Introduction
Atherosclerosis is a lipid-driven chronic disease
that leads to plaque formation in the arterial wall through intimal
inflammation, necrosis, fibrosis and calcification. As one of the
critical factors of ischemic stroke, an atherosclerotic carotid
plaque contains a lipid-rich necrotic core (LRNC), areas of
intraplaque hemorrhage (IPH), calcification and fibrous components,
and vulnerable carotid plaques tend to consist of an LRNC and areas
of IPH, rather than of calcification and fibrous components. Of
note, growing evidence indicates that vulnerable plaque rupture,
beyond perfusion defects caused by luminal stenosis, is an
important cause of stroke (1). Two
distinct studies reported that in patients with mild to moderate
stenosis, vulnerable plaques were associated with the occurrence of
subsequent cerebrovascular events, which was markedly higher than
in patients with severe stenosis (>70%) (2,3).
Furthermore, a previous histopathologic assessment also suggested
that certain plaque elements known as hallmarks of unstable plaque,
independent of arterial narrowing, were more likely to cause
cerebrovascular or cardiovascular disorders (4). Thus, when designing therapeutic
interventions for effective stroke prevention, it is crucial to
identify carotid atherosclerotic plaques and high-risk determinants
as early as possible.
So far, various plaque imaging techniques
[ultrasound, computed tomography (CT) and magnetic resonance
imaging (MRI)] and serological biomarkers of vulnerability
[high-sensitivity C-reactive protein (Hs-CRP), MMP-9 and sCD40
ligand] have been adopted to predict the risk of cerebrovascular
events (5). In addition, as a key
chemokine, monocyte chemotactic protein-1 (MCP-1) has been
demonstrated to have important roles in atherosclerosis by
promoting the migration and infiltration of monocytes into the
plaque through its receptor C-C motif chemokine receptor 2(6). Among the neuroimaging technologies, a
novel gemstone spectral imaging (GSI) technique that incorporates
CT angiography (CTA) with a gemstone detector has received
increasing attention in recent years (7). Although conventional CT and CTA have
been widely used for quantitative measurement of tissue composition
and grading of the severity of stenosis in patients with stroke,
they exhibit deficiencies in the ability to characterize different
components of mixed or noncalcified plaques, particularly in
smaller plaques and vessels. Unlike conventional CT and CTA, GSI
further improves the characterization and quantification of plaque
components, since it collects data using multiple single-source
spectra to generate additional material-differentiating information
via the combination of CT techniques with the measurement of
spectral attenuation properties of the examined tissue to achieve
material decomposition (MD).
Hence, GSI holds great potential to better
characterize and quantify the concentration of plaque components,
such as lipids, calcification and fibrous tissues (8), in order to accurately distinguish
vulnerable plaques from stable plaques in carotid arteries. A
recent study by Shinohara et al (9) compared the applicability of GSI to
that of 3D time-of-flight magnetic resonance angiography (MRA) in
patients with carotid artery stenosis, revealing that the effective
Z-value of noncalcified carotid plaques was markedly lower in the
group with high signal intensity than that in the group with low
signal intensity on MRA. Taken together with another study
revealing a high concordance between GSI and virtual
histology-intravascular ultrasound in the assessment of carotid
plaque composition using effective Z-maps (10), these findings indicate the
potential use of GSI for the identification of vulnerable carotid
plaques. Therefore, the aim of the present study was to provide a
comprehensive evaluation of carotid atherosclerotic plaques using
both GSI imaging biomarkers and serological biomarkers, and further
explore their possible roles in the atherogenic process. GSI-CTA
data, as well as serum Hs-CRP and MCP-1 levels were analyzed in
patients with this disease in an attempt to establish a novel
strategy for the comprehensive assessment of vulnerable carotid
plaques by integrating the GSI variables along with the serological
parameters.
Patients and methods
Subjects
A total of 42 cases of asymptomatic carotid plaque
(27 males and 15 females; mean age, 63.6±10.4 years) were enrolled
in the present study. All patients were diagnosed based on their
medical history, clinical examination and results of carotid duplex
sonography, brain MRI and MRA scans. Ultrasound assessment was
performed for carotid artery segmentation, in which the carotid
artery was divided into four different segments: i) Common carotid
artery, ii) carotid bulb, iii) external carotid artery and iv)
internal carotid artery. Carotid plaques were detected by
ultrasound and characterized according to the Mannheim consensus as
focal structures encroaching into the arterial lumen by ≥0.5 mm or
50% of the surrounding intima-media thickness value, or a thickness
≥1.5 mm (11). Furthermore, these
patients with carotid plaques underwent GSI scan and were then
further divided into an unstable plaque group (n=17; 5 males and 12
females; mean age, 63.29±11.91 years) and stable plaque group
(n=25; 10 males and 15 females; mean age, 63.80±9.57 years). A
total of 19 healthy individuals (10 males and 9 females; mean age,
60.3±11.8 years), who received carotid ultrasound examination and
exhibited no large-vessel atherosclerosis, were included as normal
controls (NCs). The study protocol was reviewed and approved by the
Ethics Committee of Beijing Anzhen Hospital (Beijing, China). All
diagnostic procedures were performed in accordance with relevant
guidelines and regulations, and written informed consent was
obtained from all of the participants of the study.
None of the participating individuals had i) stroke,
myocardial infarction, brain tumors, blood diseases or other
abnormal coagulation diseases, rheumatic heart disease, infectious
endocarditis, arrhythmia, serious liver or kidney diseases, acute
or chronic inflammatory diseases or autoimmune diseases; ii)
disturbance of consciousness or severe cognitive impairment; nor
iii) other reasons to prevent them from undergoing GSI. The
following diagnostic tests were performed in both patients and NCs:
Complete blood count, blood chemistry, Hs-CRP, electrocardiogram,
posterior-anterior chest radiography, transthoracic cardiac
echocardiography, transcranial Doppler ultrasonography and carotid
duplex sonography.
Sample collection
Whole blood samples were drawn between 9:00 a.m. and
12:00 p.m. After centrifugation, the serum was collected and stored
at -70˚C in small aliquots for further use.
CT protocols
Upon injection of nonionic contrast agent
(iopamidol; Bracco Imaging), GSI-CTA was performed with a scan
delay of 5 sec using a 64-slice spiral CT scanner with a gemstone
detector (GE Discovery CT 750 HD; GE Healthcare). The flow rates
and volume of contrast material were determined based on a fixed
duration (12 sec) of injection and dose tailored to the bodyweight
of each patient (252 mgI/kg). A total of 65 ml iopamidol (30 gI/100
ml) was intravenously injected at a flow rate of 3.5 ml/sec,
followed by administration of 30 ml saline chaser at the same flow
rate. Scanning was performed using the 0.6x0.625 mm GSI mode. The
scanning parameters employed in GSI mode were as follows: Tube
voltage, 80/140 kV and 0.5-msec instantaneous switch; tube current,
600 mA; slice thickness, 0.625 mm; rotation speed, 0.8 sec; helical
pitch, 1.375; and matrix, 512x512.
Image evaluation
After scanning, GSI viewer 4.5 (GE Healthcare) was
utilized for further analysis. GSI plaque imaging was initially
quantified for carotid atherosclerotic plaque characterization. The
images were reviewed in a blinded fashion by two radiologists who
are experts in carotid plaque imaging. They participated in the
interpretation of the data, discussing and reaching a consensus if
any disagreement occurred. To avoid potential radiation damage to
X-ray-sensitive organs such as the lens and thyroid gland in the
head and neck, the middle part of the ascending aorta was selected
as the region of interest (ROI), where a threshold value of 100
Hounsfield units (HU) was established for triggering the
contrast-enhanced scan (12). The
area of ROI is ~10 mm2. The minimum ROI volume was set
on the plaque through post-processing techniques and a
characteristic spectral attenuation curve in HU of the
corresponding region was acquired. The points on the curve
represent the average CT values of tissues at different keV
levels.
The ROI was then placed in the fat tissue, muscle
fiber tissue and bone structure, and three distinctive attenuation
curves with different colors were obtained to determine the nature
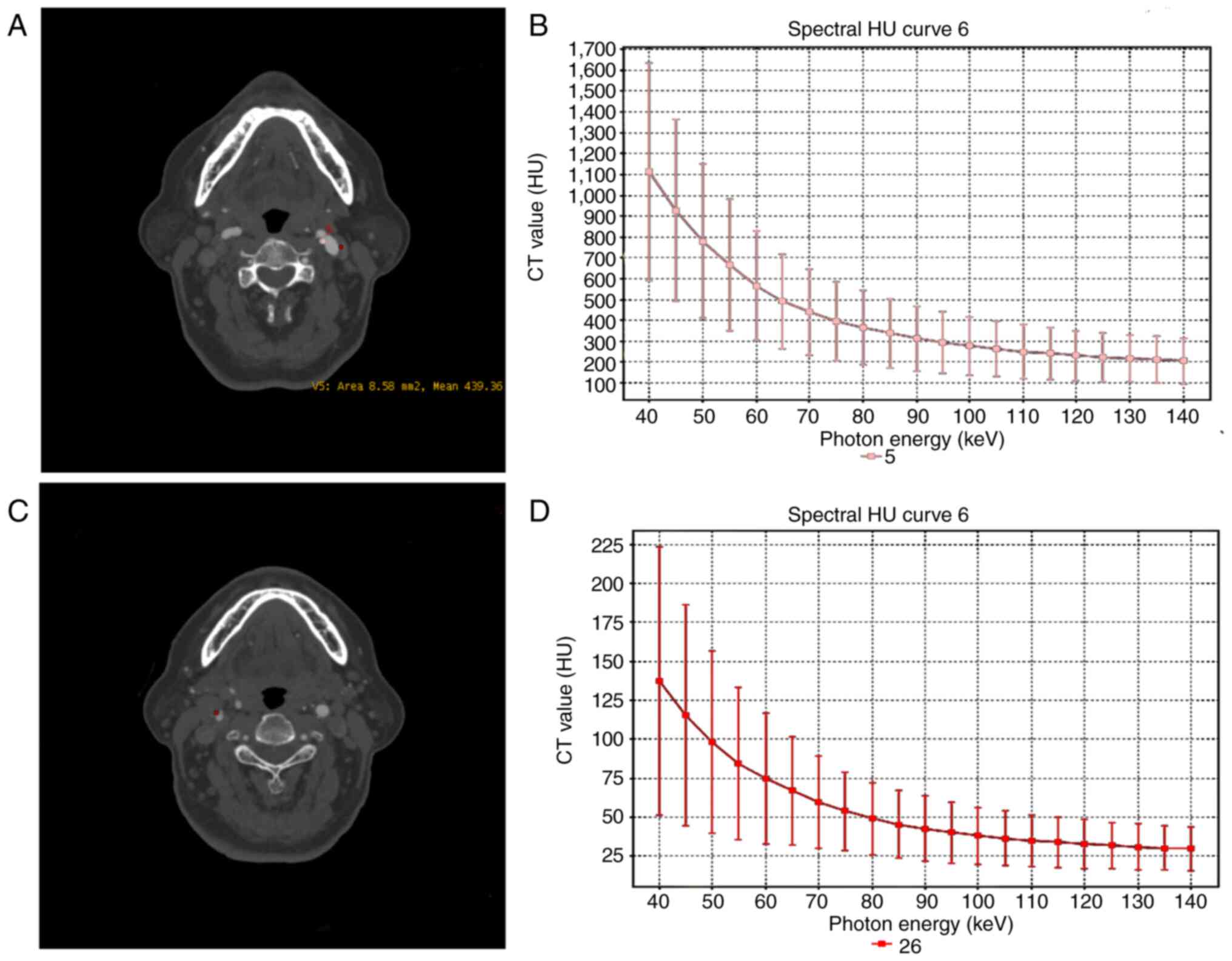
of the plaques (Fig. 1). Three
categories of plaques were defined according to their average CT
values: i) Lipid cores plaques, non-calcified plaques (NCPs)<30
HU; ii) fibrous plaques, 30 HU<NCP<150 HU; and iii) calcified
plaques, >220 HU. Furthermore, the calcified plaques were
divided into spotty or large calcification. The former refers to
plaques of <3 mm in size on curved multiplanar reformation
images and 1-sided on axial images, while the latter refer to
plaques with calcification greater than the spotty calcification
(13). Large calcified and fibrous
plaques were recognized as stable plaques, whereas lipid and spotty
calcified plaques were regarded as unstable plaques (13,14).
Upon qualitative analysis of the atherosclerotic
plaques, MD analysis was selected for measurement of the
intraplaque calcium content. In brief, the energy range was set at
65 keV, followed by placing of the minimum ROI volume on the target
plaque, ensuring that the ROI was placed on the images with the
original scanning layer thickness, particularly those with a
relatively uniform density, in order to detect the tissue content
(g/l) of the corresponding intraplaque structure. Calcium
(water)-based MD analysis was performed as described previously
(15) to measure intraplaque
calcium content in the selected ROI using an extracted calcium map.
In addition, the slope of the spectral curve associated with the CT
value of the plaque was calculated and the difference between the
CT values of two measured points on the energy spectrum curve was
divided by the energy difference between these two points. In the
present study, since the single-energy CT values at 40 and 110 keV
were selected as reference points, the value of the slope K of the
spectrum curve was equivalent to (CTa-CTb)/70.
Immunoturbidimetry
Serum MCP-1 levels were determined with a commercial
immunoturbidimeter (cat. no. EK0441; Wuhan Boster Biological
Technology, Co. Ltd.) according to the supplier's instructions,
with a detection limit of 15.6 pg/ml. All assays were performed
simultaneously in a blinded manner.
Follow-up
To explore the difference in short-term prognosis
between stable and unstable plaques, trained investigators
collected patients' data through telephone or face-to-face
interviews at 1, 3 and 12 months after baseline measurements.
Clinical events and medical therapy were evaluated at each
follow-up visit among all patients. The primary outcome measures
were a composite of stroke (either ischemic or hemorrhagic) or
transient ischemic attack (TIA), whichever occurred first. All of
the primary outcome events and medical therapies were reviewed by
TTN and LM.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 8 (GraphPad Software Inc.). Quantitative data are presented
as the mean ± standard deviation (age, leukocyte, monocyte, calcium
content, MCP-1 level) or as the median with interquartile range
(Hs-CRP level and spectral curve slope). Normally distributed data
were analyzed by one-way ANOVA for multiple-group comparison and
the Student-Newman-Keuls post-hoc test for intergroup comparison,
or with a Pearson's correlation test. Non-normally distributed data
were analyzed with the Kruskal-Wallis test or Spearman's
correlation analysis. The χ2 test was used to compare
differences in qualitative data (sex, diabetes, coronary artery
disease, dyslipidemia, hypertension, smoking, peripheral artery
disease and carotid stenosis) between groups. Receiver operating
characteristic (ROC) curve analysis was also performed for
quantitative slope of spectral curve, calcium content, serum Hs-CRP
and MCP-1 level, and areas under the ROC curve (AUC) were
calculated. P<0.05 was considered to indicate statistical
significance.
Results
Baseline characteristics
Patient characteristics, including risk factors and
laboratory data, obtained on the first day of sampling, are
presented in Table I. No
significant differences were observed between NCs and either of the
carotid atherosclerosis groups.
 | Table IBaseline characteristics of patients
and NC. |
Table I
Baseline characteristics of patients
and NC.
| Parameter | Unstable plaque
(n=17) | Stable plaque
(n=25) | NC (n=19) | P-value |
|---|
| Age, years | 63.30±11.90 | 63.80±9.60 | 60.20±11.80 | 0.555 |
| Female sex | 12.00 (70.60) | 15.00 (60.00) | 10.00 (52.60) | 0.543 |
| Diabetes | 3.00 (17.70) | 5.00 (20.00) | - | 0.849 |
| Coronary artery
disease | 3.00 (17.70) | 2.00 (8.00) | - | 0.343 |
|
Hypercholesterolemia | 10.00 (58.80) | 9.00 (36.00) | 4.00 (21.10) | 0.064 |
| Hypertension | 7.00 (41.20) | 9.00 (36.00) | - | 0.735 |
| Smoking | 7.00 (41.20) | 7.00 (28.00) | 4.00 (21.10) | 0.665 |
| Peripheral artery
disease | 7.00 (41.20) | 8.00 (32.00) | - | 0.542 |
| Leukocytes,
x103/µl | 7.01±2.12 | 7.06±1.65 | 6.86±1.92 | 0.935 |
| Carotid stenosis,
% | | | | 0.318 |
| >50 | 6.00 (35.30) | 11.00 (44.00) | - | |
| ≤50 | 11.00 (64.70) | 14.00 (56.00) | | |
| Monocytes,
x103/µl | 0.39±0.11 | 0.38±0.09 | 0.35±0.10 | 0.450 |
Carotid atherosclerotic plaque
characterization by using GSI
CTA source image presents a stable plaque in the
left carotid bulb, and the energy spectrum curve of plaque displays
a bow-down curve with a negative slope (Fig. 1). Among the 42 patients with
carotid atherosclerosis, a total of 22 unstable plaques were
detected in 17 cases, while a total of 30 stable plaques were
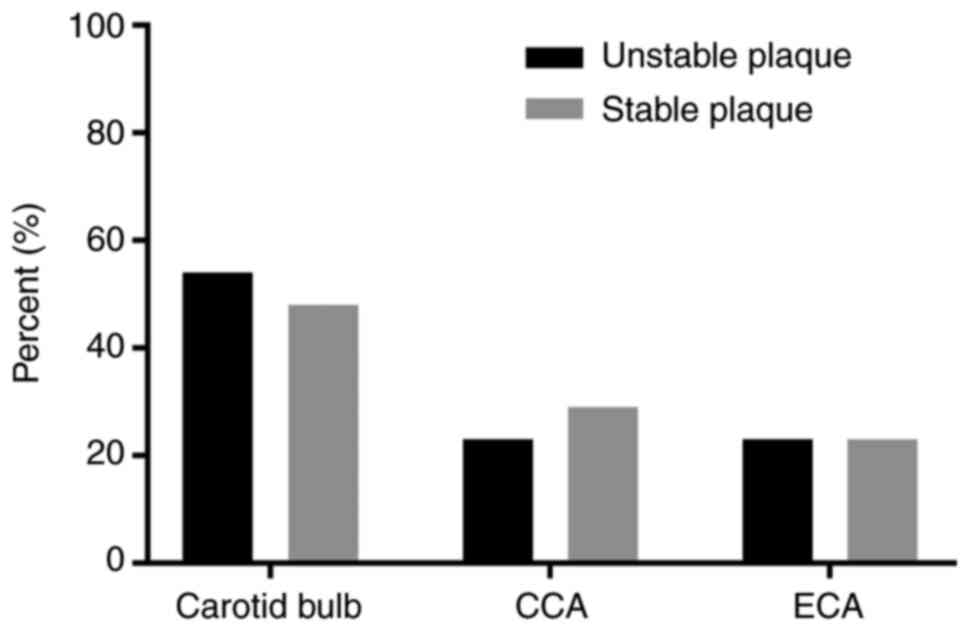
detected in 25 cases by using GSI. In the unstable plaque group,
the position of the atherosclerosis plaque was the carotid bulb
(12/22, 54.5%), common carotid artery (5/22, 22.7%) and external
carotid artery (5/22, 22.7%). In the stable plaque group, there
were 15 cases in the carotid bulb (15/30, 50%), 8 cases in the
common carotid artery (8/30, 26.7%) and 7 cases in the external
carotid artery (7/30, 23.3%). However, no plaques were identified
in the internal carotid artery in both carotid plaque groups. No
significant differences in the location of the plaques in the
different carotid segments were observed between the stable and
unstable plaque groups (Fig.
2).
Calcium content in plaques and slope
of spectral curve via GSI
In the selected ROI, the calcium content was
significantly lower in the unstable plaque group (47.53±37.17 g/l)
than in the stable plaque group (147.85±49.54 g/l; P<0.001),
while the slope of the spectral curve was significantly higher in
the unstable plaque group [0.29 (interquartile range: -3.86, 3.00)]
than in the stable plaque group [-6.55 (interquartile range:
-14.55, -4.50); P<0.001] (Fig.
3). Additional data are presented in Table SI.
Serum Hs-CRP and MCP-1 levels
Patients with unstable plaques exhibited increased
Hs-CRP levels [15.33 (interquartile range: 10.65, 17.35) mg/l]
compared with those of the stable plaque and NC groups [2.38
(interquartile range: 1.23, 3.75) mg/l and 1.21 (interquartile
range: 0.76, 1.50) mg/l, respectively; both P<0.001]. Similarly,
there were significantly elevated MCP-1 levels in patients with
unstable plaques (467.13±66.28 pg/ml) compared with those in the
stable plaque and NC groups (351.84±81.89 and 153.64±49.79 pg/ml,
respectively; both P<0.001) (Fig.
3). Additional data are presented in Table SI.
ROC of calcium content, spectral curve
slope and serum levels of Hs-CRP and MCP-1 in the plaque
groups
In general, the ROC curves were constructed for
calculation of the AUC at the optimal cut-off value along with
maximum sensitivity and specificity. As a result, the generated AUC
of the calcium content in carotid plaque was 0.938 and the assumed
cut-off calcium content for discriminating stable and unstable
plaque was 101.5 pg/ml, with a sensitivity of 73.33% and a
specificity of 100%. The generated AUC of the slope of the spectral
curve was 0.942 and the assumed cut-off slope of the spectral curve
for differentiating stable from unstable plaque was 3.835, with a
sensitivity of 96.67% and a specificity of 77.27%. The generated
AUC of serum Hs-CRP was 1 and the optimal Hs-CRP threshold cutoff
was determined to be 7.14 mg/l, with a sensitivity and specificity
of 100 and 100%, respectively. The generated AUC of serum MCP-1 was
0.901 and the optimal MCP-1 threshold cutoff was determined to be
392.3 pg/ml, with a sensitivity and specificity of 84 and 100%,
respectively (Fig. 4). Additional
data are presented in Table
SII.
 | Figure 4ROC curve analysis of spectral curve
slope, calcium content in plaque and serum levels of Hs-CRP, MCP-1
in the plaque groups. Quantitative analysis for curve slope of
plaque (green line), calcium content in plaque (orange line), serum
MCP-1 level (blue line) and serum Hs-CRP level (red line) in plaque
groups was performed using ROC curve analysis. The AUC for the
calcium content in the plaque was 0.938 and the assumed cut-off for
the calcium content for discriminating between stable and unstable
plaque was 101.5 (g/l), with a sensitivity of 73.33% and a
specificity of 100%. The AUC of the slope of the spectral curve was
0.942 and the assumed cut-off for the slope of the spectral curve
for differentiating stable from unstable plaque was 3.835, with a
sensitivity of 96.67% and a specificity of 77.27%. The AUC of serum
Hs-CRP levels was 1 and the optimal Hs-CRP threshold cutoff was
determined to be 7.14 mg/l, with associated sensitivity and
specificity of 100 and 100%, respectively. The AUC of serum MCP-1
levels was 0.901 and the ideal MCP-1 threshold cutoff was
determined to be 392.3 pg/ml, with associated sensitivity and
specificity of 84 and 100%, respectively. ROC, receiver operating
characteristic; AUC, area under the curve; Hs-CRP, high-sensitivity
C-reactive protein; MCP-1, monocyte chemotactic protein-1. |
Correlation analysis
Age, complete blood counts (leukocytes and
monocytes) and calcium content were not significantly correlated
with the serum levels of Hs-CRP or MCP-1 in either the patients or
NC groups. No obvious correlation was observed between the calcium
content, serum levels of Hs-CRP or MCP-1 in either patients with
unstable plaques nor those with stable plaques (data not shown). No
obvious correlation was obtained between the calcium content and
spectrum curve slope in patients with unstable plaques (r=-0.236,
P=0.291; data not shown), but the calcium content was significantly
negatively correlated with spectrum curve slope in patients with
stable plaques (r=-0.494, P=0.006) (Fig. S1).
Medication use and cerebrovascular
events
During the 1-year follow-up, no significant
differences were observed in the medication use between the two
carotid atherosclerosis groups, with the exception of higher use of
lipid-lowering drugs in the unstable plaque group than in the
stable plaque group (P<0.001). No deaths occurred in the present
study cohort. Ischemic stroke occurred in 2 patients with unstable
plaques (2/17, 11.8%) due to artery-to-artery embolism from the
carotid artery origin and in 2 patients with stable plaques (2/25,
8%) in the absence of TIA or intracranial hemorrhage (Table II), but no statistically
significant difference in the occurrence rate of ischemic stroke
was obtained between these two groups (P>0.05).
 | Table IIMedication use and cerebrovascular
events during 1-year follow-up in the unstable and stable plaque
groups. |
Table II
Medication use and cerebrovascular
events during 1-year follow-up in the unstable and stable plaque
groups.
| Item | Unstable plaque
(n=17) | Stable plaque
(n=25) | P-value |
|---|
| ≥1 antiplatelet
agent | 15/17 | 24/25 | 0.556 |
| ≥1 antihypertensive
agent | 7/17 | 7/25 | 0.508 |
| ≥1 lipid-lowering
agent | 16/17 | 10/25 | <0.001 |
| ≥1 glucose-lowering
agent | 3/17 | 4/25 | >0.999 |
| ≥1 anticoagulant
agent | 0 | 0 | |
| Quitting
smoking | 0 | 0 | |
| CEA/CAS | 0 | 0 | |
| Outcome of stroke
or TIA | 2/17 | 2/25 | >0.999 |
| Ischemic
stroke | 2/17 | 2/25 | |
| TIA | 0 | 0 | |
| Intracranial
hemorrhage | 0 | 0 | |
Discussion
In the present study, patients with unstable plaques
exhibited a decrease in calcium content compared with that of the
calcified plaques group. Together with a previous study suggesting
that less calcification was associated with clinically symptomatic
plaques rather than with asymptomatic plaques (16), this finding indicates that the GSI
calcium content may be associated with plaque instability. This is
noteworthy because recent data have demonstrated that
atherosclerotic plaque calcification is a complex, active
biological process involving plaque vulnerability to rupture,
consequently leading to major cardiovascular events such as
myocardial infarction. At present, clinical imaging modalities,
including non-invasive methods such as CT or invasive methods such
as intravascular US or optical coherence tomography, are utilized
for the characterization of calcified carotid plaques (17). To the best of our knowledge, the
present study is the first time the GSI technique has been adopted
to analyze the calcium content in carotid atherosclerotic plaque.
In the present study, this novel technique was selected because,
inheriting the detection sensitivity of CT towards calcium, GSI
uses X-rays and expresses the absorption of the energy spectrum
based on tissue composition and lesions, performing quantitative
analysis via the MD technique, where the calcium map displays only
calcium density and enables measurement of calcium content in
plaques (7,8). Of note, the present study also
indicated that the spectral curve slope of the CT value of the
plaque in patients with stable plaques was significantly lower than
that of patients with unstable plaques, which is in agreement with
a previous study by Karçaaltıncaba and Aktaş (18), who reported that vulnerable plaques
were rich in lipid cores and their energy spectrum curve exhibited
a bow-up curve with a positive slope, whereas stable plaques had a
bow-down curve with a negative slope. Of note, in the present
study, ROC curve analyses were performed, which revealed the
optimal diagnostic threshold values of calcium content and spectral
curve slope for differentiating vulnerable from stable plaques, as
well as their distinctive specificity and sensitivity, indicative
of different roles of these parameters in the assessment of carotid
plagues. Indeed, an obvious correlation was observed between the
calcium content and spectrum curve slope in the patients with
stable plaques, but not in the patients with unstable plaques,
further supporting the present viewpoint. Taken together, the
present results indicated that these two GSI parameters may serve
as potential imaging biomarkers relevant to plaque vulnerability or
disease progression.
As the most promising indicator for vascular
inflammation, CRP is one of the acute-phase proteins mainly produced
in the liver during episodes of acute inflammation or infection.
Hs-CRP assay methods are capable of detecting small changes in CRP
concentrations. Hs-CRP is currently considered a predictor of
future cardiovascular events and was classified as class III B
level of evidence in the 2016 European guidelines on cardiovascular
disease prevention (19), albeit
controversy on the validity of this biomarker still exists. Various
studies have provided strong evidence that CRP inhibits endothelial
nitric oxide production and contributes to plaque instability by
activating NF-κB, inducing the expression of MMP-2 and -9(20). In line with these previous studies,
the present study indicated that, compared with those in the stable
plaque or NC groups, patients with unstable plaques had markedly
elevated serum Hs-CRP levels. Furthermore, optimal diagnostic
threshold values were also obtained for separating vulnerable from
stable plaques with higher specificity and sensitivity than either
calcium content or spectral curve slope, supporting the view that
alterations in this inflammatory biomarker are closely associated
with the formation or development of vulnerable carotid plaques.
This is noteworthy, since a novel strategy for multiplying
individual profiles has been proposed by Nederkoorn (21) for the selection of patients with
the highest risk and for the selection of the best treatment. For
instance, along with the Reynolds risk score (22), the addition of Hs-CRP as well as
family history and traditional risk factors was reported to
efficiently improve the overall future risk prediction of
cardiovascular events (23).
However, further studies are required to address these issues.
To date, limited data are available concerning the
roles of MCP-1 in vulnerable carotid plaques. Of note, in an in
vivo animal study on apolipoprotein E-/- mice,
site-specific delivery of adenoviral-mediated small hairpin RNA
targeting mouse MCP-1 downregulated MCP-1 expression, which turned
a vulnerable plaque into a more stable plaque phenotype and
prevented plaque disruption, suggesting its detrimental effects on
plaque stability (24). In
parallel with these findings, the present study suggested that
patients with unstable plaques had higher serum MCP-1 levels than
patients with stable plaques or NCs. Together with the ROC analysis
of calcium content or spectral curve slope, the present findings
strongly suggest that MCP-1 may potentially be involved in carotid
plaque instability and therefore utilized for the assessment of
plaque vulnerability.
Although CT is relatively inexpensive as compared to
MRI, the ionizing radiation of CT scanning has raised increasing
concern in recent years. To reduce the risk of CT radiation, two
strategies are proposed: One is justification, which refers to CT
scans only when medically necessary; another is optimization, which
refers to adjusting and operating a CT scanner to obtain images
adequate for diagnosis at the lowest possible dose. Considering the
great potential of GSI in detecting carotid atherosclerotic plaque
components, appropriate use on patients requiring screening is
thought to be necessary and beneficial. In addition, compared to
conventional CT, the dose of X-ray radiation and contrast agent is
decreased since GSI may achieve comparable contrast-to-noise ratios
with lower dose efficiency. In any case, when choosing to perform
CTA, the most suitable patients should be selected to gain the
greatest benefit with minimum risk (25).
In the present study, although the incidence of
ischemic stroke in the unstable plaque group was slightly higher
than that in the stable plaque group (11.8 vs. 8.0%), no
statistically significant difference was observed between these two
groups during the 1-year follow-up, possibly due to the better
compliance in the administration of lipid-lowering drugs in the
former group (26). However, the
association between these two GSI parameters and the risk of
ischemic stroke was not investigated in the present study, mainly
due to the small sample size and a short-term follow-up (at 1
year). However, a multicenter prospective study using a larger
sample size will help determine the role of plaque features by GSI
as possible clinical predictors of future ipsilateral
cerebrovascular events.
In conclusion, the present study suggested that
patients with unstable plaque exhibited a significantly lower
calcium content and higher spectral curve slope than those of the
stable plaque group. A marked alteration in GSI calcium content and
spectral curve slope in patients with unstable plaque was observed,
reflecting a close link between calcification and plaque
instability. These two imaging parameters are of powerful
diagnostic value in the determination of unstable plaques with
different threshold values, indicating that they may serve as
valuable biomarkers related to atherosclerosis and plaque
vulnerability in clinical practice. However, the small sample size
is a major limitation of the present study and larger-scale
comparisons with histopathological specimens are required to
validate the reliability of GSI-based CT carotid plaque imaging in
the future. The present study also demonstrated altered serum
levels of Hs-CRP and MCP-1 proteins in patients with unstable
plaques, as well as their optimal diagnostic threshold values for
determining unstable plaques, thus supporting the hypothesis that
these pro-inflammatory molecules may be implicated in the process of
plaque instability and may therefore serve as potential serological
biomarkers for the prediction of vulnerable carotid plaques. In
summary, the present findings support the feasibility of using
these serological and imaging parameters as multiple potential
biomarkers relevant to plaque vulnerability or stroke progression.
However, these findings of GSI calcium content and spectral curve
slope in vulnerable carotid plaques pave the way for identifying
valuable candidate biomarkers in atherothrombotic stroke and for
exploring novel therapeutic strategies for effective stroke
prevention.
Supplementary Material
Correlation analysis between the
calcium content and spectral curve slope in the stable plaque
group. The calcium content was negatively correlated with the
spectral curve slope (r=.0.494, P=0.006).
Serum levels of Hs-CRP, MCP-1,
spectral curve slope of plaque and calcium content in carotid
plaques in the three groups.
ROC curve analysis of calcium content
in plaque and curve slope of plaque, serum levels of Hs-CRP, MCP-1
in plaque groups. The ROC curves were constructed for calculation
of AUC, optimal cut-off value with maximum sensitivity and
specificity.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GZL and LW conceived the experiments. ZXF, XQL, TTY
and KS performed the experiments. SJY, TTN and LM analyzed the
results. ZXF and GZL confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Review Board of Beijing Anzhen Hospital
(Beijing, China) examined and approved the study protocol in
accordance with the Declaration of Helsinki. Informed consent was
obtained from all individual participants included in the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brinjikji W, Huston J III, Rabinstein AA,
Kim GM, Lerman A and Lanzino G: Contemporary carotid imaging: From
degree of stenosis to plaque vulnerability. J Neurosurg. 124:27–42.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gao T, Zhang Z, Yu W, Zhang Z and Wang Y:
Atherosclerotic carotid vulnerable plaque and subsequent stroke: A
high-resolution MRI study. Cerebrovasc Dis. 27:345–352.
2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Takaya N, Yuan C, Chu B, Saam T, Underhill
H, Cai J, Tran N, Polissar NL, Isaac C, Ferguson MS, et al:
Association between carotid plaque characteristics and subsequent
ischemic cerebrovascular events: A prospective assessment with
MRI-initial results. Stroke. 37:818–823. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Stary HC, Chandler AB, Dinsmore RE, Fuster
V, Glagov S, Insull W Jr, Rosenfeld ME, Schwartz CJ, Wagner WD and
Wissler RW: A definition of advanced types of atherosclerotic
lesions and a histological classification of atherosclerosis. A
report from the committee on vascular lesions of the council on
arteriosclerosis, American heart association. Circulation.
92:1355–1374. 1995.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lusis AJ: Atherosclerosis. Nature.
407:233–241. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Moriya J: Critical roles of inflammation
in atherosclerosis. J Cardiol. 73:22–27. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tatsugami F, Higaki T, Nakamura Y, Honda Y
and Awai K: Dual-energy CT: Minimal essentials for radiologists.
Jpn J Radiol: Jan 4, 2022 (Epub ahead of print).
|
|
8
|
Lorsakul A, Fakhri GE, Worstell W, Ouyang
J, Rakvongthai Y, Laine AF and Li Q: Numerical observer for
atherosclerotic plaque classification in spectral computed
tomography. J Med Imaging (Bellingham). 3(035501)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shinohara Y, Sakamoto M, Kuya K, Kishimoto
J, Yamashita E, Fujii S, Kurosaki M and Ogawa T: Carotid plaque
evaluation using gemstone spectral imaging: Comparison with
magnetic resonance angiography. J Stroke Cerebrovasc Dis.
26:1535–1540. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shinohara Y, Sakamoto M, Kuya K, Kishimoto
J, Iwata N, Ohta Y, Fujii S, Watanabe T and Ogawa T: Assessment of
carotid plaque composition using fast-kV switching du-al-energy CT
with gemstone detector: Comparison with extracorporeal and virtual
histology-intravascular ultra-sound. Neuroradiology. 57:889–895.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Touboul PJ, Hennerici MG, Meairs S, Adams
H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S,
Hernandez Hernandez R, et al: Mannheim carotid intima-media
thickness and plaque consensus (2004-2006-2011). An update on
behalf of the advisory board of the 3rd, 4th and 5th watching the
risk symposia, at the 13th, 15th and 20th European stroke
conferences, Mannheim, Germany, 2004, Brussels, Belgium,. 2006, and
Hamburg, Germany, 2011. Cerebrovasc Dis. 34:290–296.
2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ma G, Yu Y, Duan H, Dou Y, Jia Y, Zhang X,
Yang C, Chen X, Han D, Guo C and He T: Subtraction CT angiography
in head and neck with low radiation and contrast dose dual-energy
spectral CT using rapid kV-switching technique. Br J Radiol.
91(20170631)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Motoyama S, Kondo T, Sarai M, Sugiura A,
Harigaya H, Sato T, Inoue K, Okumura M, Ishii J, Anno H, et al:
Multislice computed tomographic characteristics of coronary lesions
in acute coronary syndromes. J Am Coll Cardiol. 50:319–326.
2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Vancheri F, Longo G, Vancheri S, Danial
JSH and Henein MY: Coronary artery microcalcification: Imaging and
clinical implications. Diagnostics (Basel). 9(125)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yue D, Li Fei S, Jing C, Ru Xin W, Rui
Tong D, Ai Lian L and Luo YH: The relationship between calcium
(water) density and age distribution in adult women with spectral
CT: Initial result compared to bone mineral density by dual-energy
X-ray absorptiometry. Acta Radiol. 60:762–768. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kwee RM: Systematic review on the
association between calcification in carotid plaques and clinical
ischemic symptoms. J Vasc Surg. 51:1015–1025. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Barrett HE, Van der Heiden K, Farrell E,
Gijsen FJH and Akyildiz AC: Calcifications in atherosclerotic
plaques and impact on plaque biomechanics. J Biomech. 87:1–12.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Karçaaltıncaba M and Aktaş A: Dual-energy
CT revisited with multidetector CT: Review of principles and
clinical applications. Diagn Interv Radiol. 17:181–194.
2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Piepoli MF, Hoes AW, Agewall S, Albus C,
Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C, et
al: ESC scientific document group. 2016 European guidelines on
cardiovascular disease prevention in clinical practice: The sixth
joint task force of the European society of cardiology and other
societies on cardiovascular disease prevention in clinical practice
(constituted by representatives of 10 societies and by invited
experts) developed with the special contribution of the European
association for cardiovascular prevention and rehabilitation
(EACPR). Eur Heart J. 37:2315–2381. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cimmino G, Ragni M, Cirillo P, Petrillo G,
Loffredo F, Chiariello M, Gresele P, Falcinelli E and Golino P:
C-reactive protein induces expression of matrix
metalloproteinase-9: A possible link between inflammation and
plaque rupture. Int J Cardiol. 168:981–986. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nederkoorn PJ: Vulnerable carotid plaque
and biomarkers: Multiplying individual risk profiles? Eur J Neurol.
26(1425)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ridker PM, Buring JE, Rifai N and Cook NR:
Development and validation of improved algorithms for the
assessment of global cardiovascular risk in women: The reynolds
risk score. JAMA. 297:611–619. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Emerging Risk Factors Collaboration.
Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P,
Walker M, Thompson A, Sarwar N, et al: C-reactive protein,
fibrinogen, and cardiovascular disease prediction. N Engl J Med.
367:1310–1320. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liu XL, Zhang PF, Ding SF, Wang Y, Zhang
M, Zhao YX, Ni M and Zhang Y: Local gene silencing of monocyte
chemoattractant protein-1 prevents vulnerable plaque disruption in
apolipoprotein E-knockout mice. PLoS One. 7(e33497)2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Schmidt CW: CT scans: Balancing health
risks and medical benefits. Environ Health Perspect. 120:A118–A121.
2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Amarenco P, Bogousslavsky J, Callahan A
III, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic
L, Szarek M, Welch KM, et al: High-dose atorvastatin after stroke
or transient ischemic attack. N Engl J Med. 355:549–559.
2006.PubMed/NCBI View Article : Google Scholar
|