Introduction
Temporomandibular disease (TMD) is one of the most
common dental diseases and is characterized by temporomandibular
joint (TMJ) pain, snapping and difficulty in opening the mouth,
which seriously affect quality of life. There are several causes of
TMJ inflammation, including abnormal biomechanical stress, injury,
or systematic disease, such as rheumatoid arthritis, which result
in TMD (1). TM joint
osteoarthritis (TMJ-OA) is one of the most common cases of TMD and
it is typically characterized by cartilage degradation and is very
difficult to cure. At present, the main pathological changes of
TMJ-OA include an increase in inflammatory factors, chondrocyte
degeneration and apoptosis, matrix collagen decomposition and
abnormal remodeling of subchondral bone (2). Chondrocytes are the only cell
component in cartilage tissue (3).
When the balance of the joint microenvironment is disturbed by
factors such as abnormal stress, ageing and genetic factors,
inflammatory factors such as IL-1β, TNF-α and nitric oxide (NO)
increase (4), chondrocytes become
irritated and secrete excessive MMPs, e.g. MMP-13, aggrecanases
(ADAMTS-4, -5), nitric oxide synthase (iNOS) and cyclooxygenase-2
(COX-2), which leads to extracellular matrix (ECM) decomposition
(5,6). Moreover, chondrocytes in an
inflammatory state also secrete various inflammatory factors, such
as IL-1β, TNF-α, IL-6 and IL-8, further leading to the aggravation
of inflammation in the joint microenvironment (7). This vicious cycle of inflammation
will eventually lead to cartilage destruction and the development
of OA (8).
A previous study emphasizes that the altered joint
mechanics that cause OA are addressed in first-line therapy, which
stresses the rehabilitation of normal biomechanical and
non-inflammation microenvironments (9). Unfortunately, there are currently no
pharmacological treatments or effective interventions that can
alter the joint mechanics to halt or reverse the progression of OA
in the long term. As OA involves a number of pathways and risk
factors, personalized therapy is the ultimate goal, which requires
different targeted disease-modifying OA drugs (DMOADs) for suitable
therapeutic options (10). The
pool of DMOADs is always in need of growth.
Strontium ranelate (SrR) is an anti-osteoporosis
drug that has the dual effect of promoting bone formation and
inhibiting bone resorption and has been recently considered a
possible DMOAD (11). As a
promising DMOAD, SrR has some special advantages. First, SrR has a
good tolerability and safety profile and is well tolerated by the
majority of patients in long-term treatment (12). Second, SrR can modify subchondral
bone turnover, thus indirectly modifying chondrocytes in cartilage
via factors released from bone (13,14).
Third, in our previous study, it was shown that SrR has a
chondrogenic induction effect on bone mesenchymal stem cells
(BMSCs) that promotes cartilage regeneration and suppresses
cartilage degradation by inhibiting the formation of MMPs in
vitro and in vivo (15). However, the anti-inflammatory
effect of SrR has not yet been fully elucidated.
Evidence of IL-1β in progressing OA is well
established and is also an essential factor when simulating the OA
environment in vitro (6).
Increased IL-1β leads to abnormal regulation of MMPs, induces
chondrocytes to produce large amounts of NO, causes abnormal
mitochondrial function and leads to chondrocyte apoptosis and
promotes chondrocytes to produce PEG-2 and other inflammatory
mediators, leading to degradation of PG and collagen (16). However, few studies of the
anti-inflammation effect of SrR have been conducted. Alves et
al (17) published research on
antinociceptive effects of SrR; that orally taken SrR could reduce
TNF-α levels in periarticular tissues and trigeminal ganglion, but
did not decrease IL-1β expression, nor inhibit HO-1 pathway. As the
gastrointestinal barrier would block ranelate acid outside the
blood serum, it was not possible to predict the same result in an
in vitro study. The in vitro study of Henrotin et
al (18) showed that SrR had a
significant inhibitory effect on MMPs and simulated PG synthesis
even under an inflammatory environment, but that study did not
advance mechanistic investigations. Above all, whether SrR could
suppress the inflammation level of IL-1β, MMPs and stabilize ECM
proteins directly on chondrocytes and the underlying mechanism
remain to be elucidated.
The treatment of TMJ-OA involves the regeneration of
cartilage, which, using the method of tissue engineering, requires
plenty of cells. The chondrocytes from healthy TMJ are limited and
it is reasonable to obtain chondrocytes from other site of
cartilage (19). The present study
employed the chondrocytes from rat femurs and aimed to investigate
whether SrR exerted a protective effect by reducing rat chondrocyte
inflammation caused by IL-1β. To explore its effect on chondrocyte
cell viability, ECM matrix synthesis, the expression of
cartilage-forming or inflammation genes and proteins and the
involvement of the molecular mechanism of SrR in Wnt/β-catenin
signaling pathways was examined.
Materials and methods
Isolation and culture of rat
chondrocytes
Rat chondrocytes cells were isolated from
Sprague-Dawley (SD) rats. Briefly, 12 male SD rats of 4-6 weeks
(weight 100-120 g) were purchased from Vital River Laboratories.
Rats were sacrificed on receipt by sodium pentobarbital at a dosage
of 150 mg/kg intraperitoneal injection and followed with strict
disinfection, the cartilage tissue on the top of the metaphysis of
the knee side was cut with a knife and washed twice with PBS and
twice with Hanks' Balanced Salt Solution (HBSS). After washing,
cartilage tissues were cut into small pieces and incubated in HBSS
(containing Ca2+ and Mg2+) with 200 U/ml type
II collagenase (Gibco; Thermo Fisher Scientific, Inc.; cat. no.
17101-015) at 37˚C and 5% CO2 for 12 h. The cells were
centrifuged at 300 x g for 5 min (room temperature), resuspended in
high-glucose DMEM (Gibco; Thermo Fisher Scientific, Inc.) with 10%
FBS (Gibco; Thermo Fisher Scientific, Inc.) and then subjected to
routine cell culture procedures. Chondrocytes with 3-5 passages
were used for the present study.
The present study was performed strictly in
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals of the National Institutes of Health
(20). All experiments were
approved by the Animal Research Committee of the Shanghai
Stomatological Hospital and Shanghai Research Center of Model
Animal Organization (IACUC no. 2020-0010-06).
Cell treatment with SrR and IL-1β
Chondrocytes were treated with different
concentrations of SrR and induced by IL-1β to simulate
inflammation. As described in our previous study (15,21),
51.35 mg of SrR (MilliporeSigma) was dissolved in 50 ml of culture
medium to obtain a maximum soluble concentration of 2.0 mmol/l.
Then, the samples were diluted to different concentrations of 1.0,
0.5, 0.25 and 0.125 mmol/l.
IL-1β-treated chondrocytes or cartilage tissues have
been widely adopted as in vitro models to study OA (6). The recombinant rat IL-1β protein was
purchased from BioVision, Inc. (cat. no. 4130-10) and a final
concentration of 10 ng/ml was used for the present study.
Chondrocytes cell proliferation
assay
The proliferation of chondrocytes under different
concentrations of SrR was detected by CCK-8 assay. Briefly, the
chondrocytes were inoculated in 96-well plates (initial cell
density of 3x103 cells/well) and treated with SrR (0,
0.125, 0.25, 0.5, 1.0 and 2.0 mmol/l) for 1, 3, 5 and 7 days. CCK-8
(10 µl) solution in 5% CO2 was added to each plate and
incubated at 37˚C for 1 h under dark conditions. Optical density
(OD) values reflecting cell viability were measured by a microplate
reader (BioTek Instruments, Inc.; ELX800) at a wavelength of 450
nm.
Toluidine blue staining
The chondrocytes were seeded on 24-well plates at an
initial density of 1x104 cells/well with DMEM added with
SrR at 0.125, 0.25 and 0.5 mmol/l for 14 days induction, while
initial cell density of 1x105 cells/well for the
cultural medium with IL-1 and 0.25 mmol/l SrR, and for 3 days, at
37˚C. Then, the chondrocytes were fixed with 4% paraformaldehyde at
4˚C for 30 min and stained with a toluidine blue solution at room
temperature for 30 min (Beijing Solarbio Science & Technology
Co., Ltd.) following 3 or 14 days of induction. Images were
captured with an inverted light microscope (Leica DMI 3000B; Leica
Microsystems GmbH) at x200 magnification.
Hydroxyproline (Hyp) assay
An Hyp assay revealed the degradation condition of
collagen. Hyp is the characteristic amino acid that is composed of
collagen and does not exist in other human tissues (22). A hydroxyproline test kit (Abcam;
cat. no. ab222941) was used in accordance with the manufacturer's
instructions. Cell culture supernatant, ddH2O and
standard protein samples were prepared and an equal volume of NaOH
was added, evaporated, cooled and neutralized with an equal amount
of HCl. Then, the supernatant was centrifuged at 10,000 x g for 5
min (room temperature) and collected into a new tube. An oxidation
reagent was added and the solution was incubated at room
temperature for 20 min. A developer was added and the solution was
incubated at 37˚C for 5 min. DMAB concentrate was added and the
solution was incubated at 65˚C for 45 min. The OD value of each
group was measured by microplate reader at 560 nm wavelength. The
test was repeated for three times and the hydroxyproline
concentration was calculated by the standard curve method.
Immunofluorescence staining assay
The chondrocytes were cultured in DMEM with or
without IL-1 and SrR at 0.125, 0.25 and 0.5 mmol/l for 14 days at
37˚C. First, the cells were rinsed with PBS and fixed at 4˚C with
4% paraformaldehyde for 15 min. Second, the cells were treated with
PBST for 10 min under room temperature for permeability and rinsed
with PBS several times (5 min; room temperature). Then,
non-specific interactions were blocked with donkey serum and
incubated with primary antibodies against β-catenin (Abcam; cat.
no. ab16051), collagen (Col)-II (ProteinTech Group, Inc.; cat. no.
15943-1-AP) and MMP-13 (Novus Biologicals, LLC; cat. no.
NBP2-17310). Cyanine 3-conjugated donkey anti-rabbit IgG (cat. no.
GB21403; Wuhan Servicebio Technology Co., Ltd.) was used as a
secondary antibody and nuclei were stained with DAPI
(MilliporeSigma; cat. no. D9642). Images were captured by a
fluorescence microscope at x200 magnification.
Reverse transcription-quantitative
(RT-q) PCR
The chondrocytes were cultured in 6-well plates at a
density of 5x104 cells/well with DMEM added with SrR at
0.125, 0.25 and 0.5 mmol/l for 14 days induction and total RNA
extracted at days 1, 7 and 14. With initial cell density of
5x105 cells/well for the cultural medium with IL-1 and
0.25 mmol/l SrR the total RNA was extracted at days 1, 2 and 3
using TRIzol® reagent (Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. RNA purity and
quantification were tested by NanoDrop One Microvolume
Spectrophotometer (Thermo Fisher Scientific, Inc.). The RNA was
used as a template for cDNA reverse by Tiangen FastKing cDNA
Dispelling RT SuperMix (cat. no. KR118; Tiangen Biotech Co., Ltd.)
following the manufacturer's instructions. The resultant cDNA (100
ng) was amplified in a 20 µl reaction system containing 10 µl 2X
SuperReal PreMix Plus (cat. no. FP205; KR118), 1.2 µl
forward/reverse primer, 2 µl cDNA template and 6.8 µl RNase-free
ddH2O. The PCR cycling conditions were as follow:
Initial denaturation at 95˚C for 15 min, followed by 40 cycles of
denaturation at 95˚C for 10 sec, annealing at 60˚C for 20 sec and
extension at 72˚C for 20 sec. All experiments were repeated three
times and the relative fold-change of gene expression
(2-ΔΔCq method) was calculated by the Ct value (23) (LightCycler 96 PCR system; Roche
Diagnostics GmbH). The primer sequences are in Table I. All tests were performed in
triplicate.
 | Table IPrimer sequences used for the rat
chondrocytes. |
Table I
Primer sequences used for the rat
chondrocytes.
| Gene | Forward | Reverse |
|---|
| Col-II |
ATCGCCACGGTCCTACAATG |
GGCCCTAATTTTCGGGCATC |
| Aggrecan |
CAAGTCCCTGACAGACACCC |
GTCCACCCCTCCTCACATTG |
| MMP-9 |
GATCCCCAGAGCGTTACTCG |
GTTGTGGAAACTCACACGCC |
| MMP-13 |
TGCTGCATACGAGCATCCAT |
TGTCCTCAAAGTGAACCGCA |
| β-catenin |
ACTCCAGGAATGAAGGCGTG |
GAACTGGTCAGCTCAACCGA |
| GAPDH |
AGTGCCAGCCTCGTCTCATA |
GATGGTGATGGGTTTCCCGT |
Western blotting assay
The chondrocytes were cultured in 6-well plates at a
density of 5x104 cells/well and treated with or without
SrR at 0.125, 0.25 and 0.5 mmol/l for 14 days, an initial density
of 5x105 cells/well treated with or without IL-1 and
0.25 mmol/l SrR for 3 days at 37˚C. After rinsing with PBS several
times, total proteins were collected by RIPA buffer (Beyotime
Institute of Biotechnology) on ice and measured by a BCA protein
assay kit (Beyotime Institute of Biotechnology). Equivalent amounts
of protein (20 µg/lane) were transferred to PCDF membranes on 12%
SDS-PAGE gels. Following blocking with 5% skimmed milk at room
temperature for 1 h, the membrane was exposed to primary antibodies
at 4˚C overnight, including Col-II (1:500; cat. no. 28459-1-AP;
ProteinTech Group, Inc.), aggrecan (1:500; cat. no. 13880-1;
ProteinTech Group, Inc.), β-catenin (1:1,000; cat. no. ab16051;
Abcam), MMP-9 (1:500; cat. no. 10375-2; ProteinTech Group, Inc.),
MMP-13 (1:500; cat. no. Nbp2-17310; Novus Biologicals) and β-actin
(1:1,000; cat. no. 4970; Cell Signaling Technology, Inc.). Then,
PBST was washed and incubated with HRP-labelled goat anti-rabbit
IgG (1:1,000; cat. no. A0208; Beyotime Institute of Biotechnology)
or HRP-labelled goat anti-mouse IgG (1:1,000; cat. no. A0216;
Beyotime Institute of Biotechnology) at room temperature for 2 h.
The membrane was thoroughly cleaned and visualized using ECL
reagents (Pierce; Thermo Fisher Scientific, Inc.). The bands were
imaged and measured by the Quantity One Analysis system (version
4.6.6.; Bio-Rad Laboratories, Inc.).
Statistical analysis
The results are shown as the mean ± standard
deviation of three repeated experiments and were analyzed by SPSS
26.0 software (IBM Corp.). One-way analysis of variance (ANOVA)
with a subsequent post hoc Tukey's test was used to determine the
statistical significance of the differences among groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
Effects of SrR on chondrocyte
viability
Cells isolated from rat femur cartilage were
positively stained with Col-II and aggrecan, but negatively with
Col-I and 11-fibrau, which made them identify as chondrocytes
(Fig. S1).
The influence of SrR on rat chondrocyte viability
was tested by a CCK-8 assay. As illustrated in Fig. 1, SrR at low concentrations,
including 0.125, 0.25 and 0.5 mmol/l, did not suppress cell
viability at days 1, 3, 5 and 7 and no significant differences were
observed between the control group and these groups. High
concentrations of SrR (1.0 and 2.0 mmol/l) significantly inhibited
cell proliferation. The results indicated that the application of
SrR on chondrocytes was safer when the concentration is <0.5
mmol/l (Fig. 1).
Effects of SrR on chondrocyte PG
synthesis, hydroxyproline (Hyp) concentration and gene
expression
The chondrocytes were treated with 0, 0.125, 0.25
and 0.50 mmol/l SrR for 14 days and toluidine blue staining
revealed the PG content, which reflects the normal synthesis
function of chondrocytes. The level of Hyp in extracellular fluid
can reversely reflect the degradation of collagen caused by
inflammation or other reasons. PCR tests were performed on days 1,
7 and 14 to determine the gene expression of marker genes.
As shown in Fig.
2A, every SrR group had a darker stain than that of the control
group and the 0.25 and 0.50 mmol/l SrR groups were more intense
than the 0.125 mmol/l group. Fig.
2B shows the hydroxyproline (Hyp) concentration results. A
significantly lower Hyp concentration was found in all SrR groups
compared with the control group at days 7 and 14, which indicated
the protective effect of SrR against collagen degradation. Fig. 2C and D shows the relative gene expression of
aggrecan and Col-II. Significantly higher expression of aggrecan
and Col-II was found in the SrR groups than in the control groups
and higher concentrations of SrR resulted in an improved trend.
Fig. 2E-G presents the gene
expression of β-catenin, MMP-9 and MMP-13 and SrR significantly
suppressed gene expression.
Effects of SrR on chondrocyte Col-II,
aggrecan, β-catenin, MMP-9 and MMP-13 protein synthesis
The chondrocytes were treated with 0, 0.125, 0.25
and 0.50 mmol/l SrR for 14 days, which was followed by
immunofluorescence staining to visualize the location and
concentration of β-catenin, Col-II and MMP-13 protein. The results
showed a clear dose-dependent effect of SrR on promoting
chondrocyte function. β-catenin was highly expressed and located
inside the nucleus in the control group, while SrR treatment
resulted in lighter staining and a significantly lower expression
of β-catenin in the 0.50 mmol/l treatment group (Fig. 3A) compared to that of the control.
The same situation could be seen in Fig. 3C: MMP-13 was positively stained in
cytoplasm around the nucleus and its expression was lower in the
higher concentration group. Col-II located in cytoplasm and
extracellular area and had the opposite expression trend: the
control group had the lowest expression and a higher concentration
of SrR resulted in higher expression (Fig. 3B). Fig. 3D presents the WB results, which
provided a clearer view of the relative quantified protein content.
There was an increasing amount of Col-II and aggrecan in the SrR
groups and β-catenin, MMP-13 and MMP-9 were decreased in the SrR
groups at higher concentrations (Fig.
3E).
Effects of SrR on IL-1β-inflamed
chondrocyte gene and protein expression
To understand whether SrR has an anti-inflammatory
effect, 10 ng/l IL-1β was applied to chondrocyte culture medium and
the cells treated with or without SrR for 3 days. Toluidine blue
chondrocytes staining showed a significant inhibitory effect of
IL-1β on PG synthesis and SrR reversed this effect (Fig. 4A). Fig. 4B, D and E
shows that the relative gene expression of β-catenin, MMP-13 and
MMP-9 had similar tendencies. IL-1β could significantly promote the
expression of MMPs and activate β-catenin. SrR could partially
offset this activation. Fig. 4C
shows that IL-1β also depressed Col-II gene expression and that SrR
slightly increased Col-II expression but was still significantly
lower than that of the control. Fig.
4F shows the western blotting analysis of Col-II, β-catenin,
MMP-13 and MMP-9. The results were consistent with the PCR assay
results showing that Col-II was expressed at lower levels in the
IL-1β group, but it was clear that β-catenin, MMP-9 and MMP-13 were
highly expressed and SrR reversed these trends (Fig. 4G).
Effects of SrR on IL-1β-inflamed
chondrocyte β-catenin, Col-II and MMP-13 protein synthesis
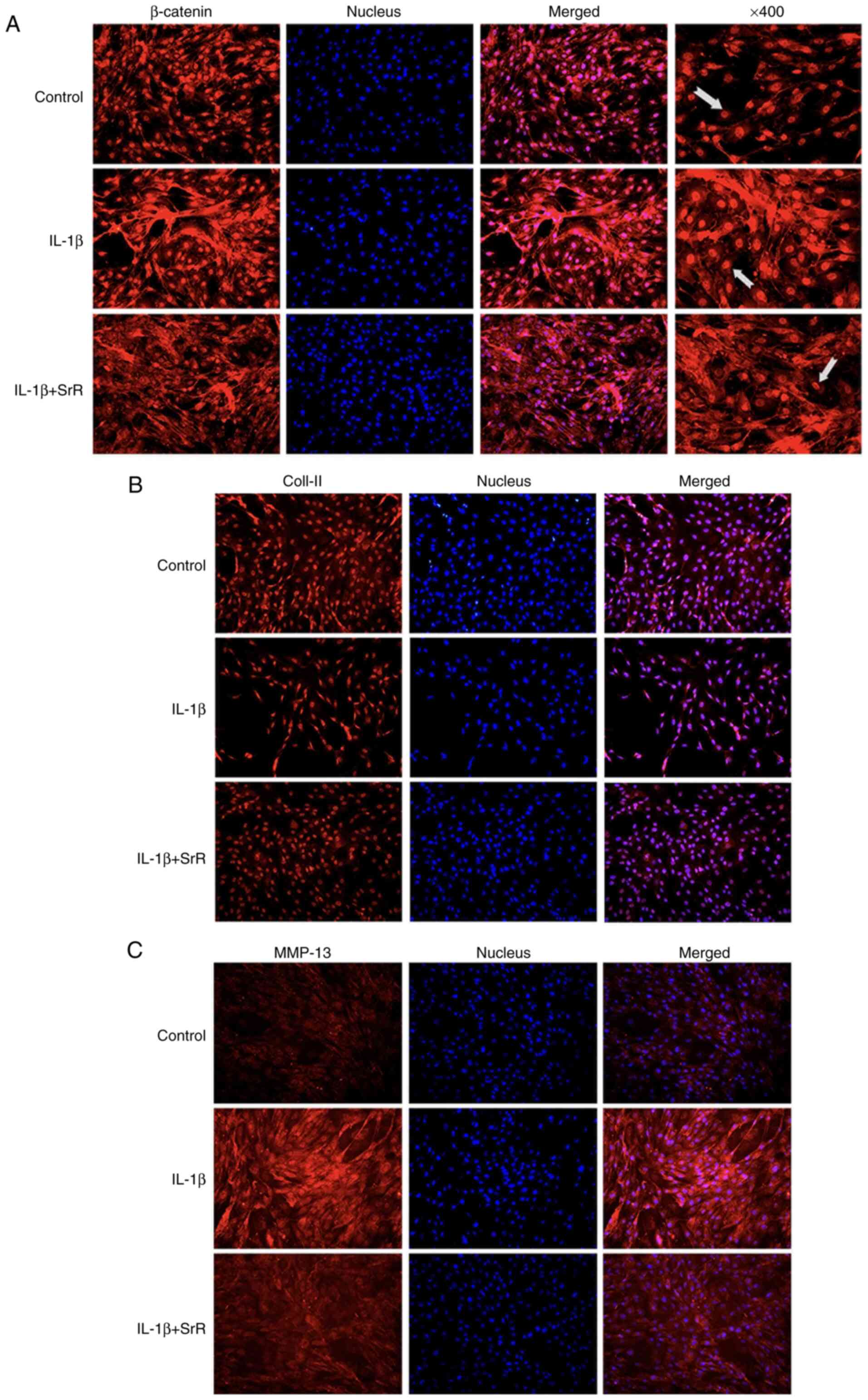
The chondrocytes were treated with IL-1β and 0 or
0.50 mmol/l SrR for 3 days, followed by immunofluorescence
staining. As shown in Fig. 5A,
β-catenin had a strong stain and accumulated inside the nucleus in
the IL-1β group and SrR treatment resulted in slightly shallower
staining, but the staining was still darker compared with the
control. Col-II was light colored in the IL-1β group but gained a
darker stain after being treated with SrR (Fig. 5B). MMP-13 had a remarkably stronger
stain in the IL-1β group and SrR could lower its expression
(Fig. 5C).
Discussion
SrR, a classic anti-osteoporosis drug, has been
gradually recognized to have an anti-osteoarthritis effect and the
scope of its clinical application has gradually expanded in recent
years. The role of SrR in promoting osteogenesis (18,24-26),
chondrogenesis (14,15,18,27)
and angiogenesis (21,28) has been experimentally confirmed,
but its anti-inflammatory effect remains to be elucidated. The
results demonstrated the anti-inflammatory effect of SrR and
evidenced a possible role for the Wnt/β-catenin pathway.
Cartilage tissue is composed of cartilage ECM, which
mostly contains Col type II and PG, with 15-22 and 4-7% of the
cartilage wet weight, respectively (29) and chondrocytes are the only cell
component (3). Cartilage
degradation is directly reflected in the increased expression of
inflammatory factors represented by the MMP family in chondrocytes
(30) and the reduction in ECM
protein synthesis, such as Col-II, PG and aggrecan (31). In the study of the
anti-inflammatory and cartilage stabilizing effects of drugs,
Col-II and PG were used as indicators of cartilage synthesis
function and MMPs were key indicators of inflammation levels. The
results of the present study showed that SrR could significantly
promote the synthesis and secretion of Col-II, PG and aggrecan and
inhibit the expression of MMP-9 and MMP-13 inflammatory factors,
which means that SrR can promote cartilage synthesis and inhibit
cartilage degradation. So is the result of Hyp test, which is a
marker of collagen degradation, that SrR exhibited a protective
effect in a normal environment. These results are consistent with
previous studies, Tat et al (13) treated subchondral osteoblasts or OA
patients with SrR and found that SrR could downregulate the mRNA
transcription levels of MMP-2 and MMP-9 and upregulate the
expression levels of osteoprotegerin (OPG) and RANK-Ligand (RANKL)
to inhibit bone resorption. Similarly, Pelletier et al
(32) demonstrated that SrR can
also downregulate the expression levels of MMP-1, MMP-13 and
cathepsin K, which play an anti-OA role. In addition, Yu et
al (14) showed that SrR could
also increase the synthesis of type II collagen and
chondroproteoglycan by upregulating Sox-9, a marker protein of
cartilage differentiation, to weaken subchondral bone remodeling,
improve local bone microstructure and alleviate articular cartilage
degeneration and poor subchondral bone remodeling (33-35).
At present, researchers define SrR as a possible
DMOAD due to its stabilizing subchondral bone resorption (11,36).
Rodrigues et al (36) noted
in a systematic review that, at present, there is only moderate
clinical evidence to confirm the positive effect of SrR on OA
treatment, especially its anti-inflammatory effect, which needs to
be confirmed by more studies. The results of the present study
clearly demonstrated the role of SrR in protecting chondrocytes in
an environment containing the inflammatory factor IL-1β. IL-1β has
been proven to be a direct pathogenic factor of OA and can trigger
the synthesis of MMPs such as MMP-1, 3, 9 and 13, resulting in
cartilage damage (9,10). Chondrocytes in an environment with
a high level of IL-1β showed a significant reduction in PG and
Col-II synthesis and ECM degradation and a significant increase in
MMP-9 and MMP-13, while SrR partially reversed this trend (Figs. 4 and 5). Henrotin et al (18) employed chondrocytes from healthy
humans and OA patients (relatively high IL-1β expression) and
treated them with SrR. The results also showed strongly stimulated
PG and insulin-like growth factor I synthesis, improved cartilage
matrix synthesis and effective inhibition of MMPs. These results
suggest that SrR has an anti-inflammatory effect at least at the
chondrocyte level.
The present study investigated whether the
Wnt/β-catenin pathway, especially β-catenin, was involved in the
anti-inflammatory effect of SrR. The Wnt/β-catenin pathway serves a
crucial role in chondrocyte proliferation, differentiation and
apoptosis (37) and aggravates OA
by promoting MMPs (38).
Wnt/β-catenin signaling in human chondrocytes has an unexpected
anticatabolic role by counteracting NF-κB-mediated MMP expression
induced by IL-1β in a negative feedback loop (39). A previous study has shown that in
animals, IL-1β indirectly activates canonical Wnt signaling by
upregulating Wnt ligands (40).
Subsequently, the transcription complex of β-catenin with TCF/lEF
induces the expression of MMP-3 and MMP-13, leading to cartilage
destruction (41). On the other
hand, activation of the Wnt/β-catenin pathway may lead to abnormal
osteogenesis but depress chondrogenesis (42). The reciprocal inhibitory effect
between β-catenin and Sox-9 was observed by Akiyama et al
(43), in which Sox-9 represses
β-catenin/Tcf/lef complex activities and shows an inhibitory effect
on β-catenin when the cells were in the chondrogenic
differentiation trend but a controversial trend when osteogenesis
was dominant. The upregulation of β-catenin led to the inhibition
of Col-II and PG synthesis. Taken together, cartilage degradation
is correlated to the promotion of β-catenin activity. The present
study showed that SrR could inhibit β-catenin synthesis and
accumulation. In normal chondrocytes, β-catenin and MMP synthesis
decreased with increasing SrR concentration (≤0.50 mmol/l). The
addition of IL-1β significantly activated β-catenin expression and
SrR attenuated the increase in β-catenin induced by IL-1β. However,
further studies with more bio-markers, including in vivo
experiments, are required to further determine the mechanism and
confirm the role of β-catenin in the anti-inflammatory effect of
SrR.
SrR has specific characteristics, such as a regular
effect on the RANK/RANKL/OPG system that promotes bone formation
and inhibits bone resorption (44), increases the vascularization of new
bone tissue (21), encourages
chondrogenesis in cartilage tissue (35) and reduces the expression of
inflammatory factors. All these results made SrR a promising drug
for DMOADs. However, more reliable evidence, especially
well-designed clinical trials, is needed to confirm the anti-OA
effects.
In conclusion, SrR decreased MMPs but promoted
Col-II, aggrecan and PG synthesis in rat chondrocytes with or
without the presence of IL-1β and SrR attenuated the increase in
β-catenin induced by IL-1β, thus reducing the inflammatory
reaction.
Supplementary Material
Immunofluorescence staining assay.
Chondrocytes were positively stained with Col-II and aggrecan, but
negatively with Col-I and 11-fibrau. The images were captured under
x200 magnification. Col, collagen.
Acknowledgements
The authors would like to thank Dr Xinxin Han and Dr
Shangfeng Liu (Shanghai Key Laboratory of Craniomaxillofacial
Development and Diseases, Fudan University, Shanghai, China), and
Mr. Xiaolong Feng and Mr. Longyu Li (Shanghai Research Centre of
Model Organisms, Shanghai, China) for the assistance and execution
of the experiments.
Funding
Funding: The authors acknowledge the financial support from the
Shanghai Commission of Science and Technology (grant no.
19YF1442400).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HY performed most of the cell molecular biological
tests. YL and XY helped HY with the primary chondrocytes cell
isolation and culture. JH performed the statistical analysis. QZ
was responsible for the study design and revised the manuscript. XG
performed the study design, statistical analysis and wrote the
manuscript. XG and QZ confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participant
All experiments involving animals were approved by
the Animal Research Committee of the Shanghai Stomatological
Hospital and Shanghai Research Center of Model Animal Organization
(IACUC No. 2020-0010-06).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Joseph R, Rahena A, Hassan N, Glen H,
James W and Soichiro I: Epidemiology of temporomandibular disorder
in the general population: A systematic review. Adv Dent Oral
Health. 10(555787)2019.
|
|
2
|
Wang XD, Zhang JN, Gan YH and Zhou YH:
Current understanding of pathogenesis and treatment of TMJ
osteoarthritis. J Dent Res. 94:666–673. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Charlier E, Deroyer C, Ciregia F, Malaise
O, Neuville S, Plener Z, Malaise M and de Seny D: Chondrocyte
dedifferentiation and osteoarthritis (OA). Biochem Pharmacol.
165:49–65. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wojdasiewicz P, Poniatowski ŁA and
Szukiewicz D: The role of inflammatory and anti-inflammatory
cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm.
2014(561459)2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Loeser RF: Molecular mechanisms of
cartilage destruction: Mechanics, inflammatory mediators, and aging
collide. Arthritis Rheum. 54:1357–1360. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Johnson CI, Argyle DJ and Clements DN: In
vitro models for the study of osteoarthritis. Vet J. 209:40–49.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Samavedi S, Diaz-Rodriguez P, Erndt-Marino
JD and Hahn MS: A three-dimensional chondrocyte-macrophage
coculture system to probe inflammation in experimental
osteoarthritis. Tissue Eng Part A. 23:101–114. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gu YT, Chen J, Meng ZL, Ge WY, Bian YY,
Cheng SW, Xing CK, Yao JL, Fu J and Peng L: Research progress on
osteoarthritis treatment mechanisms. Biomed Pharmacother.
93:1246–1252. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Xia B, Chen D, Zhang J, Hu S, Jin H and
Tong P: Osteoarthritis pathogenesis: A review of molecular
mechanisms. Calcif Tissue Int. 95:495–505. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Grässel S and Muschter D: Recent advances
in the treatment of osteoarthritis. F1000Res. 9(325)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pelletier JP, Roubille C, Raynauld JP,
Abram F, Dorais M, Delorme P and Martel-Pelletier J:
Disease-modifying effect of strontium ranelate in a subset of
patients from the phase III knee osteoarthritis study SEKOIA using
quantitative MRI: Reduction in bone marrow lesions protects against
cartilage loss. Ann Rheum Dis. 74:422–429. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tenti S, Cheleschi S, Guidelli GM,
Galeazzi M and Fioravanti A: What about strontium ranelate in
osteoarthritis? Doubts and securities. Mod Rheumatol. 24:881–884.
2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tat SK, Pelletier JP, Mineau F, Caron J
and Martel-Pelletier J: Strontium ranelate inhibits key factors
affecting bone remodeling in human osteoarthritic subchondral bone
osteoblasts. Bone. 49:559–567. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yu DG, Ding HF, Mao YQ, Liu M, Yu B, Zhao
X, Wang XQ, Li Y, Liu GW, Nie SB, et al: Strontium ranelate reduces
cartilage degeneration and subchondral bone remodeling in rat
osteoarthritis model. Acta Pharmacol Sin. 34:393–402.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yu H, Liu Y, Yang X, He J, Zhang F, Zhong
Q and Guo X: Strontium ranelate promotes chondrogenesis through
inhibition of the Wnt/β-catenin pathway. Stem Cell Res Ther.
12(296)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sun Y, Zhou L, Lv D, Liu H, He T and Wang
X: Poly(ADP-ribose) polymerase 1 inhibition prevents
interleukin-1β-induced inflammation in human osteoarthritic
chondrocytes. Acta Biochim Biophys Sin (Shanghai). 47:422–430.
2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Alves SM, Abreu SC, Lemos JC, Gomes FI,
Alves SM, do Val DR, Freitas RS, Pereira KM, de Paulo Teixeira
Pinto V, de Castro Brito GA, et al: Anti-inflammatory and
anti-nociceptive effects of strontium ranelate on the
zymosan-induced temporomandibular joint inflammatory
hypernociception in rats depend on TNF-α inhibition. Pharmacol Rep.
69:764–772. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Henrotin Y, Labasse A, Zheng SX, Galais P,
Tsouderos Y, Crielaard JM and Reginster JY: Strontium ranelate
increases cartilage matrix formation. Bone Miner Res. 16:299–308.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang L, Lazebnik M and Detamore MS:
Hyaline cartilage cells outperform mandibular condylar cartilage
cells in a TMJ fibrocartilage tissue engineering application.
Osteoarthritis Cartilage. 17:346–353. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
National Research Council. Guide for the
care and use of laboratory animals: Eighth edition. Washington, DC:
The National Academies Press, 2011. https://doi.org/10.17226/12910.
|
|
21
|
Guo X, Wei S, Lu M, Shao Z, Lu J, Xia L,
Lin K and Zou D: Dose-dependent effects of strontium ranelate on
ovariectomy rat bone marrow mesenchymal stem cells and human
umbilical vein endothelial cells. Int J Biol Sci. 12:1511–1522.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
da Silva CM, Spinelli E and Rodrigues SV:
Fast and sensitive collagen quantification by alkaline
hydrolysis/hydroxyproline assay. Food Chem. 173:619–623.
2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Aimaiti A, Maimaitiyiming A, Boyong X, Aji
K, Li C and Cui L: Low-dose strontium stimulates osteogenesis but
high-dose doses cause apoptosis in human adipose-derived stem cells
via regulation of the ERK1/2 signaling pathway. Stem Cell Res Ther.
8(282)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mao Z, Fang Z, Yang Y, Chen X, Wang Y,
Kang J, Qu X, Yuan W and Dai K: Strontium ranelate-loaded PLGA
porous microspheres enhancing the osteogenesis of MC3T3-E1 cells.
RSC Adv. 7:24607–24615. 2017.
|
|
26
|
Pilmane M, Salma-Ancane K, Loca D, Locs J
and Berzina-Cimdina L: Strontium and strontium ranelate: Historical
review of some of their functions. Mater Sci Eng C Mater Biol Appl.
78:1222–1230. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Deepthi S, Abdul Gafoor AA, Sivashanmugam
A, Nair SV and Jayakumar R: Nanostrontium ranelate incorporated
injectable hydrogel enhanced matrix production supporting
chondrogenesis in vitro. J Mater Chem B. 4:4092–4103.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Pan FY, Li ZM, Liu XW, Luo Y, Ma Z, Feng
SX and Xu N: Effect of strontium ranelate on rabbits with
steroid-induced osteonecrosis of femoral head through TGF-β1/BMP2
pathway. Eur Rev Med Pharmacol Sci. 24:1000–1006. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jackson A and Gu W: Transport properties
of cartilaginous tissues. Curr Rheumatol Rev. 5(40)2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Troeberg L and Nagase H: Proteases
involved in cartilage matrix degradation in osteoarthritis. Biochim
Biophys Acta. 1824:133–145. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu-Bryan R and Terkeltaub R: Emerging
regulators of the inflammatory process in osteoarthritis. Nat Rev
Rheumatol. 11:35–44. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pelletier JP, Kapoor M, Fahmi H,
Lajeunesse D, Blesius A, Maillet J and Martel-Pelletier J:
Strontium ranelate reduces the progression of experimental dog
osteoarthritis by inhibiting the expression of key proteases in
cartilage and of IL-1β in the synovium. Ann Rheum Dis. 72:250–257.
2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lefebvre V, Behringer RR and de
Crombrugghe B: L-Sox5, Sox6 and Sox9 control essential steps of the
chondrocyte differentiation pathway. Osteoarthritis Cartilage. 9
(Suppl A):S69–S75. 2001.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Oh CD, Lu Y, Liang S, Mori-Akiyama Y, Chen
D, de Crombrugghe B and Yasuda H: SOX9 regulates multiple genes in
chondrocytes, including genes encoding ECM proteins, ECM
modification enzymes, receptors and transporters. PLoS One.
9(e107577)2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kim HJ and Im GI: Electroporation-mediated
transfer of SOX trio genes (SOX-5, SOX-6, and SOX-9) to enhance the
chondrogenesis of mesenchymal stem cells. Stem Cells Dev.
20:2103–2114. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Rodrigues TA, Freire AO, Bonfim BF,
Cartágenes MS and Garcia JB: Strontium ranelate as a possible
disease-modifying osteoarthritis drug: A systematic review. Braz J
Med Biol Res. 51(e7440)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Sassi N, Laadhar L, Allouche M, Achek A,
Kallel-Sellami M, Makni S and Sellami S: WNT signaling and
chondrocytes: From cell fate determination to osteoarthritis
physiopathology. J Recept Signal Transduct Res. 34:73–80.
2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Xia H, Cao D and Yang F, Yang W, Li W, Liu
P, Wang S and Yang F: Jiawei Yanghe decoction ameliorates cartilage
degradation in vitro and vivo via Wnt/β-catenin signaling pathway.
Biomed Pharmacother. 122(109708)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ma B, van Blitterswijk CA and Karperien M:
A Wnt/β-catenin negative feedback loop inhibits
interleukin-1-induced matrix metalloproteinase expression in human
articular chondrocytes. Arthritis Rheum. 64:2589–2600.
2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yoshida Y, Yamasaki S, Oi K, Kuranobu T,
Nojima T, Miyaki S, Ida H and Sugiyama E: IL-1β enhances Wnt signal
by inhibiting DKK1. Inflammation. 41:1945–1954. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yun K and Im SH: Transcriptional
regulation of MMP13 by Lef1 in chondrocytes. Biochem Biophys Res
Commun. 364:1009–1014. 2007.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C,
Rosier RN, O'Keefe RJ, Zuscik M and Chen D: Activation of
beta-catenin signaling in articular chondrocytes leads to
osteoarthritis-like phenotype in adult beta-catenin conditional
activation mice. J Bone Miner Res. 24:12–21. 2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Akiyama H, Lyons JP, Mori-Akiyama Y, Yang
X, Zhang R, Zhang Z, Deng JM, Taketo MM, Nakamura T, Behringer RR,
et al: Interactions between Sox9 and beta-catenin control
chondrocyte differentiation. Genes Dev. 18:1072–1087.
2004.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Han W, Fan S, Bai X and Ding C: Strontium
ranelate, a promising disease modifying osteoarthritis drug. Expert
Opin Investig Drugs. 26:375–380. 2017.PubMed/NCBI View Article : Google Scholar
|