Introduction
Obesity is a public health hazard worldwide
(1). A previous study revealed
that the prevalence of obesity (defined as body mass index ≥30
kg/m2) increased from 3.2 to 10.8% in adult men and from
6.4 to 14.9% in adult women between 1975 and 2014(2). Obesity can result in insulin
resistance, which substantially increases the risk of type-2
diabetes (T2D) (3,4). For patients with obesity and T2D,
sleeve gastrectomy (SG), one of the most popular procedures of
bariatric surgery (5), is
effective in reducing body fat, improving hepatic insulin
resistance and eventually controlling hyperglycemia (6-9).
Furthermore, reduced fat accumulation following SG is specifically
associated with the amelioration of hepatic insulin resistance
(10). However, the concrete
mechanism is not fully understood. Previous studies have mainly
focused on the effects of food intake and satiety induced by
post-operative gut hormone changes on metabolism (11-13).
It should be noted that adipose metabolism plays an important role
in reducing body weight and improving glycemic control. Thus,
further research on the effect of SG on lipid metabolism in adipose
tissue is required.
White adipose tissue (WAT) has been recognized as an
immunoendocrine organ that regulates energy balance and metabolism
(14). Expansion of WAT leads to
obesity with a subsequent imbalance in the production and signaling
of adipokines (15). The levels of
leptin, an adipose tissue-derived hormone, are increased in both
circulation and WAT in obesity, but reduced following SG (16-19).
Since leptin is an adipocyte effector protein, its expression is a
marker of pre-adipocyte differentiation (20). Recently, Palhinha et al
(21) suggested that leptin was an
inducer of adipogenesis and associated inflammation. Src homology 2
domain-containing phosphatase 2 (Shp2) is encoded by the Ptpn11
gene and involved in leptin signaling downstream of the leptin
receptor long form (LepRb) in the hypothalamus (22). This protein plays a notable role in
balancing food intake and energy (23), which is a potential target for the
treatment of obesity (24).
Several studies have demonstrated that Shp2 deficiency in the
forebrain causes obesity and diabetes due to disrupted leptin
signaling (22,23,25).
Moreover, Tao et al (26)
reported that Shp2 suppresses the early stages of adipogenic
differentiation in 3T3-L1 cells. However, to the best of our
knowledge, there have been no previous studies examining the role
of Shp2 in the lipid metabolism of WAT following SG and adipogenic
differentiation of white pre-adipocytes.
In the present study, obese rats with T2D were used
to perform vertical SG to investigate the fat metabolism in
inguinal WAT (ingWAT) and its underlying mechanism in the
regulation of obesity.
Materials and methods
Animals
All animal experiments were approved by the Animal
Care and Use Committee of Nanjing Drum Tower Hospital, Nanjing
Medical University (approval no. 2019AE02013; Nanjing, China). The
present study complies with all relevant ethical regulations of
Nanjing Drum Tower Hospital Ethics Committee.
A total of 16 male Sprague-Dawley rats (weight,
150±10 g; age, 4 weeks) were purchased from the animal core
facility of Nanjing Medical University (Jiangsu, China). The rats
were housed in specific pathogen-free units of the Animal Center at
Nanjing Drum Tower Hospital maintained at 60-70% relative humidity
and 23±1˚C, with a 12-h light/dark cycle and ad libitum
access to a 60% high-fat diet (HFD; cat. no. D12492; Research
Diets, Inc.) and water. After 8 weeks on HFD, T2D was induced by
intraperitoneal injection of 30 mg/kg streptozotocin (STZ;
MilliporeSigma). Random blood glucose levels were measured 72 h
following STZ injection with a hand-held glucose meter (OneTouch
UltraVue; Johnson & Johnson). Rats with random blood glucose
levels >16.0 mmol/l on three consecutive days were considered
diabetic. Diabetic rats were randomly assigned to the sham or the
SG group (n=8/group). Body weight, food intake and fasting glucose
(FG) levels were measured pre-operatively and post-operatively at
week 2, 4, 6 and 8. An oral glucose tolerance test (OGTT) was
performed pre-operatively and at week 8 post-surgery. The body fat
content and distribution were also measured at week 8 using
dual-energy X-ray absorptiometry (DEXA). The ingWAT was then
harvested for subsequent experiments.
SG surgical procedure
Rats were fasted overnight and then anesthetized
using isoflurane (3% for induction; 2% for maintenance). Under
sterile conditions, a single 3-cm epigastric laparotomy was
performed. As presented in Fig.
1A, the greater curvature of the stomach from the antrum to the
fundus and glandular stomach was dissected, ~70% of the gastric
volume was excised and the gastroesophageal junction and the
pylorus were preserved. For the sham group, a similar length
incision was made in the anterior gastric wall, then sutured using
a 6-0 absorbable thread. Abdominal closure was performed using 3-0
silk suture with a continuous suture technique. The rats received
ibuprofen (15 mg/kg of body weight daily) to minimize
post-operative pain for 2 days. After allowing 24 h for the
anastomoses to heal, the animals were kept on a liquid diet for 3
days before being returned to normal chow for an additional 8
weeks.
 | Figure 1SG alleviates obesity and glucose
metabolic disorders in a rat T2D model. (A) Illustration of the
sham and SG operations. (B) Appearance of rats in the sham and SG
groups were recorded 8 weeks after surgery. Changes in (C) body
weight, (D) food intake and (E) fasting glucose levels following
surgery. (F) Pre-operative and (G) post-operative OGTT results. (H)
AUC for glucose levels. (I and J) Body fat content after SG was
measured using DEXA. (K) Adipose tissue in the inguinal region 8
weeks post-operation. Scale bar, 10 mm. (L) H&E staining images
of ingWAT were obtained using a light microscope. Scale bar, 100
µm. (M) Diameter of adipocytes of ingWAT in the sham and SG groups.
(N) Triglyceride concentration in the ingWAT from the the sham and
SG groups. (O) Leptin concentration in the ingWAT from the sham and
SG groups. (P) Western blot images and (Q) semi-quantitative
analysis of PPARγ and Cebpα protein levels in ingWAT after surgery.
The data are presented as the mean ± SD. *P<0.05,
**P<0.01 and ***P<0.001 vs. the sham
group; ###P<0.001 vs. the SG preoperative level. SG,
sleeve gastrectomy; T2D, type-2 diabetes; OGTT, oral glucose
tolerance test; AUC, area under the curve; DEXA, dual-energy X-ray
absorptiometry; H&E, hematoxylin and eosin; ingWAT, inguinal
white adipose tissue; Cebpα, CCAAT/enhancer-binding protein alpha;
PPARγ, peroxisome proliferator-activated receptor γ. |
Primary pre-adipocytes isolation and
culture
Primary pre-adipocytes were isolated from the ingWAT
of 4-week-old SD rats. In brief, adipose tissue samples were
collected from the inguinal region under sterile conditions and
washed in PBS three times. The tissue mass was cut with scissors
into ~1-mm sections and digested with 0.1% type-I collagenase at
37˚C for 1 h in a shaking water bath. High-glucose (4.5 g/l) DMEM
(Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) was added to stop digestion. To
remove undigested tissue fragments and large cell aggregates, the
digested tissue suspension was filtered through 100-µm nylon mesh
(Falcon). The cells were collected by centrifugation at 233 x g for
10 min at room temperature (RT). The cells were then resuspended in
high-glucose DMEM supplemented with 10% FBS and 1% penicillin and
streptomycin (Gibco; Thermo Fisher Scientific, Inc.) at 37˚C with
5% CO2.
For white adipocyte in vitro differentiation,
fully confluent pre-adipocytes were kept in DMEM + 10% FBS growth
medium for 2 days, then treated with induction medium (DMEM + 10%
FBS) supplemented with 10 µg/ml insulin, 1 µM dexamethasone and 0.5
mM isobutylmethylxanthine (MilliporeSigma). After 2 days, the cells
were kept in medium supplemented with 10 µg/ml insulin for a
further 2 days. The culture medium was then replaced with fresh
DMEM containing 10% FBS every 2-3 days. The cells were fully
differentiated into mature adipocytes on day 9.
Oil Red O staining
Differentiated adipocytes were washed three times
with phosphate-buffered saline and were subsequently fixed in 4%
paraformaldehyde at RT for 30 min. Afterwards, adipocytes were
exposed to Oil Red O for 1 h at RT. To remove the staining
solution, cells were washed several times with distilled water.
Representative images were taken using a light microscope (Zeiss
AG).
Small interfering RNA (siRNA)
transient transfection
The negative control (NC) and specific siRNA against
Shp2 were purchased from HIPPOBIO, Inc. Pre-adipocytes were seeded
in 6-well plates at a density of 60-70% confluence and transfected
with 50 nM siRNA using Lipofectamine® 3000 transfection
reagent (Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions. After 6 h of transfection, the medium
was replaced with normal medium and the cells were cultured for
another 18 h at 37˚C with 5% CO2, and then harvested for
transfection efficiency evaluation using western blot analysis. The
transfected cells were collected to perform subsequent experiments
at least 48 h after transfection and transfection was performed
every 72 h during differentiation. The NC siRNA consisted of a
scrambled sequence that would not cause the specific degradation of
any known cellular mRNA. The siRNA sequences are presented in
Table I.
 | Table ISequences of siRNA. |
Table I
Sequences of siRNA.
| Genes | Direction | Sequences
(5'-3') |
|---|
| NC siRNA | Sense |
UUCUCCGAACGUGUCACGU |
| | Antisense |
ACGUGACACGUUCGGAGAA |
| Shp2 siRNA | Sense |
GUUCCUAAAACCAUUCAGATG |
| | Antisense |
UCUGAAUGGUUUUAGGAACGT |
Lentiviral transduction
Lentivirus-containing expression cassettes were
purchased from Syngentech Co. Ltd., and lentivirus preparation and
packaging were performed by the company. Lentiviral transduction
was performed following the manufacturer's instructions. Briefly,
5x105 ingWAT pre-adipocytes were seeded in T25 flasks.
The next day, these cells were infected with Shp2 lentiviral
expression vector (pLV-hef1a-mNeongreen-P2A-Puro-WPRE-CMV-Ptpn11)
or control lentiviral expression vector
(pLV-hef1a-mNeongreen-P2A-Puro-WPRE-CMV-MCS) at MOI values of 50 at
37˚C with 5% CO2. The culture media were replaced at 12
h post-transfection, and the cells were incubated for a further 48
h. At 72 h post-transfection, the transfected cells showing green
fluorescence were observed under a fluorescence microscope and
transduction efficiency was evaluated using western blot analysis.
The transfected cells were collected to perform subsequent
experiments 4 days after transfection. Stably transduced cells were
selected using 2 µg/ml puromycin (Beyotime Institute of
Biotechnology) for 3 days.
Histological analysis
At week 8 post-surgery or if a humane endpoint,
including listlessness, infection or eating cessation or drinking
cessation could not be relieved, rats were euthanized by
CO2 inhalation. The flow rate of CO2 was
20-30% of the chamber volume per minute. Death was confirmed based
on the absence of a heartbeat, respiration and corneal reflex.
After the rats were sacrificed, their ingWAT was fixed in 4%
paraformaldehyde at RT for 24 h. The samples were then embedded in
paraffin and cut into 5-µm coronal slides using a microtome (Thermo
Fisher Scientific, Inc.). After at 60˚C for 30 min, the tissue
slides from the sham and SG groups were deparaffinized in xylene,
rehydrated in gradient ethanol (each for 5 min) and then washed
under running water for 3 min. Tissue slides were stained using
hematoxylin and eosin (Beyotime Institute of Biotechnology). The
slides were immersed in hematoxylin at RT for 3 min and rinsed with
running water, sequentially followed by differentiation for 30 sec
in 1% acid-alcohol (hydrochloric acid and ethanol) at RT and then
rinsed again with running water. The slides were then stained with
eosin at RT for 30 sec and rinsed again with water. The slides were
air-dried at RT. Subsequently, the slides were sequentially
immersed in 75, 85, 95 and 100% ethanol, a solution of 50% ethanol
and 50% xylene, and 100% xylene, each for 1 min. The slides were
then observed under a light microscope (Zeiss AG). The diameter of
the adipocytes was measured using ImageJ (version 1.8.0; National
Institutes of Health).
Immunofluorescence staining
After heating at 60˚C for 30 min, histological
slides of ingWAT were deparaffinized in xylene, then rehydrated
using decreasing concentrations of ethanol ranging from 100 to 50%.
The slides were blocked with 5% bovine serum albumin
(MilliporeSigma) for 1 h at RT, then incubated with a primary
antibody against Shp2 (1:200 dilution; cat. no. sc-7384; Santa Cruz
Biotechnology, Inc.) overnight at 4˚C. The slides were washed with
PBS and incubated with tetramethylrhodamine-conjugated secondary
antibody (1:200 dilution; cat. no. ab6786; Abcam) for 1 h at RT in
the dark. The slides were counterstained with DAPI at RT in the
dark, and fluorescence images of randomly selected fields (n=3)
were obtained using a fluorescence microscope (Zeiss AG).
ELISA
The triglyceride (cat. no. 1025; Applygen
Technologies, Inc.) and leptin (cat. no. ml002969; Mlbio) levels in
ingWAT samples were measured using ELISA kits according to the
manufacturer's protocols. The absorbance was read at 450 nm using a
microplate reader (Thermo Fisher Scientific, Inc.).
Western blotting
WAT and adipocytes were lysed with RIPA lysis buffer
(Beijing Solarbio Science & Technology Co., Ltd.) containing 1
mM phenylmethanesulfonyl fluoride (Beijing Solarbio Science &
Technology Co., Ltd.) and 1 mM phosphatase inhibitor cocktail
(Bimake). Lysate protein concentrations were determined using a BCA
protein assay kit (Thermo Fisher Scientific, Inc.). Equal
quantities of protein (10 µg/well) were separated using 10% (w/v)
SDS-PAGE (Epizyme, Inc.) and were subsequently transferred to PVDF
membranes (MilliporeSigma) according to standard procedures. After
the membranes were blocked with 5% (w/v) milk (Biofroxx) for 1 h at
RT, the membranes were incubated with primary antibodies (1:1,000
dilution) against Shp2 (cat. no. 3397), β-catenin (cat. no. 84441)
(both Cell Signaling Technology, Inc.), peroxisome
proliferator-activated receptor γ (PPARγ; cat. no. AP0686; Bioworld
Technology, Inc.), CCAAT/enhancer-binding protein alpha (Cebpα;
cat. no. ab40764; Abcam), adiponectin (cat. no. 2789; Cell
Signaling Technology, Inc.), leptin (cat. no. DF8583; Affinity
Biosciences) and β-actin (cat. no. 4970; Cell Signaling Technology,
Inc.) overnight at 4˚C. A horseradish peroxidase-conjugated goat
anti-rabbit/mouse IgG (cat. no. BL003A/BL001A; 1:5,000; Biosharp
Life Sciences) was used as a secondary antibody for 1 h at RT. All
signals were detected using the ChemiDocXRS+ Imaging
System (Tanon Science and Technology Co., Ltd.). Quantitative
analysis of protein densitometry was performed using Image J
(version 1.8.0; National Institutes of Health).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from WAT and adipocytes
using the RNA-quick Purification Kit (ES Science) according to the
manufacturer's instructions. cDNA synthesis was performed using the
High-Capacity cDNA Reverse Transcription Kit (Vazyme Biotech Co.,
Ltd.) in accordance with the manufacturer's instructions. qPCR was
performed using a SYBR Green QPCR kit (Vazyme Biotech Co., Ltd.) on
a LightCycler 480 PCR System (Roche Diagnostics). The following
thermocycling conditions were used for the qPCR: Initial
denaturation at 95˚C for 2 min, followed by 40 cycles of 95˚C for
20 sec, 60˚C for 20 sec and 72˚C for 20 sec, and then a final step
at 78˚C for 5 min. The fold changes in the expression levels of
each gene were calculated using the 2-ΔΔCq method
(27). The mRNA expression levels
for each target gene were normalized to those of β-actin. The
primer sequences are presented in Table II.
 | Table IIPrimer sequences for reverse
transcription-PCR. |
Table II
Primer sequences for reverse
transcription-PCR.
| Genes | Direction | Sequences
(5'-3') |
|---|
| Shp2 | Forward |
CCGAGAGAAAGGTGTGGACT |
| | Reverse |
TGAACCGGTACTGTGCTTCT |
| PPARγ | Forward |
CTCCAGCATTTCTGCTCCAC |
| | Reverse |
CGCAGGCTCTACTTTGATCG |
| Cebpα | Forward |
GCAAGAGCCGAGATAAAGCC |
| | Reverse |
CCTTGACCAAGGAGCTCTCA |
| Adiponectin | Forward |
GACAAGGCCGTTCTCTTCAC |
| | Reverse |
CCCATACACTTGGAGCCAGA |
| Fabp4 | Forward |
ATGTGCAGAAGTGGGATGGA |
| | Reverse |
GTCACGCCTTTCATGACACA |
| Leptin | Forward |
TGTCCAAGATGGACCAGACC |
| | Reverse |
GAGCTATCTGCAGCACGTTT |
| β-actin | Forward |
CCTCTATGCCAACACAGTGC |
| | Reverse |
CACAGAGTACTTGCGCTCAG |
Cell Counting Kit-8 (CCK-8) assay
The viability of pre-adipocytes was assessed using
CCK-8 (Dojindo Laboratories, Inc.) assays according to the
manufacturer's instructions following treatment with recombinant
leptin rat (0, 5, 25, 50, 100 and 200 ng/ml) (Bioworld Technology,
Inc.), SHP099 (0, 1, 5, 10, 50, 100 and 200 µM) or PNU-74654 (0,
0.5, 1, 10, 25, 50 and 100 µM) (MedChemExpress) at 37˚C with 5%
CO2 for 24 h. The absorbance at 450-nm wavelength was
detected using a microplate reader (Thermo Fisher Scientific,
Inc.).
Statistical analysis
SPSS (version 26.0; IBM, Corp.) and GraphPad Prism
software (version 8.4; GraphPad Software, Inc.) were used for
statistical analysis. Quantitative data are representative of at
least three independent experiments. Unpaired two-tailed Student's
t-tests were used to compare the means of two groups. Differences
between multiple groups were detected using one-way analysis of
variance (ANOVA) followed by Bonferroni's post-hoc test as
appropriate. The data are presented as the mean ± SD. P<0.05 was
considered to indicate a statistically significant difference.
Results
SG alleviates obesity and associated
metabolic disorders
The body shapes of rats 8 weeks after surgery are
presented in Fig. 1B. There were
no significant differences in body weight or food intake between
the sham and the SG group pre-operatively. Throughout the
post-operative period, the SG group had a significantly lower body
weight and food intake compared with those of the sham group
(P<0.01; Fig. 1C and
D). During the post-operative
period, the FG levels declined significantly in the SG group
compared with the sham group (P<0.05; Fig. 1E). Compared with week 0, the FG
levels in the SG group were significantly reduced (70.8±10.1%
reduction; P<0.001) at week 8 post-surgery. Compared with
the pre-operative results (Fig.
1F), the SG group demonstrated marked improvements in glucose
control following surgery (Fig.
1G). Moreover, the SG group revealed significantly improved
glucose control compared with the sham group post-surgery
(P<0.001; Fig. 1H). The
pre- and post-operative results of the sham group did not differ
significantly.
SG suppresses fat accumulation in vivo
and downregulates leptin levels in adipose tissue
The SG group had significantly lower body fat
compared with the sham group (P<0.01; Fig. 1I and J). As presented in Fig. 1K, the volume of adipose tissue of
the SG group was smaller than those of the sham group. Histological
analysis of adipose tissue sections revealed smaller adipocyte
sizes in rats from the SG group compared with the sham
(P<0.001; Fig. 1L and
M). At week 8 post-surgery, the SG
group exhibited significantly lower triglyceride
(P<0.001) and leptin (P<0.001) levels in
adipose tissue than the sham group (Fig. 1N and O). Furthermore, the expression of the
adipogenic markers, PPARγ and Cebpα, were significantly
downregulated in ingWAT from the SG group compared with the sham
group (P<0.05; Fig. 1P
and Q).
Leptin downregulates the expression of
Shp2 and β-catenin and upregulates the expression of adipogenic
markers
To investigate the effect of leptin on the
expression of Shp2, ingWAT pre-adipocytes were treated with
different concentrations of leptin (0, 5, 25, 50, 100 and 200
ng/ml) during differentiation. The results indicated that Shp2 and
β-catenin protein levels were markedly reduced by leptin in a
dose-dependent manner (Fig. 2A).
Marked changes in Shp2 and β-catenin protein expression were
observed after 100 and 200 ng/ml leptin treatment. CCK-8 assay was
performed to evaluate the cell viability, the results of which
suggested that there was no effect on cell viability at 100 ng/ml
leptin (Fig. 2B). Subsequently,
100 ng/ml leptin was used to stimulate ingWAT pre-adipocytes during
differentiation. As presented in Fig.
2C, leptin treatment markedly increased the lipid accumulation
of adipocytes. In addition, the mRNA expression level of Shp2 and
fatty acid-binding protein 4 (Fabp4), and the mRNA or protein
expression levels of the other adipogenic markers, including PPARγ,
Cebpα, adiponectin and leptin significantly increased (all
P<0.05; Fig. 2D-F).
 | Figure 2Leptin downregulates Shp2/β-catenin
protein levels and promotes adipogenic differentiation. (A) Western
blot analysis of Shp2 and β-catenin protein levels in ingWAT
adipocytes treated with 0, 5, 25, 50, 100 or 200 ng/ml leptin. (B)
IngWAT pre-adipocytes were treated with different concentrations of
leptin, and cell viability was measured using Cell Counting Kit-8
assays. (C) Oil Red O staining following 100 ng/ml leptin
supplementation at differentiation day 9. Scale bar, 500 µm. (D)
Reverse transcription-quantitative PCR analysis of mRNA levels of
adipogenic markers in adipocytes treated with leptin. (E) Western
blotting and (F) quantitative analysis of PPARγ, Cebpα and
adiponectin protein levels in adipocytes treated with leptin. The
data are presented as the mean ± SD (n=3). *P<0.05,
**P<0.01 and ***P<0.001 vs. control
group. Shp2, Src homology 2 domain-containing phosphatase 2;
ingWAT, inguinal white adipose tissue; PPARγ, peroxisome
proliferator-activated receptor γ; Cebpα, CCAAT/enhancer-binding
protein α; Fabp4, fatty acid-binding protein 4. |
SG upregulates Shp2 and β-catenin
levels in ingWAT
The mRNA and protein expression levels of Shp2 and
β-catenin were significantly upregulated following SG
(P<0.01 and P<0.001; Fig.
3A-C). Moreover, the fluorescence intensity of Shp2 in ingWAT
was significantly increased in the SG group compared with the sham
group (P<0.05; Fig. 3D
and E).
Expression pattern of Shp2 in ingWAT
pre-adipocyte differentiation
To confirm that the observed changes in Shp2 were
associated with inhibition of pre-adipocyte differentiation at
day-2 post-confluence, growth-arrested ingWAT pre-adipocytes were
induced into mature adipocytes. The differentiation status of
ingWAT pre-adipocytes was monitored by Oil Red O staining, which is
indicative of terminal differentiation (Fig. 4A). The results suggested that the
mRNA and protein levels of Shp2 were significantly reduced
following treatment with inducers (P<0.001), and the mRNA
expression levels of adipogenic markers significantly increased
(P<0.01 and P<0.001; Fig.
4B and C).
Knockdown or inhibition of Shp2
accelerates ingWAT pre-adipocyte differentiation
To examine the role of Shp2 during ingWAT
pre-adipocyte differentiation, siRNA was used to knock-down the
expression of Shp2. The results demonstrated that Shp2 knockdown
resulted in increased lipid accumulation throughout differentiation
(Fig. 5A). Additionally, Shp2
siRNA significantly inhibited both the mRNA and protein expression
of Shp2 at day 9 post-induction (P<0.05 and P<0.001;
Fig. 5B-D). Triglyceride levels
were also significantly increased (P<0.001; Fig. 5E) and there were significant
increases in the mRNA levels of adipogenic markers
(P<0.01; Fig. 5F).
Analysis of protein expression confirmed the adipogenic effects of
Shp2 knockdown. PPARγ, Cebpα and adiponectin protein levels were
significantly increased at differentiation day 9 in the Shp2
knockdown group compared with the NC group (P<0.05;
Fig. 5G and H). Although the protein expression levels
of leptin increased following Shp2 knockdown, this was not
statistically significant. Furthermore, the expression of
β-catenin, a protein involved in the Wnt signaling pathway, was
significantly reduced in the Shp2 knockdown group compared with the
NC group (P<0.05; Fig. 5G and
H).
 | Figure 5Shp2 knockdown accelerates ingWAT
pre-adipocyte differentiation. (A) Oil Red O staining of Shp2
knockdown at differentiation day 9. Scale bar, 500 µm. (B) Western
blotting and (C) semi-quantitative analysis of Shp2 knockdown. (D)
RT-qPCR analysis of mRNA level of Shp2 knockdown. (E) Triglyceride
concentration of adipocytes with Shp2 knockdown. (F) RT-qPCR
analysis of mRNA levels of adipogenic markers in Shp2 knockdown
adipocytes. (G) Western blotting and (H) semi-quantitative analysis
of adipogenic markers and β-catenin in Shp2 knockdown adipocytes.
The data are presented as the mean ± SD (n=3).
*P<0.05, **P<0.01 and
***P<0.001 vs. NC groups. Shp2, Src homology 2
domain-containing phosphatase 2; ingWAT, inguinal white adipose
tissue; siRNA/siR, small interfering RNA; RT-qPCR, reverse
transcription-quantitative PCR; NC, negative control; PPARγ,
peroxisome proliferator-activated receptor γ; Cebpα,
CCAAT/enhancer-binding protein α; Fabp4, fatty acid-binding protein
4. |
Furthermore, similar experiments were carried out in
ingWAT pre-adipocytes in the presence of the Shp2 inhibitor,
SHP099, in the induction medium at different time points. IngWAT
pre-adipocytes were treated with different concentrations of
SHP099, and cell viability was measured using CCK-8 assays, with
the results showing that cell viability decreased with increasing
SHP099 concentration (Fig. 6A).
The optimal protective effect was conferred by 10 µM, so this
concentration was selected for subsequent experiments. As presented
in Fig. 6B-F, SHP099 had a
significantly positive effect on cellular lipid accumulation
(P<0.001; Fig. 6B and C) and significantly increased the mRNA
and protein levels of adipogenic markers compared with the NC group
(P<0.05; Fig. 6D-F).
Moreover, the protein expression of β-catenin was decreased
significantly compared with that in the NC group (P<0.05;
Fig. 6E and F). These results suggested that knockdown
or inhibition of Shp2 promoted ingWAT adipocyte differentiation,
and these adipogenic effects may be mediated by the Wnt/β-catenin
signaling.
Shp2 inhibits ingWAT pre-adipocyte
differentiation by activating the Wnt/β-catenin signaling
pathway
To determine whether the Wnt/β-catenin signaling
pathway was involved in the effects of Shp2 on pre-adipocyte
differentiation, a small-molecule inhibitor of the Wnt/β-catenin
pathway, PNU-74654 (28,29), was used to test this hypothesis.
The pre-adipocytes were treated with different concentrations for
24 h, cell viability was measured using CCK-8 assays. When the
concentration reached 10 µM, cell viability was not significantly
affected, and an optimal concentration of 10 µM was selected for
subsequent experiments (Fig. 7A).
Shp2 was overexpressed in ingWAT pre-adipocytes using lentiviral
transduction. At day 9 of differentiation, lipid accumulation
markedly decreased in the Shp2-overexpressing cells (Fig. 7B). Moreover, the triglyceride
levels of Shp2-overexpressing adipocytes were significantly reduced
compared with the vector group (P<0.01; Fig. 7C). Furthermore, the overexpression
of Shp2 decreased the mRNA and protein levels of adipogenic markers
(P<0.05; Fig. 7D-F),
while the protein expression level of β-catenin was significantly
upregulated (P<0.05; Fig.
7E and F). These results
suggested that Shp2 may suppress adipocyte differentiation through
the Wnt/β-catenin pathway. Next, PNU-74654 was added to the
inducing medium at different time points, and the protein
expression of β-catenin was significantly decreased compared with
the Shp2 overexpression group (P<0.05; Fig. 7E and F). As presented in Fig. 7B, Shp2-overexpressing adipocytes
treated with PNU-74654 displayed markedly increased lipid
accumulation. In addition, triglyceride levels were also
significantly increased compared with the Shp2 overexpression group
(P<0.01; Fig. 7C).
Furthermore, the mRNA and protein expression of adipogenic markers
were significantly increased compared with the Shp2 overexpression
group following treatment with PNU-74654 (P<0.05;
Fig. 7D-F). These results
suggested that Shp2 suppressed ingWAT adipocyte differentiation by
activating the Wnt/β-catenin signaling pathway.
 | Figure 7Shp2 inhibits ingWAT pre-adipocyte
differentiation by activating the Wnt/β-catenin signaling pathway.
(A) IngWAT pre-adipocytes were treated with different
concentrations of the β-catenin inhibitor PNU-74654, and cell
viability was measured using Cell Counting Kit-8 assays. (B) Oil
Red O staining of adipocytes following Shp2 overexpression with or
without PNU-74654 treatment at differentiation day 9. Scale bar,
500 µm. (C) Triglyceride concentration in adipocytes following Shp2
overexpression with or without PNU-74654 treatment. (D) Reverse
transcription-quantitative PCR analysis of mRNA levels of
adipogenic markers in adipocytes following Shp2 overexpression with
or without PNU-74654 treatment. (E) Western blotting images and (F)
semi-quantitative analysis of adipogenic markers and β-catenin in
adipocytes following Shp2 overexpression with or without PNU-74654
treatment. The data are presented as the mean ± SD (n=3).
*P<0.05, **P<0.01 and
***P<0.001 vs. the vector group; ^P<0.05,
^^P<0.01 and ^^^P<0.001 vs. the Shp2 overexpression group.
Shp2, Src homology 2 domain-containing phosphatase 2; ingWAT,
inguinal white adipose tissue; PNU, PNU-74654; PPARγ, peroxisome
proliferator-activated receptor γ; Cebpα, CCAAT/enhancer-binding
protein α; Fabp4, fatty acid-binding protein 4. |
Discussion
The present study demonstrated that SG resulted in
significant improvements in body weight, glucose control and fat
accumulation. The major findings of the present study were as
follows: i) Leptin expression was decreased and Shp2 expression
increased in ingWAT following SG; ii) leptin inhibited Shp2
expression and promoted adipogenic differentiation in white
pre-adipocytes; iii) both Shp2 knockdown and inhibition promoted
the lipid accumulation of white adipocytes, while overexpression of
Shp2 reduced it; and iv) Shp2 inhibited the adipogenic
differentiation of ingWAT pre-adipocytes by activating the
Wnt/β-catenin signaling pathway. These findings revealed a novel
role of Shp2 in lipid metabolism in WAT, which may provide insight
into the mechanism through which SG sustains long-term weight
loss.
Gut hormones, bile acid and gut flora are considered
major causes of the effects of SG on body weight and metabolic
changes (13). Moreover, reduced
fat accumulation after SG also results in body weight and metabolic
changes (30,31). Obesity is characterized by
excessive accumulation of body fat, especially in WAT. Adipocytes
play a notable role in lipid storage and metabolism (32). The suppression of adipogenesis can
affect the formation of WAT, which is conducive to alleviating the
accompanying metabolic disorders (33). The present study identified an
important role for SG in this process.
The present findings indicated that a HFD increases
fat accumulation and that SG could reverse this phenomenon. This
suggests that SG contributes to weight loss by reducing fat
accumulation. Moreover, the SG group had improved glucose control
and lipid metabolism compared with the sham group, which was
consistent with previous reports (34,35).
A significant post-operative reduction in leptin levels was also
observed in the ingWAT. Leptin is an adipokine that acts centrally
to regulate food intake and metabolism by activating LepRb in the
hypothalamus (36,37). With WAT expansion in patients with
obesity, high leptin levels are detectable in the circulation,
which impairs the hypothalamus and leads to leptin resistance
(38). A partial reduction in
plasma leptin level in obesity restores hypothalamic leptin
sensitivity, which contributes to weight loss and improves insulin
sensitivity (39). Thus, decreased
leptin levels after SG contributes to fat consumption and weight
loss.
Reduced fat accumulation allows sustained weight
loss. In the present study, continuous leptin supplementation had a
positive effect on the differentiation of pre-adipocytes from
ingWAT. The expression levels of the adipogenic factors PPARγ,
Cebpα, adiponectin, Fabp4 and leptin were upregulated in mature
adipocytes. It was also observed that leptin downregulated the
expression of Shp2 in ingWAT pre-adipocytes. Previous studies have
indicated that Shp2 participates in leptin signaling immediately
downstream of LepRb in the hypothalamus, which could alleviate
leptin resistance in obesity (22,40).
However, only a few have documented the role of Shp2 in leptin
signaling in adipocytes, and its involvement in the regulation of
adipogenic differentiation remains controversial.
A previous study suggested that Shp2 suppressed
adipogenic differentiation in 3T3-L1 cells (26). By contrast, He et al
(41) revealed that Shp2 knockout
in embryonic stem cells could inhibit adipocyte differentiation and
that adipose tissue-specific deletion of Shp2 resulted in
lipodystrophy. In addition, Bettaieb et al (42) demonstrated that adipose
tissue-specific Shp2 deletion did not affect the development of
adipose tissue. These results indicate that Shp2 may play different
roles in adipocyte differentiation and adipose tissue formation.
The present study demonstrated that the knockdown of Shp2 promoted
ingWAT pre-adipocyte differentiation. Consistent with this finding,
SHP099, a Shp2 inhibitor, also promoted adipogenic differentiation.
Conversely, overexpression of Shp2 markedly inhibited ingWAT
pre-adipocyte differentiation. These findings led to the hypothesis
that Shp2 may be an inhibitor of adipogenesis and that the observed
increase in Shp2 expression in ingWAT following SG may represent an
integral part of the reduced fat accumulation. Notably, the
elaborate mechanism between leptin and Shp2 remains to be clarified
in future studies.
The Wnt/β-catenin signaling pathway plays a
significant role in various biological processes, especially in the
regulation of cell proliferation and differentiation, which has
been demonstrated to inhibit adipocyte differentiation in
vitro (43,44). Shp2 has been reported to signal via
various kinases, including Erk, MAPK and Wnt/β-catenin (41,45,46).
However, to the best of our knowledge, the association between Shp2
and the Wnt/β-catenin pathway in adipogenic differentiation has not
been reported previously. In the present study, SG upregulated
β-catenin expression levels in ingWAT. β-catenin expression after
adipogenic differentiation of ingWAT pre-adipocytes was reduced
following the knockdown or inhibition of Shp2, but increased
following Shp2 overexpression. Moreover, when using the β-catenin
inhibitor PNU-74654, the adipogenic differentiation of ingWAT
pre-adipocytes was enhanced compared with Shp2 overexpression
alone. These results suggested that Shp2 may serve as a positive
regulator of the Wnt/β-catenin pathway. And more key proteins of
Wnt/β-catenin signaling will be investigated in future studies.
There are some limitations to address for the
present study. First, a restricted diet group, in which the food
intake was equivalent to that of SG group to rule out the effect of
diet on fat reduction was not used. However, previous studies have
confirmed that the reduced fat accumulation caused by SG is not due
to reduced food intake alone (10,17).
Secondly, the post-operative outcomes were only observed at week 8.
Furthermore, only ingWAT was used as a representative WAT for ease
of morphological comparison and for consistency in vivo and
in vitro, and more types of WAT are needed for further
investigation. Finally, we did not measure the protein expression
level of Fabp4 due to the lack of antibody. Fabp4 regulates leptin
secretion and increases with leptin synergistically during adipose
inflammation process (47). The
expression level of Fabp4 may be reflected by leptin level
partially. The protein expression level of Fabp4 could be
investigated in future studies via western blotting.
Nevertheless, the results of the present study
demonstrated that the Shp2/Wnt/β-catenin pathway was important for
adipogenesis and sustained weight loss following SG. This study
provides further insight into the mechanisms through which SG
modulates body weight through reduced fat accumulation. Meanwhile,
decreased triglyceride levels through fat consumption may
contribute to glucose and lipid metabolism.
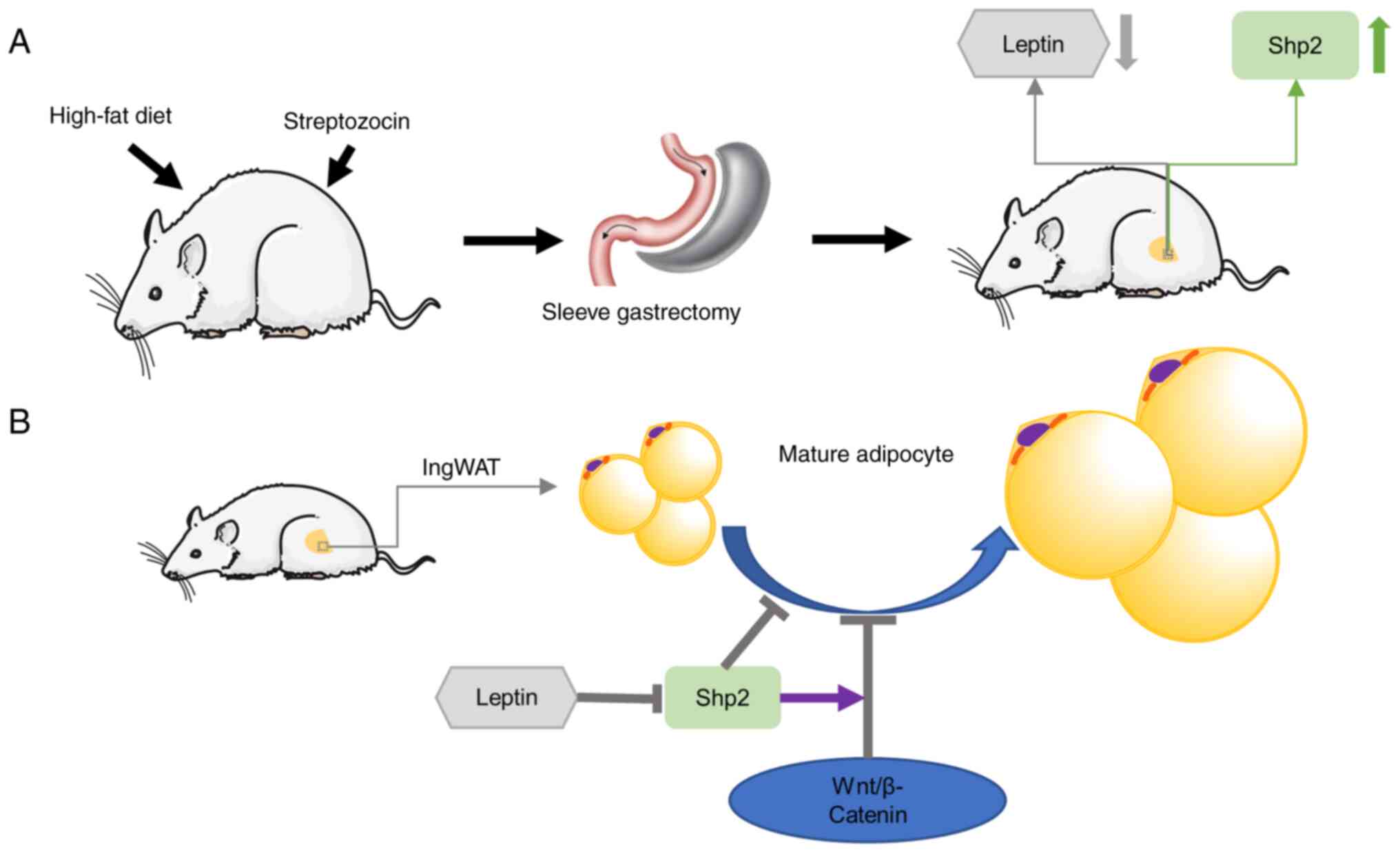
In the present study, SG was considered to alleviate
obesity and related metabolic disorders. Moreover, it inhibited fat
accumulation and upregulated Shp2 level in ingWAT (Fig. 8A). SG reduced leptin levels in
ingWAT, which in turn upregulated Shp2/β-catenin levels. The
findings further revealed that Shp2 suppressed ingWAT adipocyte
differentiation by activating the Wnt/β-catenin signaling pathway,
while the inhibition of β-catenin reversed the effects of Shp2 on
adipogenic differentiation (Fig.
8B). These observations raise the possibility that Shp2 and
Shp2-associated signaling may serve as potential therapeutic
targets for the treatment of obesity and T2D.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the Natural Science
Foundation of Jiangsu Province (grant no. BK20181155), the
Changzhou Sci&Tech Program (grant no. CJ20200097) and the Key
Project supported by Medical Science and Technology Development
Foundation (grant no. ZKX16034).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XYQ and ZS conceived the study and drafted the
manuscript. XL, YJ and JQ performed the in vivo experiments
and analyzed the data. SC and PS performed the in vitro
experiments. JQ and ZQ carried out the statistical analysis. XSQ
and LT supervised the experiments, analyzed the data and revised
the manuscript. XYQ and ZS confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by and
performed in accordance with the Animal Care and Use Committee of
Nanjing Drum Tower Hospital, Nanjing Medical University (approval
no. 2019AE02013). All animal care and use protocols were approved
by the Committee on the Ethics of Animal Experiments.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bhupathiraju SN and Hu FB: Epidemiology of
obesity and diabetes and their cardiovascular complications. Circ
Res. 118:1723–1735. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
NCD Risk Factor Collaboration (NCD-RisC).
Trends in adult body-mass index in 200 countries from 1975 to 2014:
A pooled analysis of 1698 population-based measurement studies with
19·2 million participants. Lancet. 387:1377–1396. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bell GI and Polonsky KS: Diabetes mellitus
and genetically programmed defects in beta-cell function. Nature.
414:788–791. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Blüher M: Obesity: Global epidemiology and
pathogenesis. Nat Rev Endocrinol. 15:288–298. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Debédat J, Amouyal C, Aron-Wisnewsky J and
Clément K: Impact of bariatric surgery on type 2 diabetes:
Contribution of inflammation and gut microbiome? Semin
Immunopathol. 41:461–475. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Puzziferri N, Roshek TB III, Mayo HG,
Gallagher R, Belle SH and Livingston EH: Long-term follow-up after
bariatric surgery: A systematic review. JAMA. 312:934–942.
2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Schauer PR, Bhatt DL, Kirwan JP, Wolski K,
Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE,
Nissen SE, et al: Bariatric surgery versus intensive medical
therapy for diabetes-5-year outcomes. N Engl J Med. 376:641–651.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Albaugh VL, Sharma G, Tu C and Aminian A:
Clinical significance of diabetes control before metabolic surgery.
Surg Obes Relat Dis. 17:1271–1278. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hofsø D, Fatima F, Borgeraas H, Birkeland
KI, Gulseth HL, Hertel JK, Johnson LK, Lindberg M, Nordstrand N,
Cvancarova Småstuen M, et al: Gastric bypass versus sleeve
gastrectomy in patients with type 2 diabetes (Oseberg): A
single-centre, triple-blind, randomised controlled trial. Lancet
Diabetes Endocrinol. 7:912–924. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Angelini G, Castagneto Gissey L, Del Corpo
G, Giordano C, Cerbelli B, Severino A, Manco M, Basso N, Birkenfeld
AL, Bornstein SR, et al: New insight into the mechanisms of ectopic
fat deposition improvement after bariatric surgery. Sci Rep.
9(17315)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Eickhoff H: Central modulation of energy
homeostasis and cognitive performance after bariatric surgery. Adv
Neurobiol. 19:213–236. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Manning S and Batterham RL: The role of
gut hormone peptide YY in energy and glucose homeostasis: Twelve
years on. Annu Rev Physiol. 76:585–608. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pucci A and Batterham RL: Mechanisms
underlying the weight loss effects of RYGB and SG: Similar, yet
different. J Endocrinol Invest. 42:117–128. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Vegiopoulos A, Rohm M and Herzig S:
Adipose tissue: Between the extremes. EMBO J. 36:1999–2017.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Unamuno X, Gómez-Ambrosi J, Rodríguez A,
Becerril S, Frühbeck G and Catalán V: Adipokine dysregulation and
adipose tissue inflammation in human obesity. Eur J Clin Invest.
48(e12997)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Otero M, Lago R, Lago F, Casanueva FF,
Dieguez C, Gómez-Reino JJ and Gualillo O: Leptin, from fat to
inflammation: Old questions and new insights. FEBS Lett.
579:295–301. 2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dohmen J, Praktiknjo M, Rudeloff A,
Uschner FE, Klein S, Plamper A, Matthaei H, Rheinwalt KP, Wehner S,
Kalff JC, et al: Impact of sleeve gastrectomy and dietary change on
metabolic and hepatic function in an obesity rat model-experimental
research. Int J Surg. 75:139–147. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bužga M, Zavadilová V, Holéczy P, Švagera
Z, Švorc P, Foltys A and Zonča P: Dietary intake and ghrelin and
leptin changes after sleeve gastrectomy. Wideochir Inne Tech
Maloinwazyjne. 9:554–561. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Farey JE, Preda TC, Fisher OM,
Levert-Mignon AJ, Stewart RL, Karsten E, Herbert BR, Swarbrick MM
and Lord RV: Effect of laparoscopic sleeve gastrectomy on fasting
gastrointestinal, pancreatic, and adipose-derived hormones and on
non-esterified fatty acids. Obes Surg. 27:399–407. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Catalioto RM, Maggi CA and Giuliani S:
Chemically distinct HDAC inhibitors prevent adipose conversion of
subcutaneous human white preadipocytes at an early stage of the
differentiation program. Exp Cell Res. 315:3267–3280.
2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Palhinha L, Liechocki S, Hottz ED, Pereira
JA, de Almeida CJ, Moraes-Vieira PM, Bozza PT and Maya-Monteiro CM:
Leptin induces proadipogenic and proinflammatory signaling in
adipocytes. Front Endocrinol (Lausanne). 10(841)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang EE, Chapeau E, Hagihara K and Feng
GS: Neuronal Shp2 tyrosine phosphatase controls energy balance and
metabolism. Proc Natl Acad Sci USA. 101:16064–16069.
2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
do Carmo JM, da Silva AA, Ebaady SE,
Sessums PO, Abraham RS, Elmquist JK, Lowell BB and Hall JE: Shp2
signaling in POMC neurons is important for leptin's actions on
blood pressure, energy balance, and glucose regulation. Am J
Physiol Regul Integr Comp Physiol. 307:R1438–R1447. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Feng GS: Shp2 as a therapeutic target for
leptin resistance and obesity. Expert Opin Ther Targets.
10:135–142. 2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
He Z, Zhang SS, Meng Q, Li S, Zhu HH,
Raquil MA, Alderson N, Zhang H, Wu J, Rui L, et al: Shp2 controls
female body weight and energy balance by integrating leptin and
estrogen signals. Mol Cell Biol. 32:1867–1878. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tao J, Zheng L, Meng M, Li Y and Lu Z:
Shp2 suppresses the adipogenic differentiation of preadipocyte
3T3-L1 cells at an early stage. Cell Death Discov.
2(16051)2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Durand J, Lampron A, Mazzuco TL, Chapman A
and Bourdeau I: Characterization of differential gene expression in
adrenocortical tumors harboring beta-catenin (CTNNB1) mutations. J
Clin Endocrinol Metab. 96:E1206–E1211. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wu L, Zhou Z, Han S, Chen J, Liu Z, Zhang
X, Yuan W, Ji J and Shu X: PLAGL2 promotes epithelial-mesenchymal
transition and mediates colorectal cancer metastasis via
β-catenin-dependent regulation of ZEB1. Br J Cancer. 122:578–589.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Shen Y, Liu Y, Zheng SQ, Han J, Pei EL, Li
ZH, Xie XY, Li ZQ and Luo M: Effects of left gastric artery
ligation versus sleeve gastrectomy on obesity-induced adipose
tissue macrophage infiltration and inflammation in diet-induced
obese rats. Med Sci Monit. 25:6719–6726. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang X, Zhu C, Gao J, Mei F, Yin J, Bu L,
Cheng X, Sheng C and Qu S: Gender difference in the relationship
between serum uric acid reduction and improvement in body fat
distribution after laparoscopic sleeve gastrectomy in Chinese obese
patients: A 6-month follow-up. Lipids Health Dis.
17(288)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Church C, Horowitz M and Rodeheffer M: WAT
is a functional adipocyte? Adipocyte. 1:38–45. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ghaben AL and Scherer PE: Adipogenesis and
metabolic health. Nat Rev Mol Cell Biol. 20:242–258.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Schauer PR, Kashyap SR, Wolski K,
Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE
and Bhatt DL: Bariatric surgery versus intensive medical therapy in
obese patients with diabetes. N Engl J Med. 366:1567–1576.
2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Feng Y, Zhong C, Niu J, Zhang L, Zhao Y,
Wang W, Hu Z, Wang H, He P, Ning Q, et al: Effects of sleeve
gastrectomy on lipid and energy metabolism in ZDF rats via PI3K/AKT
pathway. Am J Transl Res. 10:3713–3722. 2018.PubMed/NCBI
|
|
36
|
Myers MG, Cowley MA and Münzberg H:
Mechanisms of leptin action and leptin resistance. Annu Rev
Physiol. 70:537–556. 2008.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Friedman JM and Halaas JL: Leptin and the
regulation of body weight in mammals. Nature. 395:763–770.
1998.PubMed/NCBI View
Article : Google Scholar
|
|
38
|
Myers MG Jr, Leibel RL, Seeley RJ and
Schwartz MW: Obesity and leptin resistance: Distinguishing cause
from effect. Trends Endocrinol Metab. 21:643–651. 2010.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhao S, Zhu Y, Schultz RD, Li N, He Z,
Zhang Z, Caron A, Zhu Q, Sun K, Xiong W, et al: Partial leptin
reduction as an insulin sensitization and weight loss strategy.
Cell Metab. 30:706–719.e6. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Carpenter LR, Farruggella TJ, Symes A,
Karow ML, Yancopoulos GD and Stahl N: Enhancing leptin response by
preventing SH2-containing phosphatase 2 interaction with Ob
receptor. Proc Natl Acad Sci USA. 95:6061–6066. 1998.PubMed/NCBI View Article : Google Scholar
|
|
41
|
He Z, Zhu HH, Bauler TJ, Wang J, Ciaraldi
T, Alderson N, Li S, Raquil MA, Ji K, Wang S, et al: Nonreceptor
tyrosine phosphatase Shp2 promotes adipogenesis through inhibition
of p38 MAP kinase. Proc Natl Acad Sci USA. 110:E79–E88.
2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Bettaieb A, Matsuo K, Matsuo I, Nagata N,
Chahed S, Liu S and Haj FG: Adipose-specific deletion of Src
homology phosphatase 2 does not significantly alter systemic
glucose homeostasis. Metabolism. 60:1193–1201. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Moseti D, Regassa A and Kim WK: Molecular
regulation of adipogenesis and potential anti-adipogenic bioactive
molecules. Int J Mol Sci. 17(124)2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Huang WQ, Lin Q, Zhuang X, Cai LL, Ruan
RS, Lu ZX and Tzeng CM: Structure, function, and pathogenesis of
SHP2 in developmental disorders and tumorigenesis. Curr Cancer Drug
Targets. 14:567–588. 2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Feng GS: Shp-2 tyrosine phosphatase:
Signaling one cell or many. Exp Cell Res. 253:47–54.
1999.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Gan L, Liu Z, Cao W, Zhang Z and Sun C:
FABP4 reversed the regulation of leptin on mitochondrial fatty acid
oxidation in mice adipocytes. Sci Rep. 5(13588)2015.PubMed/NCBI View Article : Google Scholar
|