mPTP and reduced the release of apoptotic
factors cytochrome c into the cytoplasm, so as to prevent
apoptosis. DOX can directly activate cardiomyocyte apoptosis by
reducing Bcl-2/Bax ratio (49).
Previous studies have noted that the high expression of Bcl-2 can
enhance the resistance of cells to most DNA damage factors and
inhibit the apoptosis of target cells caused by most
chemotherapeutic drugs and that the Bax gene, which inhibits Bcl-2,
is the main apoptosis gene in human body (50,51).
The proportional relationship between Bax/Bcl-2 protein is one of
the key factors to determine the inhibitory effect on apoptosis
(5,52). However, whether WG also has the
ability to change the increase of Bax/Bcl-2 ratio caused by DOX to
resist cardiomyocyte apoptosis remains to be elucidated.
In conclusion, the present study showed that WG
significantly reduced DOX-induced cardiac toxicity in rats. The
anti-apoptotic activity may be partly responsible for the
cardioprotective effect of WG. The present study reported that WG
reduces the release of cytochrome c by changing
mitochondrial permeability and serve an anti-apoptotic role to
achieve cardiac protection. However, more studies are required to
determine the specific mechanism of WG's anti-apoptotic effect and
promote the clinical application of WG in the future. The present
study also lacked the corresponding positive drug control of
mitochondrial protective agents and the specific difference between
WG and traditional cardiovascular protective drugs is not clear;
this will also be the further direction of research.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW, JZ, JX, JCha, XY, JuW, JChe and JiW
substantially contributed to the conception and the design of the
study, and in the acquisition, analysis and interpretation of the
data. JZ and JX contributed to manuscript drafting or critical
revisions on intellectual content. YW and JZ approved the final
manuscript version to be published. YW and JZ agreed to be
accountable for all aspects of the work, so that any questions
relating to research integrity or scientific accuracy in any part
of the study are appropriately investigated and resolved. YW and JZ
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of Hubei Medical College.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Speth PA, van Hoesel QG and Haanen C:
Clinical pharmacokinetics of doxorubicin. Clin Pharmacokinet.
15:15–31. 1988.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nam J, Son S, Ochyl LJ, Kuai R,
Schwendeman A and Moon JJ: Chemo-photothermal therapy combination
elicits anti-tumor immunity against advanced metastatic cancer. Nat
Commun. 9(1074)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Koleini N and Kardami E: Autophagy and
mitophagy in the context of doxorubicin-induced cardiotoxicity.
Oncotarget. 8:46663–46680. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tadokoro T, Ikeda M, Ide T, Deguchi H,
Ikeda S, Okabe K, Ishikita A, Matsushima S, Koumura T, Yamada KI,
et al: Mitochondria-dependent ferroptosis plays a pivotal role in
doxorubicin cardiotoxicity. JCI Insight. 5(e132747)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shi H, Tang H, Ai W, Zeng Q, Yang H, Zhu
F, Wei Y, Feng R, Wen L, Pu P and He Q: Schisandrin B antagonizes
cardiotoxicity induced by pirarubicin by inhibiting mitochondrial
permeability transition pore (mPTP) opening and decreasing
cardiomyocyte apoptosis. Front Pharmacol. 12(733805)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Han Y, Cai Y, Lai X, Wang Z, Wei S, Tan K,
Xu M and Xie H: lncRNA RMRP prevents mitochondrial dysfunction and
cardiomyocyte apoptosis via the miR-1-5p/hsp70 axis in LPS-induced
sepsis mice. Inflammation. 43:605–618. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Songbo M, Lang H, Xinyong C, Bin X, Ping Z
and Liang S: Oxidative stress injury in doxorubicin-induced
cardiotoxicity. Toxicol Lett. 307:41–48. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wenningmann N, Knapp M, Ande A, Vaidya TR
and Ait-Oudhia S: Insights into doxorubicin-induced cardiotoxicity:
Molecular mechanisms, preventive strategies, and early monitoring.
Mol Pharmacol. 96:219–232. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fang X, Wang H, Han D, Xie E, Yang X, Wei
J, Gu S, Gao F, Zhu N, Yin X, et al: Ferroptosis as a target for
protection against cardiomyopathy. Proc Natl Acad Sci USA.
116:2672–2680. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sangweni NF, Moremane M, Riedel S, van
Vuuren D, Huisamen B, Mabasa L, Barry R and Johnson R: The
prophylactic effect of pinocembrin against doxorubicin-induced
cardiotoxicity in an in vitro H9c2 cell model. Front Pharmacol.
11(1172)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Khan NM, Haseeb A, Ansari MY, Devarapalli
P, Haynie S and Haqqi TM: Wogonin, a plant derived small molecule,
exerts potent anti-inflammatory and chondroprotective effects
through the activation of ROS/ERK/Nrf2 signaling pathways in human
osteoarthritis chondrocytes. Free Radic Biol Med. 106:288–301.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lucas CD, Dorward DA, Sharma S, Rennie J,
Felton JM, Alessandri AL, Duffin R, Schwarze J, Haslett C and Rossi
AG: Wogonin induces eosinophil apoptosis and attenuates allergic
airway inflammation. Am J Respir Crit Care Med. 191:626–636.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shi X, Zhang B, Chu Z, Han B, Zhang X,
Huang P and Han J: Wogonin inhibits cardiac hypertrophy by
activating Nrf-2-mediated antioxidant responses. Cardiovasc Ther.
2021(9995342)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chang WT, Shao ZH, Vanden Hoek TL, McEntee
E, Mehendale SR, Li J, Becker LB and Yuan CS: Cardioprotective
effects of grape seed proanthocyanidins, baicalin and wogonin:
Comparison between acute and chronic treatments. Am J Chin Med.
34:363–365. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bei W, Jing L and Chen N: Cardio
protective role of wogonin loaded nanoparticle against
isoproterenol induced myocardial infarction by moderating oxidative
stress and inflammation. Colloids Surf B Biointerfaces.
185(110635)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hydock DS, Lien CY, Jensen BT, Parry TL,
Schneider CM and Hayward R: Rehabilitative exercise in a rat model
of doxorubicin cardiotoxicity. Exp Biol Med (Maywood).
237:1483–1492. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hiensch AE, Bolam KA, Mijwel S, Jeneson
JAL, Huitema ADR, Kranenburg O, van der Wall E, Rundqvist H,
Wengstrom Y and May AM: Doxorubicin-induced skeletal muscle
atrophy: Elucidating the underlying molecular pathways. Acta
Physiol (Oxf). 229(e13400)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Badawy AM, El-Naga RN, Gad AM, Tadros MG
and Fawzy HM: Wogonin pre-treatment attenuates cisplatin-induced
nephrotoxicity in rats: Impact on PPAR-γ, inflammation, apoptosis
and Wnt/β-catenin pathway. Chem Biol Interact. 308:137–146.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Luo S, Wang T, Qin H, Lei H and Xia Y:
Obligatory role of heat shock protein 90 in iNOS induction. Am J
Physiol Cell Physiol. 301:C227–C233. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shi H, Zeng Q, Wei Y, Yang H, Tang H, Wang
D, Pu P and Feng R: Canagliflozin is a potential cardioprotective
drug but exerts no significant effects on pirarubicin-induced
cardiotoxicity in rats. Mol Med Rep. 24(703)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang K, Guo QL, You QD, Yang Y, Zhang HW,
Yang L, Gu HY, Qi Q, Tan Z and Wang X: Wogonin induces the
granulocytic differentiation of human NB4 promyelocytic leukemia
cells and up-regulates phospholipid scramblase 1 gene expression.
Cancer Sci. 99:689–695. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yang D, Guo Q, Liang Y, Zhao Y, Tian X, Ye
Y, Tian J, Wu T and Lu N: Wogonin induces cellular senescence in
breast cancer via suppressing TXNRD2 expression. Arch Toxicol.
94:3433–3447. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang C and Cui C: Inhibition of lung
cancer proliferation by wogonin is associated with activation of
apoptosis and generation of reactive oxygen species. Balkan Med J.
37:29–33. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kalyanaraman B: Teaching the basics of the
mechanism of doxorubicin-induced cardiotoxicity: Have we been
barking up the wrong tree? Redox Biol. 29(101394)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ruggeri C, Gioffré S, Achilli F, Colombo
GI and D'Alessandra Y: Role of microRNAs in doxorubicin-induced
cardiotoxicity: An overview of preclinical models and cancer
patients. Heart Fail Rev. 23:109–122. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Swain SM, Whaley FS and Ewer MS:
Congestive heart failure in patients treated with doxorubicin: A
retrospective analysis of three trials. Cancer. 97:2869–2879.
2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nagy L, Szabó F, Iványi J, Németh L,
Kovács GL, Palatka J, Tarján J, Tóth K and Roth E: A method for
detection of doxorubicin-induced cardiotoxicity: Flow-mediated
vasodilation of the brachial artery. Exp Clin Cardiol. 6:87–92.
2001.PubMed/NCBI
|
|
29
|
Zhang X, Hu C, Kong CY, Song P, Wu HM, Xu
SC, Yuan YP, Deng W, Ma ZG and Tang QZ: FNDC5 alleviates oxidative
stress and cardiomyocyte apoptosis in doxorubicin-induced
cardiotoxicity via activating AKT. Cell Death Differ. 27:540–555.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zheng X, Zhong T, Ma Y, Wan X, Qin A, Yao
B, Zou H, Song Y and Yin D: Bnip3 mediates doxorubicin-induced
cardiomyocyte pyroptosis via caspase-3/GSDME. Life Sci.
242(117186)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Minotti G, Menna P, Salvatorelli E, Cairo
G and Gianni L: Anthracyclines: Molecular advances and
pharmacologic developments in antitumor activity and
cardiotoxicity. Pharmacol Rev. 56:185–229. 2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yun CH, Chae HJ, Kim HR and Ahn T:
Doxorubicin- and daunorubicin-induced regulation of Ca2+
and H+ fluxes through human bax inhibitor-1
reconstituted into membranes. J Pharm Sci. 101:1314–1326.
2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang Y, Ma XY, Zhang T, Qin M, Sun B, Li
Q, Hu DW and Ren LQ: Protective effects of apocynum venetum against
pirarubicin-induced cardiotoxicity. Am J Chin Med. 47:1075–1097.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Irfan A, Reichlin T, Twerenbold R, Fischer
C, Ballarino P, Nelles B, Wildi K, Zellweger C, Rubini Gimenez M,
Mueller M, et al: Cardiomyocyte injury induced by hemodynamic
cardiac stress: Differential release of cardiac biomarkers. Clin
Biochem. 48:1225–1229. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lee CF, Chavez JD, Garcia-Menendez L, Choi
Y, Roe ND, Chiao YA, Edgar JS, Goo YA, Goodlett DR, Bruce JE and
Tian R: Normalization of NAD+ redox balance as a therapy
for heart failure. Circulation. 134:883–894. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Mazat JP, Devin A and Ransac S: Modelling
mitochondrial ROS production by the respiratory chain. Cell Mol
Life Sci. 77:455–465. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Poljsak B, Šuput D and Milisav I:
Achieving the balance between ROS and antioxidants: When to use the
synthetic antioxidants. Oxid Med Cell Longev.
2013(956792)2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zorov DB, Juhaszova M and Sollott SJ:
Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS
release. Physiol Rev. 94:909–950. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Bartlett JJ, Trivedi PC and Pulinilkunnil
T: Autophagic dysregulation in doxorubicin cardiomyopathy. J Mol
Cell Cardiol. 104:1–8. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bauer TM and Murphy E: Role of
mitochondrial calcium and the permeability transition pore in
regulating cell death. Circ Res. 126:280–293. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Rottenberg H and Hoek JB: The path from
mitochondrial ROS to aging runs through the mitochondrial
permeability transition pore. Aging Cell. 16:943–955.
2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Halestrap AP: What is the mitochondrial
permeability transition pore? J Mol Cell Cardiol. 46:821–831.
2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Baechler BL, Bloemberg D and Quadrilatero
J: Mitophagy regulates mitochondrial network signaling, oxidative
stress, and apoptosis during myoblast differentiation. Autophagy.
15:1606–1619. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ow YP, Green DR, Hao Z and Mak TW:
Cytochrome c: Functions beyond respiration. Nat Rev Mol Cell Biol.
9:532–542. 2008.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Bock FJ and Tait SWG: Mitochondria as
multifaceted regulators of cell death. Nat Rev Mol Cell Biol.
21:85–100. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Jiang X and Wang X: Cytochrome C-mediated
apoptosis. Annu Rev Biochem. 73:87–106. 2004.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kwong JQ and Molkentin JD: Physiological
and pathological roles of the mitochondrial permeability transition
pore in the heart. Cell Metab. 21:206–214. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Javadov S and Karmazyn M: Mitochondrial
permeability transition pore opening as an endpoint to initiate
cell death and as a putative target for cardioprotection. Cell
Physiol Biochem. 20:1–22. 2007.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Chang WT, Lin YW, Ho CH, Chen ZC, Liu PY
and Shih JY: Dapagliflozin suppresses ER stress and protects
doxorubicin-induced cardiotoxicity in breast cancer patients. Arch
Toxicol. 95:659–671. 2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ashkenazi A, Fairbrother WJ, Leverson JD
and Souers AJ: From basic apoptosis discoveries to advanced
selective BCL-2 family inhibitors. Nat Rev Drug Discov. 16:273–284.
2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Adams JM and Cory S: The Bcl-2 protein
family: Arbiters of cell survival. Science. 281:1322–1326.
1998.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Song S, Chu L, Liang H, Chen J, Liang J,
Huang Z, Zhang B and Chen X: Protective effects of dioscin against
doxorubicin-induced hepatotoxicity via regulation of
Sirt1/FOXO1/NF-κb signal. Front Pharmacol. 10(1030)2019.PubMed/NCBI View Article : Google Scholar
|


















