Introduction
Traumatic brain injury (TBI) is one of the primary
causes of disability and death in young adults and children
worldwide in a 2020 study (1,2). TBI
not only causes major mechanical injury of cerebral tissues but
also induces secondary mood changes, including anxiety, that
persist for years post-injury and severely impair the quality of
life for patients (3,4). The amygdala is prominently involved
in neurobiological models of anxiety (5,6). To
date, several pathological processes responsible for neuronal
death, such as excitotoxicity, oxidative stress, inflammation and
apoptosis, have been revealed to be involved in the secondary
damage (cell death, excitotoxicity, oxidative stress and
inflammation) of TBI (7,8). Neuroinflammation is characterized by
glial activation (such as the microglia and astrocytes) and
increased release of inflammatory substances within the brain, and
it is important in short- and long-term neuronal injuries post-TBI
(9). However, only a few effective
strategies have been proven to improve the clinical prognosis of
TBI (10).
Melatonin (N-acetyl 5-methoxytryptamine), a major
product of the pineal gland, has been demonstrated to generate
neuroprotective effects against a series of central nervous system
(CNS) disease models, including cerebral ischemia (11), brain and spinal cord trauma
(12) and subarachnoid and
intracerebral hemorrhage (13). It
possesses fundamental characteristics such as low neurotoxicity and
high permeability in the blood-brain barrier (14). Previous studies have reported that
melatonin ameliorates secondary injury (for example, by decreasing
apoptosis, inflammation and oxidative stress) (15-17)
and also improves functional deficits (such as memory, learning and
motor activities). However, to the best of our knowledge, there is
no clear information on the clinical therapeutic effects of
melatonin in patients with TBI. Based on the aforementioned
observations, the present study hypothesized that melatonin reduces
neuronal injury induced by TBI.
The cyclic adenosine monophosphate/protein kinase A
(cAMP/PKA) signaling pathway plays an important role in controlling
the proliferation and differentiation of progenitor cells and the
production of cytokines (18). It
has been revealed that PKA phosphorylation is associated with CNS
injury in vivo and in vitro (19). Furthermore, PKA plays an important
role in the activation of the cAMP-response element-binding protein
(CREB) (20). Activation of the
PKA/CREB signaling pathway has been suggested to exert
neuroprotective effects and to inhibit apoptotic cells in brain
tissues (21). Therefore,
regulation of amygdaloid PKA may reduce social avoidance and
anxiety-like phenotypes (22).
Moreover, melatonin administration ameliorates cerebral damage
through the modulation of the phosphorylated (p)-AMPK/p-CREB
signaling pathways and induces CREB activation correlated with
long-term memory and cognition in vitro (23). p-CREB reduces neuroinflammation by
regulating p-NF-κB and reducing the transcription of inflammatory
mediators (24). However, the
roles of the PKA/CREB signaling pathway in mood changes post-TBI,
including depression and anxiety, remain obscure. Moreover, whether
melatonin has the potential to be developed as a therapeutic
strategy against TBI mediated by the PKA/CREB signaling pathway
required investigation.
The present study was conducted to investigate the
effects of melatonin on anxiety-like behaviors and the pathological
changes in a rodent model of TBI. The present study also examined
whether the PKA/CREB signaling pathway was involved in the
neuroprotective effects of melatonin.
Materials and methods
Experimental animals
Adult male Sprague-Dawley rats (n=228; 6-8 weeks
old) weighing 300-350 g were purchased from Liaoning Changsheng
Biotechnology and raised at a stable temperature (25±1˚C), humidity
(50±10%) in a 12-h light/12-h dark alternating cycle with free
access to food and water. The present study was approved by the
Animal Review Board of Cangzhou Central Hospital [Cangzhou, China;
approval no. 2020-013-02(Z)]). All animal experiments were
performed according to the standards of the Institutional Animal
Care and Use Committee at the Cangzhou Central Hospital. The animal
health and behavior were monitored every day.
Once behavioral tests were finished, the rats were
euthanized by intraperitoneal injection (i.p) of sodium
pentobarbital (200 mg/kg). If rats were still alive after the
experiments, euthanasia was conducted. If the rats lost their
appetite for 5 days or stopped drinking for 3 days, lost >20% of
their body weight and were unable to eat and drink before the
scheduled experimental endpoint, euthanasia was also conducted.
Electrocardiographic monitoring (straight lines) was used to detect
the death of rats.
Group assignment and drug
treatment
In experiment series 1, adult male Sprague-Dawley
rats were randomly divided (computer-based randomization) into one
of the following four groups: i) Sham; ii) TBI; iii) TBI + Mel
(melatonin); and iv) TBI + S (saline). Melatonin (10 mg/kg) was
dissolved in saline containing 2% ethanol was administered via i.p
immediately after TBI (25). An
equal volume of saline containing 2% ethanol was administered
through i.p immediately after TBI in the TBI + S group.
In experiment series 2, adult male Sprague-Dawley
rats were randomly assigned to one of the following groups: i) TBI
+ Mel + S; ii) TBI + Mel + H89; and iii) TBI + Mel + dibutyryl-cAMP
(dbcAMP). H-89 (0.2 mg/100 g; cat. no. HY-15979A; MedChemExpress)
is a potent and selective inhibitor of PKA (26). DbcAMP (1 mg/100 g; cat. no.
HY-B0764A; MedChemExpress) selectively activates cAMP-dependent
protein kinase (PKA) (27). H89
and dbcAMP were dissolved in saline (containing 10% dimethyl
sulfoxide and 20% sulfobutylether β-cyclodextrin) and were applied
by stereotactic injection 15 min before surgery. After anesthesia
with sevoflurane (3-4%), a hole was made with microbore dental
drill, then stainless-steel guide cannulae (28-gauge) were
stereotactically injected to bilaterally target the amygdala (2.8
mm posterior to the bregma, 5.3 mm lateral from the midline and
6.25 mm ventral from the skull surface). To observe the interaction
of H89 or dbcAMP on melatonin, the Sham, Sham + Mel + H89 and Sham
+ Mel + dbcAMP groups were established. Melatonin, H89, and dbcAMP
were administered as aforementioned (Fig. 1). The number of animals required
for each experiment in each group and the total number of animals
were detailed in Table I.
 | Table INumber of rats in each experimental
phase and experimental group. |
Table I
Number of rats in each experimental
phase and experimental group.
| A, Experimental
series 1 (n=120) |
|---|
| Experimental
group | OFT + EPM | IF | ELISA | Western
blotting |
|---|
| Sham (n=30) | 12 | 6 | 6 | 6 |
| TBI (n=30) | 12 | 6 | 6 | 6 |
| TBI + Mel
(n=30) | 12 | 6 | 6 | 6 |
| TBI + S (n=30) | 12 | 6 | 6 | 6 |
| B, Experimental
series 2 (n=108) |
| Experimental
group | OFT + EPM | IF | ELISA | Western
blotting |
| TBI + Mel + S
(n=24) | 12 | - | 6 | 6 |
| TBI + Mel + H89
(n=24) | 12 | - | 6 | 6 |
| TBI + Mel + dbcAMP
(n=24) | 12 | - | 6 | 6 |
| Sham (n=12) | 12 | - | - | - |
| Sham + Mel + H89
(n=12) | 12 | - | - | - |
| Sham + Mel + dbcAMP
(n=12) | 12 | - | - | - |
TBI model
TBI was established using the weight-drop (WD)
method (9,28). The animals were placed on a
platform covered with foam and anesthetized with sevoflurane (7-8%
for induction, and 3-4% for maintenance). A bone window with a
diameter of 6-mm was drilled (3.5 mm behind the bregma and 2.5 mm
to the right of the midline). A stainless-steel planchet (diameter,
6-mm; thickness, 5-mm) was attached. An iron weight (20 g) was
freely dropped from a height of 25 cm, and the dura was kept
intact. In sham-operated animals, the skull was exposed under
anesthesia and skin incisions were then closed using silk sutures
without TBI. Rats in the TBI or Sham group were treated by another
experimenter blinded to behavioral tests.
Open field test (OFT)
The OFT was performed to evaluate anxiety and
inquiry behavior (29). The site
was a large open field (60x60x40 cm), which was segmented into 16
equal squares. The test was conducted at 30 days post-TBI. The site
was cleaned with 75% alcohol to prevent foreign smells that may
influence behavior. The rat was positioned in the site, and the
distance, speed, the number of squares crossed (with four paws),
the time spent in the center and the rearing times were recorded
for 90 sec. The trajectory images were analyzed using a
computerized tracking system (supermaze XR-Xmaze; Shanghai XinRuan
Information Technology Co. Ltd.
Elevated plus maze (EPM)
The EPM consisted of two closed arms (50 cm L x 10
cm W x 40 cm H) and two open arms with the same size at 90˚ angles
to each other. The maze was 70-cm high from the floor. At 30 days
post-TBI the rat was placed in the center zone of the maze with its
nose directed toward the same open arm and allowed to explore the
maze for 5 min. The maze was cleaned with 75% ethanol after each
test. The time spent on the open and closed arms, the distance
traveled and the number of entries were recorded and analyzed using
the software (XR-XG2011; Shanghai XinRuan Information Technology
Co. Ltd.). An entry was calculated when all four paws were entered
into an arm. The anxiety level was evaluated by counting the
percentage of open arm entries (number of open arm entries/number
of all arm entries) and the percentage of open arm time (time spent
in the open arms/total time spent in all arms). The locomotor
activity was evaluated using the total moving distance in the maze.
Once behavioral tests (OFT and EPM) were finished, euthanasia was
conducted by intraperitoneal injection of sodium pentobarbital (200
mg/kg).
Immunofluorescence
At 24 h after TBI, rats were anaesthetized with
sevoflurane (8%) and transcardially perfused with cold PBS followed
by 4% formaldehyde. The cerebral tissues containing the amygdala
were fixed in 10% neutral-buffered formalin at 25˚C for 24 h,
embedded in solid paraffin and cut into a thickness of 4-µm (n=6).
The paraffin sections were dewaxed, washed with ethanol for 2 min
at 25˚C and hydrated (100% ethanol for 3 min, 95% ethanol for 2
min, 80% ethanol for 2 min, 75% ethanol for 2 min, H2O
for 1 min) at room temperature, blocked using
QuickBlock™ Blocking Buffer (P0222; Beyotime
Biotechnology) for 1 h at room temperature and then incubated
overnight at 4˚C with the primary mouse antibody against glial
fibrillary acidic protein antibody (GFAP; 1:100; cat. no. ab4648;
Abcam). On the next day, the pathological sections were washed
three times in phosphate buffer saline (PBS) and incubated with the
corresponding secondary antibodies (Cy3-conjugated goat anti-mouse
IgG; 1:500; cat. no. A0521; Beyotime Institute of Biotechnology)
for 1 h at 25˚C in the dark.
Neuronal apoptosis was detected using TUNEL
staining. After dewaxing and hydration as aforementioned), the
slices were washed three times in PBS before adding 20 µg/ml of
Proteinase K (cat. no. st533; Beyotime Institute of Biotechnology)
at 37˚C for 35 min. The slices were then incubated overnight at 4˚C
with the primary polyclonal mouse antibody against neuronal nuclei
(NeuN, 1:200, ab104224; Abcam). After washing with PBS, the
sections were incubated with TDT enzyme including a fluorescent
labeling solution [One-step TUNEL cell apoptosis detection kit
(fluorescent green); cat. no. C1088; Beyotime Institute of
Biotechnology] at 37˚C for 60 min in the dark. Subsequently, 5
µg/ml of Antifade Mounting Medium with
4',6-diamidino-2-phenylindole (DAPI; cat. no. P0131; Beyotime
Institute of Biotechnology) was used for 3 min at 25˚C to label the
cell nuclei locations. Immunofluorescence images were captured
using a fluorescence microscope (Olympus Corporation). A total of 6
fields of view were evaluated in each tissue section (x400
magnification). The position of amygdala was defined according to
the corresponding brain anatomical map stained with DAPI (Fig. S2), which was ~5.3 mm from the
midline and 6.25 mm from the parietal cortex. The number of
GFAP-positive cells, the number of activated astrocytes and the
percentage of NeuN- and TUNEL-positive cells were calculated by
Image-pro plus 6.0 software (Media Cybernetics, Inc.).
Western blotting
At 24 h post-TBI, rats were anaesthetized with
sevoflurane (8%) and transcardially perfused with cold PBS, total
protein was extracted from the amygdala tissues and lysed in lysis
buffer (cat. no. P0013; Beyotime Institute of Biotechnology).
Subsequently, 30 µg protein per lane was electrophoresed on 12%
SDS-PAGE, and the separated proteins were transferred onto
polyvinylidene fluoride membranes and blocked in Quickblock™
sealing fluid (cat. no. P0252; Beyotime Institute of Biotechnology)
at 25˚C for 10 min, followed by incubation overnight at 4˚C with
the indicated primary antibodies as follows: P-CREB (1:500; cat.
no. AF5785), CREB (1:500; cat. no. AF1018), p-PKA (1:500; cat. no.
AF1942), p-NF-κB (1:500; cat. no. AF5878), NF-κB (1:500; cat. no.
AF7569) (all Beyotime Institute of Biotechnology) and GAPDH
(1:2,000; cat. no. K106389P; Beijing Solarbio Science &
Technology Co., Ltd.). The next day, the membranes were incubated
with horseradish peroxidase AffiniPure goat anti-rabbit IgG (H + L;
1:1,000; cat. no. A0208; Beyotime Institute of Biotechnology) at
37˚C for 1 h. Anti-GAPDH Polyclonal Antibody (1:2,000; cat. no.
K106389P; Beijing Solarbio Science & Technology Co., Ltd.) was
used as an internal reference. The polyvinylidene fluoride
membranes were incubated with ECL reagent (cat. no. P0018FM;
Beyotime Institute of Biotechnology) for 5 min at room temperature,
and protein bands were detected by a western blot detection system
(Gel Doc XRS; Bio-Rad Laboratories, Inc.). The gray value of the
protein bands was analyzed using the Image-pro plus 6.0 software
(Media Cybernetics, Inc.).
Enzyme-linked immunosorbent assay
(ELISA)
At 24 h post-TBI, the rats were euthanized using 200
mg/kg sodium pentobarbital (i.p.) and the amygdala was separated.
The TNF-α Assay (cat. no. PT516) and IL-6 Assay (cat. no. PI328)
ELISA kits (both from Beyotime Institute of Biotechnology) were
used to measure the levels of TNF-α and IL-6 in the amygdala
according to the manufacturer's instructions.
Statistical analysis
All statistical analyses were conducted using SPSS
Statistics (version 20.0; IBM Corp.). Data are presented as mean ±
standard deviation (n=12 rats/group in behavioral test, n=6
rats/group in molecular and pathological tests). Levene's test was
used to test the homogeneity of variance hypothesis. When
identifying data heteroscedasticity, the logarithmic transformation
was performed and the heteroscedasticity was corrected. The
statistical significance between groups was evaluated using one-way
analysis of variance, followed by Tukey's post hoc test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Melatonin alleviates anxiety-like
behaviors post-TBI
Behavioral tests (OFT and EPM) were performed to
evaluate the effects of melatonin on anxiety-related behaviors. The
behavioral assessments of all rats at 30 days after TBI are
presented in Fig. 2. First, the
results of OFT indicated that rats in all the TBI groups exhibited
a decreased amount of movement; namely, a significantly shorter
total distance, slower average speed, less rearing, fewer number of
squares crossed and less time spent in the center compared with
those in the Sham group (P<0.0001 vs. Sham; Fig. 2A-F). However, melatonin
administration after TBI exposure resulted in significantly
increased moving distance, greater average speed, more time spent
in the center, more squares crossed and increased rearing
(P<0.0001 vs. TBI; Fig. 2A-F).
No significant differences were observed in the total moving
distance, average speed, rearing, number of squares crossed and
time spent in the center between rats in the TBI and TBI + S
groups.
Next, the results of EPM indicated that rats in the
TBI group exhibited a significant decrease in the percentage of
open arm entries and the percentage of open arm time compared with
rats in the Sham group (P<0.0001 vs. Sham; Fig. 2G-I). Melatonin could significantly
increase the percentage of open arm entries and the percentage of
open arm times (P<0.0001 vs. TBI; Fig. 2G-I). There were no significant
differences in the total moving distance among the groups (Fig. 2J), which suggested that the
locomotor activity of rats among the different groups in this test
was not altered.
Melatonin reduces neuronal apoptosis
in the region of amygdala post-TBI
At 24 h post-TBI, neuronal apoptosis was further
evaluated by TUNEL staining combined with NeuN staining. As
presented in Fig. 3A and B, there were few TUNEL-positive cells in
the Sham group. Compared with the Sham group, TBI caused a
significant increase in the number of TUNEL- and NeuN-positive
cells (P<0.0001 vs. Sham group; Fig. 3A and B). However, melatonin administration
significantly reduced neuronal apoptosis in the amygdala and
provided neuroprotective effects after TBI (P<0.0001 vs. TBI
group; Fig. 3A and B). In addition, there were no statistical
differences in neuronal apoptosis between the TBI and TBI + S
groups.
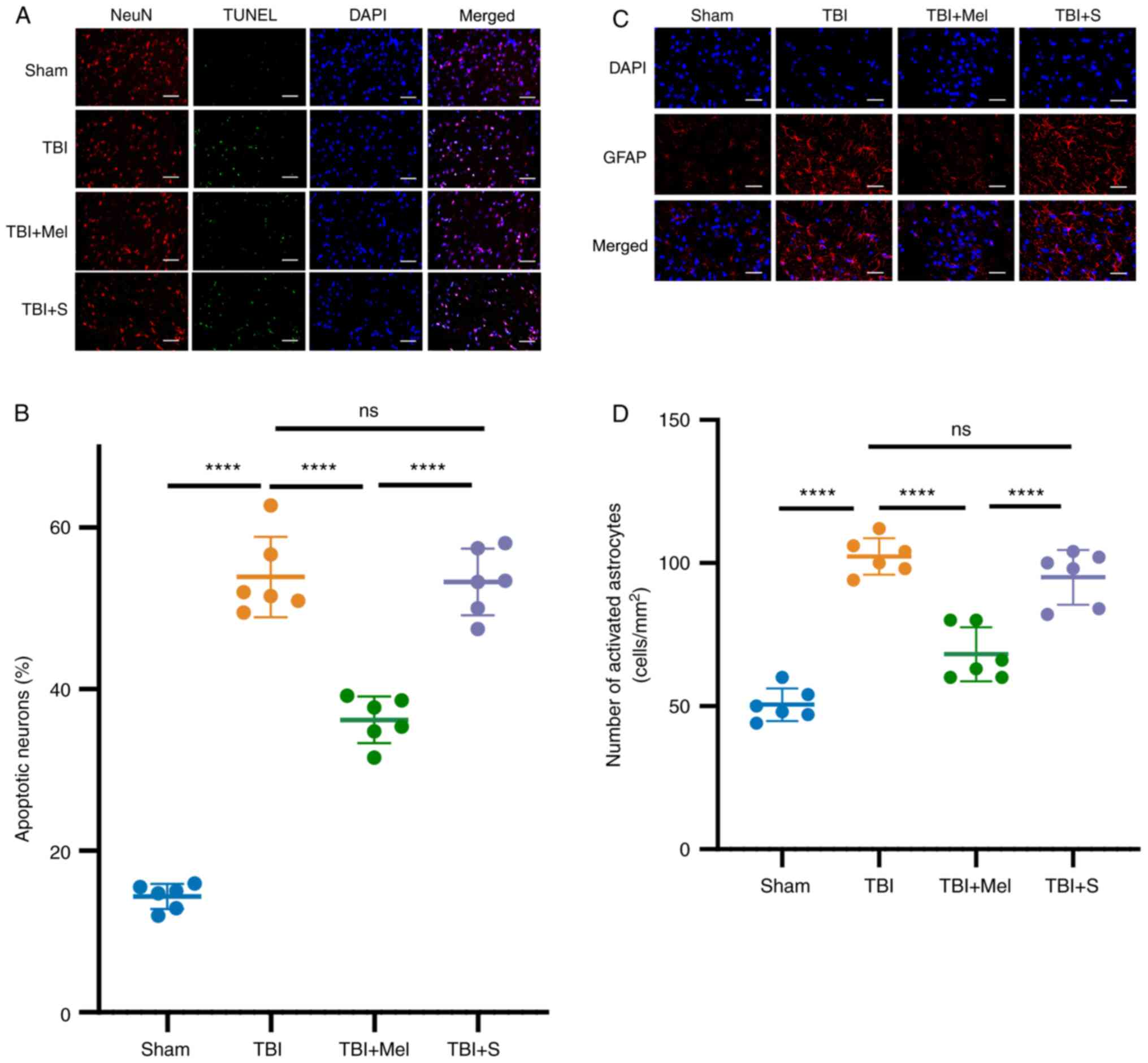 | Figure 3Melatonin reduces neuronal apoptosis
and upregulates the activation of astrocytes in the region of
amygdala post-TBI. (A) Representative images of NeuN (specificity
marker of neurons) plus TUNEL staining in the amygdala (NeuN, red;
TUNEL, green; DAPI, blue). Magnification, x400; scale bar=50 µm.
(B) Apoptosis was quantitatively observed by TUNEL staining (n=6).
(C) Representative images of GFAP (specificity marker of astrocyte
activation) in the amygdala (GFAP, red; DAPI, blue). Magnification,
x400; scale bar=50 µm. (D) Quantification of changes in the number
of activated astrocytes. n=6 rats per group.
****P<0.0001. TBI, traumatic brain injury; Mel,
melatonin; S, saline; ns, not significant; NeuN, neuronal nuclei;
DAPI, 4',6-diamidino-2-phenylindole; GFAP, glial fibrillary acidic
protein. |
Melatonin upregulates the activation
of astrocytes in the region of amygdala post-TBI
GFAP is a marker of mature and activated astrocytes.
The results demonstrated that the number of GFAP-positive cells was
significantly elevated at 24 h post-TBI compared with that in the
Sham group (P<0.0001 vs. Sham, Fig.
3C and D). However, the number
of GFAP-positive cells significantly decreased in the TBI + Mel
group (P<0.001 vs. TBI; Fig. 3C
and D). Moreover, no statistical
difference was observed in the number of GFAP-positive cells in the
region of amygdala between rats in the TBI and TBI + S groups.
Melatonin reduces the level of
inflammatory cytokines and activates the PKA/CREB signaling pathway
in the amygdala post-TBI
The effects of melatonin on inflammatory cytokine
levels were evaluated by ELISA. The results indicated that the
levels of TNF-α and IL-6 in the TBI group were significantly
increased compared with those in the Sham group (P<0.0001 vs.
Sham; Fig. 4A and B). Melatonin administration significantly
downregulated the expression levels of TNF-α and IL-6 compared with
those in the TBI group (P<0.01 vs. TBI; Fig. 4A and B).
 | Figure 4Melatonin reduces the level of
inflammatory cytokines and activates the PKA/CREB signaling pathway
in the amygdala post-TBI. (A) Expression levels of TNF-α in the
amygdala of rats. (B) Expression levels of IL-6 in the amygdala.
(C) Representative results of immunoblotting of PKA, p-PKA, CREB,
p-CREB, NF-κB and p-NF-κB in the amygdala tissue. Relative protein
expression levels of (D) p-PKA/PKA, (E) p-CREB/CREB and (F)
p-NF-κB/NF-κB. n=6 rats per group. *P<0.05,
**P<0.01, ***P<0.001,
****P<0.0001. TBI, traumatic brain injury; Mel,
melatonin; S, saline; ns, not significant; PKA, protein kinase A;
CREB, cAMP-response element-binding protein; p-, phosphorylated;
t-, total. |
To further understand the mechanism of action of
melatonin on anxiety-like behaviors, the protein expression levels
of PKA, CREB and NF-κB and the phosphorylation levels of the three
proteins in the amygdala tissues were detected using western
blotting. The expression levels of p-PKA, p-CREB and p-NF-κB
significantly increased in the TBI groups compared with those in
the Sham groups (P<0.001 vs. Sham; Fig. 4C-F). The p-PKA/t-PKA ratio and the
p-CREB/t-CREB ratio were also significantly higher in the TBI + Mel
group compared with the TBI group (P<0.01 vs. Sham; Fig. 4C-E). In addition, the expression of
p-NF-κB/t-NF-κB ratio was significantly decreased in the TBI + Mel
group compared with that in the TBI group (P<0.0001 vs. Sham;
Fig. 4C and F). There was no difference between the
TBI and TBI + S groups in the expression of each protein.
Melatonin may alleviate TBI-induced
anxiety-like behaviors through the PKA/CREB signaling pathway
Whether the exact mechanism of action of melatonin
against anxiety-like behaviors post-TBI was associated with the
PKA/CREB pathway was also determined. H89 and dbcAMP were
administered 15 min before TBI exposure. There was no difference
among the Sham, Sham + Mel + H89 and Sham + Mel + dbcAMP groups in
terms of open field and elevated maze tests (Fig. S1). Therefore, it was hypothesized
that there was no interaction of H89 or dbcAMP with melatonin, and
the three groups were eliminated in following experiment. There was
a significant decrease in total moving distance, average speed,
time spent in the center, number of squares crossed and rearing
times in the OFT in the rats of the TBI + Mel + H89 group
(P<0.0001 vs. TBI + Mel + S; Fig.
5A-F). The EPM results revealed that the rats in the TBI + Mel
+ H89 group exhibited a significant decrease in the percentage of
open arm entries and the percentage of open arm times (P<0.0001
vs. TBI + Mel + S; Fig. 5G-I).
However, after the activation of PKA with dbcAMP, the protective
effect of melatonin on the nervous system was strengthened and
exhibited the opposite effect of H89 application.
The effects of melatonin on inflammatory cytokine
levels in the amygdala were measured by ELISA. Results revealed
that the levels of TNF-α and IL-6 in the TBI + Mel + H89 group were
significantly increased compared with those in the TBI + Mel + S
group (P<0.05 vs. TBI + Mel + S; Fig. 6). DbcAMP application (TBI + Mel +
dbcAMP) significantly downregulated the expression levels of
inflammatory cytokines compared with those in the TBI + Mel + S
(P<0.05; Fig. 6) and TBI + Mel
+ H89 groups (P<0.0001; Fig.
6).
Furthermore, the results revealed that H89
pretreatment before TBI in the TBI + Mel + H89 group induced
significant decreases in the levels of p-PKA (P<0.05 vs. TBI +
Mel + S; Fig. 7A and B) and p-CREB (P<0.0001 vs. TBI + Mel +
S; Fig. 7A and C) in the amygdala compared with those in
the TBI + Mel + S group. It was also revealed that the expression
levels of p-NF-κB were significantly increased in the TBI + Mel +
H89 group compared with those in the TBI + Mel + S group
(P<0.0001 vs. TBI + Mel + S; Fig.
7A and D). Although the
expression levels of p-PKA and p-CREB were significantly elevated
(P<0.001), the expression level of p-NF-κB significantly
declined in the TBI + Mel + dbcAMP group compared with that in the
TBI + Mel + S and TBI + Mel + H89 groups (P<0.001; Fig. 7A-D). These findings revealed that
melatonin could activate the PKA/CREB signaling pathway, whereas
PKA inhibitors could partially reverse the neuroprotective effects
of melatonin, which further aggravated the anxiety-like behavior
and the release of inflammatory cytokines compared with those in
the TBI + Mel + S group.
 | Figure 7Melatonin alleviates TBI-induced
anxiety-like behaviors through the PKA/CREB signaling pathway. (A)
Representative results of immunoblotting of PKA, p-PKA, CREB,
p-CREB, NF-κB and p-NF-κB in the amygdala tissue. Relative protein
expression levels of (B) p-PKA/t-PKA, (C) p-CREB/t-CREB and (D)
p-NF-κB/t-NF-κB. n=6 rats per group. *P<0.05,
***P<0.001, ****P<0.0001. TBI,
traumatic brain injury; Mel, melatonin; S, saline; dbcAMP,
dibutyryl-cAMP; PKA, protein kinase A; CREB, cAMP-response
element-binding protein; p-, phosphorylated; t-, total. |
Discussion
TBI, represented by acute neurological injury, is
considered to be a trigger for chronic traumatic disorders,
including Alzheimer's disease and Parkinson's disease. However, no
drug is currently available to effectively prevent these
complications (30-32).
Although previous studies have reported the involvement of
anti-inflammation in the neuroprotective effects of melatonin
(25,33), the underlying molecular mechanisms
remain unclear. The present study demonstrated the following
primary novel findings: i) Melatonin decreased neuronal apoptosis
in the amygdala post-TBI; ii) melatonin alleviated anxiety-like
behaviors post-TBI; and iii) inhibition of p-PKA using H89 revealed
that the protective effects of melatonin could be partially
reversed post-TBI. To the best of our knowledge, the present study
is the first to support the significance and important functions of
PKA inhibition in the development of anxiety-like behaviors after
TBI exposure to date.
TBI is likely linked to long-term emotional
disorders such as depression and anxiety (34). A previous report indicated that in
the developing brain, mild-to-moderate TBI results in early
behavioral and metabolomics changes (35). The present study revealed that TBI
caused anxiety-like behaviors in rats. The results demonstrated
that the amount of rearing and number of squared crossed, as well
as the average speed and total moving distance, were significantly
decreased in the OFT. Moreover, there was a significant increase in
the amount of time spent stationary in corners. Melatonin
administration could relieve anxiety-like behaviors in rodents, but
the protective effects of melatonin could be partially eliminated
after the administration of PKA inhibitors. The amygdala is part of
the limbic system of brain tissue that produces, recognizes and
regulates emotions (36). The
amygdala is prominently involved in a neurobiological model of
anxiety (37). Therefore, the
present study selected the amygdala as the focus of investigation
in the primary research.
Recently, there is growing evidence indicating that
melatonin is a potential antioxidant considered as a protectant
against TBI (15). Due to this
beneficial role, the present study explored the underlying
mechanism by which melatonin protects the brain. The dose of
melatonin (10 mg/kg, i.p) in the present study was based on a
previous study (10), as well as
our preliminary experiments. Intervention should be initiated as
soon as possible after TBI, preferably within 4 h after injury, to
achieve maximum neuroprotective effects (38); therefore, the time point of
melatonin treatment used in the present study was 0 h post-TBI.
Astrocytes are the most abundant glial cells in the
CNS and are traditionally considered as the only structural
elements supporting the structure of the brain (39). However, the importance of
astrocytes in the survival of neurons has been proposed in recent
years (40,41). In addition, drastically increased
GFAP and allograft inflammatory factor 1 expression levels have
been reported in the WD model of TBI (42). Astrocytes respond to TBI by
activating the NF-κB pathway, which can result in the secretion of
cytokines (43). For example, a
study has demonstrated that reactive astrocytes may intensify
inflammatory processes, if astrocytes exposed to an inflammatory
stimulus produce proinflammatory cytokines such as IL-1β and TNF-α
(44). IL-6-knockout can
significantly reduce the neurological damage after TBI (45). Moreover, inhibition of TNF-α
signaling can decrease neuronal apoptosis (46).
There are two main sources of inflammatory
cytokines: One is the secretion of neutrophils and macrophages in
the peripheral circulatory system, and the other is the microglia,
astrocytes, oligodendrocytes and damaged neurons from the CNS. The
present study aimed to explore the effects of melatonin on
anxiety-like behaviors, neuronal apoptosis and astrocyte activation
induced by TBI in rats. It was hypothesized that astrocyte
activation might release more inflammatory cytokines, so the
expression levels of TNF-α and IL-6 in cerebral tissues were
tested. The present study revealed that astrocyte activation was
increased in rats with TBI accompanied by increased neuronal
apoptosis; moreover, after melatonin administration, the numbers of
apoptotic neurons and activated astrocytes post-TBI were decreased.
Furthermore, melatonin alleviated the anxiety-like behaviors
induced by TBI. Therefore, it was hypothesized that the improvement
of emotional changes induced by melatonin was associated with
reduction of neuronal apoptosis and astrocyte activation.
Several previous studies have reported that the
PKA/CREB signaling pathway is involved in apoptosis and correlates
with cognitive function (47,48).
Activation of PKA/CREB signaling plays an important role in
reducing neuronal death or neurotoxicity (21). Another previous study suggested
that CREB activation also decreases the apoptosis of neuroblastoma
cells (49). The present study
demonstrated that pretreatment with H89, a special inhibitor of
PKA, significantly decreased the expression levels of p-PKA and
CREB, increased the expression levels of IL-6 and TNF-α and
reversed the improvement of anxiety-like behaviors induced by
melatonin. However, pretreatment with dbcAMP, a PKA activator,
further improved the anxiety-like behavior of rats. Overall, these
results indicated that melatonin could alleviate the neuronal
damage induced by TBI through the PKA/CREB signaling pathway.
However, cAMP acts directly on three main effectors: PKA, protein
directly activated by c-AMP (EPAC) and cyclic nucleotide-gated
channels (50). In the
hippocampus, EPAC contributes to the control of neuronal growth and
differentiation and has been implicated in memory and learning as
well as in anxiety and depression (51). Therefore, the role of EPAC in the
findings in the present study cannot be ruled out.
The present study had several limitations. The
experiments were limited only to the amygdala region, and changes
in the ventromedial prefrontal cortex and hippocampus remain
unexplored. The specific pathway of apoptosis, such as the
expression of Bcl-2 and Bak, should be investigated in future
experiments. CREB is a downstream factor of PKA, and the
phosphorylation of PKA will directly affect the activation and
phosphorylation of CREB. Lack of CREB inhibitor or knockdown
experiments is another limitation of the present study.
To summarize, melatonin could alleviate TBI-induced
anxiety-like behaviors in rats, and the underlying mechanism may be
associated with activation of the PKA/CREB signaling pathway.
Supplementary Material
Interaction of H89 or dbcAMP on
melatonin. (A) Trajectory map. (B) Total moving distance. (C)
Average speed. (D) Rearing times. (E) Number of squares crossed.
(F) Percentage of time spent in the center. (G) Elevated plus maze
at 30 days post-TBI of the experimental rats. (H) Percentage of
open arm entries (%). (I) Percentage of time spent in open arms
(%). (J) Total moving distance in EPM. n=12. dbcAMP,
dibutyryl-cAMP; TBI, traumatic brain injury; Mel, melatonin; S,
saline; ns, not significant.
Brain anatomical map stained with
DAPI. The corresponding brain anatomical map stained with DAPI
(blue). The position indicated by the white box is the amygdala.
DAPI, 4',6-diamidino-2-phenylindole.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Medical Science
Research Project of Hebei Province (grant no. 20211745).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LLX and ZZL performed the experiments and drafted
the manuscript. SSL, YJF and MMQ helped to conduct the design of
study and acquired the data. ZZL performed the statistical
analyses. LLX conceived the study and revised the manuscript. MMQ
and ZZL confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Review
Board of Cangzhou Central Hospital [Cangzhou, China; approval no.
2020-013-02(Z)]). All animal experiments were performed according
to the standards of the Institutional Animal Care and Use Committee
at the Cangzhou Central Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Studlack PE, Keledjian K, Farooq T,
Akintola T, Gerzanich V, Simard JM and Keller A: Blast-induced
brain injury in rats leads to transient vestibulomotor deficits and
persistent orofacial pain. Brain Inj. 32:1866–1878. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Capizzi A, Woo J and Verduzco-Gutierrez M:
Traumatic brain injury: An overview of epidemiology,
pathophysiology, and medical management. Med Clin North Am.
104:213–238. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yatsiv I, Grigoriadis N, Simeonidou C,
Stahel PF, Schmidt OI, Alexandrovitch AG, Tsenter J and Shohami E:
Erythropoietin is neuroprotective, improves functional recovery,
and reduces neuronal apoptosis and inflammation in a rodent model
of experimental closed head injury. FASEB J. 19:1701–1703.
2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Adibhatla RM and Hatcher JF: Lipid
oxidation and peroxidation in CNS health and disease: From
molecular mechanisms to therapeutic opportunities. Antioxid Redox
Signal. 12:125–169. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Adhikari A, Lerner TN, Finkelstein J, Pak
S, Jennings JH, Davidson TJ, Ferenczi E, Gunaydin LA, Mirzabekov
JJ, Ye L, et al: Basomedial amygdala mediates top-down control of
anxiety and fear. Nature. 527:179–185. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jayakar R, Tone EB, Crosson B, Turner JA,
Anderson PL, Phan KL and Klumpp H: Amygdala volume and social
anxiety symptom severity: Does segmentation technique matter?
Psychiatry Res Neuroimaging. 295(111006)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ji J, Kline AE, Amoscato A, Samhan-Arias
AK, Sparvero LJ, Tyurin VA, Tyurina YY, Fink B, Manole MD, Puccio
AM, et al: Lipidomics identifies cardiolipin oxidation as a
mitochondrial target for redox therapy of brain injury. Nat
Neurosci. 15:1407–1413. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Hill RL, Singh IN, Wang JA, Kulbe JR and
Hall ED: Protective effects of phenelzine administration on
synaptic and non-synaptic cortical mitochondrial function and lipid
peroxidation-mediated oxidative damage following TBI in young adult
male rats. Exp Neurol. 2020(113322)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chiu CC, Liao YE, Yang LY and Wang JY,
Tweedie D, Karnati HK, Greig NH and Wang JY: Neuroinflammation in
animal models of traumatic brain injury. J Neurosci Methods.
272:38–49. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Maas AI, Roozenbeek B and Manley GT:
Clinical trials in traumatic brain injury: Past experience and
current developments. Neurotherapeutics. 7:115–126. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Koh PO: Melatonin regulates the
calcium-buffering proteins, parvalbumin and hippocalcin, in
ischemic brain injury. J Pineal Res. 53:358–365. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ding K, Wang H, Xu J, Li T, Zhang L, Ding
Y, Zhu L, He J and Zhou M: Melatonin stimulates antioxidant enzymes
and reduces oxidative stress in experimental traumatic brain
injury: The Nrf2-ARE signaling pathway as a potential mechanism.
Free Radic Biol Med. 73:1–11. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang Z, Ma C, Meng CJ, Zhu GQ, Sun XB, Huo
L, Zhang J, Liu HX, He WC, Shen XM, et al: Melatonin activates the
Nrf2-ARE pathway when it protects against early brain injury in a
subarachnoid hemorrhage model. J Pineal Res. 53:129–137.
2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Osier N, McGreevy E, Pham L, Puccio A, Ren
D, Conley YP, Alexander S and Dixon CE: Melatonin as a therapy for
traumatic brain injury: A review of published evidence. Int J Mol
Sci. 19(1539)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rehman SU, Ikram M, Ullah N, Alam SI, Park
HY, Badshah H, Choe K and Kim MO: Neurological Enhancement effects
of melatonin against brain injury-induced oxidative stress,
neuroinflammation, and neurodegeneration via AMPK/CREB signaling.
Cells. 8(760)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cheung RT, Tipoe GL, Tam S, Ma ES, Zou LY
and Chan PS: Preclinical evaluation of pharmacokinetics and safety
of melatonin in propylene glycol for intravenous administration. J
Pineal Res. 41:337–343. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kaur C, Sivakumar V, Robinson R, Foulds
WS, Luu CD and Ling EA: Neuroprotective effect of melatonin against
hypoxia-induced retinal ganglion cell death in neonatal rats. J
Pineal Res. 54:190–206. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zyuz'kov GN, Miroshnichenko LA, Polyakova
TY, Stavrova LA, Simanina EV, Agafonov VI and Zhdanov VV:
Participation of cAMP/PKA-Mediated signaling pathways in functional
activity of regeneration-competent cells in the nervous tissue
under conditions of ethanol-induced neurodegeneration. Bull Exp
Biol Med. 167:723–727. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gao X, Zhang X, Cui L, Chen R, Zhang C,
Xue J, Zhang L, He W, Li J, Wei S, et al: Ginsenoside Rb1 promotes
motor functional recovery and axonal regeneration in post-stroke
mice through cAMP/PKA/CREB signaling pathway. Brain Res Bull.
154:51–60. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ye J, Yin Y, Liu H, Fang L, Tao X, Wei L,
Zuo Y, Yin Y, Ke D and Wang JZ: Tau inhibits PKA by nuclear
proteasome-dependent PKAR2α elevation with suppressed CREB/GluA1
phosphorylation. Aging Cell. 19(e13055)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ma CL, Li L, Yang GM, Zhang ZB, Zhao YN,
Zeng XF, Zhang DX, Yu Y, Shi ZJ, Yan QW, et al: Neuroprotective
effect of gastrodin in methamphetamine-induced apoptosis through
regulating cAMP/PKA/CREB pathway in cortical neuron. Hum Exp
Toxicol. 39:1118–1129. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yang L, Shi LJ, Yu J and Zhang YQ:
Activation of protein kinase A in the amygdala modulates
anxiety-like behaviors in social defeat exposed mice. Mol Brain.
9(3)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sung JY, Bae JH, Lee JH, Kim YN and Kim
DK: The melatonin signaling pathway in a long-term memory in vitro
study. Molecules. 23(737)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li C, Chen T, Zhou H, Feng Y, Hoi MPM, Ma
D, Zhao C, Zheng Y and Lee SMY: BHDPC is a novel neuroprotectant
that provides anti-neuroinflammatory and neuroprotective effects by
inactivating NF-κB and activating PKA/CREB. Front Pharmacol.
25(614)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ding K, Xu J, Wang H, Zhang L, Wu Y and Li
T: Melatonin protects the brain from apoptosis by enhancement of
autophagy after traumatic brain injury in mice. Neurochem Int.
91:46–54. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Song J, Cheon SY, Lee WT, Park KA and Lee
JE: PKA Inhibitor H89
(N-[2-p-bromocinnamylamino-ethyl]-5-isoquinolinesulfonamide)
attenuates synaptic dysfunction and neuronal cell death following
ischemic injury. Neural Plast. 2015(374520)2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Salehi F, Hosseini-Zare MS, Aghajani H,
Seyedi SY, Hosseini-Zare MS and Sharifzadeh M: Effect of
bucladesine, pentoxifylline, and H-89 as cyclic adenosine
monophosphate analog, phosphodiesterase, and protein kinase A
inhibitor on acute pain. Fundam Clin Pharmacol. 31:411–419.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shishido H, Ueno M, Sato K, Matsumura M,
Toyota Y, Kirino Y, Tamiya T, Kawai N and Kishimoto Y: Traumatic
brain injury by weight-drop method causes transient amyloid-β
deposition and acute cognitive deficits in mice. Behav Neurol.
2019(3248519)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Oh HM, Lee JS, Kim SW, Oh YT, Kim WY, Lee
SB, Cho YR, Jeon YJ, Cho JH and Son CG: Uwhangchungsimwon, A
standardized herbal drug, exerts an anti-depressive effect in a
social isolation stress-induced mouse model. Front Pharmacol.
10(1674)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kondo A, Shahpasand K, Mannix R, Qiu J,
Moncaster J, Chen CH, Yao Y, Lin YM, Driver JA, Sun Y, et al:
Antibody against early driver of neurodegeneration cis P-tau blocks
brain injury and tauopathy. Nature. 523:431–436. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tajiri N, Acosta SA, Shahaduzzaman M,
Ishikawa H, Shinozuka K, Pabon M, Hernandez-Ontiveros D, Kim DW,
Metcalf C, Staples M, et al: Intravenous transplants of human
adipose-derived stem cell protect the brain from traumatic brain
injury-induced neurodegeneration and motor and cognitive
impairments: Cell graft biodistribution and soluble factors in
young and aged rats. J Neurosci. 34:313–326. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yu S, Kaneko Y, Bae E, Stahl E, Wang Y,
van Loveren H, Sanberg PR and Borlongan CV: Severity of controlled
cortical impact traumatic brain injury in rats and mice dictates
degree of behavioral deficits. Brain Res. 1287:157–163.
2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chen H, Sun X, Yang X, Hou Y, Yu X, Wang
Y, Wu J, Liu D, Wang H, Yu J and Yi W: Dexmedetomidine reduces
ventilator-induced lung injury (VILI) by inhibiting Toll-like
receptor 4 (TLR4)/nuclear factor (NF)-κB signaling pathway. Bosn J
Basic Med Sci. 18:162–169. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Popovitz J, Mysore SP and Adwanikar H:
Long-term effects of traumatic brain injury on anxiety-like
behaviors in mice: Behavioral and neural correlates. Front Behav
Neurosci. 13(6)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chitturi J, Li Y, Santhakumar V and
Kannurpatti SS: Early behavioral and metabolomic change after mild
to moderate traumatic brain injury in the developing brain.
Neurochem Int. 120:75–86. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Šimić G, Tkalčić M, Vukić V, Mulc D,
Španić E, Šagud M, Olucha-Bordonau FE, Vukšić M and R Hof P:
Understanding emotions: Origins and roles of the amygdala.
Biomolecules. 11(823)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Herrington JD, Miller JS, Pandey J and
Schultz RT: Anxiety and social deficits have distinct relationships
with amygdala function in autism spectrum disorder. Soc Cogn Affect
Neurosci. 11:907–914. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sullivan PG, Sebastian AH and Hall ED:
Therapeutic window analysis of the neuroprotective effects of
cyclosporine A after traumatic brain injury. J Neurotrauma.
28:311–318. 2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Neal M and Richardson JR: Epigenetic
regulation of astrocyte function in neuroinflammation and
neurodegeneration. Biochim Biophys Acta Mol Basis Dis.
1864:432–443. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chávez CE, Oyarzún JE, Avendaño BC,
Mellado LA, Inostroza CA, Alvear TF and Orellana JA: The opening of
connexin 43 hemichannels alters hippocampal astrocyte function and
neuronal survival in prenatally LPS-Exposed adult offspring. Front
Cell Neurosci. 13(460)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Fujita A, Yamaguchi H, Yamasaki R, Cui Y,
Matsuoka Y, Yamada KI and Kira JI: Connexin 30 deficiency
attenuates A2 astrocyte responses and induces severe
neurodegeneration in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
hydrochloride Parkinson's disease animal model. J
Neuroinflammation. 15(227)2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Witcher KG, Bray CE, Dziabis JE, McKim DB,
Benner BN, Rowe RK, Kokiko-Cochran ON, Popovich PG, Lifshitz J,
Eiferman DS and Godbout JP: Traumatic brain injury-induced neuronal
damage in the somatosensory cortex causes formation of rod-shaped
microglia that promote astrogliosis and persistent
neuroinflammation. Glia. 66:2719–2736. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Gao TL, Yuan XT, Yang D, Dai HL, Wang WJ,
Peng X, Shao HJ, Jin ZF and Fu ZJ: Expression of HMGB1 and RAGE in
rat and human brains after traumatic brain injury. J Trauma Acute
Care Surg. 72:643–649. 2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Hirsch EC, Breidert T, Rousselet E, Hunot
S, Hartmann A and Michel PP: The role of glial reaction and
inflammation in Parkinson's disease. Ann N Y Acad Sci. 991:214–228.
2003.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ley EJ, Clond MA, Singer MB, Shouhed D and
Salim A: IL6 deficiency affects function after traumatic brain
injury. J Surg Res. 170:253–256. 2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Baratz R, Tweedie D, Wang JY, Rubovitch V,
Luo W, Hoffer BJ, Greig NH and Pick CG: Transiently lowering tumor
necrosis factor-alpha synthesis ameliorates neuronal cell loss and
cognitive impairments induced by minimal traumatic brain injury in
mice. J Neuroinflammation. 12(45)2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Xing J, Han D, Xu D, Li X and Sun L: CREB
Protects against temporal lobe epilepsy associated with cognitive
impairment by controlling oxidative neuronal damage. Neurodegener
Dis. 19:225–237. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Yang Y, Ma S, Wei F, Liang G, Yang X,
Huang Y, Wang J and Zou Y: Pivotal role of cAMP-PKA-CREB signaling
pathway in manganese-induced neurotoxicity in PC12 cells. Environ
Toxicol. 34:1052–1062. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Ciani E, Guidi S, Della Valle G, Perini G,
Bartesaghi R and Contestabile A: Withdrawal: Nitric oxide protects
neuroblastoma cells from apoptosis induced by serum deprivation
through cAMP-response element-binding protein (CREB) activation. J
Biol Chem. 295(3391)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Purves GI, Kamishima T, Davies LM, Quayle
JM and Dart C: Exchange protein activated by cAMP (Epac) mediates
cAMP-dependent but protein kinase A-insensitive modulation of
vascular ATP-sensitive potassium channels. J Physiol.
587:3639–3650. 2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Aesoy R, Muwonge H, Asrud KS, Sabir M,
Witsoe SL, Bjornstad R, Kopperud RK, Hoivik EA, Doskeland SO and
Bakke M: Deletion of exchange proteins directly activated by cAMP
(Epac) causes defects in hippocampal signaling in female mice. PLoS
One. 13(e0200935)2018.PubMed/NCBI View Article : Google Scholar
|





















