Introduction
Acute hepatic injury is a common liver disease that
may lead to hepatic failure. This life-threatening condition may
irreversibly affect the normal liver function (1). Several harmful substances can cause
acute hepatic injury, including carbon tetrachloride
(CCl4), alcohol and acetaminophen-based medicines
(2). CCl4 is a chemical
hepatic toxin that induces liver cell damage via the metabolic
activation of cytochrome P4502E1(3). In addition, CCl4 triggers
metabolism to form free radicals, which in turn can promote the
generation of lipid peroxidation products, thus promoting
hepatocyte apoptosis and necrosis (4). Therefore, it has been reported that
chemicals or drugs with antioxidant activities may possess
significant potential for alleviating CCl4-induced
hepatic damage (5). The current
therapeutic approaches for treating liver diseases primarily focus
on the immediate discontinuation of drugs that affect the nature of
hepatic damage and the administration of agents with
anti-inflammatory properties, acting against free radicals, and
those that promote liver cell metabolism, repair and regeneration
(6). However, specific and
effective treatment strategies against liver diseases are still
lacking. Due to its poor prognosis and high mortality, hepatic
injury has become a focus in international research (7).
Rosiglitazone (RSG), an insulin sensitizer, has been
used to treat type 2 diabetes in clinical practice (8). It can increase the insulin
sensitivity of tissues such as skeletal muscle and liver, and
improve the utilization of glucose (9). In addition, RSG is a highly selective
synthetic ligand of peroxisome proliferator-activated receptor γ
(PPARγ); binding of RSG with PPARγ has been shown to upregulate the
expression of PPARγ to exert its biological effects (10). A previous study on animal models of
status epilepticus showed that treatment with RSG could activate
PPARγ, which in turn may significantly inhibit the production of
reactive oxygen species (ROS) and lipid peroxidation, while
increasing the antioxidant effects of superoxide dismutase (SOD)
and glutathione (GSH) (11). In
addition, pretreatment of thrombin-induced microglial cells with
RSG has been reported to increase the expression of PPARγ and heme
oxygenase 1 (HO-1), thereby increasing their antioxidant ability
(12). Furthermore, another study
demonstrated that RSG could upregulate nuclear factor erythroid
2-related factor 2 (Nrf2) and heme oxygenase 1 (HO-1) in a
PPARγ-dependent manner, eliminate excess ROS production and
attenuate the oxidative stress response of glucose-induced liver
cells (13). The aforementioned
studies indicated that PPARγ and its agonist RSG could serve a
significant role in regulating the redox state both in vitro
and in vivo.
The use of CCl4 as an inducer for
establishing a hepatic injury research model has been widely
applied and certified (14).
Therefore, in the present study, CCl4 was used to induce
acute hepatic injury in mice. Subsequently, the effects of RSG on
hepatic injury and its underlying mechanism of action were
explored. The findings of the current study could provide a novel
direction for the treatment of hepatic injury.
Materials and methods
Animals and reagents
All animal experiments were performed in accordance
with the National Institutes of Health Guidelines for the Handling
of Laboratory Animals (15) and
were approved by the Ethics Committee of The Eighth Affiliated
Hospital of Xinjiang Medical University (Urumqi, China; approval
no. 2020-035). A total of 40 male Kunming (KM) mice (weight, 20±2
g; 4 weeks-old; Beijing Vital River Laboratory Animal Technology
Co., Ltd.) were acclimated for 1 week in an environment of 25˚C
with a 12-h light/dark cycle, relative humidity of 55%, and free
access to food and water. Subsequently, 1 ml CCl4
(Sinopharm Chemical Reagent Co., Ltd.) was dissolved in 100 ml
olive oil and mixed well to prepare a 1% CCl4 solution.
RSG was purchased from Chengdu Hengrui Pharmaceutical Co., Ltd.
Establishment of the mouse model
KM mice were divided into the following four groups:
i) Control; ii) RSG; iii) CCl4; and iv) RSG +
CCl4 groups. Mice in the control and CCl4
group were separately treated with an intraperitoneal injection of
a single dose of saline or 1% CCl4 (10 ml/kg),
respectively. Prior to CCl4 injection, mice in the RSG +
CCl4 group were treated with 10 mg/kg RSG by oral gavage
for 5 consecutive days. Mice in the RSG group were treated as
outlined in the RSG + CCl4 group, except with an
additional (10 ml/kg) saline injection in place of CCl4.
All mice were fasted and had only free access to water for 24 h
after modeling. Subsequently, mice were anesthetized using sodium
pentobarbital (100 mg/kg; intraperitoneal) and the abdominal cavity
was opened through an incision along the midline of the abdomen to
expose the abdominal aorta. Following blood collection (0.5 ml)
with a syringe, the mice were sacrificed by cervical dislocation.
The liver tissues were immediately obtained, washed with PBS and
were then stored at -80˚C.
Hematoxylin and eosin (H&E)
staining
The liver tissue samples were fixed in 4%
paraformaldehyde at 4˚C for 6 h, dehydrated with gradient alcohol
and embedded in paraffin. Subsequently, the tissue samples were cut
into 5-µm slices. Sections were immersed in xylene, rehydrated with
gradient alcohol and water, and stained with hematoxylin for 3 min
and eosin for 3 min (Beijing Solarbio Science & Technology Co.,
Ltd.) at room temperature. Following dehydration with gradient
alcohol, the tissues were treated with xylene for 3 min.
Physiological changes in the tissue samples were observed under a
light microscope (magnification, x400; Olympus Corporation).
Detection of alanine aminotransferase
(ALT) and aspartate aminotransferase (AST)
The blood was allowed to stand at 4˚C for 30 min and
the serum was collected following centrifugation at 1,000 x g for
10 min at 4˚C. The serum levels of ALT and AST were measured using
ALT (cat. no. C009-1-1) and AST assay kits (cat. no. C010-1-1; both
from Nanjing Jiancheng Bioengineering Institute), according to the
manufacturer's instructions. Briefly, the serum sample was added to
a tube, mixed with ALT or AST matrix solution for 30 min in a 37˚C
water bath followed by the addition of a color developing agent.
The reaction was terminated following incubation of the samples
with stop solution for 20 min in a water bath at 37˚C. After
incubation for 5 min at room temperature, a microplate reader
(Thermo Fisher Scientific, Inc.) was used to measure the optical
density (OD) value of each well at a wavelength of 505 nm.
Detection of indices of hepatic
function
The mass of tissue samples used to measure indices
of hepatic function was calculated according to the formula: Liver
mass (g): volume (ml)=1:9. Subsequently, the tissue samples were
added into pre-cooled physiological saline and homogenized on ice.
The homogenate was then centrifuged at 1,500 x g at 4˚C for 10 min
and the supernatant was collected. SOD (cat. no. A001-1), catalase
(CAT; cat. no. A007-1-1), GSH (cat. no. A005-1), NO (cat. no.
A012-1-2) and malondialdehyde (MDA; cat. no. A003-1-1) assay kits
(all from Nanjing Jiancheng Bioengineering Institute) were used to
assess the content of the corresponding indices, according to the
manufacturer's instructions. The OD values were measured using a
microplate reader (Thermo Fisher Scientific, Inc.) at a wavelength
of 550 (for SOD and NO), 405 (for CAT), 412 (for GSH) and 532 nm
(for MDA).
The determination of ROS was performed using a ROS
assay kit (cat. no. HR7814; Beijing BioRab Technology Co. Ltd.).
The tissue sections were soaked in the cleaning solution for 15 sec
and then the residual solution on the surfaces were wiped off. The
sections were incubated in the staining working solution for 30 min
at 37˚C in the dark. After washing twice with PBS, the results were
observed under a fluorescence microscope (magnification, x100;
Olympus Corporation).
ELISA
Serum samples were collected as aforementioned. The
secretion levels of TNF-α (cat. no. 88-7324), IL-6 (cat. no.
88-7064) and IL-1β (cat. no. 88-7013; all from Thermo Fisher
Scientific, Inc.) in the serum were determined using the
corresponding ELISA kits according to the manufacturer's
instructions. Finally, a microplate reader (Thermo Fisher
Scientific, Inc.) was used to measure the OD values in each well at
a wavelength of 450 nm.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from tissues using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). The extracted RNA was reverse transcribed into cDNA using
the PrimeScript RT reagent kit (Takara Bio, Inc.) according to the
manufacturer's protocol. The mRNA expression levels were quantified
using the QuantiTect SYBR Green PCR kit (Qiagen, Inc.) on the ABI
7500 system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The qPCR amplification conditions were as follows: 95˚C for 10 min,
followed by 40 cycles at 95˚C for 30 sec, 64˚C for 34 sec and 72˚C
for 30 sec. The relative gene expression levels were normalized to
that of the housekeeping gene, GAPDH. The 2-ΔΔCq method
was utilized (16). The primer
sequences used for qPCR are listed in Table I.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene | Sequence |
|---|
| TNF-α | F:
5'-GCTGAGCTCAAACCCTGGTA-3' |
| | R:
5'-CGGACTCCGCAAAGTCTAAG-3' |
| IL-6 | F:
5'-CCAGTTGCCTTCTTGGGACTG-3' |
| | R:
5'-CAGGTCTGTTGGGAGTGGTATCC-3' |
| IL-1β | F:
5'-TGCCACCTTTTGACAGTGATG-3' |
| | R:
5'-AGTCACAGAGGATGGGCTCT-3' |
| Nrf2 | F:
5'-ACAGTGCTCCTATGCGTGAA-3' |
| | R:
5'-GAGCCTCTAAGCGGCTTGAA-3' |
| NQO1 | F:
5'-GTCCATTCCAGCTGACAACCA-3' |
| | R:
5'-TTGCCCTGAGGCTCCTAATC-3' |
| HO-1 | F:
5'-TGCTAGCCTGGTGCAAGATA-3' |
| | R:
5'-GCCAACAGGAAGCTGAGAGT-3' |
| GAPDH | F:
5'-TGTTTCCTCGTCCCGTAGA-3' |
| | R:
5'-ATCTCCACTTTGCCACTGC-3' |
Western blot analysis
Total proteins were extracted from liver tissue
samples using RIPA lysis buffer (Beyotime Institute of
Biotechnology). The protein concentration was measured using a BCA
protein assay kit (cat. no. A045-3-2; Nanjing Jiancheng
Bioengineering Institute). Total protein extracts (20 µg/lane) were
then separated by SDS-PAGE on 10% gels and were transferred onto
PVDF membranes. After blocking with 5% skimmed milk at room
temperature for 2 h, the membranes were incubated with primary
antibodies against cleaved caspase-8 (cat. no. 9748; dilution,
1:1,000), caspase-8 (cat. no. 4927; dilution, 1:1,000; both from
Cell Signaling Technology, Inc.), cleaved caspase-3 (cat. no.
ab32042; dilution, 1:1,000), caspase-3 (cat. no. ab184787;
dilution, 1:2,000), cleaved poly(ADP-ribose) polymerase (PARP; cat.
no. ab32064; dilution, 1:1,000), PARP (cat. no. ab191217; dilution,
1:1,000), PPARγ (cat. no. ab178860; dilution, 1:1,000), Nrf2 (cat.
no. ab62352; dilution, 1:1,000), NAD(P)H quinone oxidoreductase 1
(NQO1; cat. no. ab213239; dilution, 1:500), HO-1 (cat. no. ab52947;
dilution, 1:2,000), NOD-like receptor protein 3 (NLRP3; cat. no.
ab263899; dilution, 1:1,000), caspase-1 (cat. no. ab138483;
dilution, 1:1,000), IL-1β (cat. no. ab234437; dilution, 1:1,000),
apoptosis-associated speck-like protein (ASC; cat. no. ab283684;
dilution, 1:1,000) and the loading control, β-actin (cat. no.
ab8226; dilution, 1:1,000; all from Abcam) at 4˚C overnight.
Following incubation with the primary antibodies, the membranes
were incubated with HRP-conjugated anti-rabbit (cat. no. ab97051;
dilution, 1:10,000) or anti-mouse (cat. no. ab6728; dilution,
1:2,000; both from Abcam) antibodies at 37˚C for 2 h. The bands
were visualized with an ECL reagent (MilliporeSigma) at room
temperature for 2 min. The density of each band was semi-quantified
using ImageJ software (v1.8; National Institutes of Health).
Statistical analysis
All experiments were repeated three times. Data are
expressed as the mean ± SD. One-way ANOVA followed by Tukey's post
hoc test was carried out to compare the differences among multiple
groups. Statistical analysis was performed using GraphPad Prism 8.0
software (GraphPad Software, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
RSG attenuates hepatic injury
The changes in liver histology among the four groups
of mice were evaluated by H&E staining. The results showed that
hepatocytes in the control and RSG groups were a normal shape and
were tightly arranged. In the CCl4 group, the cell
arrangement was disordered, and inflammatory cell infiltration and
cell necrosis were observed. However, pretreatment of
CCl4-treated mice with RSG markedly improved
inflammatory infiltration. In addition, the cell arrangement was
more compact in the RSG + CCl4 group compared with that
in the CCl4 group (Fig.
1A). Furthermore, no differences were observed in the serum
levels of ALT and AST between the control and RSG groups. However,
in the CCl4 group, the levels of both transaminases were
significantly increased, whereas the levels were reduced following
pretreatment with RSG (Fig. 1B).
Subsequently, the levels of the hepatic biochemical indicators SOD,
CAT, GSH, MDA, NO and ROS were determined in each group of mice
using the corresponding kits. No statistically significant
differences in the activity of the aforementioned indicators were
observed between the control and RSG groups. However, the levels of
SOD, CAT and GSH were reduced, whereas those of MDA and NO were
increased in the CCl4 group. This effect was abrogated
following pretreatment with RSG (Fig.
1C). In addition, the ROS fluorescence of the CCl4
group was significantly enhanced compared with that in the control
group and RSG pretreatment reduced the ROS fluorescence intensity
(Fig. 1D). These findings
suggested that pretreatment of mice with RSG could reduce the
severity of CCl4-induced hepatic injury.
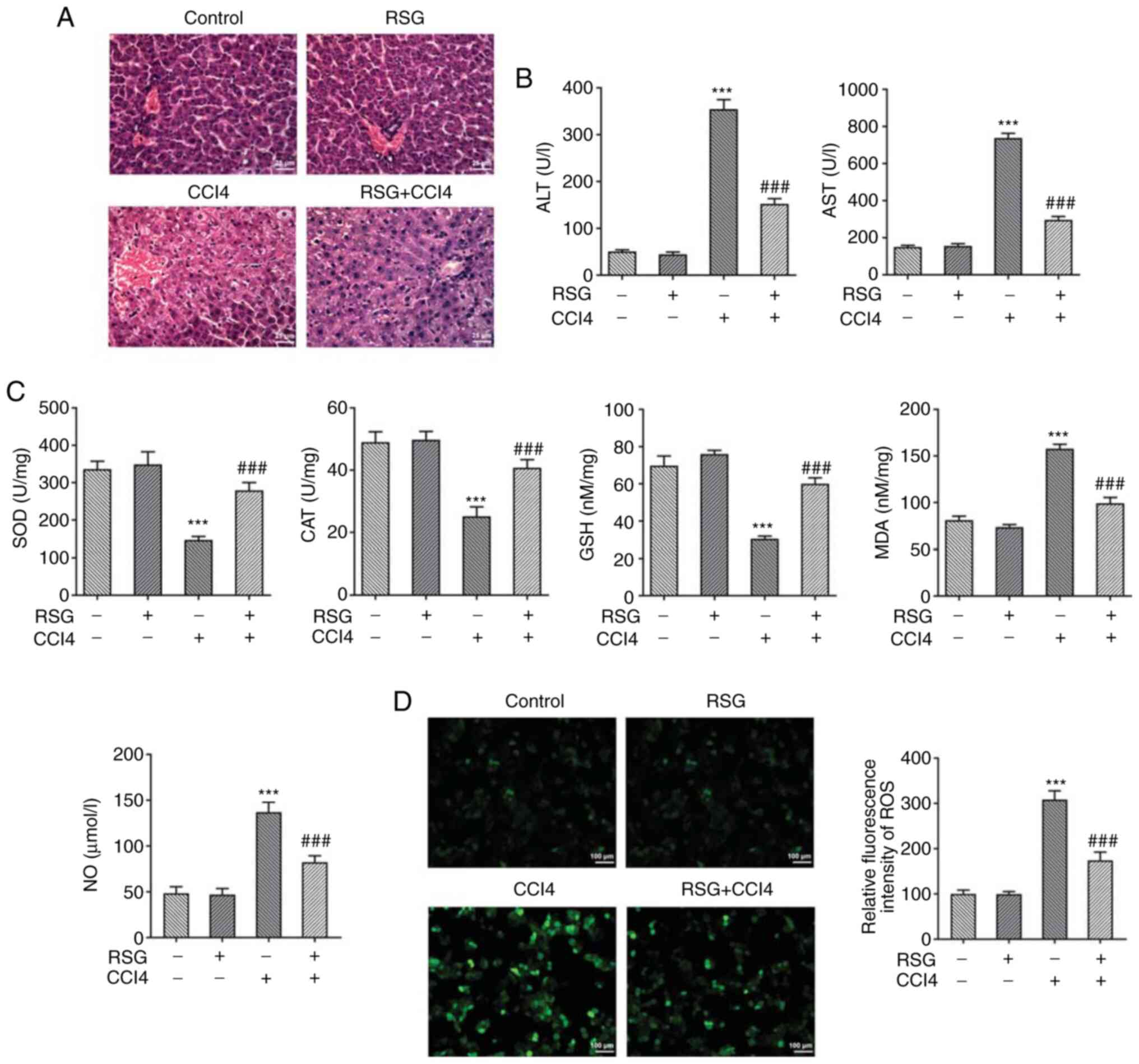 | Figure 1(A) Hepatic tissue samples from the
four groups of mice were evaluated by hematoxylin and eosin
staining (magnification, x400). (B) ALT and AST levels in the serum
samples from each group of mice were determined using the
corresponding kits. (C) Hepatic biochemical indicator levels (SOD,
CAT, GSH, MDA and NO) in each group of mice were measured using the
corresponding kits. (D) ROS levels in each group of mice were
measured using an ROS assay kit. ***P<0.001 vs. the
control group; ###P<0.001 vs. the CCl4
group. RSG, rosiglitazone; CCl4, carbon tetrachloride;
ALT, alanine transaminase; AST, aspartate transaminase; SOD,
superoxide dismutase; CAT, catalase; GSH, glutathione; MDA,
malondialdehyde; ROS, reactive oxygen species. |
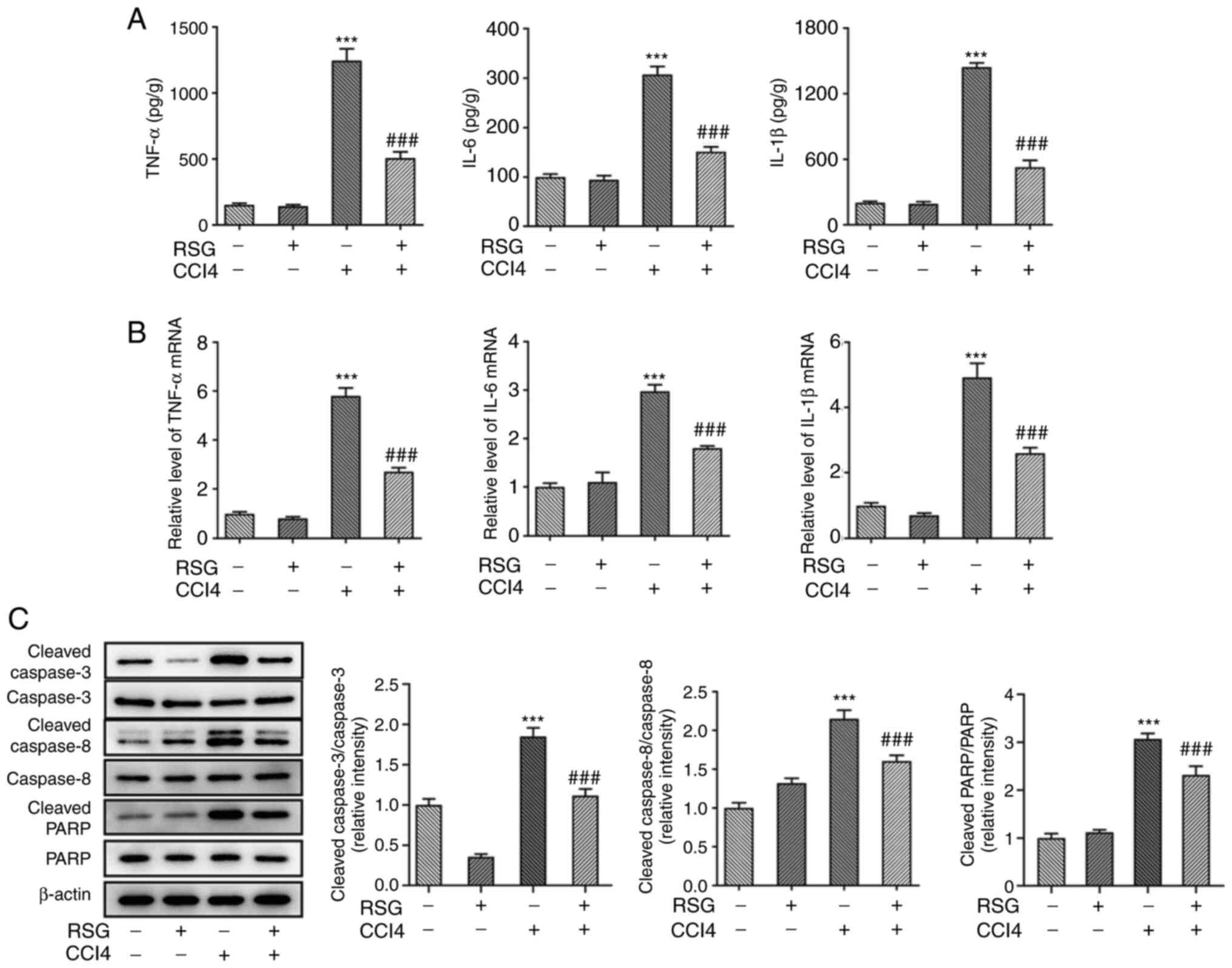
RSG may abrogate the effects of
CCl4 on hepatocyte inflammation and apoptosis
Since hepatic injury is closely associated with
inflammation, the present study aimed to determine the expression
levels of inflammatory factors in serum samples and liver tissues
isolated from mice in different groups. Therefore, the secretion
levels of TNF-α, IL-6 and IL-1β in the serum were measured using
ELISA. The secretion levels of the aforementioned inflammatory
factors were all enhanced in the CCl4 group compared
with those in the control group. However, the levels of TNF-α, IL-6
and IL-1β were reduced in the RSG + CCl4 group compared
with the CCl4 group (Fig.
2A). The mRNA expression levels of TNF-α, IL-6 and IL-1β in the
liver tissues were detected using RT-qPCR. No statistically
significant differences were observed in the expression levels of
the inflammatory factors between the control and RSG groups,
whereas the increase in the expression of these factors was less
prominent in the RSG + CCl4 group compared with the
CCl4 group (Fig. 2B).
Additionally, the expression levels of apoptosis-related proteins
were evaluated by western blot analysis. Cleaved capase-3, cleaved
caspase-8 and cleaved PARP were all upregulated in the
CCl4 group compared with in the control group. In
addition, the expression levels of the apoptosis-related proteins
were reduced in the RSG + CCl4 group compared with those
in the CCl4 group, thus suggesting the RSG may protect
hepatocytes from apoptosis to a certain degree (Fig. 2C).
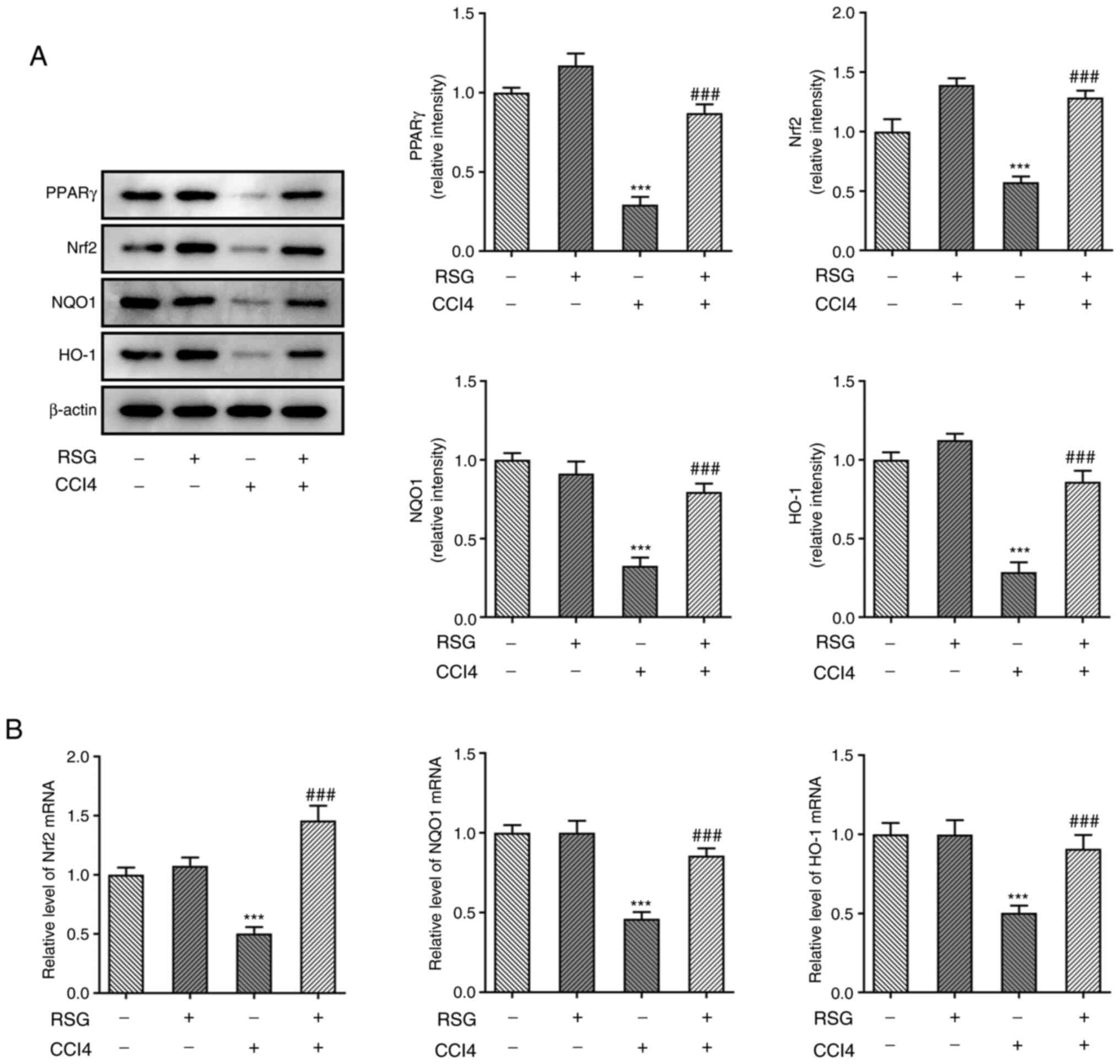
RSG exerts its effects on hepatocyte
injury via the Nrf2 and NLRP3 signaling pathways
The aforementioned results indicated that RSG could
alleviate hepatic injury; therefore, subsequent experiments focused
on uncovering the possible mechanism underlying the effects of RSG
on hepatic injury. The expression levels of PPARγ and of proteins
associated with the Nrf2 signaling pathway were determined using
western blot analysis. The results showed that PPARγ, Nrf2, NQO1
and HO-1 were downregulated in the CCl4 group compared
with in the control group. However, pretreatment with RSG reversed
the CCl4-mediated decreased expression of these factors
(Fig. 3A). The mRNA expression
levels of Nrf2, NQO1 and HO-1 were measured by RT-qPCR.
Consistently, the expression levels of Nrf2, NQO1 and HO-1 were
downregulated in the CCl4 group and were restored
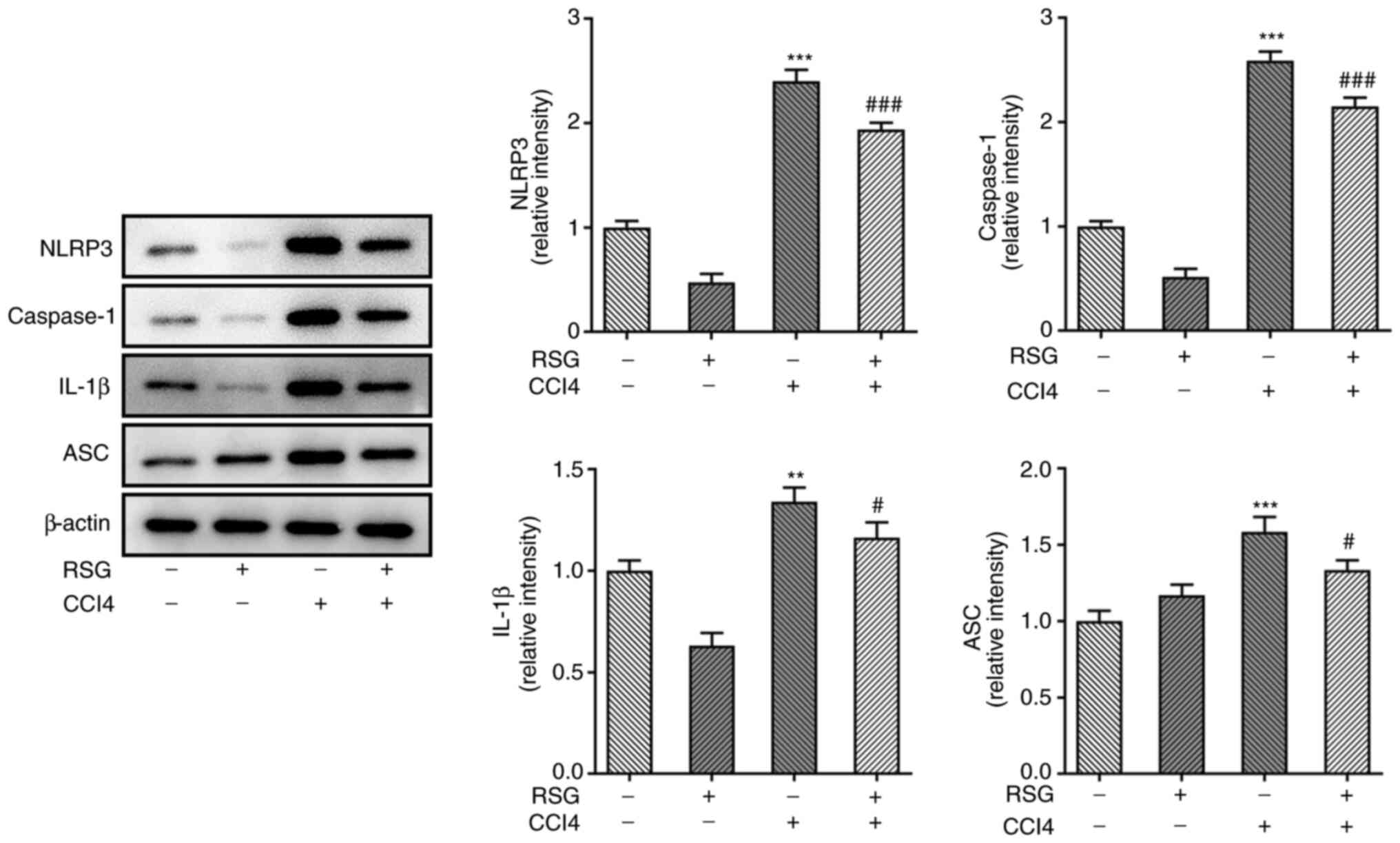
following pretreatment with RSG (Fig.
3B). Additionally, the expression levels of the NLRP3 signaling
pathway-related proteins were detected using western blot analysis.
NLRP3, caspase-1, IL-1β and ASC were significantly upregulated in
the CCl4 group compared with those in the control group.
However, the expression these factors was significantly decreased
in the RSG + CCl4 group compared with in the
CCl4 group (Fig.
4).
Discussion
Currently, inhibiting oxidative stress and
inflammation is considered a significant strategy for treating
hepatic injury (17). It has been
reported that the CCl4-induced chemical hepatic injury
model mimics the pathology of human viral hepatic injury (18). Therefore, CCl4 was
selected to establish a mouse model of hepatic injury. In the
present study, following histopathological examination, the serum
levels of ALT and AST were measured. ALT and AST are two enzymes
that play a significant role in amino acid metabolism and are
widely present in hepatocytes. The inflammation-, necrosis- or
poisoning-mediated hepatocyte injury can change the permeability of
hepatic cellular membrane, thus promoting the overflow of ALT and
AST from the cells into the blood circulation (19). In the present study, the serum
levels of ALT and AST were significantly increased in
CCl4-treated mice, whereas pretreatment with RSG
abrogated this effect. Furthermore, the levels of several hepatic
indicators were determined, including SOD, CAT, GSH, MDA, NO and
ROS. The activity of SOD, an active protease containing metal
elements, reflects the organismal ability to scavenge oxygen free
radicals (20). CAT, a scavenger
enzyme removes hydrogen peroxide from the body (21). In addition, GSH, a small peptide
composed of three amino acids, transforms free radicals to acidic
substances, thereby accelerating the excretion of free radicals
(22). MDA, the final product of
lipid peroxidation, can destroy the structural and functional
integrity of cell membranes, and the levels of MDA indirectly
reflect the degree of oxidative stress (23). The results of the current study
showed that RSG could reverse the CCl4-mediated decrease
in the levels of SOD, CAT and GSH, and the increase in levels of
MDA, NO and ROS, thus suggesting that RSG could exert an inhibitory
effect on oxidative stress. In addition, the reduced levels of
inflammatory factors (24) and
apoptosis-related proteins (25)
indicated that RSG could likewise alleviate inflammation and
apoptosis in the liver.
The liver is rich in mitochondria and is the
dominant organ for the generation of ROS (26). Under normal conditions, ROS is
produced in hepatocytes as a by-product of normal metabolism and
detoxification. On the other hand, the antioxidant system is
involved in the immediate degradation of the newly generated ROS in
the liver (27). However, when the
production of ROS is continuous, the excessive amounts of ROS can
cause cell damage, eventually leading to the development of several
hepatic diseases (28). Nrf2 is a
key transcription factor that regulates the antioxidant stress
response in cells and activates the endogenous antioxidant response
(29). Under oxidative stress,
Nrf2 dissociates from kelch-like ECH-associated protein 1 and
dimerizes with several transcription factors to translocate to the
nucleus. In the nucleus, Nrf2 regulates the expression of
downstream factors, such as HO-1, NQO1 and other antioxidant
enzymes (30). It has been
reported that the Nrf2 signaling pathway is involved in the
antioxidant defense system of the liver via regulating liver
metabolism and detoxification, and promoting liver cell
regeneration (31). More
importantly, Nrf2 signaling is also involved in the pathogenesis of
numerous hepatic diseases, such as drug-induced hepatic injury
(32). A previous study
demonstrated that excessive use of drugs may cause oxidative injury
to the liver, thereby activating the Nrf2 pathway and promoting the
rapid translocation of Nrf2 to the nucleus. In turn, activated Nrf2
could regulate the expression of its downstream target genes to
exert its protective role in the liver (33). It has also been identified that the
activation of NLRP3 signaling serves a significant role in acute
hepatic injury (34). A previous
study on mouse models indicated that NLRP3 may serve a key role in
the modulation of hepatic inflammation and fibrosis (35). Additionally, NLRP3 may activate the
proteolytic cleavage of pro-caspase-1 into activated caspase-1 and
is involved in the mature IL-1β- and IL-18-mediated inflammatory
process. Mature IL-1β and IL-18 arise from the cytokine precursors
pro-IL-1β and pro-IL-18, respectively (36). The results of the present study
demonstrated that RSG could activate PPARγ and Nrf2, and inhibit
the activation of the NLRP3 inflammasome.
In conclusion, the present study revealed that RSG
could reduce the serum levels of ALT and AST, and indicated that it
may inhibit inflammation, oxidative stress and hepatocyte apoptosis
in a mouse model of hepatic injury. Notably, RSG could activate the
Nrf2 signaling pathway and inhibit activation of the NLRP3
inflammasome, thus exerting a protective effect against acute
hepatic injury. The current study may improve the understanding of
the mechanism underlying the effect of RSG on hepatic injury, thus
supporting the potential application of RSG in clinical practice.
However, the present study is limited, as it only represents a
preliminary mechanistic study; therefore, the mechanism of action
of the downstream factors involved in the aforementioned pathways
is worthy of further investigation.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Eighth
Affiliated Hospital of Xinjiang Medical University (grant no.
2020022).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MM and LM contributed to the conception of the
study. MM wrote the manuscript. LM, YM, BXM and MM all performed
the experiments. YM analyzed the data and BXM critically revised
the manuscript. LM and MM confirm the authenticity of all raw data.
All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
All animal experimental procedures were performed
according to the ethical guidelines of The Eighth Affiliated
Hospital of Xinjiang Medical University. All efforts were made to
minimize animal suffering. This study was approved by the
Institutional Animal Care and Use Committee of The Eighth
Affiliated Hospital of Xinjiang Medical University (approval no.
2020-035).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Coccolini F, Coimbra R, Ordonez C, Kluger
Y, Vega F, Moore EE, Biffl W, Peitzman A, Horer T, Abu-Zidan FM, et
al: Liver trauma: WSES 2020 guidelines. World J Emerg Surg.
15(24)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zou Y, Xiong JB, Ma K, Wang AZ and Qian
KJ: Rac2 deficiency attenuates CCl4-induced liver injury
through suppressing inflammation and oxidative stress. Biomed
Pharmacother. 94:140–149. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhang X, Kuang G, Wan J, Jiang R, Ma L,
Gong X and Liu X: Salidroside protects mice against CCl4-induced
acute liver injury via down-regulating CYP2E1 expression and
inhibiting NLRP3 inflammasome activation. Int Immunopharmacol.
85(106662)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cichoż-Lach H and Michalak A: Oxidative
stress as a crucial factor in liver diseases. World J
Gastroenterol. 20:8082–8091. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li S, Tan HY, Wang N, Zhang ZJ, Lao L,
Wong CW and Feng Y: The role of oxidative stress and antioxidants
in liver diseases. Int J Mol Sci. 16:26087–26124. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cong M, Zhao W, Liu T, Wang P, Fan X, Zhai
Q, Bao X, Zhang D, You H, Kisseleva T, et al: Protective effect of
human serum amyloid P on CCl4-induced acute liver injury in mice.
Int J Mol Med. 40:454–464. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tsai TH, Tam K, Chen SF, Liou JY, Tsai YC,
Lee YM, Huang TY and Shyue SK: Deletion of caveolin-1 attenuates
LPS/GalN-induced acute liver injury in mice. J Cell Mol Med.
22:5573–5582. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Stage TB, Christensen MH, Jørgensen NR,
Beck-Nielsen H, Brøsen K, Gram J and Frost M: Effects of metformin,
rosiglitazone and insulin on bone metabolism in patients with type
2 diabetes. Bone. 112:35–41. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li J, Xue YM, Zhu B, Pan YH, Zhang Y, Wang
C and Li Y: Rosiglitazone Elicits an Adiponectin-Mediated
Insulin-Sensitizing Action at the Adipose Tissue-Liver Axis in
Otsuka Long-Evans Tokushima Fatty Rats. J Diabetes Res.
2018(4627842)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ahn KO, Lim SW, Yang HJ, Li C, Sugawara A,
Ito S, Choi BS, Kim YS, Kim J and Yang CW: Induction of PPAR gamma
mRNA and protein expression by rosiglitazone in chronic
cyclosporine nephropathy in the rat. Yonsei Med J. 48:308–316.
2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yu X, Shao XG, Sun H, Li YN, Yang J, Deng
YC and Huang YG: Activation of cerebral peroxisome
proliferator-activated receptors gamma exerts neuroprotection by
inhibiting oxidative stress following pilocarpine-induced status
epilepticus. Brain Res. 1200:146–158. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gong P, Stewart D, Hu B, Li N, Cook J, Nel
A and Alam J: Activation of the mouse heme oxygenase-1 gene by
15-deoxy-Delta(12,14)-prostaglandin J(2) is mediated by the stress
response elements and transcription factor Nrf2. Antioxid Redox
Signal. 4:249–257. 2002.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang X, Wang Z, Liu JZ, Hu JX, Chen HL, Li
WL and Hai CX: Double antioxidant activities of rosiglitazone
against high glucose-induced oxidative stress in hepatocyte.
Toxicol In Vitro. 25:839–847. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ravan AP, Bahmani M, Ghasemi Basir HR,
Salehi I and Oshaghi EA: Hepatoprotective effects of Vaccinium
arctostaphylos against CCl4-induced acute liver injury in rats. J
Basic Clin Physiol Pharmacol. 28:463–471. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
National Research Council of the National
Academies: Guide for the Care and Use of Laboratory Animals. 8th
edition. The National Academies Press, Washington, DC, 2011.
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Farzanegi P, Dana A, Ebrahimpoor Z, Asadi
M and Azarbayjani MA: Mechanisms of beneficial effects of exercise
training on non-alcoholic fatty liver disease (NAFLD): Roles of
oxidative stress and inflammation. Eur J Sport Sci. 19:994–1003.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wei PC, Chang AN, Kao J, Du Z, Meyers RM,
Alt FW and Schwer B: Long Neural Genes Harbor Recurrent DNA Break
Clusters in Neural Stem/Progenitor Cells. Cell. 164:644–655.
2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Melchart D, Hager S, Albrecht S, Dai J,
Weidenhammer W and Teschke R: Herbal Traditional Chinese Medicine
and suspected liver injury: A prospective study. World J Hepatol.
9:1141–1157. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dormandy TL: Free-radical pathology and
medicine. A review. J R Coll Physicians Lond. 23:221–227.
1989.PubMed/NCBI
|
|
21
|
Popović B, Velimirović M, Stojković T,
Brajović G, De Luka SR, Milovanović I, Stefanović S, Nikolić D,
Ristić-Djurović JL, Petronijević ND and Trbovich AM: The influence
of ageing on the extrapineal melatonin synthetic pathway. Exp
Gerontol. 110:151–157. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
D'Agostino J, Zhang H, Kenaan C and
Hollenberg PF: Mechanism-based inactivation of human cytochrome
P450 2B6 by chlorpyrifos. Chem Res Toxicol. 28:1484–1495.
2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cui X, Gong J, Han H, He L, Teng Y, Tetley
T, Sinharay R, Chung KF, Islam T, Gilliland F, et al: Relationship
between free and total malondialdehyde, a well-established marker
of oxidative stress, in various types of human biospecimens. J
Thorac Dis. 10:3088–3097. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ohara M, Ohnishi S, Hosono H, Yamamoto K,
Yuyama K, Nakamura H, Fu Q, Maehara O, Suda G and Sakamoto N:
Extracellular vesicles from amnion-derived mesenchymal stem cells
ameliorate hepatic inflammation and fibrosis in rats. Stem Cells
Int. 2018(3212643)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen MF, Huang SJ, Huang CC, Liu PS, Lin
KI, Liu CW, Hsieh WC, Shiu LY and Chen CH: Saikosaponin d induces
cell death through caspase-3-dependent, caspase-3-independent and
mitochondrial pathways in mammalian hepatic stellate cells. BMC
Cancer. 16(532)2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mansouri A, Gattolliat CH and Asselah T:
Mitochondrial dysfunction and signaling in chronic liver diseases.
Gastroenterology. 155:629–647. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Okoye CN, MacDonald-Jay N and Kamunde C:
Effects of bioenergetics, temperature and cadmium on liver
mitochondria reactive oxygen species production and consumption.
Aquat Toxicol. 214(105264)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhu R, Wang Y, Zhang L and Guo Q:
Oxidative stress and liver disease. Hepatol Res. 42:741–749.
2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
No JH, Kim YB and Song YS: Targeting nrf2
signaling to combat chemoresistance. J Cancer Prev. 19:111–117.
2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bataille AM and Manautou JE: Nrf2: A
potential target for new therapeutics in liver disease. Clin
Pharmacol Ther. 92:340–348. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Dodson M, de la Vega MR, Cholanians AB,
Schmidlin CJ, Chapman E and Zhang DD: Modulating NRF2 in disease:
Timing is everything. Annu Rev Pharmacol Toxicol. 59:555–575.
2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Shin SM, Yang JH and Ki SH: Role of the
Nrf2-ARE pathway in liver diseases. Oxid Med Cell Longev.
2013(763257)2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chan K, Han XD and Kan YW: An important
function of Nrf2 in combating oxidative stress: Detoxification of
acetaminophen. Proc Natl Acad Sci USA. 98:4611–4616.
2001.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Xu Y, Tang Y, Lu J and Zhang W, Zhu Y,
Zhang S, Ma G, Jiang P and Zhang W: PINK1-mediated mitophagy
protects against hepatic ischemia/reperfusion injury by restraining
NLRP3 inflammasome activation. Free Radic Biol Med. 160:871–886.
2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wree A, McGeough MD, Inzaugarat ME, Eguchi
A, Schuster S, Johnson CD, Peña CA, Geisler LJ, Papouchado BG,
Hoffman HM and Feldstein AE: NLRP3 inflammasome driven liver injury
and fibrosis: Roles of IL-17 and TNF in mice. Hepatology.
67:736–749. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Strowig T, Henao-Mejia J, Elinav E and
Flavell R: Inflammasomes in health and disease. Nature.
481:278–286. 2012.PubMed/NCBI View Article : Google Scholar
|