Introduction
Cardiac arrest (CA) is a global public health
concern with a low resuscitation rate and a high mortality rate
(1,2). Despite improvements in
cardiopulmonary resuscitation (CPR) and post-resuscitation care in
recent years, the post-discharge survival rate of patients with
return of spontaneous circulation (ROSC) is less than one-third of
all cases (3-6).
Myocardial dysfunction along with the systemic ischemia/reperfusion
(I/R) injury that occurs during CPR is a primary cause for the poor
prognosis of patients after ROSC (7). Thus, protection of myocardial
function after CA is essential and of significant value.
Although the mechanism of CPR-induced myocardial
injury is not fully understood, systemic I/R-induced reactive
oxygen species (ROS) has been widely demonstrated as a critical
factor (8-10).
CA/ROSC is a global I/R event accompanied by ROS generation.
Moreover, the accumulation of ROS is further exacerbated after ROSC
owing to oxygenated blood returning to the tissues, which generates
oxidation of cell macromolecular substances (9,11).
Generally, oxidative stress events, featuring excessive production
of ROS, have been recognized as serving a key role in cell damage,
mitochondrial dysfunction, and ultimately cell apoptosis and death
(12).
Restoring spontaneous circulation and preventing
hypoxic ischemic tissue injury are the goals of CPR (13). As sufficient oxygen delivery is
required to restore and maintain the energy state of the heart, the
use of maximal inspired oxygen (O2) concentrations
during CPR and of earlier post-resuscitation are recommended in the
European Resuscitation Council Guidelines 2021(14). However, a considerable amount of
data has emerged challenging the appropriateness of the use of 100%
O2 during and after resuscitation from CA (15,16).
Hyperoxia has been shown to increase the generation of ROS,
resulting in aggravated reperfusion myocardial injury and worsened
CPR outcomes (17).
Hydrogen (H2) gas is a type of endogenous
gas transmitter produced by the intestinal flora during the
fermentation of nondigestible carbohydrates (18). Previous, studies have demonstrated
that molecular H2 is a new type of safe and effective
therapeutic agent (19,20). In addition, studies to date have
found that H2 therapy significantly protects against
oxidative stress and inflammation-related diseases, such as cancer,
atherosclerosis, diabetes, I/R injury, neurodegenerative diseases,
arthritis, hepatitis and pancreatitis (21-24).
Based on its safety and wide effectiveness, H2 therapy
is attracting increasing attention and is undergoing an important
evolution from bench to bedside (19,25).
In previous studies, H2 inhalation without hyperoxia
after resuscitation improved the neurological outcome in rat models
of CA (26,27). However, the effects of
H2 inhalation on CA/CPR-induced myocardial injury remain
poorly understood. Therefore, the present study used H2
inhalation in post-resuscitation care to investigate its effect on
myocardial injury induced by CA in rats.
Materials and methods
Animals
A total of 60 adult male Wistar rats (age, 10-12
weeks; weight, 400-450 g) were purchased from the Experimental
Animal Center of Shandong University (Jinan, China). All animals
were housed together in a room maintained at 40-60% relative
humidity and 23±2˚C, with 12-h light/dark cycles and ad
libitum access to food and water. All animal experiments were
approved by the Animal Care and Use Committee of the Qilu Hospital
of Shandong University (Jinan, China; approval no.
KYLL-2020-ZM-122), and adhered to the Care and Use of Laboratory
Animals guidelines and to the Animal Research: Reporting of In
Vivo Experiments guidelines. All animal experiments took place
at Shandong Provincial Engineering Laboratory for Emergency and
Critical Care Medicine, Qilu Hospital of Shandong University. All
animals were anesthetized with phenobarbital sodium and then
sacrificed with carbon dioxide release devices.
Rat model of asphyxial CA
The 10-min asphyxial CA/CPR model was used in this
study. Rats were randomly assigned to the following four groups
(n=15 per group): i) Sham + normoxia (anesthetized with 4%
isoflurane mixed with room air and normoxia inhalation by
ventilator for 2 h); ii) Sham + H2 (anesthetized with 4%
isoflurane mixed with room air and H2 inhalation by
ventilator for 2 h); iii) CPR + normoxia (anesthetized with 4%
isoflurane mixed with room air, CA/CPR treatment and normoxia
inhalation by ventilator for 2 h after ROSC); and iv) CPR +
H2 (anesthetized with 4% isoflurane mixed with room air,
CA/CPR treatment and H2 inhalation using a ventilator
for 2 h after ROSC). Rats were anesthetized with 4% isoflurane
mixed with room air and were under tracheal intubation with
connection to a ventilator. Intravascular catheters were placed
into the right femoral artery and right vein for blood pressure
monitoring and drug administration, respectively. After
stabilization for 20 min, the ventilator was disconnected to induce
CA. Circulatory arrest was determined by cessation of the arterial
pulse and a mean arterial pressure (MAP) of <20 mmHg. After 10
min of asphyxia, CPR was performed, with inhalation of air with a
ventilator and intravenous administration of epinephrine (0.01
mg/kg). In addition, the rate of artificial chest compressions was
~200 per min. Epinephrine (0.02 mg/kg) was administered at 2-min
intervals until ROSC was achieved. ROSC was defined by an MAP of
>60 mmHg that lasted for at least 10 min. A total of 3 rats with
ROSC failure within 5 min or those that could not be disengaged
from the ventilator after observation for 1 h were excluded from
the study. The core temperature of each rat was maintained at
37.0±0.5˚C. After ROSC, gas inhalation was continued for 2 h
(Fig. 1A): Premixed gas (1.3%
H2 and 26% O2) was used in the H2
therapy groups, and normoxia (26% O2) was used as the
control (27). Following this, the
rats were euthanized by exposure to a gradually increasing
concentration of CO2 (the flow rate was 50% of the
chamber volume/min).
 | Figure 1Inhalation of H2 gas after
ROSC improves post-resuscitation survival and cardiac function. (A)
The experimental process for CPR and post-resuscitation care in the
rat model of asphyxial CA/CPR. (B and C) The MAP and heart rate of
rats during asphyxial CA/CPR. (D) The survival rate of rats in each
group were recorded for 7 days after asphyxial CA/CPR (n=10). (E-G)
Representative images and quantitative assessment of LVFS and LVEF
evaluated by echocardiography (n=8-10). (H and I) The serum levels
of CKMB and cTnT (n=5). *P<0.05,
**P<0.01 and ***P<0.001. ROSC, return
of spontaneous circulation; MAP, mean arterial pressure;
H2, hydrogen molecule; HR, heart rate; CA, cardiac
arrest; CPR, cardiopulmonary resuscitation; LVFS, left ventricular
fraction shortening; LVEF, left ventricular ejection fraction;
CKMB, creatine kinase-MB; cTnT, cardiac troponin T. |
Cell culture
H9C2 cells were purchased from the American Type
Culture Collection and cultured in Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 100 U/ml
penicillin + 100 mg/ml streptomycin in a cell incubator (95% air
and 5% CO2) at 37˚C. In the cell hypoxia/reoxygenation
(H/R) model, H9C2 cells were exposed to hypoxia (5% CO2
and 1% O2 at 37˚C) for 24 h and reoxygenation (95% air
and 5% CO2) for 4 or 12 h.
H2 treatment in vitro
In the H2 treatment group,
H2-rich medium was used to culture the H92C cells
instead of normal DMEM. H2 was diluted into cell culture
medium to produce an H2-rich culture medium (0.6 mmol/l)
(28). The H2-rich
medium was freshly prepared for each experiment. DMEM was used as
the vehicle.
Transmission electron microscopy
(TEM)
Part of the left ventricle (LV) was obtained from
the rats and fixed quickly in 2% glutaraldehyde and 2%
paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) at room
temperature for 2 h. After 3 washes in phosphate-buffered saline,
tissues were fixed and stained using 1% osmium tetroxide on 4˚C for
2 h and washed 3 times in phosphate-buffered saline. After ethanol
dehydration, samples were embed in LX112 resin (Ladd Research
Industries) at room temperature for 2 h. Ultrathin sections (~70
nm) were obtained with a MT-X ultramicrotome (Leica EM UC7; Leica
Microsystems, Inc.) and observed using an electron microscope
(H-7650; Hitachi, Ltd.). Images of sections were assessed using
ImageJ (V1.8.0.112; National Institutes of Health).
Mitochondrial mass quantification
The TEM images were processed using ImageJ
V1.8.0.112. The outline of each mitochondrion was precisely drawn
using a Surface Pro 6 tablet equipped with a touch pen (Microsoft
Corporation), and filled with a solid bright color. The image with
color-filled mitochondria was converted into a binary black and
white image using the ‘color threshold’ command, and areas of
mitochondria were then generated using the ‘analyze particles’
command. Briefly, this command recognized the black objects
(mitochondria) in the binary images and outlined them such that the
area of each outlined object was automatically computed. Fractional
area was calculated as total mitochondrial area divided by image
area for the cardiomyocytes. Mitochondrial number and average
mitochondrial area (an indication of mitochondrial size) were also
measured (29).
Western blotting
Samples (left ventricle tissues from rats and H9C2
cells) were lysed with RIPA buffer (Beyotime Institute of
Biotechnology) for 30 min on ice. After centrifugation at 12,000
rpm for 10 min at 4˚C, the supernatant was transferred to a new
tube. PMSF (1:100) was added, and a BCA kit (Beyotime Institute of
Biotechnology) was used to determine protein concentration.
Proteins (20 µg) were separated using 10% SDS-PAGE and transferred
onto PVDF membranes. After blocking with 5% milk in PBS at room
temperature for 2 h, the membranes were incubated with primary
antibodies at 4˚C overnight. The membranes were washed three times
with TBS-Tween-20 (TBS-T) and incubated with secondary antibody
(dissolved in 1% BSA; 1:5,000) at room temperature for 1 h. After
being washed with TBS-T three times, the membranes were exposed to
ECL substrate (Beyotime Institute of Biotechnology; catalog no.
P0018FS) and detected by the chemiluminescence method in an AI600
gel imaging system (GE Healthcare). The following primary
antibodies were used: Anti-Beclin-1 (catalog no. ab223348;
1:1,000), anti-LC3B (catalog no. ab51520; 1:1,000), anti-p62
(catalog no. ab109012; 1:1,000) and anti-β-actin (catalog no.
ab8226; 1:1,000) (all Abcam). The secondary antibodies were goat
anti-rabbit IgG H&L (HRP) (catalog no. ab205718; 1:5,000) and
goat anti-mouse IgG H&L (HRP) (catalog no. ab6789; 1:5,000)
(both Abcam).
Immunohistochemical analysis
Rats tissues were fixed with 4% polyformaldehyde at
room temperature for 24 h, then dehydrated with alcohol and washed
with xylene. The tissues were embedded in a wax block and sliced to
a 5-µm thickness. Dewaxing agent was used to dewax the sections at
55˚C for 1 h, and different concentrations of alcohol (100, 95, 90,
80, 70, 60 and 50%, for 30 min each) were used to wash the
sections. According to the instructions of the SABC-POD kit (Boster
Biological Technology; catalog no. SA1028), sections were incubated
with 3% H2O2 at room temperature for 5 min,
and infiltrated into 0.01 M Citrate Antigen Retrieval solution
(Wuhan Servicebio Technology Co., Ltd.; catalog no. G1201-1L) at
100˚C for 20 min. To cool them down to room temperature, sections
were washed three times with PBS (phosphate-buffered saline). After
blocking with 5% BSA (Wuhan Servicebio Technology Co., Ltd.;
catalog no. SW3015) at 37˚C for 30 min, sections were incubated
with primary antibodies at 4˚C overnight. Next, the sections were
washed three times with PBS, and incubated with anti-rabbit IgG
H&L (HRP; 1:1,000; cat. no. ab205718; Abcam) and goat
anti-mouse IgG H&L (HRP; 1:1,000; cat. no. ab6789; Abcam) at
37˚C for 30 min. After washing three times with PBS, the sections
were incubated with SABC at room temperature for 20 min. Finally,
sections were incubated with DAB substrate (Boster Biological
Technology; catalog no. AR1022) and washed with ddH2O.
Sections were redyed with hematoxylin for 30 sec, dehydrated with
inversed different concentrations of alcohol and sealed with
Permount™ Mounting Medium (Sangon Biotech, Co., Ltd.;
catalog no. E675007). Immunohistochemical staining was performed
with anti-LC3B (1:1,000). Images of sections were captured (IX53;
Olympus Corporation) and assessed (ImageJ V1.8.0.112) according to
the percentage of stained cells (total original magnification, x40;
area, 250x250 µm).
Immunofluorescence analysis and
confocal microscopy
H9C2 cells were incubated with the aforementioned
primary antibodies to evaluate the levels of Beclin-1 (1:100) and
LC3B (1:100). Samples were incubated with DAPI after washing three
times with PBS at room temperature, and then sealed with coverslips
immediately. Images of cells were taken using confocal laser
scanning fluorescence microscopy (SP8; Leica Microsystems
GmbH).
Evaluation of fluorescent LC3
punctae
The changing fluorescent punctae of LC3 in H9C2
cells were observed with a tandem red fluorescent protein
(RFP)-green fluorescent protein (GFP)-LC3 construct
(Ad-RFP-GFP-LC3). Ad-RFP-GFP-LC3 adenovirus was purchased from
ViGene Biosciences (Charles River Laboratories, Inc.). H9C2 cells
(American Type Culture Collection; cat. no. CRL-1446) were
transfected with Ad-RFP-GFP-LC3 at 50 MOI. In brief, H9C2 cells
were inoculated into a 24-well plate at a density of
1x105 cells/well. A total of 250 µl of DMEM containing
1% FBS (both Thermo Fisher Scientific, Inc.), 100 µl adenovirus
(106 PFU/ml; ViGene Biosciences; Charles River
Laboratories, Inc.) and 100 µl Lipofectamine 2000®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was added to
each well. After mixing, the cells were cultured for 6 h in an
incubator containing 5% CO2 at 37˚C. After 6 h, the
adenovirus was moved off and the transfected cells were cultured
for 48 h continuously. The green and red fluorescence intensities
were assessed under laser scanning fluorescence microscopy (SP8;
Leica Microsystems GmbH). Images of sections were assessed (ImageJ
software V1.8.0.112; National Institutes of Health) according to
the numbers of red and yellow dots in each cell (total original
magnification, x63).
Ultrasonic cardiogram
Under isoflurane anesthesia, the spontaneous
breathing of the rats was maintained. The two-dimensional images of
the LV [left ventricular fraction shortening (LVFS) and left
ventricular ejection fraction (LVEF)] were collected using
ultrasonic cardiogram equipment (Vevo2100; VisualSonics, Inc.) on
the short-axis and long-axis section of the parasternal papillary
muscles. The two-dimension-guided M-mode or B-mode ultrasonic
cardiogram of 10 cardiac cycles was obtained. Images were assessed
using Vevo2100 software.
Creatine kinase-MB (CKMB) and cardiac
troponin-T (cTnT) measurement
Blood was collected at sacrifice and then the serum
was isolated using centrifugation (1,000 g; 4˚C for 15 min). The
serum concentrations of CKMB (Cloud-Clone Corp.; catalog no.
SEA479Ra) and cTnT (Cloud-Clone Corp.; catalog no. SED232Ra) were
measured by ELISA kit following the manufacturer's
instructions.
Statistical analysis
The statistical significance was performed using
GraphPad Prism 8 (GraphPad Software, Inc.) Differences between
groups were estimated using one-way ANOVA followed by Tukey's post
hoc test. Comparisons across two variables were used a two-way
ANOVA followed by Tukey's post hoc test. P<0.05 was considered
to indicate a statistically significant difference. All data are
expressed as the mean ± standard error.
Results
Inhalation of H2 gas after
ROSC improves post-resuscitation survival and cardiac function
As shown in Fig. 1B
and C, there were no statistical
differences in MAP and heart rate during post-resuscitation care
whether using inhalation of H2 gas or not. The survival
rate of the rats was recorded continuously for 7 days after CA/CPR
(Fig. 1D). In the Sham groups, the
survival rate was 100% whether using inhalation of H2
gas or not. However, compared with that in the normoxia groups, the
survival rate at 7 days after ROSC was significantly higher
following inhalation of H2 gas during post-resuscitation
care.
Ultrasonic cardiogram detection was used to further
evaluate the effect of H2 on the cardiac function of
rats after ROSC. As shown in Fig.
1E, cardiac function was evaluated by echocardiography at 4 and
72 h post-ROSC. Echocardiograms were analyzed by the LV trace of M
mode images using VevoStrain software. Compared with the Sham
group, the LVFS and LVEF were significantly decreased within 4 h
following ROSC. However, the LVFS and LVEF significantly increased
after ROSC in the CPR + H2 group (Fig. 1F and G). However, there was no significant
difference in levels of LVFS and LVEF between CPR and CPR +
H2 groups at 72 h. Moreover, compared with inhalation
normoxia, H2 therapy also markedly decreased the levels
of myocardial injury biomarker CKMB and cTnT in serum after CPR at
4 and 72 h (Fig. 1H and I). Thus, inhalation of H2
after ROSC significantly improved the cardiac function of rats.
Inhalation of H2 gas after
ROSC improves mitochondrial mass and decreases the number of
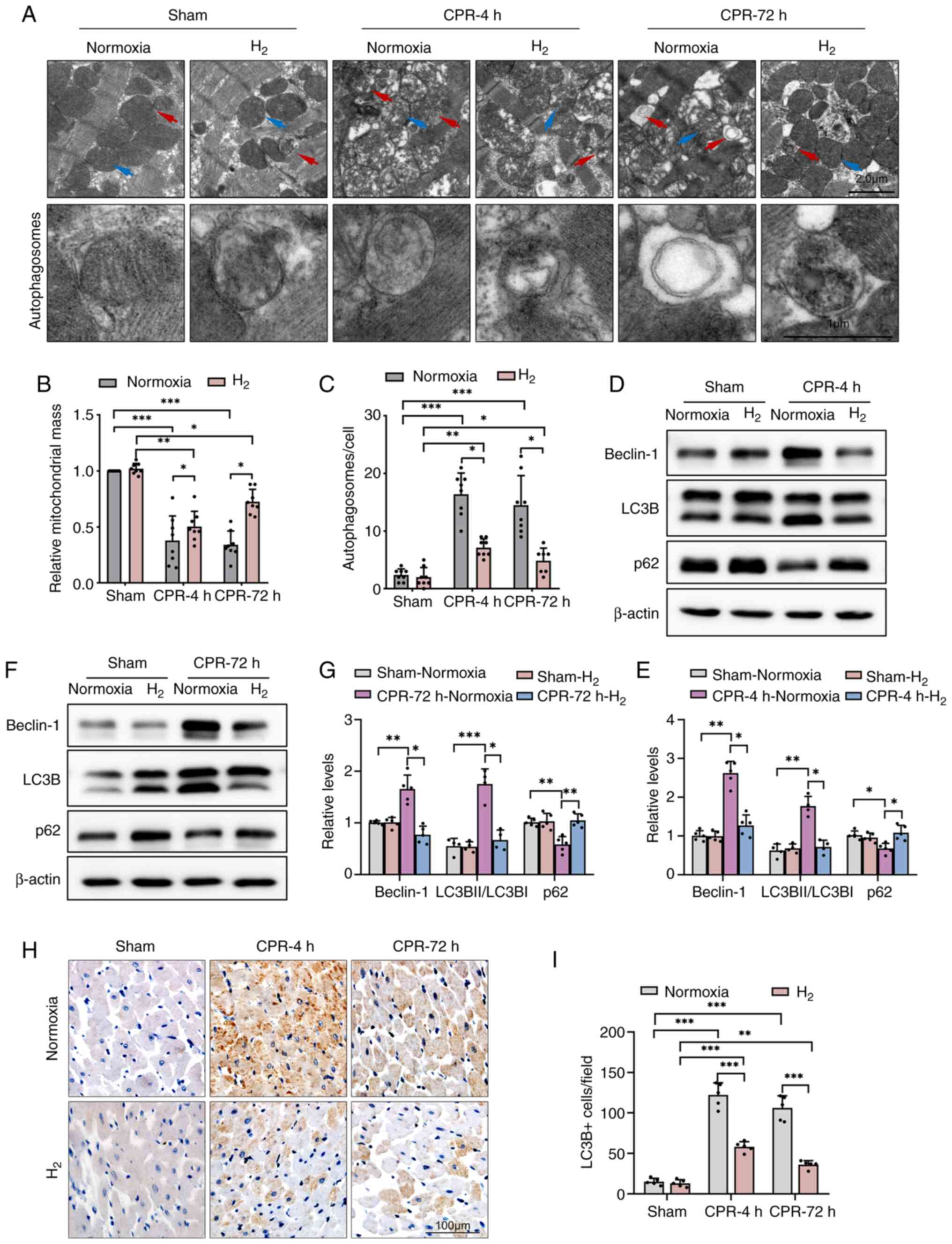
autophagosomes in rat cardiomyocytes
To investigate the potentially cardioprotective
mechanism of the inhalation of H2 after ROSC, the
cross-section and TEM images of LV cardiomyocytes were observed. As
shown in Fig. 2A and B, TEM analysis revealed the presence of
extensive mitochondrial abnormalities, such as swelling,
disorganization and loss of cristae, and the relative mitochondrial
mass were significantly deceased in post-resuscitation rat LV
cardiomyocytes. However, inhalation of H2 gas after ROSC
significantly decreased the number of abnormal mitochondria and
increased the relative mitochondrial mass in rat LVs, and cardiac
tissue seemed to have returned to its original morphology at 72
h.
Moreover, autophagic lysosomal structures were
common in post-resuscitation rat LV cardiomyocytes, suggesting the
activation of autophagic cell death mechanisms. In the CPR groups,
a significant decrease in autophagic vesicles was observed at 4 and
72 h in post-resuscitation rats after inhalation of H2
gas compared with normoxia (Fig.
2A and C).
Inhalation of H2 gas after
ROSC decreases the expression levels of Beclin-1 and suppresses
autophagy activation in rat cardiomyocytes
The study next investigated how H2
affects autophagy in the heart. As show in Fig. 2D-G, western blot analysis revealed
increased expression levels of the autophagy promotor protein
Beclin1 in normoxic post-resuscitation rat hearts. The expression
levels of LC3B were also higher in the CPR + Normoxia groups at 4
and 72 h compared with that in the Sham-Normoxia group, and the
ratio of LC3BII/I was also significantly higher, indicating an
enrichment of LC3BII. However, the expression levels of p62 were
significantly lower in the same groups. There results suggested
excessive autophagy activation. However, compared with the CPR +
Normoxia groups, H2 treatment suppressed autophagy
activation with significantly lower Beclin-1 and LC3B levels, and a
higher p62 protein level. Furthermore, immunohistochemical staining
revealed that LC3B levels were significantly higher in
cardiomyocytes from rats after ROSC, while H2 therapy
significantly decreased the LC3B protein levels (Fig. 2H and I).
H2 treatment suppresses
H/R-induced autophagy activation in H9C2 cells
All H/R-induced injury experiments were performed on
the rat heart embryonic H9C2 cell line. The present study data
(Figs. 1 and 2) had shown that H2-treatment
protected cardiomyocytes from CA/resuscitation by inhibiting
autophagy activation. To clarify the role of H2 therapy
in CPR-induced myocardial I/R injury, H9C2 cells were subjected to
24 h of hypoxia followed by 4 or 12 h of reoxygenation. According
to a previous study protocol, H2-rich culture medium was
used as H2 treatment for cells in vitro (28).
The expression levels of autophagic markers
Beclin-1, LC3B and p62 were measured by western blot analysis in
H/R-treated H9C2 cells. After H/R, H9C2 cells showed an increase in
expression levels of autophagic proteins Beclin-1, LC3B and p62,
compared with the control (Fig. 3A
and B). H2
significantly decreased Beclin-1 and LC3B expression in the
H/R-treated cardiomyocytes, suggesting the inhibition of autophagy.
However, the p62 expression was increased significantly in these
H/R + H2 groups.
Consistent with the western blot analysis, the
immunofluorescence staining also indicated that the expression of
Beclin-1 and LC3B was increased in H/R-treated cells, while
H2 administration significantly decreased the expression
of these proteins (Fig. 3C-F).
These data support the proposal that H2 protects against
H/R-induced injury by decreasing H/R-mediated autophagy.
H2 treatment suppresses
H/R-induced accumulation of autophagosomes and autolysosomes in
H9C2 cells
In order to study the formation and process of
autophagy, H9C2 cells were transfected with Ad-monomeric RFP
(mRFP)-GFP-LC3 and observed at different time points after H/R. As
shown in Fig. 4, the generation of
both GFP- and mRFP-positive autophagosomes was significantly
increased in the H9C2 cells after H/R, while H2
treatment resulted in a significant decrease in both autophagosome
types.
Discussion
The present study investigated the cardioprotective
effect of H2 inhalation after ROSC in a rat CPR model.
The results revealed that H2 treatment ameliorated
animal survival and myocardial abnormalities. The echocardiography
at 4 and 72 h after ROSC revealed improved cardiac function in
H2-treated animals compared with the CPR group.
Consistent with previous studies (30-33),
using electron microscopy analysis, extensive mitochondrial
abnormalities and autophagosomes were observed in myocardial cells
at 4 and 72 h after ROSC in rats subjected to asphyxial CA/CPR.
However, H2 treatment after ROSC improved mitochondrial
morphology and decreased the numbers of autophagosomes. These data
demonstrated that the excessive activation of autophagy might exist
for a long period in rats subjected to asphyxial CA/CPR.
H2 treatment significantly suppressed autophagy
activation.
The present data have defined the role of autophagy
in myocardial survival/death after ROSC in an asphyxial CA/CPR
model. Furthermore, the role of H2 in the regulation of
autophagy against myocardial injury has not been investigated in
asphyxial CA/CPR. In previous studies, autophagy activation was
considered as a double-edged sword with both pro-survival and
death-causing potential in myocardial I/R injury (34,35).
Excessive activation of autophagy leads to degeneration of
organelles and drives cell death after reperfusion. Additionally,
excess autophagosome clearance has been determined as a major cause
of cardiomyocyte death as a result of the observation of
autophagosome generation in necrotic cardiomyocytes (36).
In the present study, the data showed that
autophagosomes significantly accumulated in cardiomyocytes after
ROSC, indicating excessive activation of autophagy. Moreover, the
expression levels of Beclin-1 and LC3B were also increased in the
LV from 4 to 72 h after asphyxial CA/CPR. Beclin-1, a component of
phosphatidylinositol type III kinase complex, has been confirmed to
serve a crucial role in regulating autophagosome formation
(34). Previous studies have
reported the association between Beclin-1 and autophagy-associated
cell death in cerebral ischemia (37-40).
The present data also revealed that H2 treatment
significantly inhibited autophagy, with decreased Beclin-1 and LC3B
expression in cardiomyocytes. Moreover, compared with those in the
CPR or H/R groups, the expression levels of p62 significantly
decreased in the H2 treatment groups in vitro and
in vivo, indicating the inhibition of autophagy. However, in
contrast to the results in the animal experiments, the expression
levels of p62 were significantly increased in the H/R groups in
cells. These data may be related to the different amounts of time
suffering hypoxia in vitro and in vivo. In
H/R-induced injury experiments, a longer period of hypoxia
increased the expression levels of p62 in the H9C2 cells. In
summary, these results evaluated the potential cardioprotection of
H2 treatment in CA/CPR.
In vitro, Ad-mRFP-GFP-LC3 were used to
observe the formation and process of autophagy. After transfection
with Ad-mRFP-GFP-LC3, H9C2 cells ubiquitously express the
autophagosome-building microtubule-associated protein LC3 linked
with both mRFP and GFP. With autophagy activation, fluorescent
signals of mRFP and GFP significantly increase with the formation
of phagosomes. Moreover, GFP signals are quenched where
autophagosomes eventually fuse with lysosomes (41). In the in vitro H/R
experiments of the present study, a decrease in
autophagy-associated proteins (Beclin-1 and LC3B) and
autophagosomes was observed after H2 treatment, which
suggested that the cell homeostasis mechanisms of H2
therapy in cardioprotection are associated with the inhibition of
autophagy. In previous studies, the anti-apoptotic properties of
H2 have also been demonstrated, with the alleviation of
hyperoxia inducing lung epithelial cell apoptosis via the induction
of Bcl-2 and the suppression of Bax expression (42-44).
These results revealed a potential mechanism of
H2-mediated cell fate under stress.
In conclusion, the present study demonstrated that
H2 inhalation after resuscitation suppressed autophagy
activation and improved cardiac function and survival in a rat
model of CA. These findings suggest a potentially novel and easily
applicable treatment for cardiac dysfunction in post-cardiac arrest
syndrome; however, further investigation is required to confirm the
cell homeostasis mechanisms of H2 therapy for
cardioprotection.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the State Key Program of
the National Natural Science Foundation of China (grant no.
82030059), the National Natural Science Foundation of China (grant
nos. 81772036, 82072144, 81671952, 81873950 and 81873953), the
National Key R&D Program of China (grant nos. 2020YFC1512700,
2020YFC1512705, 2020YFC1512703 and 2020YFC0846600), the National
S&T Fundamental Resources Investigation Project (grant no.
2018FY100600 and 2018FY100602), the Taishan Pandeng Scholar Program
of Shandong Province (grant no. tspd20181220), the Taishan Young
Scholar Program of Shandong Province (grant nos. tsqn20161065 and
tsqn201812129), the Youth Top-Talent Project of National Ten
Thousand Talents Plan, Qilu Young Scholar Program and the
Fundamental Research Funds of Shandong University (grant no.
2018JC011) and the Inner Mongolia Department of Education (grant
no. NJZY22143).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XG, TX, XH and YC conceived and designed the study.
XG, XF, XY, TX, JL, JG and XZ performed the study. XG, TX, SW, QY,
JW and XF contributed to the data analysis. XG, TX, XH and YC wrote
the manuscript. XG and TX confirm the authenticity of all the raw
data. All authors have read and approved the manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Care and Use Committee of the Qilu Hospital of Shandong University
(Jinan, China; approval no. KYLL-2020-ZM-122), and adhered to the
guidelines from the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brady WJ, Mattu A and Slovis CM: Lay
Responder care for the adult victim of out-of-hospital cardiac
arrest. Reply. N Engl J Med. 382(e24)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dankiewicz J, Cronberg T, Lilja G,
Jakobsen JC, Levin H, Ullén S, Rylander C, Wise MP, Oddo M, Cariou
A, et al: Hypothermia versus normothermia after out-of-hospital
cardiac arrest. N Engl J Med. 384:2283–2294. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Couzin-Frankel J: Clinical trials test
potential CPR upgrade. Science. 363:913–914. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
He M, Gong Y, Li Y, Mauri T, Fumagalli F,
Bozzola M, Cesana G, Latini R, Pesenti A and Ristagno G: Combining
multiple ECG features does not improve prediction of defibrillation
outcome compared to single features in a large population of
out-of-hospital cardiac arrests. Crit Care. 19(425)2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shao F, Li CS, Liang LR, Li D and Ma SK:
Outcome of out-of-hospital cardiac arrests in Beijing, China.
Resuscitation. 85:1411–1417. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bray JE, Bernard S, Cantwell K, Stephenson
M and Smith K: VACAR Steering Committee. The association between
systolic blood pressure on arrival at hospital and outcome in
adults surviving from out-of-hospital cardiac arrests of presumed
cardiac aetiology. Resuscitation. 85:509–515. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chenoune M, Lidouren F, Adam C, Pons S,
Darbera L, Bruneval P, Ghaleh B, Zini R, Dubois-Randé JL, Carli P,
et al: Ultrafast and whole-body cooling with total liquid
ventilation induces favorable neurological and cardiac outcomes
after cardiac arrest in rabbits. Circulation. 124:901–911, 1-7.
2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xu H, Li Y, Liu R, Wu L, Zhang C, Ding N,
Ma A, Zhang J and Xie X: Protective effects of ghrelin on brain
mitochondria after cardiac arrest and resuscitation. Neuropeptides.
76(101936)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nguyen Thi PA, Chen MH, Li N, Zhuo XJ and
Xie L: PD98059 protects brain against cells death resulting from
ROS/ERK activation in a cardiac arrest rat model. Oxid Med Cell
Longev. 2016(3723762)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Penna C, Perrelli MG and Pagliaro P:
Mitochondrial pathways, permeability transition pore, and redox
signaling in cardioprotection: Therapeutic implications. Antioxid
Redox Signal. 18:556–599. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cui R, Liu S, Wang C, Liu T, Ren J, Jia Y,
Tong Y, Liu C and Zhang J: Methane-rich saline alleviates CA/CPR
brain injury by inhibiting oxidative stress, microglial
activation-induced inflammatory responses, and ER stress-mediated
apoptosis. Oxid Med Cell Longev. 2020(8829328)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang R, Liu B, Fan X, Wang W, Xu T, Wei
S, Zheng W, Yuan Q, Gao L, Yin X, et al: Aldehyde dehydrogenase 2
protects against post-cardiac arrest myocardial dysfunction through
a novel mechanism of suppressing mitochondrial reactive oxygen
species production. Front Pharmacol. 11(373)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lellouche F and L'Her E: Usual and
advanced monitoring in patients receiving oxygen therapy. Respir
Care. 65:1591–1600. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Soar J, Böttiger BW, Carli P, Couper K,
Deakin CD, Djärv T, Lott C, Olasveengen T, Paal P, Pellis T, et al:
European resuscitation council guidelines 2021: Adult advanced life
support. Resuscitation. 161:115–151. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chu DK, Kim LH, Young PJ, Zamiri N,
Almenawer SA, Jaeschke R, Szczeklik W, Schünemann HJ, Neary JD and
Alhazzani W: Mortality and morbidity in acutely ill adults treated
with liberal versus conservative oxygen therapy (IOTA): A
systematic review and meta-analysis. Lancet. 391:1693–1705.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Allardet-Servent J, Sicard G, Metz V and
Chiche L: Benefits and risks of oxygen therapy during acute medical
illness: Just a matter of dose! Rev Med. Interne. 40:670–676.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Vereczki V, Martin E, Rosenthal RE, Hof
PR, Hoffman GE and Fiskum G: Normoxic resuscitation after cardiac
arrest protects against hippocampal oxidative stress, metabolic
dysfunction, and neuronal death. J Cereb Blood Flow Metab.
26:821–835. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhou G, Goshi E and He Q:
Micro/nanomaterials-augmented hydrogen therapy. Adv Healthc Mater.
8(e1900463)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Buret AG, Allain T, Motta JP and Wallace
JL: Effects of hydrogen sulfide on the microbiome: From toxicity to
therapy. Antioxid Redox Signal. 36:211–219. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hardeland R: Hydrogen therapy: A future
option in critical care? Crit Care Med. 40:1382–1383.
2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ohsawa I, Ishikawa M, Takahashi K,
Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S
and Ohta S: Hydrogen acts as a therapeutic antioxidant by
selectively reducing cytotoxic oxygen radicals. Nat Med.
13:688–694. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Liu CL, Zhang K and Chen G: Hydrogen
therapy: From mechanism to cerebral diseases. Med Gas Res. 6:48–54.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Durante W: Hydrogen sulfide therapy in
diabetes-accelerated atherosclerosis: A whiff of success. Diabetes.
65:2832–2834. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang L, Yu H, Tu Q, He Q and Huang N: New
approaches for hydrogen therapy of various diseases. Curr Pharm
Des. 27:636–649. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sano M, Suzuki M, Homma K, Hayashida K,
Tamura T, Matsuoka T, Katsumata Y, Onuki S and Sasaki J: Promising
novel therapy with hydrogen gas for emergency and critical care
medicine. Acute Med Surg. 5:113–118. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hayashida K, Sano M, Kamimura N, Yokota T,
Suzuki M, Ohta S, Fukuda K and Hori S: Response to letter regarding
article, ‘hydrogen inhalation during normoxic resuscitation
improves neurological outcome in a rat model of cardiac arrest
independently of targeted temperature management’. Circulation.
132(e148)2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hayashida K, Sano M, Kamimura N, Yokota T,
Suzuki M, Ohta S, Fukuda K and Hori S: Hydrogen inhalation during
normoxic resuscitation improves neurological outcome in a rat model
of cardiac arrest independently of targeted temperature management.
Circulation. 130:2173–2180. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen H, Xie K, Han H, Li Y, Liu L, Yang T
and Yu Y: Molecular hydrogen protects mice against polymicrobial
sepsis by ameliorating endothelial dysfunction via an Nrf2/HO-1
signaling pathway. Int Immunopharmacol. 28:643–654. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhou Z, Vidales J, González-Reyes JA,
Shibata B, Baar K, Rutkowsky JM and Ramsey JJ: A 1-month ketogenic
diet increased mitochondrial mass in red gastrocnemius muscle, but
not in the brain or liver of middle-aged mice. Nutrients.
13(2533)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lu Y, Zeng X, Jing X, Yin M, Chang MMP,
Wei H, Yang Y, Liao X, Dai G and Hu C: Pre-arrest hypothermia
improved cardiac function of rats by ameliorating the myocardial
mitochondrial injury after cardiac arrest. Exp Biol Med (Maywood).
244:1186–1192. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ji X, Bradley JL, Zheng G, Ge W, Xu J, Hu
J, He F, Shabnam R, Peberdy MA, Ornato JP, et al: Cerebral and
myocardial mitochondrial injury differ in a rat model of cardiac
arrest and cardiopulmonary resuscitation. Biomed Pharmacother.
140(111743)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Huang Y, Gao X, Zhou X, Xie B, Zhang Y,
Zhu J and Zhu S: Mitophagy in the hippocampus is excessive
activated after cardiac arrest and cardiopulmonary resuscitation.
Neurochem Res. 45:322–330. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Cao S, Sun Y, Wang W, Wang B, Zhang Q, Pan
C, Yuan Q, Xu F, Wei S and Chen Y: Poly (ADP-ribose) polymerase
inhibition protects against myocardial ischaemia/reperfusion injury
via suppressing mitophagy. J Cell Mol Med. 23:6897–6906.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Shi B, Ma M, Zheng Y, Pan Y and Lin X:
mTOR and beclin1: Two key autophagy-related molecules and their
roles in myocardial ischemia/reperfusion injury. J Cell Physiol.
234:12562–12568. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng
YL, Cheng PW, Li CY and Li CJ: Current mechanistic concepts in
ischemia and reperfusion injury. Cell Physiol Biochem.
46:1650–1667. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kroemer G and Levine B: Autophagic cell
death: The story of a misnomer. Nat Rev Mol Cell Biol. 9:1004–1010.
2008.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Rami A, Langhagen A and Steiger S: Focal
cerebral ischemia induces upregulation of beclin 1 and
autophagy-like cell death. Neurobiol Dis. 29:132–141.
2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Grishchuk Y, Ginet V, Truttmann AC, Clarke
PG and Puyal J: Beclin 1-independent autophagy contributes to
apoptosis in cortical neurons. Autophagy. 7:1115–1131.
2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Guo D, Ma J, Yan L, Li T, Li Z, Han X and
Shui S: Down-regulation of lncrna MALAT1 attenuates neuronal cell
death through suppressing beclin1-dependent autophagy by regulating
Mir-30a in cerebral ischemic stroke. Cell Physiol Biochem.
43:182–194. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Xu T, Guo J, Wei M, Wang J, Yang K, Pan C,
Pang J, Xue L, Yuan Q, Xue M, et al: Aldehyde dehydrogenase 2
protects against acute kidney injury by regulating autophagy via
the Beclin-1 pathway. JCI Insight. 6(e138183)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kaizuka T, Morishita H, Hama Y, Tsukamoto
S, Matsui T, Toyota Y, Kodama A, Ishihara T, Mizushima T and
Mizushima N: An autophagic flux probe that releases an internal
control. Mol Cell. 64:835–849. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kawamura T, Wakabayashi N, Shigemura N,
Huang CS, Masutani K, Tanaka Y, Noda K, Peng X, Takahashi T,
Billiar TR, et al: Hydrogen gas reduces hyperoxic lung injury via
the Nrf2 pathway in vivo. Am J Physiol Lung Cell Mol Physiol.
304:L646–L656. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ge L, Yang M, Yang NN, Yin XX and Song WG:
Molecular hydrogen: A preventive and therapeutic medical gas for
various diseases. Oncotarget. 8:102653–102673. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ohta S: Molecular hydrogen as a preventive
and therapeutic medical gas: Initiation, development and potential
of hydrogen medicine. Pharmacol Ther. 144:1–11. 2014.PubMed/NCBI View Article : Google Scholar
|