Introduction
A spinal cord injury (SCI) can lead to the
deterioration of various physiological functions, especially
bladder function (1). After an
SCI, nerve impulses from the bladder and sphincter do not reach the
brain, and cannot be felt by the affected individual. This type of
bladder dysfunction is referred to a neurogenic bladder (NB)
(2,3). A significant pathological feature of
NB is detrusor fibrosis, which occurs when a large number of
collagen fibers are deposited between the muscle bundles (4). This collage deposition leads to
detrusor contracture, detrusor weak contraction, bladder storage
and emptying disorders, upper urinary tract damage and serious
complications, such as renal failure (5,6).
The treatment strategies for SCI-induced NB include
psychotherapy, electrical stimulation, chemotherapy, intermittent
catheterization and surgery (7,8).
Bladder fibrosis has been reported to occur over time in patients
with NB (9), suggesting that
fibrosis is an important factor affecting the outcome of therapy.
Studies have shown that both TGF-β1 and basic fibroblast growth
factor (bFGF) are important for the differentiation and
proliferation of smooth muscle cells, and activation of these
signaling pathways contributes to fibrosis and sclerosis of the
bladder wall after an SCI (10-12).
Some researchers have found that microRNA-101b downregulates the
TGF-β signaling pathway by inhibiting TGF-β receptor 1, thereby
inhibiting fibrosis. These results indicate that TGF-β1 is involved
in the fibrosis of bladder tissue. An in vitro study showed
that bFGF upregulates the expression of Collagen I (13). However, the mechanism underlying NB
fibrosis is not fully understood.
Current treatment strategies for bladder fibrosis
include the use of antifibrotic drugs, such as relaxin (14), and the application of stem cell and
gene therapy (15,16); however, none of these treatments
have been effective. Therefore, developing additional therapies for
NB is important. Telmisartan is a new type of antihypertensive drug
and a specific angiotensin II receptor antagonist (17). A recent study has shown that
angiotensin II can promote cardiac fibrosis by binding to Ang II
type I receptors and further promoting the synthesis of
TGF-β1(18). However, no clinical
guidelines have recommended the use of telmisartan for the
treatment of NB. In the present study, the L6-S1 spinal nerves of
rats were bilaterally dissected to construct an NB rat model to
observe the effects of telmisartan on maximum cystometric capacity,
residual urine volume, bladder wet weight, bladder compliance,
detrusor pressure and fibrosis-related gene expression. The results
of the present study may provide rationale for using telmisartan
during the early treatment of NB.
Materials and methods
Experimental animals and
groupings
To rule out potential confounding effects, only 30
adult male Sprague-Dawley rats (weight, 260±10 g; age, 13 weeks)
were obtained from Shanghai Sippr-BK Laboratory Animal Co. Ltd. The
rats were housed together in a room maintained at 40-60% relative
humidity and 23±2˚C with 12-h light/dark cycles and ad
libitum access to food and water. The rats were randomly
assigned to the following five groups (n=6 per group): i) Sham
treatment (epidural exposure only); ii) spinal cord transection
treatment; iii) combined treatment with 0.5 mg/kg/day telmisartan
(cat. no. S1738; Selleck Chemicals) administered by gavage for 14
consecutive days, plus spinal cord transection; iv) combined
treatment with 3 mg/kg/day telmisartan administered by gavage for
14 consecutive days, plus spinal cord transection; and v) combined
treatment with 6 mg/kg/day telmisartan administered by gavage for
14 consecutive days, plus spinal cord transection. The sample size
was determined using GPower software (version 3.1.9, University of
Dusseldorf, Dusseldorf, Germany). The health and behavior of the
animals were monitored every 2 days. After cystometric analysis at
the 3rd week or if a humane endpoint, including listlessness or
cessation of eating or drinking cessation, was reached, the rats
were euthanized by the intraperitoneal injection of sodium
pentobarbital (200 mg/kg body weight) and decapitation. All
experimental protocols were approved by the Institutional Animal
Care and Use Committee of Shandong University Hospital [approval
no. KYLL-2021(LW)013]. All procedures were performed in compliance
with guidelines issued by the National Institutes of Health.
NB model preparation
After 1 week of adaptation, the rats were
anesthetized and fixed on an operating table in the prone position.
A 2 cm median incision was made in the back skin covering the L6-S1
region. The lamina at the L6-S1 level was removed and the spinal
cord was completely severed. In the Sham group, the spinal cord was
only exposed and not transected. A gelatin sponge (Ethicon, Inc.)
was placed between the severed ends to stop bleeding and prevent
healing. Next, the muscle layer and skin layer were separately
sutured. After surgery, ampicillin sodium (100 mg/kg; cat. no.
S3170; Selleck Chemicals) was intramuscularly injected once a day
for 5 consecutive days.
Cystometric analysis
For evaluation of bladder function by cystometry,
the rats were anesthetized by inhalation of 1.5-2.0% isoflurane
(cat. no. R510-22; RWD Life Science Inc.) for maintenance and
induction at 14 days after the operation. A midline abdominal
incision was made and a catheter was inserted via the bladder dome.
The catheter was connected to a dual-channel syringe pump (cat. no.
HK-400A; Shenzhen Hawk Medical Instrument Co., Ltd.) and a pressure
transducer in a urodynamic measurement system (Dantec Menuet).
Cystometry was performed as described in a previous study (19). The following parameters were
measured: Maximum cystometric capacity, residual urine volume,
bladder wet weight, bladder compliance and detrusor pressure.
Masson and H&E staining
The rat bladder was cut from the bladder neck, and
the connective tissue surrounding the bladder wall was removed.
Next, the bladder tissues were fixed with 4% paraformaldehyde (cat.
no. P0099; Beyotime Institute of Biotechnology) at room temperature
(RT) for 24 h, embedded in paraffin and sectioned into 3-mm thick
sections. For Masson staining, the paraffin-embedded sections were
deparaffinized with an alcohol gradient and xylene, and then washed
with distilled water. The sections were then stained with
hematoxylin (cat. no. S2384; Selleck Chemicals) at RT for 5 min,
washed with tap water, immersed in a 1% hydrochloric acid alcohol
solution for several seconds and rinsed with running water for
several minutes. Next, the sections were stained with ponceau red
dye (cat. no. S4497; Selleck Chemicals) at RT for 5-10 min; after
which, the tissues were rinsed with distilled water and an aqueous
phosphomolybdic acid (Electron Microscopy Sciences) solution for
3-5 min. The sections were then treated with aniline blue solution
(cat. no. A9540; Beijing Solarbio Science & Technology Co.,
Ltd.) at RT for 5 min, followed by treatment with 1% glacial acetic
acid (Guangzhou Jinhuada Chemical Reagent Co., Ltd.) for 1 min.
Finally, the sections were dehydrated using an alcohol gradient and
xylene, and then sealed with neutral gum (cat. no. N116470-100 g,
Shanghai Aladdin Biochemical Technology Co., Ltd.). Blue collagen
fibers, red muscle fibers, red cellulose and red blood cells were
observed under a microscope (Olympus Corporation). For H&E
staining, the slides were immersed in hematoxylin at RT for 30 sec,
rinsed with running water until transparent, stained with eosin at
RT for 30 sec and then rinsed again with water. The slides were
then air-dried at RT. Subsequently, the slides were sequentially
immersed twice in 95% ethanol solution, twice in 100% ethanol,
twice in a solution of 50% ethanol and 50% xylene and twice in 100%
xylene. The slides were then observed under a light microscope
(Olympus Corporation).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using TRIzol®
reagent (cat. no. 15596018; Thermo Scientific, Inc.) according to
the manufacturer's instructions. The amount of RNA was quantified
by spectrophotometry. Subsequently, reverse transcription was
performed using the Bestar™ qPCR RT kit according to the
manufacturer's protocol (cat. no. 2220; DBI Bioscience), followed
by qPCR that was performed by using Bestar™ qPCR MasterMix (cat.
no. 2043; DBI Bioscience, Shanghai, China) on an Mx3000P qPCR
instrument (Stratagene; Agilent Technologies, Inc.). The following
thermocycling conditions were used for qPCR: Melting at 95˚C for 2
min; followed by 40 cycles of denaturation at 94˚C for 20 sec,
annealing at 58˚C for 20 sec, elongation at 72˚C for 20 sec and
72˚C complete elongation for 5 min. The primers used for qPCR were
synthesized by Sangon Biotech Co., Ltd. and are listed in Table I. mRNA expression levels were
quantified using the 2-ΔΔCq method (20) and normalized to the internal
reference gene GAPDH.
 | Table ISequences of primers used for reverse
transcription-quantitative PCR. |
Table I
Sequences of primers used for reverse
transcription-quantitative PCR.
| Gene | Sequence (5'-3') |
|---|
| H-GAPDH | F:
TGTTCGTCATGGGTGTGAAC |
| | R:
ATGGCATGGACTGTGGTCAT |
| H-bFGF | F:
AGAAGAGCGACCCTCACATCA |
| | R:
CGGTTAGCACACACTCCTTTG |
| H-TGF-β1 | F:
GGCCAGATCCTGTCCAAGC |
| | R:
GTGGGTTTCCACCATTAGCAC |
| H-α-SMA | F:
AAAAGACAGCTACGTGGGTGA |
| | R:
GCCATGTTCTATCGGGTACTTC |
| H-Collagen I | F:
GAGGGCCAAGACGAAGACATC |
| | R:
CAGATCACGTCATCGCACAAC |
| H-Collagen III | F:
GGAGCTGGCTACTTCTCGC |
| | R:
GGGAACATCCTCCTTCAACAG |
Western blotting
Freshly harvested bladders were homogenized in RIPA
lysis buffer (cat. no. P0013C; Beyotime Institute of Biotechnology)
and the amount of soluble protein in each homogenate was quantified
using a BCA Protein Assay kit (cat. no. 23225; Thermo Fisher
Scientific, Inc.). Subsequently, soluble protein (30 µg) was
separated by 10% SDS-PAGE and transferred nitrocellulose membranes.
Following blocking with 5% skimmed milk for 1 h at RT, the
membranes were incubated overnight at 4˚C with the following
primary rabbit antibodies: Anti-bFGF (1:1,000; cat. no. F3393;
Sigma-Aldrich; Merck KGaA), anti-TGF-β1 (1:1,000; cat. no. 3711;
Cell Signaling Technology, Inc.), anti-α-SMA (1:1,000; cat. no.
19245; Cell Signaling Technology, Inc.), anti-Collagen I (1:1,000;
cat. no. ab255809; Abcam), anti-Collagen III (1:1,000; cat. no.
ab7778; Abcam) and anti-GAPDH (1:1,000; cat. no. 5174; Cell
Signaling Technology, Inc.). The membranes were then incubated with
a horseradish peroxidase-labeled goat anti-rabbit antibody
(1:2,000; cat. no. SA00001-2; ProteinTech Group, Inc.) at RT for 2
h. The protein bands were visualized with a chemiluminescent
development agent (Chemistar™ High-sig ECL Western Blotting
Substrate; Tanon Science & Technology Co., Ltd.). Protein
expression was semi-quantified using GAPDH as the loading control
by Image J software (2.0; National Institutes of Health).
Immunohistochemistry (IHC)
The paraffin-embedded sections were prepared
according to the protocol described in the ‘Masson and H&E
staining’ section. After deparaffinization and hydration, the
paraffin-embedded sections were placed in a microwave for antigen
retrieval at 120˚C for 20 min in an autoclave, followed by washing
with xylene and gradual rehydration in graded ethanol. Then, the
sections were blocked with 3% H2O2 at RT for
15 min, and then further blocked with PBS containing 3% BSA (cat.
no. ST023; Beyotime Institute of Biotechnology) at RT for 30 min.
Next, the sections were incubated overnight at 4˚C with the
following rabbit antibodies: Anti-rat α-SMA (1:150; cat. no. 41550;
Signalway Antibody LLC), anti-rat Collagen I (1:200; cat. no.
ab270993; Abcam) and anti-rat Collagen III (1:100; cat. no. ab7778;
Abcam). Subsequently, the sections were incubated with a
horseradish peroxidase-labeled goat anti-rabbit secondary antibody
(1:4,000; cat. no. ab205718; Abcam) for 1 h at RT. The sections
were developed using a DAB reagent kit (cat. no. DAB-1031; Fuzhou
Maixin Biotech Co., Ltd.) for ~15 min, and then counterstained with
hematoxylin for 4 min at RT. After three washes with PBS, the
sections were mounted onto slides with neutral gum and then
observed under a CX43 light microscope (Olympus Corporation). Brown
particles were regarded as positive staining. The number of
positive cells per high power field was counted using Image J
software (2.0; National Institutes of Health).
Statistical analysis
All statistical analyses were performed using
GraphPad Prism software (version 9.00; GraphPad Software, Inc.).
All experiments were repeated 3 times and data are presented as the
mean ± standard deviation. Comparisons among multiple groups were
analyzed using one-way ANOVA followed by Tukey's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
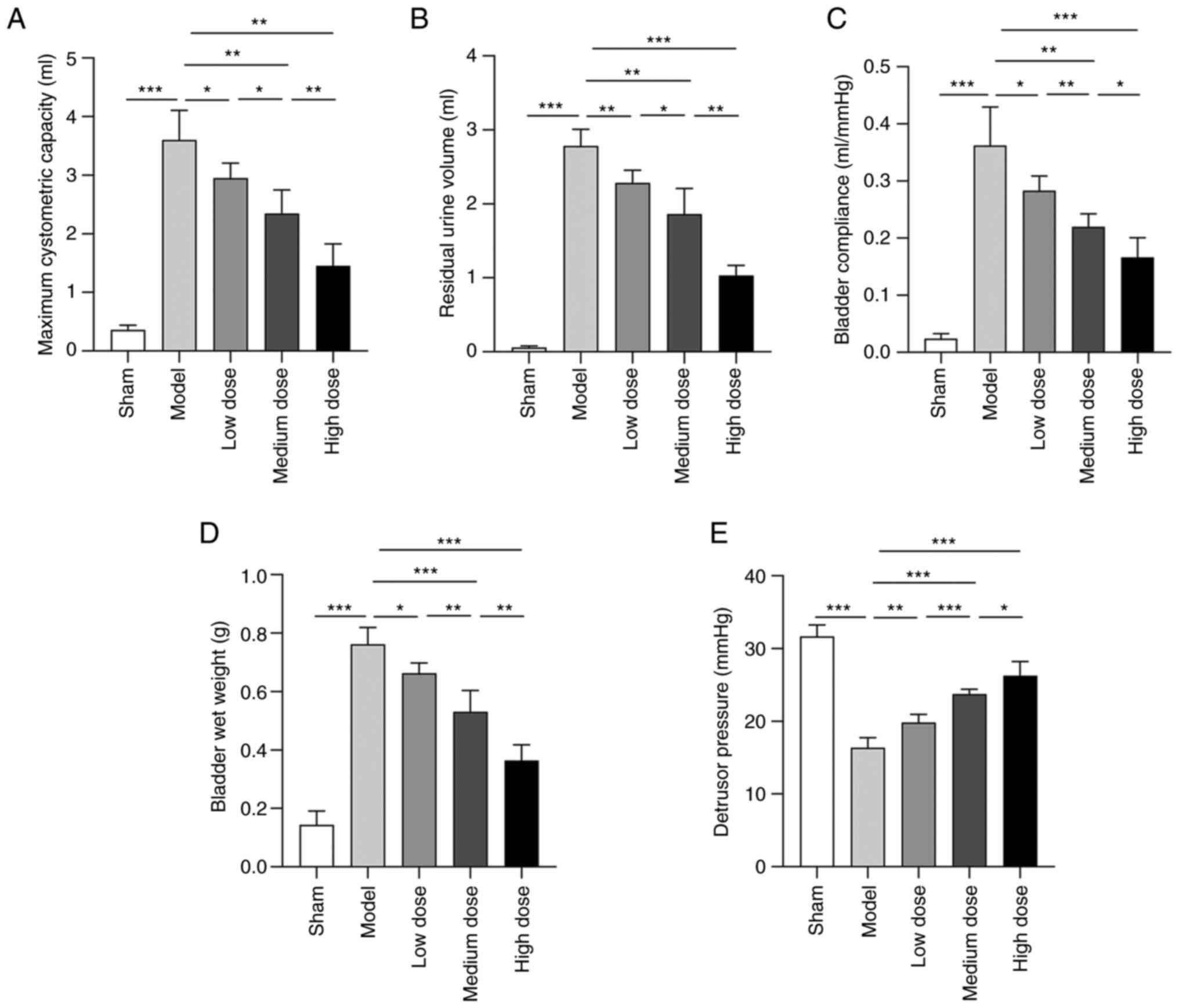
Impaired bladder function in rats
receiving spinal cord transection is improved by telmisartan
After spinal cord transection, the cystometry test
results were altered; rats in the model group displayed
significantly higher bladder compliance, increased maximum
cystometric capacity, increased residual urine volume, increased
bladder wet weight and decreased detrusor pressure compared with
that in the sham group (Fig. 1).
After treatment with telmisartan, the values of the aforementioned
parameters were significantly recovered, and the degree of recovery
increased in a dose-dependent manner.
Disrupted bladder structure in rats
receiving spinal cord transection is restored by telmisartan
The antifibrotic effect of telmisartan was evaluated
b(1)y comparing changes in the
content of collagen fibers and smooth muscle fibers in the bladder
wall as determined by Masson and H&E staining. The Masson
staining results demonstrated that the bladder walls of rats that
received spinal cord transection had disordered fibrous connective
tissue, a distorted layered structure, increased thickness, reduced
lamina propria, smooth muscle hypertrophy and increased numbers of
intermuscular fibers compared with the sham group (Fig. 2A). Moreover, the H&E staining
results showed that the bladder detrusor cells in the sham group
had a long spindle shape and were uniformly distributed,
structurally tight and arranged in parallel (Fig. 2B). Compared with the sham group,
rats that received spinal cord transection showed thickened bladder
propria, hypertrophic and disordered detrusor cells, decreased
numbers of muscle cells and increased amounts of intermuscular
connective tissue. Telmisartan treatment relieved bladder tissue
fibrosis and detrusor cell hypertrophy in rats that received spinal
cord transection.
Increased fibrosis-related gene
expression in rats receiving spinal cord transection is rescued by
telmisartan
Western blotting and RT-qPCR were used to
investigate changes in fibrosis-related gene expression. The
results showed significantly increased expression levels of bFGF,
TGF-β1, Collagen I, Collagen III and α-SMA expression in the smooth
muscle cells of rat bladder tissue after spinal cord transection
compared with those in the sham group (Fig. 3). Furthermore, telmisartan reversed
the effects of spinal cord transection on the expression of
fiber-related genes to various degrees. The IHC results showed the
expression of target proteins in tissue in situ. The results
demonstrated that the expression of Collagen I, Collagen III and
α-SMA was significantly increased after spinal cord transection
compared with that in the sham group, whereas telmisartan treatment
limited these effects (Fig.
4).
 | Figure 3bFGF, TGF-β, α-SMA, Collagen I, and
Collagen III mRNA and protein expression levels in each group of
rats. (A) Reverse transcription-quantitative PCR detection of bFGF,
TGF-β, α-SMA, Collagen I and Collagen III mRNA expression levels.
bFGF, TGF-β, α-SMA, Collagen I and Collagen III protein expression
levels were (B) determined by western blotting and (C)
semi-quantified. *P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001. bFGF,
basic fibroblast growth factor; α-SMA, α-smooth muscle actin; ns,
not significant. |
Discussion
An impairment of normal nervous system function that
occurs due to an injury can be difficult to recover and affects the
function of target organs, such as the bladder detrusor muscle
(1). Although there is a link
between hypertension and abnormal bladder function (21), to the best of our knowledge, the
effect of ARB drugs on NB after an SCI has not been reported. The
present study showed that treatment with telmisartan significantly
reduced maximum cystometric capacity, residual urine volume,
bladder wet weight and bladder compliance, and increased detrusor
pressure in NB model rats. Telmisartan treatment also inhibited
bladder tissue fibrosis and decreased the expression levels of
bFGF, TGF-β1, Collagen I, Collagen III and α-SMA. Therefore, the
present study provided supporting evidence for the use of
telmisartan in treating NB after an SCI.
Current reports concerning changes that occur in
bladder compliance after an SCI are inconsistent (22-24).
A previous study showed that the bladder compliance of rats
increased at 4 weeks after an SCI, but later decreased at 8 weeks
after the SCI (25). This
suggested that with a prolonged injury time, bladder compliance
initially increases and then decreases. A potential explanation
might be that in the early stage of injury, the bladder detrusor
muscle had a low degree of fibrosis and only a small amount of
collagen deposition. This allows for a compensatory enlargement of
the bladder, which increases bladder capacity, lowers detrusor
pressure and increases bladder compliance. Due to the long injury
time, the bladder detrusor muscle may have become hypertrophic,
fibrotic and degenerated, leading to a high degree of detrusor
fibrosis, increased numbers of collagen fibers and decreased
numbers of elastic fibers. These changes may result in a
significant increase in detrusor muscle pressure and a gradual
decrease in bladder compliance, which could provide an explanation
for the results obtained in the present study.
It is generally believed that bladder wall fibrosis
plays an important role in NB (26). In the present study, the Masson and
H&E staining results showed thickening of the bladder wall,
disordered and hypertrophic detrusor cells, and proliferative
collagen fibers in NB model rats. However, the short duration of
the SCIs in the present study allowed for compensatory increases in
maximum cystometric capacity and decreases in detrusor pressure.
The collagen fibers in the bladder wall are primarily type 1 and 3,
and type 3 collagen is the determinant of bladder compliance
(27). The present study indicated
that the expression levels of Collagen I and Collagen III in the
bladder detrusor muscles in the model group were not significantly
increased, thus bladder compliance did not decrease. In addition,
the protein expression levels of bFGF and TGF-β1 in the model group
were significantly higher compared with those in the sham group,
indicating that the TGF-β1 signaling pathway was activated in the
NB model rats, leading to bladder fibrosis. Previous studies have
shown that angiotensin II can promote cardiac fibrosis by binding
to AngII type I receptors and further promoting the synthesis of
TGF-β1 (28-30).
Telmisartan is a specific angiotensin II receptor antagonist. We
hypothesized that telmisartan might have antifibrotic effects,
which was confirmed by our experimental results. Therefore, the
effect of the angiotensin II pathway on the TGF-β pathway requires
further investigation.
The Masson staining results showed that the bladder
smooth muscles of rats that received spinal cord transection were
hypertrophic and thickened, and displayed increased numbers of
collagen fibers. We speculated that smooth muscle hypertrophy and
thickening might be related to increased pressure in the bladder.
High pressure is a harmful stimulus to smooth muscle cells, and may
activate certain protein kinases in the cells and initiate abnormal
cell proliferation (31,32). This change is similar to that
observed during liver fibrosis, as a previous study showed that
pressure activates protein kinases, regulates gene transcription
and initiates cell proliferation (33).
The present study had a number of limitations.
Firstly, the experiments were conducted at 14 days after an SCI,
which is at an early stage. Secondly, only the effects of three
selected telmisartan doses were assessed, thus the dose-response
relationship for further doses of telmisartan should be
investigated in future studies. Thirdly, NB has several subtypes,
meaning the results of the present study should be verified in
further NB subtypes. Therefore, it is not clear whether the results
of the present study can be directly applied to human NB. Finally,
additional human bladder tissue samples should be analyzed to
confirm the present findings. However, the present study suggested
that potential strategies for preventing bladder fibrosis should be
implemented as soon as possible for the treatment of NB, and
telmisartan may serve as a useful therapeutic drug.
Acknowledgements
Not applicable.
Funding
Funding: This study was funded by the Jinan Science and
Technology Development Plan (grant no. 201907074).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WC and QL conceived and designed the experiments.
QL, RW and NM performed the experiments. CW analyzed the data. QL
wrote the first draft. WC made the amendments and provided
financial support. All authors read and approved the final
manuscript. WC and QL confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
All experimental protocols were performed according
to guidelines developed by the National Institutes of Health and
were approved by the Institutional Animal Care and Use Committee of
Hospital of Shandong University [approval no. KYLL-2021 (LW)
013].
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Hamid R, Averbeck MA, Chiang H, Garcia A,
Al Mousa RT, Oh SJ, Patel A, Plata M and Del Popolo G: Epidemiology
and pathophysiology of neurogenic bladder after spinal cord injury.
World J Urol. 36:1517–1527. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Przydacz M, Chlosta P and Corcos J:
Recommendations for urological follow-up of patients with
neurogenic bladder secondary to spinal cord injury. Int Urol
Nephrol. 50:1005–1016. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shang Z, Jia C, Yan H, Cui B, Wu J, Wang
Q, Gao W, Cui X, Li J and Ou T: Injecting RNA interference
lentiviruses targeting the muscarinic 3 receptor gene into the
bladder wall inhibits neurogenic detrusor overactivity in rats with
spinal cord injury. Neurourol Urodyn. 38:615–624. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ge Q, Wang M, Lin Y, Xu C, Xiao J and Shen
Z: Establishment of animal model manifested as bladder neurogenic
changes generated by bilateral pelvic nerve injury in male rats.
Int Urol Nephrol. 53:421–429. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Doyle C, Cristofaro V, Sack BS, Mahmood F,
Sullivan MP and Adam RM: The role of the mucosa in modulation of
evoked responses in the spinal cord injured rat bladder. Neurourol
Urodyn. 37:1583–1593. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Soebadi MA, Bakula M, Hakim L, Puers R and
De Ridder D: Wireless intravesical device for real-time bladder
pressure measurement: Study of consecutive voiding in awake
minipigs. PLoS One. 14(e0225821)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wyndaele JJ, Birch B, Borau A, Burks F,
Castro-Diaz D, Chartier-Kastler E, Drake M, Ishizuka O, Minigawa T,
Opisso E, et al: Surgical management of the neurogenic bladder
after spinal cord injury. World J Urol. 36:1569–1576.
2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Romo PGB, Smith CP, Cox A, Averbeck MA,
Dowling C, Beckford C, Manohar P, Duran S and Cameron AP:
Non-surgical urologic management of neurogenic bladder after spinal
cord injury. World J Urol. 36:1555–1568. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li YL, Wen JJ, Wen YB, He XF, Wu JW, Li
YW, Han ZJ, Feng JJ, Yan SH, Li SL, et al: Reconstruction of
bladder function and prevention of renal deterioration by means of
end-to-side neurorrhaphy in rats with neurogenic bladder. Neurourol
Urodyn. 37:1272–1280. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wada N, Shimizu T, Takai S, Shimizu N,
Tyagi P, Kakizaki H and Yoshimura N: Combinational effects of
muscarinic receptor inhibition and β3-adrenoceptor stimulation on
neurogenic bladder dysfunction in rats with spinal cord injury.
Neurourol Urodyn. 36:1039–1045. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Duan LJ, Qi J, Kong XJ, Huang T, Qian XQ,
Xu D, Liang JH and Kang J: MiR-133 modulates TGF-β1-induced bladder
smooth muscle cell hypertrophic and fibrotic response: Implication
for a role of microRNA in bladder wall remodeling caused by bladder
outlet obstruction. Cell Signal. 27:215–227. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Koeck I, Burkhard FC and Monastyrskaya K:
Activation of common signaling pathways during remodeling of the
heart and the bladder. Biochem Pharmacol. 102:7–19. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sang R, Liu Y, Kong L, Qian L and Liu C:
Effect of acellular amnion with increased TGF-β and bFGF levels on
the biological behavior of tenocytes. Front Bioeng Biotechnol.
8(446)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ikeda Y, Zabbarova IV, Birder LA, Wipf P,
Getchell SE, Tyagi P, Fry CH, Drake MJ and Kanai AJ: Relaxin-2
therapy reverses radiation-induced fibrosis and restores bladder
function in mice. Neurourol Urodyn. 37:2441–2451. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cho KJ and Kim JC: Management of urinary
incontinence with underactive bladder: A review. Int Neurourol J.
24:111–117. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Levanovich PE, Diokno A, Hasenau DL,
Lajiness M, Pruchnic R and Chancellor MB: Intradetrusor injection
of adult muscle-derived cells for the treatment of underactive
bladder: Pilot study. Int Urol Nephrol. 47:465–467. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Drugs for hypertension. Med Lett Drugs
Ther. 62:73–80. 2020.PubMed/NCBI
|
|
18
|
Liu X, Shan X, Chen H, Li Z, Zhao P, Zhang
C, Guo W, Xu M and Lu R: Stachydrine ameliorates cardiac fibrosis
through inhibition of angiotensin II/transformation growth factor
β1 fibrogenic axis. Front Pharmacol. 10(538)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen Y, Ma Y, He Y, Xing D, Liu E, Yang X,
Zhu W, Wang Q and Wen JG: The TGF-β1 pathway is early involved in
neurogenic bladder fibrosis of juvenile rats. Pediatr Res.
90:759–767. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Torimoto K, Matsumoto Y, Gotoh D, Morizawa
Y, Miyake M, Samma S, Tanaka N, Hirayama A and Fujimoto K:
Overactive bladder induces transient hypertension. BMC Res Notes.
11(196)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Abolhasanpour N, Eidi A, Hajebrahimi S,
Reyhani-Rad S and Hashim H: Effect of cerebrolysin on bladder
function after spinal cord injury in female Wistar rats. Int J
Urol. 26:917–923. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Salehi-Pourmehr H, Rahbarghazi R, Mahmoudi
J, Roshangar L, Chapple CR, Hajebrahimi S, Abolhasanpour N and
Azghani MR: Intra-bladder wall transplantation of bone marrow
mesenchymal stem cells improved urinary bladder dysfunction
following spinal cord injury. Life Sci. 221:20–28. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lin CY, Sparks A and Lee YS: Improvement
of lower urinary tract function by a selective serotonin
5-HT1A receptor agonist, NLX-112, after chronic spinal
cord injury. Exp Neurol. 332(113395)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Maciejewski CC, Tredget EE and Metcalfe
PD: Urodynamic improvements following oral medical therapy for
partial bladder outlet obstruction in an animal model. Neurourol
Urodyn. 34:286–291. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
He YL, Wen JG, Pu QS, Wen YB, Zhai RQ,
Chen Y, Ma Y, Liu EP, Xing D, Ji FP, et al: Losartan prevents
bladder fibrosis and protects renal function in rat with neurogenic
paralysis bladder. Neurourol Urodyn. 40:137–146. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hui J, Sharma S, Rajani S and Singh A: The
specific molecular composition and structural arrangement of
eleutherodactylus coqui gular skin tissue provide its high
mechanical compliance. Int J Mol Sci. 21(5593)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen R, Feng Y, Wu J, Song Y, Li H, Shen
Q, Li D, Zhang J, Lu Z, Xiao H and Zhang Y: Metformin attenuates
angiotensin II-induced TGFβ1 expression by targeting hepatocyte
nuclear factor-4-α. Br J Pharmacol. 175:1217–1229. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wipff PJ, Rifkin DB, Meister JJ and Hinz
B: Myofibroblast contraction activates latent TGF-beta1 from the
extracellular matrix. J Cell Biol. 179:1311–1323. 2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Campbell SE and Katwa LC: Angiotensin II
stimulated expression of transforming growth factor-beta1 in
cardiac fibroblasts and myofibroblasts. J Mol Cell Cardiol.
29:1947–1958. 1997.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Thenappan T, Ormiston ML, Ryan JJ and
Archer SL: Pulmonary arterial hypertension: Pathogenesis and
clinical management. BMJ. 360(j5492)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Frismantiene A, Philippova M, Erne P and
Resink TJ: Smooth muscle cell-driven vascular diseases and
molecular mechanisms of VSMC plasticity. Cell Signal. 52:48–64.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang JG, Xing ZY, Zha TT, Tian XJ, Du YN,
Chen J and Xing W: Longitudinal assessment of rabbit renal fibrosis
induced by unilateral ureteral obstruction using two-dimensional
susceptibility weighted imaging. J Magn Reson Imaging.
47:1572–1577. 2018.PubMed/NCBI View Article : Google Scholar
|