Introduction
Sickle cell disease (SCD) is one of the most
frequent and severe monogenic disorders, affecting 20-25 million
individuals worldwide. It is caused by a single point mutation
(Glu6Val) in the β-globin gene. Of note, >300,000 children with
SCD are anticipated to be born each year globally, with almost 75%
of these births occurring in Sub-Saharan Africa (1). SCD is a fatal hematological illness,
characterized by veno-occlusive events and hemolytic anemia.
Abnormal sickle-shaped erythrocytes, along with other blood cells
that form aggregates, impair blood flow in small vessels, resulting
in distal tissue ischemia and inflammation. When this process
affects the bones, it causes the typical vaso-occlusive crisis
(VOC), with the clinical manifestation of severe pain; however,
every organ can be affected by vaso-occlusion. Sickling and
persistent hemolytic anemia, even when mild or asymptomatic, induce
parenchymal injury and chronic organ damage, resulting in
significant morbidity and early mortality (2). Endothelial inflammation and
predisposition to thrombosis are also hallmarks of SCD, which has
recently been identified as a thrombophilic condition, and the
incidence of venous thromboembolism (VTE) is increased in patients
with SCD (3).
The definition of acute chest syndrome (ACS)
includes a new pulmonary density on a chest radiograph and at least
one of the following: Temperature ≥38.5˚C, tachypnea or shortness
of breath, chest pain, cough and a decrease in oxygen saturation
(SpO2) (4). ACS is the
second-most prevalent complication of SCD, accounting for ~25% of
all deaths among patients with SCD. In the majority of cases, ACS
presents following a VOC or surgery (5). A number of factors have been
implicated in the pathophysiology of ACS, including infections,
sickling and vaso-occlusion, inflammation, fat emboli caused by
bone marrow necrosis, VTE and hypoventilation related to pain or
opioids. The incidence of viral infections causing ACS in adult
patients with SCD has been reported to be <10% of cases
(6). The severe form of ACS can
lead to multi-organ failure. Transfusions or exchange transfusions
are mandatory for the treatment of ACS when respiratory failure is
developed (6).
Patients with SCD have been classified in the
‘high-risk’ category of the population during the coronavirus
disease 2019 (COVID-19) pandemic due to their weakened immune
system, caused by functional hyposplenism, as well as systemic
vasculopathy, which poses them at risk of end organ failure and
thrombosis (7). COVID-19 has been
reported to trigger the development of ACS (8).
Endothelial damage and a procoagulant state appear
to be distinct hallmarks of COVID-19(9). In COVID-19, it has been observed that
a shift in the vascular equilibrium with endothelitis with
lymphocyte infiltration and subsequent ischemia is linked to a
procoagulant condition, particularly in high-risk ethnicities, such
as African Americans (10,11). As a result, the SCD population
appears to be at an increased risk of developing severe pulmonary
vascular damage as a result of severe acute respiratory syndrome
coronavirus 2 (SARS-CoV-2) infection (12). There is a serious concern that the
overlap in the pathophysiology of COVID-19-related lung disease and
ACS will lead to unfavorable outcomes in these patients.
Furthermore, as both COVID-19 and SCD promote the incidence of
thromboembolic events, the combination of these two disorders may
significantly raise the risk of complications (13).
Reports of patients with SCD infected with COVID-19
have previously been published, with contradictory outcomes. In
some of these reports, the patients were shown to present with a
surprisingly satisfactory clinical outcome, which was unexpected
given the vulnerability of patients with SCD to infections and
vaso-occlusive complications (14,15).
The present study describes the clinical characteristics,
management and outcomes of 3 unvaccinated patients with SCD who
were hospitalized due to SARS-CoV-2 infection in a hospital in
Greece.
Case report
Case 1
The first case is that of a 54-year-old male patient
with S/β-thalassemia. His medications included hydroxyurea at 1,000
mg daily, folic acid at 5 mg daily and acetylsalicylic acid at 100
mg daily.
The patient presented to the Emergency Department
(ED) of Laiko General Hospital (Athens, Greece) with complaints of
fever over the last 14 days. He was unvaccinated for SARS-CoV-2. He
had a positive reverse transcription-polymerase reaction (RT-PCR)
test of a nasopharyngeal specimen for SARS-CoV-2 infection 10 days
prior to his visit to the ED. In the ED, the patient was noted to
have a SpO2 of 90% in room air, a blood pressure (BP) of
120/50 mmHg, a heart rate (HR) of 88 bpm and a temperature of
39.2˚C. The clinical characteristics of the patient are summarized
in Table I.
 | Table IClinical characteristics of the
patients in the present study. |
Table I
Clinical characteristics of the
patients in the present study.
| Case | Age/sex | Genotype | Blood group | Vaccination
status | Imaging findings | Complications | Management | Days of
hospitalization | Outcome |
|---|
| 1 | 54/M | S/β-thalassemia | 0 | Unvaccinated | Chest CT scan and
CTPA revealed nodular ground glass opacities in all lung fields.
Pulmonary embolism was not detected | None | Oxygen therapy; fluid
replacement; remdesivir; enoxaparin; dexamethasone; antibiotics;
transfusion of RBCs | 8 | Recovery |
| 2 | 45/M | S/β-thalassemia | 0 | Unvaccinated | Chest CT scan and
CTPA revealed nodular ground glass opacities in all lung fields and
a small left pleural effusion. Pulmonary embolism was not
detected | VOC, ACS,
hemolysis | Oxygen therapy;
fluid replacement; morphine; remdesivir; enoxaparin; dexamethasone;
antibiotics; transfusion of RBCs | 16 | Recovery |
| 3 | 51/F |
S/β-thalassemia | A | Unvaccinated | Chest CT scan and
CTPA revealed ground glass opacities and consolidation in lower
lung lobes and pulmonary embolism located in proximal subsegmental
branch of the left pulmonary artery | VOC, ACS, pulmonary
embolism | Oxygen therapy;
fluid replacement; morphine; tramadol; remdesivir; enoxaparin;
dexamethasone; antibiotics; transfusion of RBCs | 21 | Recovery |
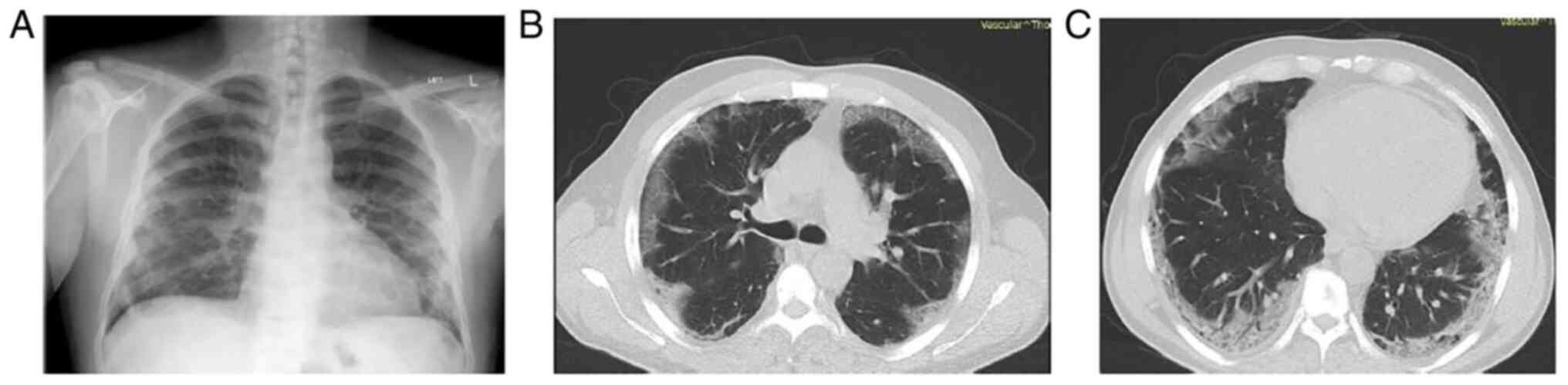
A clinical examination revealed crackles on
auscultation at the right lung base. The remaining results of the
clinical examination did not reveal any notable findings. A chest
X-ray was performed and revealed patchy infiltrates, mostly in the
lower lung fields (Fig. 1A). The
analysis of arterial blood gas revealed partial pressure of oxygen
(pO2) levels of 52 mmHg, partial pressure of carbon
dioxide (pCO2) levels at 28 mmHg, pH 7.47 and
HCO3- at 20.2 mmol/l in room air. The results of the
laboratory analysis are summarized in Table II.
 | Table IILaboratory analyses of the three
cases in the present study. |
Table II
Laboratory analyses of the three
cases in the present study.
| Parameter | Case 1 | Case 2 | Case 3 |
|---|
| Hematocrit, %
(normal range, 40-54%) | 21.3 | 22.5 | 27.6 |
| Hemoglobin (normal
13.5-18 g/dl) | 6.8 | 7.4 | 9.3 |
| White blood cell
count, K/µl (normal range, 4.5-11 K/µl) | 7.29 | 11.52 | 16.68 |
| Neutrophil count,
K/µl (normal range, 1.5-6.6 K/µl) | 5.3 | 7.8 | 11.48 |
| Lymphocyte count,
K/µl (normal range, 1.2-3.4 K/µl) | 1.49 | 2.78 | 4.1 |
| Red blood cell
count, M/µl (normal range, 4.6-6.2 M/µl) | 2.71 | 2.84 | 4.15 |
| Platelet count,
K/µl (normal range, 140-440 K/µl) | 304 | 70 | 312 |
| Aspartate
transaminase, U/l (normal range, 10-40 U/l) | 31 | 493 | 50 |
| Alanine
transaminase, U/l (normal range, <41 U/l) | 24 | 111 | 60 |
| Creatine kinase,
U/l (normal range, 38-190 U/l) | 175 | 15,003 | 40 |
| Lactate
dehydrogenase, U/l (normal range, 135-225 U/l) | 238 | 8,440 | 380 |
| Gamma-glutamyl
transferase, U/l (normal range, 8-61 U/l) | 44 | 87 | 237 |
| Alkaline
phosphatase, U/l (normal range, 40-129 U/l) | 74 | 774 | 149 |
| Ferritin, ng/ml
(normal range, 30-400 ng/ml) | 552 | >100,000 | 854 |
| C-reactive protein,
mg/l (normal range, 0-5 mg/l) | 120.51 | 339.56 | 38 |
| D-dimer, µg/ml
(normal range, <0.5 µg/ml) | 18.90 | >20 | 1.13 |
The patient underwent a chest computed tomography
(CT) scan and computed tomography pulmonary angiogram (CTPA), which
revealed nodular ground glass opacities in all lung fields
(Fig. 1B and C). Pulmonary embolism was not
detected.
The patient received oxygen therapy with a nasal
cannula delivering oxygen at a flow rate of 3 liters/min. He was
also treated with fluid replacement therapy, subcutaneous
enoxaparin, intravenous remdesivir, dexamethasone and ceftriaxone.
He received a simple transfusion of 1 unit of red blood cells
(RBCs). After 3 days of hospitalization, his clinical condition and
oxygen levels improved. No other complications occurred, and the
patient was discharged following a hospitalization duration of 8
days.
Case 2
The second case is that of a 45-year-old male
patient with S/β-thalassemia. He had a past medical history of
splenectomy and hospitalization for acute painful VOCs, 3 years
prior. His current medications included folic acid at 5 mg daily
and acetylsalicylic acid at 100 mg daily.
The patient presented to the ED of Laiko General
Hospital with complaints of low-grade fever, pain in the upper
extremities and chest pain over the last 24 h. In the ED, the
patient was noted to have a SpO2 of 93% in room air, a
BP of 140/70 mmHg, a HR 85 bpm, and a temperature of 36.7˚C. The
clinical characteristics of the patient are summarized in Table I.
A clinical examination revealed diminished breath
sounds on auscultation at the left lung base. It also revealed
yellow eyes and skin, indicating jaundice. A chest X-ray was
performed and this revealed mild infiltrates in both lungs and
blunting of the left costophrenic angle (Fig. 2A). The analysis of arterial blood
gas revealed pO2 levels of 70 mmHg, pCO2
levels of 31 mmHg, pH 7.42 and HCO3- at 20.5 mmol/l in
room air. The results of the laboratory analyses are summarized in
Table II.
The patient was tested for SARS-CoV-2 infection and
had a positive detection of SARS-CoV-2 nucleic acid in an obtained
nasopharyngeal sample using RT-PCR. He had been vaccinated against
SARS-CoV-2. The patient underwent a chest CT scan and CTPA, which
revealed nodular ground glass opacities in all lung fields and a
small left pleural effusion (Fig.
2B and C). Pulmonary embolism
was not detected.
A diagnosis of VOC complicated by ACS was made. The
patient received oxygen therapy with a nasal cannula delivering
oxygen at a flow rate of 3 liters/per min. He was also treated with
fluid replacement therapy, subcutaneous prophylactic enoxaparin,
intravenous morphine, remdesivir, dexamethasone and ceftriaxone. In
total, he received four simple top-up transfusions of 4 units of
RBCs. Following 3 days of hospitalization, the pain and oxygen
levels had improved. The biochemical abnormalities had also
gradually improved. The patient was discharged following a
hospitalization duration of 16 days.
Case 3
The second case is that of a 50-year-old female
patient with S/β-thalassemia. She had a past medical history of
splenectomy, cholecystectomy, hypothyroidism and depression. Her
medications included acetylsalicylic acid at 100 mg daily,
crizanlizumab at 250 mg every 4 weeks, levothyroxine at 50 µg daily
and sertraline at 100 mg daily.
The patient presented to the ED of Laiko General
Hospital with complaints of fever, headache, vomiting and pain in
the upper and lower extremities over the past 2 days. In the ED,
the patient was noted to have a SpO2 of 94% in room air,
a BP of 115/70 mmHg, a HR 88 bpm, and a temperature of 37.4˚C. The
clinical characteristics of the patient are summarized in Table I.
At the initial presentation, the clinical
examination revealed crackles on auscultation in both lung bases. A
chest X-ray was performed and this revealed consolidation in both
lower lung lobes (Fig. 3A). The
analysis of arterial blood gas revealed pO2 levels of 73
mmHg, pCO2 levels of 35 mmHg, pH 7.40 and
HCO3- at 20.7 mmol/l on room air. The results of the
laboratory analyses are summarized in Table II.
The patient was tested for SARS-CoV-2 infection and
had a positive detection of SARS-CoV-2 nucleic acid in an obtained
nasopharyngeal sample using RT-PCR. She had not received the
vaccine for SARS-CoV-2. The patient received oxygen therapy with a
nasal cannula delivering oxygen at a flow rate of 2 litres/min. She
was also treated with fluid replacement therapy, subcutaneous
prophylactic enoxaparin, intravenous morphine and tramadol due to
VOC, remdesivir, dexamethasone and cefipime. On the 6th day of
hospitalization, the patient presented with a deterioration in
oxygen levels and an increase in inflammatory indices with
C-reactive protein levels of 269.48 mg/l and ferritin levels of
1,142 ng/ml.
The patient underwent a chest CT scan and CTPA,
which revealed ground glass opacities and consolidation in the
lower lung lobes and pulmonary embolism located in the proximal
subsegmental branch of the left pulmonary artery (Fig. 2B and C). The diagnosis of ACS was thus
made.
Antimicrobial treatment was modified to include
meropenem and vancomycin empirically. The patient also received
therapeutic enoxaparin and oxygen therapy with a nasal cannula
delivering oxygen at a flow rate of 4 liters/min. No specific
microorganism was isolated from blood, urine or sputum cultures.
She received two simple top-up transfusions of RBCs.
The patient's condition gradually improved, the
inflammatory indices decreased within 5 days, and there was
complete resolution of the fever after 10 days. The patient was
discharged following a hospitalization duration of 21 days.
Discussion
In the Hemoglobinopathy Center of Laiko General
Hospital, ~320 patients with SCD are being treated. Since the onset
of the COVID-19 pandemic, 29 patients of these (29/320) have been
infected, with 5 of these patients requiring hospitalization. The
cases of 3 patients hospitalized in the COVID-19 Unit of Laiko
General Hospital are described in the present study.
The reports regarding hospitalization rates and
outcomes of patients with SCD with COVID-19 disease are
conflicting. It has been reported that the number of patients with
SCD requiring hospitalization due to SARS-CoV-2 infection is
unexpectedly low, raising the hypothesis that patients with SCD are
potentially not as vulnerable to this infection as had been
considered at the beginning of the pandemic, probably due to
chronic inflammation (13).
Moreover, in a retrospective study on 24 patients with SCD and
COVID-19 infection by Balanchivadze et al (16), 54% required hospitalization;
however, they were shown to have a generally a mild course of the
disease, with low rates of intubation, intensive care unit (ICU)
admission and mortality. Of note, in the case series by
Chen-Goodspeed and Idowu (17),
while the hospitalization rate was 60%, the ICU admission rate and
the mortality rate were 0%. Moreover, McCloskey et al
(7), in their case series of
patients with SCD with SARS-CoV-2 infection, reported a full
recovery in the majority of patients, with no requirement for
admission to the ICU, mechanical ventilation or non-invasive
ventilation.
On the contrary, based on other published case
series reporting the experience with COVID-19 in patients with SCD,
the disease was identified as a main risk factor for severe
COVID-19 infection. There are published case series which have
reported an increased risk of hospitalization, VOC and ICU
admission or mortality (18-21).
A previous matched cohort analysis of mortality in African American
individuals reported a higher risk of hospitalization and
development of pneumonia. The case fatality rates for those with
SCD compared with African American individuals without SCD or
sickle cell trait did not differ significantly; however, the
reported mortality was relatively high (3.2%) (22). Another large cohort study found
that patients with SCD had a 4-fold increased risk of
COVID-19-related hospitalization and a 2.6-fold increased risk of
COVID-19-related mortality (23).
The largest series of patients has been reported by the
international SECURE-SCD, including 750 COVID-19 cases of patients
with SCD. A mortality rate of 4.7% was mentioned in adult patients
with SCD with COVID-19(24).
Risk factors for hospitalization and severe COVID-19
illness in patients with SCD have been identified and are worth
mentioning. In the study by Arlet et al (19), it was found that age and the SC
genotype were strong independent risk factors for mechanical
ventilation or mortality. Mucalo et al (24), in a large series of the SECURE-SCD
Registry, reported that frequent previous acute care visits for
pain in children and adults and heart/lung and renal comorbidities
in children were risk factors for the development of severe disease
and hospitalization. Treatment with hydroxyurea decreased the risk
of presenting with pain during COVID-19(24).
Minniti et al (21), in a smaller group of 66 patients,
reported that an older age with underlying chronic organ damage to
the kidneys, heart, lungs and brain were risk factors of morbidity
regardless of the hemoglobin genotype, with pulmonary hypertension
being a factor for a higher risk of mortality. The researchers also
mentioned that elevated levels of lactate dehydrogenase and D-dimer
were associated with a higher risk of mortality (21). Menapace and Thein (25) highlighted racial health disparities
among patients with SCD, based on the higher rates of symptomatic
infection needing hospitalization and mortality in African American
and Hispanic patients.
Of note, all the patients described herein had
favorable outcomes despite the varying clinical presentations, and
different medical history and medications. More specifically, the
patient (case 1) who did not have a history of painful crises and
was receiving hydroxyurea, which exerts a protective effect against
COVID-19, had a successful outcome, similar to the other patients
(cases 2 and 3) who had a history of painful crises and were not
receiving hydroxyurea. Furthermore, the patients depicted as cases
2 and 3 had severe complications, such severe hemolysis and
pulmonary embolism. All the patients received specific treatment
for COVID-19 and required RBC transfusion.
In the Hemoglobinopathy Center of Laiko General
Hospital, 5 patients with COVID-19 disease required hospitalization
among the 29 infected patients (5/29, 17.2%) and there were no
deaths (0/29, 0%). In Greece, by March 8, 2022, there were 26,303
deaths due to COVID-19 of non-SCD patients among the 2,538,168
infected patients (mortality 1%; https://www.worldometers.info/coronavirus/country/greece/)
According to the country's (Greece) data regarding mortality of
COVID-19 (https://www.worldometers.info/coronavirus/country/greece/),
patients with SCD with SARS-CoV-2 infection do not experience a
higher mortality than thosw without SCD as a comorbidity.
Of note, all our 3 patients described herein had not
been vaccinated against SARS-CoV-2. This was an additional risk
factor for the hospitalization of these patients (26). Jan et al (27) performed the first study to explore
hesitancy of patients with SCD concerning the vaccine for
SARS-CoV-2. The researchers reported that, according to the
majority of the beliefs of unvaccinated participants, the adverse
effects of vaccination represented the most significant barrier for
this patient group to receive the vaccine (27). The positive outcome of the
hospitalized patients described in the present study may be
attributed to their relatively young age and the absence of severe
related comorbidities. The patient that was on treatment with
hydroxyurea (case 1) had the least severe clinical course. Another
protective factor may be the ABO blood group type, since it has
been reported that group O is associated with a relative protection
against SARS-CoV infection (28).
In conclusion, the patients with SCD described in
the present study that were hospitalized for COVID-19, developed
significant complications such as VOC, pulmonary embolism and ACS;
however, they did not require ICU admission and all had a favorable
outcome. Patients suffering from SCD who present with VOC, even in
the absence of the typical clinical features of COVID-19, need to
be tested for SARS-CoV-2. Recognizing the various clinical
scenarios of SARS-CoV-2 infection in patients with SCD is critical
for therapeutic interventions to be initiated promptly. The
importance of transfusions/exchange transfusions in patients with
SCD who develop ACS as a complication of COVID, along with other
therapeutic interventions for COVID-19, should be stressed for all
clinicians managing patients with COVID-19, and a hematological
consultation is critical.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CSi, AT and CSt were involved in the conception and
design of the study. VEG and MND wrote and prepared the draft of
the manuscript and advised on patient treatment. CD, SB, PS and NT
analyzed patient data and wrote and prepared the draft of the
manuscript. DAS was involved in the writing of the final draft,
provided critical revisions and was also involved in the design of
the study. SM and PP obtained the medical images. MND, SM, PP and
VEG made substantial contributions to conception, and analysis and
interpretation of data. VEG and SM confirm the authenticity of all
the data. All authors contributed to manuscript revision and
approved the final version of the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
No ethical approval was required for this
publication. Written informed consent was obtained from the
patients for the publication of this case series and accompanying
images.
Patient consent for publication
Written informed consent was obtained from the
patients for publication of this case series and accompanying
images. A copy of the written consent is available for review by
the Editor-in-Chief of this journal on request.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have not competing
interests.
References
|
1
|
Aygun B and Odame I: A global perspective
on sickle cell disease. Pediatr Blood Cancer. 59:386–390.
2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ware RE, de Montalembert M, Tshilolo L and
Abboud MR: Sickle cell disease. Lancet. 390:311–323.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shet AS, Lizarralde-Iragorri MA and Naik
RP: The molecular basis for the prothrombotic state in sickle cell
disease. Haematologica. 105:2368–2379. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Glassberg J: Evidence-based management of
sickle cell disease in the emergency department. Emerg Med Pract.
13:1–20; quiz 20. 2011.PubMed/NCBI
|
|
5
|
Paul RN, Castro OL, Aggarwal A and Oneal
PA: Acute chest syndrome: Sickle cell disease. Eur J Haematol.
87:191–207. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Vichinsky EP, Neumayr LD, Earles AN,
Williams R, Lennette ET, Dean D, Nickerson B, Orringer E, McKie V,
Bellevue R, et al: Causes and outcomes of the acute chest syndrome
in sickle cell disease. National acute chest syndrome study group.
N Engl J Med. 342:1855–1865. 2000.PubMed/NCBI View Article : Google Scholar
|
|
7
|
McCloskey KA, Meenan J, Hall R and
Tsitsikas DA: COVID-19 infection and sickle cell disease: A UK
centre experience. Br J Haematol. 190:e57–e58. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Beerkens F, John M, Puliafito B, Corbett
V, Edwards C and Tremblay D: COVID-19 pneumonia as a cause of acute
chest syndrome in an adult sickle cell patient. Am J Hematol.
95:E154–E156. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Giannis D, Ziogas IA and Gianni P:
Coagulation disorders in coronavirus infected patients: COVID-19,
SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol.
127(104362)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Varga Z, Flammer AJ, Steiger P, Haberecker
M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka
F and Moch H: Endothelial cell infection and endotheliitis in
COVID-19. Lancet. 395:1417–1418. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
White RH and Keenan CR: Effects of race
and ethnicity on the incidence of venous thromboembolism. Thromb
Res. 123 (Suppl 4):S11–S17. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Teulier M, Elabbadi A, Gerotziafas G,
Lionnet F, Voiriot G and Fartoukh M: Severe COVID-19 with acute
respiratory distress syndrome (ARDS) in a sickle cell disease adult
patient: Case report. BMC Pulm Med. 21(46)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Silva-Pinto AC, Santos-Oliveira L, Santos
FLS, Kashima Haddad S, De Santis GC and do Tocantins Calado R:
COVID-19 infection in sickle cell patients in a developing Country:
A case series. Acta Haematol. 145:1–4. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hussain FA, Njoku FU, Saraf SL, Molokie
RE, Gordeuk VR and Han J: COVID-19 infection in patients with
sickle cell disease. Br J Haematol. 189:851–852. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Arlet JB, de Luna G, Khimoud D, Odièvre
MH, de Montalembert M, Joseph L, Chantalat-Auger C, Flamarion E,
Bartolucci P, Lionnet F, et al: Prognosis of patients with sickle
cell disease and COVID-19: A French experience. Lancet Haematol.
7:e632–e634. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Balanchivadze N, Kudirka AA, Askar S,
Almadhoun K, Kuriakose P, Fadel R and Dabak V: Impact of COVID-19
infection on 24 patients with sickle cell disease. One center urban
experience, Detroit, MI, USA. Hemoglobin. 44:284–289.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen-Goodspeed A and Idowu M: COVID-19
presentation in patients with sickle cell disease: A case series.
Am J Case Rep. 22(e931758)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Telfer P, De la Fuente J, Sohal M, Brown
R, Eleftheriou P, Roy N, Piel FB, Chakravorty S, Gardner K, Velangi
M, et al: Real-time national survey of COVID-19 in hemoglobinopathy
and rare inherited anemia patients. Haematologica. 105:2651–2654.
2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Arlet JB, Lionnet F, Khimoud D, Joseph L,
de Montalembert M, Morisset S, Garou A, Cannas G, Cougoul P,
Guitton C, et al: Risk factors for severe COVID-19 in hospitalized
sickle cell disease patients: A study of 319 patients in France. Am
J Hematol. 97:E86–E91. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Panepinto JA, Brandow A, Mucalo L, Yusuf
F, Singh A, Taylor B, Woods K, Payne AB, Peacock G and Schieve LA:
Coronavirus disease among persons with sickle cell disease, United
States, March 20-May 21, 2020. Emerg Infect Dis. 26:2473–2476.
2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Minniti CP, Zaidi AU, Nouraie M, Manwani
D, Crouch GD, Crouch AS, Callaghan MU, Carpenter S, Jacobs C, Han
J, et al: Clinical predictors of poor outcomes in patients with
sickle cell disease and COVID-19 infection. Blood Adv. 5:207–215.
2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Singh A, Brandow AM and Panepinto JA:
COVID-19 in individuals with sickle cell disease/trait compared
with other Black individuals. Blood Adv. 5:1915–1921.
2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Clift AK, Saatci D, Coupland CAC,
Dambha-Miller H and Hippisley-Cox J: International Investigator
Group for Ethnicity and COVID-19. Sickle cell disorders and severe
COVID-19 outcomes: A cohort study. Ann Intern Med. 174:1483–1487.
2021.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Mucalo L, Brandow AM, Dasgupta M, Mason
SF, Simpson PM, Singh A, Taylor BW, Woods KJ, Yusuf FI and
Panepinto JA: Comorbidities are risk factors for hospitalization
and serious COVID-19 illness in children and adults with sickle
cell disease. Blood Adv. 5:2717–2724. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Menapace LA and Thein SL: COVID-19 and
sickle cell disease. Haematologica. 105:2501–2504. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Griffin JB, Haddix M, Danza P, Fisher R,
Koo TH, Traub E, Gounder P, Jarashow C and Balter S: SARS-CoV-2
infections and hospitalizations among persons aged ≥16 Years, by
vaccination status-los angeles county, California, May 1-July 25,
2021. MMWR Morb Mortal Wkly Rep. 70:1170–1176. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jan H, Waheeb A, AlAhwal H, Almohammadi A,
Al-Marzouki A, Barefah A, Bahashawan S and Radhwi O: COVID-19
vaccine perception and hesitancy among patients with sickle cell
disease in the western region of Saudi Arabia. Cureus.
14(e21026)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Guillon P, Clément M, Sébille V, Rivain
JG, Chou CF, Ruvoën-Clouet N and Le Pendu J: Inhibition of the
interaction between the SARS-CoV spike protein and its cellular
receptor by anti-histo-blood group antibodies. Glycobiology.
18:1085–1093. 2008.PubMed/NCBI View Article : Google Scholar
|