Introduction
Spinal cord injury (SCI) is a common and destructive
injury caused by external forces. SCI refers to fracture and/or
severe complications following dislocation of the spinal cord
(1). SCI is known to be mostly
caused by road traffic accidents, falls and sports-related
accidents, and could further lead to sensory disturbance, motor
dysfunction and lower extremity paralysis (2-4).
As a result of the unremitting efforts made by global researchers
and the successful translation into clinical practice, recovery
from SCI through rehabilitation therapies and orthopedic appliances
is now possible for mild cases, whereas patients with severe cases
continue to risk disability and even death (5,6).
Surgical techniques, biological therapies such as stem cell and
precursor cell transplantation, and pharmacological therapies such
as methylprednisolone (MP) and gangliosides have been approved for
the treatment of SCI; however, clinically significant effects have
not been reported (7,8). Thus, there is an extremely urgent
need to find new therapies for SCI and secondary injury following
SCI.
Lenalidomide (LEN), chemically known as
(RS)-3-(4-amino-1-oxoisoindolin-2-yl)piperidine-2,6-dione
(molecular formula,
C13H13N3O3), exerts
antitumor, anti-inflammatory, anti-angiogenic and immunomodulatory
effects (9,10). LEN also improves the motor behavior
defect caused by the striatum and ameliorates dopaminergic fiber
loss. This protective effect is accompanied by an decrease in
microgliosis in the striatum and hippocampus and reduction in NF-κB
activity in Parkinson's disease (11). LEN has also shown other protective
potential, such as exerting anti-inflammatory and neuroprotective
effects in a G93A mutant superoxide dismutase (SOD)-1 mouse model
of amyotrophic lateral sclerosis (12). The anti-inflammatory effect of LEN
is exerted via the regulation of cytokine production by human
myeloid-derived primary dendritic cells, which allows for
beneficial immunoregulation and is therefore facilitative for
treating inflammation-related diseases, including multiple myeloma
(MM) (13). LEN inhibits the
downstream genes of Notch2 signaling transduction, including
recombination signal binding protein for immunoglobulin κ J region
(also called CSL or CBF1) and hes family bHLH transcription factor
1 (HES1). Under LEN treatment, Notch2 signaling combines with the
expression of multiple drug-resistance proteins to modulate the
inhibitory effect on cancer cell proliferation (14). A previous study reported a
significant decline in the expression levels of Notch signaling
molecules, including the receptors, the ligands and the downstream
cytokines, after treatment with LEN in mesenchymal stem cells
(MSCs) from patients with MM (MM-MSCs). It was concluded that
treatment with LEN inactivated Notch signaling to restore the
osteogenic differentiation of MM-MSCs (15). These previous studies have
collectively confirmed the interaction between LEN and Notch
signaling, which may play a role in the clinical treatment for
SCI.
The present study aimed to verify this hypothesis
and the results may contribute to the exploration of the
therapeutic potentials of LEN other than for treating MM.
Materials and methods
Cell culture and treatment
The rat adrenal pheochromocytoma cell line, PC12,
acquired from the American Type Culture Collection (ATCC), was
cultured in ATCC-formulated RPMI-1640 medium (cat. no. 30-2001)
supplemented with 10% heat-inactivated horse serum and 5% FBS (all
Gibco; Thermo Fisher Scientific, Inc.) in an environment of 95% air
and 5% CO2 at 37˚C. The cells were pretreated for 24 h
with LEN (cat. no. EY0006; AMQUAR Corporation) dissolved in DMSO
(Shanghai Aladdin Biochemical Technology Co., Ltd.) at a dose of
1.25, 2.5, 5 and 10 µM. H2O2 at a dose of
100, 200, 300 and 400 µM was selected to treat PC12 cells for 1 h
after LEN treatment. Cells in the JAG + LEN +
H2O2 group were pre-treated with 50 µg/ml
Notch agonist Jagged-1 (JAG; cat. no. AS-61298; AnaSpec) peptide
before treatment with LEN and H2O2. Untreated
cells were regarded as the control group.
Cell Counting Kit-8 (CCK-8) assay
The viability of
H2O2-stimulated PC12 cells was detected using
a CCK-8 Cell Proliferation and Cytotoxicity assay (Beijing Solarbio
Science & Technology Co., Ltd.). In total, 100 µl cell
suspension was added to a 96-well plate at a density of
5x103 cells/well and precultured in an incubator at 37˚C
in 5% CO2. Subsequently, 10 µl CCK-8 solution was added
to each well and incubated with the cells for 4 h. A microplate
reader (RT-3001; Thermo Fisher Scientific, Inc.) was used to
measure the absorbance at a wavelength of 450 nm.
MTT assay
PC12 cells in the logarithmic growth phase were
collected, and 100 µl cell suspension was added to a 96-well plate
at a density of 5x103 cells/well. The cells were treated
with a concentration gradient of LEN after the formation of the
cell monolayer on the bottom of the well. Following 48 h of
incubation with LEN in 5% CO2 at 37˚C, 20 µl MTT
solution (Procell Life Science & Technology Co., Ltd.) was
added to each well and the incubation was continued for 4 h. The
incubation was then terminated, and the culture medium was
discarded before the addition of 150 µl DMSO to each well. The
plate was vibrated using a shaking bed for 10 min at low speed to
fully dissolve the crystalized substances. The optical density was
measured at a wavelength of 490 nm using a universal microplate
spectrophotometer.
Lactate dehydrogenase (LDH)
cytotoxicity assay
Cell cytotoxicity was measured by an LDH assay using
the LDH kit (cat. no. A020-1-2; Nanjing Jiancheng Bioengineering
Institute) according to the manufacturer's protocols. Briefly, the
H2O2-stimulated cells were seeded into a
96-well plate at a density of 1x104 cells/well and
received the appropriate treatments of incubation with LEN at doses
of 1.25, 2.5, 5 and 10 µM for 24 h at 37˚C. Next, the cell culture
media was collected and the LDH activity was measured at 530 nm
using a microplate reader (Benchmark; Bio-Rad Laboratories,
Inc.).
Determination of oxidative stress
level
Oxidative stress in PC12 cells was reflected by the
levels of malondialdehyde (MDA), SOD, glutathione peroxidase
(GSH-Px) and catalase (CAT), as detected using a Lipid Peroxidation
MDA Assay kit (cat. no. S0131S; Beyotime Institute of
Biotechnology), a SOD Activity Assay kit (cat. no. ab65354; Abcam),
GSH-Px Assay kit (cat. no. EKC39116; BioVision, Inc.) and a CAT
Assay kit Without Hydrogen Peroxide (cat. no. 700910; Cayman
Chemical Company), respectively. All operational procedures
followed the manufacturer's protocols.
Western blot analysis
Total protein was extracted from PC12 cells using
RIPA lysis buffer (Absin Bioscience, Inc.). The protein
concentration was determined using a BCA kit (Beyotime Institute of
Biotechnology). Complete denaturation of the samples was performed
by incubating the protein in boiling water for 5 min. After
electrophoresis using 10% SDS-PAGE (Beijing Solarbio Science &
Technology Co., Ltd.), the protein samples (30 µg per lane) were
transferred to a PVDF membrane (Corning, Inc.). Subsequently, 5%
skimmed milk (Absin Bioscience, Inc.) was used to block the
membrane for 2 h at 25˚C, followed by incubation with primary
antibodies targeting NADPH oxidase (Nox)2 (1:5,000; cat. no.
ab129068; Abcam), Nox4 (1:1,000; cat. no. ab133303; Abcam), Bcl-2
(1:1,000; cat. no. ab196495; Abcam), Bax (1:1,000; cat. no. 2772;
Cell Signaling Technology, Inc.), caspase-3 (1:1,000; cat. no.
9662; Cell Signaling Technology, Inc.), cleaved caspase-3 (1:1,000;
cat. no. 9661; Cell Signaling Technology, Inc.), caspase-9
(1:1,000; cat. no. ab184786; Abcam), cleaved caspase-9 (1:1,000;
cat. no. ab2324; Abcam), Notch2 (1:1,000; cat. no. 5732; Cell
Signaling Technology, Inc.), HES-related family bHLH transcription
factor with YRPW motif 1 (Hey1; 1:1,000; cat. no. ab154077; Abcam),
HES1 (1:1,000; cat. no. #11988; Cell Signaling Technology, Inc.)
and GAPDH (1:1,000; cat. no. ab8245; Abcam) at 4˚C overnight. After
the membrane was washed with 0.5% TBST, it was incubated with
horseradish peroxidase-labeled secondary antibodies [goat
anti-rabbit IgG (1:2,000; cat. no. 7074) or horse anti-mouse IgG
(1:2,000; cat. no. 7076); both Cell Signaling Technology, Inc.] at
room temperature for 2 h. An ECL kit (Beyotime Institute of
Biotechnology) was used to visualize the protein bands and ImageJ
software (v6; National Institutes of Health) was used to analyze
the protein bands.
TUNEL assay
A colorimetric TUNEL Apoptosis Assay kit (cat. no.
C1088; Beyotime Institute of Biotechnology) was utilized to observe
PC12 cell apoptosis. The cells (5x105/well) seeded into
a 24-well plate were fixed with 4% paraformaldehyde (Shanghai
Macklin Biochemical Co., Ltd.) at room temperature for 30 min, and
incubated with Enhanced Immunostaining Permeabilization Buffer
(Beyotime Institute of Biotechnology) at room temperature for 5
min. After incubation with 0.3% H2O2 in PBS
(Sigma-Aldrich; Merck KGaA) at room temperature for 20 min, 50 µl
biotin-dUTP was added to label the samples for 1 h at 37˚C in the
dark. To develop the colors, the samples were then incubated with
50 µl streptavidin-HRP for 30 min at 37˚C, followed by incubation
in 0.5 ml DAB solution for another 30 min at 37˚C and the
anti-fluorescence quencher was added dropwise for mounting. The
coloration was observed microscopically from three random fields of
view using a fluorescence microscope (magnification, x200; Olympus
Corporation).
Statistical analysis
The data were analyzed and graphs were generated
using GraphPad Prism 6 software (GraphPad Software, Inc.). Data are
presented as the mean ± SD. Differences among multiple groups were
analyzed using one-way ANOVA with a post hoc Bonferroni multiple
comparison test. P<0.05 was considered to indicate a
statistically significant difference. All experiments were
performed in triplicate.
Results
LEN increases the viability of
H2O2-stimulated PC12 cells
The SCI model was established by treating PC12 cells
with H2O2. The viability of PC12 cells showed
a declining trend as the dose of H2O2 was
increased from 100 to 400 µM (Fig.
1A). According to a previous experiment (16), 300 µM was selected as the final
dose of H2O2 for the SCI model in view of the
appropriate degree of cell death under this dose. Additionally, the
viability of PC12 cells treated with different doses of LEN
revealed no significant difference compared with the control group
(Fig. 1B), indicating the
non-cytotoxicity of LEN. After pre-treatment with increasing doses
of LEN, the viability of H2O2-stimulated PC12
cells was noticeably improved (Fig.
1C). In addition, the level of LDH in
H2O2-stimulated PC12 cells was notably
decreased by LEN in a dose-dependent manner (Fig. 1D). Thus, these results indicated
that treatment with LEN could revitalize
H2O2-stimulated PC12 cells.
LEN inhibits the oxidative stress
level in H2O2-stimulated PC12 cells
By examining the production of MDA, SOD, GSH-Px and
CAT, the present study investigated the effect of LEN on
H2O2-induced oxidative stress in PC12 cells.
It was identified that, while H2O2 induced an
increased production of MDA, this was notably decreased after
pre-treatment with LEN in PC12 cells (Fig. 2A). Conversely,
H2O2-induced suppression of SOD, GSH-Px and
CAT production was attenuated in a dose-dependent manner by LEN
(Fig. 2B-D). Furthermore, the
oxidative stress-related proteins, Nox2 and Nox4, were examined and
both were revealed to be upregulated under
H2O2 stimulation, but downregulated by LEN
treatment (Fig. 2E). These results
suggested an inhibitory effect of LEN on
H2O2-induced oxidative stress in PC12
cells.
 | Figure 2Effects of LEN on oxidative stress
level in H2O2-stimulated PC12 cells. (A-D)
The production of (A) MDA, (B) SOD, (C) GSH-Px and (D) CAT in
H2O2-stimulated PC12 cells in the presence
and absence of LEN at different doses, detected by corresponding
commercial kits. (E) Relative protein expression of oxidative
stress-related Nox2 and Nox4 in
H2O2-stimulated PC12 cells in the presence
and absence of LEN at different doses, detected by western
blotting. *P<0.05, **P<0.01 and
***P<0.001 vs. control; #P<0.05,
##P<0.01 and ###P<0.001 vs.
H2O2. LEN, lenalidomide; MDA,
malondialdehyde; SOD, superoxide dismutase; GSH-Px, glutathione
peroxidase; CAT, catalase; Nox, NADPH oxidase. |
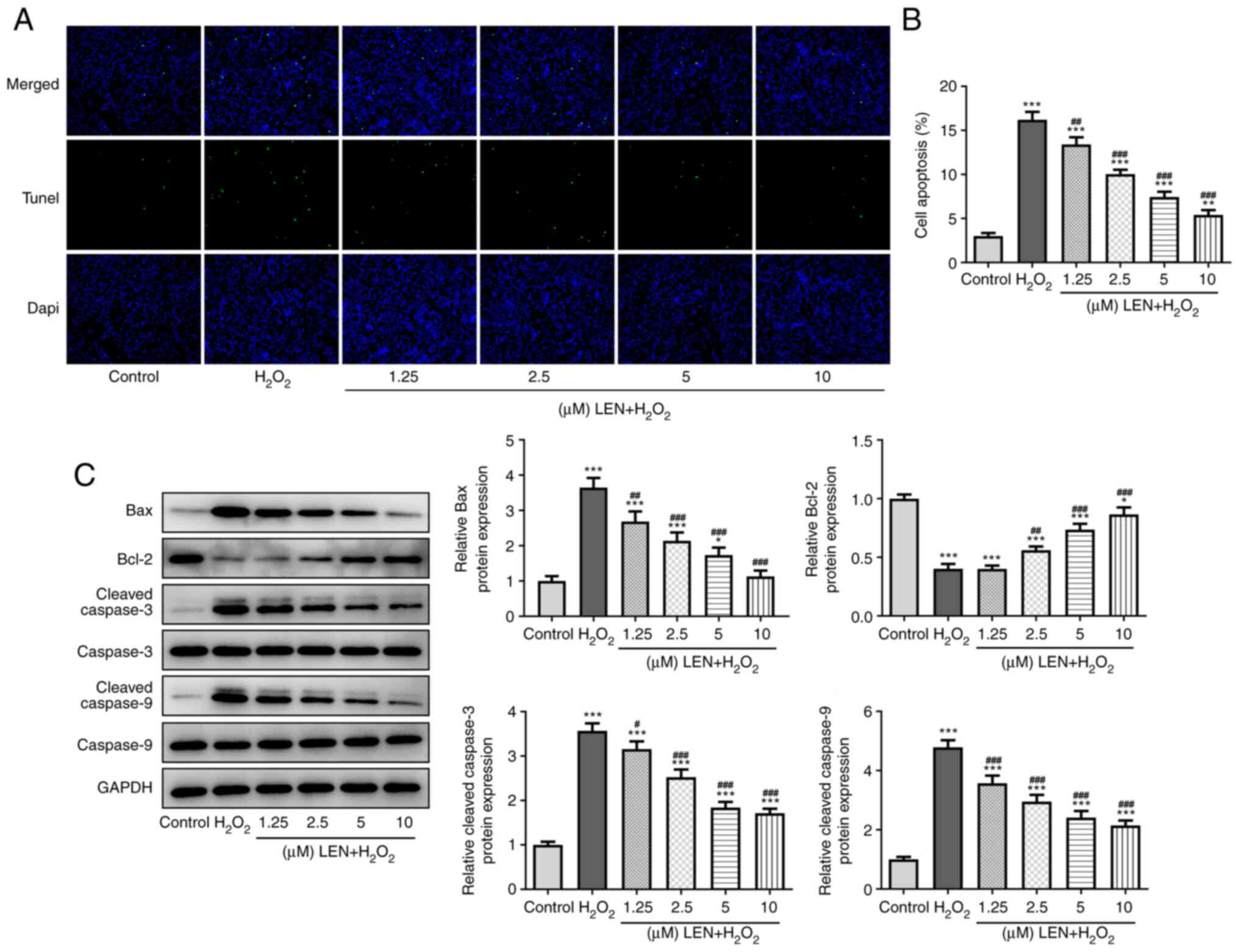
LEN extenuates the apoptosis of
H2O2-stimulated PC12 cells
H2O2-induced PC12 cell
apoptosis was detected in the absence and presence of pre-treatment
with LEN. The results of the TUNEL assay demonstrated a notable
increase in the number of apoptotic cells (green fluorescence) in
the H2O2 group, but this was significantly
decreased by pre-treatment with LEN (Fig. 3A and B). The expression levels of
apoptosis-related proteins in PC12 cells were also detected
(Fig. 3C), among which the
expression level of anti-apoptotic protein Bcl-2 was decreased by
H2O2 and dose-dependently increased after LEN
treatment, while Bax, cleaved caspase-3 and cleaved caspase-9
expression exhibited the opposite trend. These results indicated a
suppressive effect of LEN on H2O2-induced
PC12 cell apoptosis.
LEN blocks the Notch signaling pathway
in H2O2-stimulated PC12 cells
Whether LEN interacts with the Notch signaling
pathway was preliminarily examined by detecting related protein
expression levels in H2O2-stimulated PC12
cells. It was identified that the expression levels of Notch2, Hey1
and HES1 were all significantly increased in
H2O2-stimulated PC12 cells, but were
gradually decreased along with the increasing doses of LEN
(Fig. 4). These findings suggested
that treatment with LEN may block the expression of the Notch
signaling pathway in H2O2-stimulated PC12
cells.
LEN inhibits
H2O2-induced oxidative stress and apoptosis
of PC12 cells by blocking the Notch signaling pathway
To verify the involvement of Notch in the action
mechanism of LEN, rescue experiments were conducted by pre-treating
the cells with the Notch agonist Jagged-1 (JAG) peptide at a
concentration of 50 µg/ml, as previously described (17). In addition, 10 µM LEN was chosen
for these subsequent procedures. As revealed in Fig. 5A, the level of MDA formerly
downregulated by LEN in H2O2-stimulated PC12
cells was significantly upregulated by treatment with JAG. In
addition, the levels of SOD, GSH-Px and CAT in LEN-treated
H2O2-stimulated PC12 cells were significantly
decreased by JAG (Fig. 5B-D). The
expression levels of oxidative stress-related proteins in
LEN-treated H2O2-stimulated PC12 cells were
also rescued after pre-treatment with JAG, as both Nox2 and Nox4
exhibited re-upregulation (Fig.
5E). Similarly, reduced apoptosis of
H2O2-stimulated PC12 cells by LEN was
promoted after treatment with JAG (Fig. 5F and G), evidenced by the downregulation of
Bcl-2 expression and the upregulation of Bax, cleaved caspase-3 and
cleaved caspase-9 expression (Fig.
5H and I). Collectively, these
results demonstrated that the Notch agonist could reverse the
effects of LEN on the H2O2-induced oxidative
stress and apoptosis of PC12 cells.
 | Figure 5LEN suppresses
H2O2-induced oxidative stress and apoptosis
of PC12 cells by blocking the Notch signaling pathway. The
production of (A) MDA, (B) SOD, (C) GSH-Px and (D) CAT in
H2O2-stimulated PC12 cells treated with LEN
in the presence or absence of Notch agonist JAG, detected by
corresponding commercial kits. (E) Relative protein expression of
Nox2 and Nox4 in H2O2-stimulated PC12 cells
treated with LEN in the presence or absence of Notch agonist JAG,
detected by western blotting. (F and G) The apoptosis of
H2O2-stimulated PC12 cells treated with LEN
in the presence or absence of Notch agonist JAG, detected by TUNEL
(magnification, x200). (H and I) Relative protein expression of
Bax, Bcl-2, cleaved caspase-3 and cleaved caspase-9 in
H2O2-stimulated PC12 cells treated with LEN
in the presence or absence of Notch agonist JAG, detected by
western blotting. **P<0.01 and
***P<0.001 vs. control; #P<0.05,
##P<0.01 and ###P<0.001 vs.
H2O2; ∆P<0.05,
∆∆P<0.01 vs. LEN + H2O2 and
∆∆∆P<0.001 vs. LEN + H2O2. LEN,
lenalidomide; MDA, malondialdehyde; SOD, superoxide dismutase;
GSH-Px, glutathione peroxidase; CAT, catalase; Nox, NADPH oxidase;
JAG, Jagged-1. |
Discussion
The incidence of SCI has been continuously
increasing with the world's economic development, which will not
only bring serious physical and psychological harm to the patients
themselves, but also result in significant financial burden to
society (4,18). Hence, the prevention and treatment
of SCI have become major issues in the medical field. In the
present study, H2O2 was chosen for the
modeling of SCI, considering its wide use in SCI models in previous
studies (19,20), and the significantly decreased
viability in H2O2-treated PC12 cells was
confirmed.
LEN is a Food and Drug Administration-approved drug
that has immunomodulatory, antitumor and anti-angiogenic
activities, and is commonly used for the treatment of MM and
myelodysplastic syndrome (21,22).
However, little is known regarding the impact of LEN on other
diseases, including SCI. PC12 cells pre-treated with LEN in the
present study showed no indication of cytotoxicity, and their
viability was significantly improved following stimulation with
H2O2, compared with that in cells without LEN
pre-treatment. The LDH level, reflecting cytotoxic injury, in
H2O2-stimulated PC12 cells was also reduced
by LEN. It was therefore suggested that LEN treatment benefits the
survival of PC12 cells under the stimulation of
H2O2.
Previous studies have reported that an elevated
oxidative stress level characterizes the occurrence of SCI and is
considered to be a treatment target, with the overproduction of
free radicals and lipid peroxidation found in damaged spinal
neurons (23,24). LEN has been revealed to have a
regulatory effect on inflammatory cytokines as well as certain
stress signals (25,26). In addition, it has been reported
that combined treatment with LEN and nanoceria produces a
suppressive effect in vivo on central nervous system
autoimmunity-induced inflammation and oxidative stress (27). In the present study, increased MDA
and decreased SOD, GSH-Px and CAT production were observed in
H2O2-stimulated PC12 cells. Pre-treatment
with LEN effectively inhibited the production of MDA, while
promoting that of the anti-oxidants SOD, GSH-Px and CAT.
Consistently, the gene expression levels of the reactive oxygen
species, Nox2 and Nox4, were comparatively higher in
H2O2-stimulated PC12 cells in the absence of
LEN and were significantly downregulated in the presence of LEN.
These results suggested an anti-oxidative stress role of LEN in the
model of SCI.
Inflammation and apoptosis occur in the secondary
injury phase that follows the primary mechanical injury in SCI,
further resulting in the dysfunction or damage of the central
nervous system (28,29). Qu et al (30) revealed that LEN could restrict the
formation of osteoclasts and protect osteocytes from IL-1β-induced
apoptosis in a mouse model of osteoarthritis. The present study
observed a significantly increased number of apoptotic PC12 cells
after treatment with H2O2, in addition to the
downregulation of anti-apoptotic Bcl-2 protein expression and
upregulation of the pro-apoptotic proteins Bax, cleaved caspase-3
and cleaved caspase-9. However, pre-treatment with LEN decreased
the number of apoptotic cells and the expression levels of
pro-apoptotic proteins, while increasing the expression of Bcl-2.
An anti-apoptotic effect of LEN on
H2O2-stimulated PC12 cells was thus
demonstrated in the present study.
The present study also investigated the effects of
LEN by examining the underlying mechanism. Previous studies
determined the role of the interaction between LEN and Notch
signaling in inhibiting human gastric cancer cell proliferation and
promoting osteogenic differentiation in MM (14,15).
Additionally, Cai et al (31) identified that Notch inhibition
in vivo by microRNA-139-5p upregulation could potentially
attenuate the oxidative stress-induced liver injury in a diabetic
model. Notch is also known as an essential regulatory signaling
pathway in the process of cellular apoptosis in cerebrovascular
diseases (32). More importantly,
Notch expression is likely to be inhibited by resveratrol to
facilitate the recovery from SCI, according to a recent review
(33). In addition, a previous
study revealed that circular RNA_0005075 knockdown could alleviate
neuropathic pain by inactivating the Notch2 signaling pathway
(34). It was observed in the
present study that the expression levels of Notch-related proteins,
Notch2, Hey1 and HES1, were notably increased in
H2O2-stimulated PC12 cells, but were
significantly decreased by LEN treatment in a dose-dependent
manner. This result preliminarily validated the interaction of LEN
and the Notch signaling pathway. The present study further
investigated the role of Notch in the effects of LEN by introducing
the Notch agonist JAG to H2O2-stimulated PC12
cells pre-treated with LEN. It was revealed that the formerly
inhibited oxidative stress level and mitigated apoptosis caused by
LEN treatment were significantly promoted again by Notch
activation. This suggested that LEN inhibits the
H2O2-induced oxidative stress and apoptosis
of PC12 cells by blocking the Notch signaling pathway.
In conclusion, the present study demonstrated that
LEN has the ability to alleviate PC12 cell injury induced by
H2O2 stimulation, likely by blocking the
Notch signaling pathway, revealing the value of LEN in restoring
the viability of spinal cord neurons following SCI.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZL and KW designed the study, and drafted and
revised the manuscript. SY and ZC analyzed the data and searched
the literature. All authors performed the experiments. All authors
read and approved the final manuscript. ZL and SY confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eckert MJ and Martin MJ: Trauma: Spinal
cord injury. Surg Clin North Am. 97:1031–1045. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hamid R, Averbeck MA, Chiang H, Garcia A,
Al Mousa RT, Oh SJ, Patel A, Plata M and Del Popolo G: Epidemiology
and pathophysiology of neurogenic bladder after spinal cord injury.
World J Urol. 36:1517–1527. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Witiw CD and Fehlings MG: Acute spinal
cord injury. J Spinal Disord Tech. 28:202–210. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kumar R, Lim J, Mekary RA, Rattani A,
Dewan MC, Sharif SY, Osorio-Fonseca E and Park KB: Traumatic spinal
injury: Global epidemiology and worldwide volume. World Neurosurg.
113:e345–e363. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kjell J and Olson L: Rat models of spinal
cord injury: From pathology to potential therapies. Dis Model Mech.
9:1125–1137. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fan Q, Cavus O, Xiong L and Xia Y: Spinal
cord injury: How could acupuncture help? J Acupunct Meridian Stud.
11:124–132. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Varma AK, Das A, Wallace G IV, Barry J,
Vertegel AA, Ray SK and Banik NL: Spinal cord injury: A review of
current therapy, future treatments, and basic science frontiers.
Neurochem Res. 38:895–905. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cristante AF, Filho TE, Marcon RM, Letaif
OB and Rocha ID: Therapeutic approaches for spinal cord injury.
Clinics (Sao Paulo). 67:1219–1224. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ravikumar K and Sridhar B: Lenalidomide,
an antineoplastic drug, and its hemihydrate. Acta Crystallogr C.
65:o502–o505. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Weisel K and Kanz L: Lenalidomide. Recent
Results Cancer Res. 201:347–357. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Valera E, Mante M, Anderson S, Rockenstein
E and Masliah E: Lenalidomide reduces microglial activation and
behavioral deficits in a transgenic model of Parkinson's disease. J
Neuroinflammation. 12(93)2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mosley RL and Gendelman HE: Control of
neuroinflammation as a therapeutic strategy for amyotrophic lateral
sclerosis and other neurodegenerative disorders. Exp Neurol.
222:1–5. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yamamoto K, Kitawaki T, Sugimoto N, Fujita
H, Kawase Y, Takaori-Kondo A and Kadowaki N: Anti-inflammatory
modulation of human myeloid-derived dendritic cell subsets by
lenalidomide. Immunol Lett. 211:41–48. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ding W, Zeng T, Tao W, Ge W, Deng V, Lei
H, Xiao Y and Liao F: Effect of lenalidomide on the human gastric
cancer cell line SGC7901/vincristine notch signaling. J Cancer Res
Ther. 14:S237–S242. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Guo J, Fei C, Zhao Y, Zhao S, Zheng Q, Su
J, Wu D, Li X and Chang C: Lenalidomide restores the osteogenic
differentiation of bone marrow mesenchymal stem cells from multiple
myeloma patients via deactivating notch signaling pathway.
Oncotarget. 8:55405–55421. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yi G, Liu L, Lv C, Wei Y and Yan T:
Ginsenoside Rg1 defenses PC-12cells against hydrogen
peroxide-caused damage via up-regulation of miR-216a-5p. Life Sci.
236(116948)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wen F, Wong HK, Tay CY, Yu H, Li H, Yu T,
Tijore A, Boey FY, Venkatraman SS and Tan LP: Induction of myogenic
differentiation of human mesenchymal stem cells cultured on notch
agonist (Jagged-1) modified biodegradable scaffold surface. ACS
Appl Mater Interfaces. 6:1652–1661. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yue JK, Chan AK, Winkler EA, Upadhyayula
PS, Readdy WJ and Dhall SS: A review and update on the guidelines
for the acute management of cervical spinal cord injury-part II. J
Neurosurg Sci. 60:367–384. 2016.PubMed/NCBI
|
|
19
|
Siriphorn A, Chompoopong S and Floyd CL:
17β-estradiol protects Schwann cells against
H2O2-induced cytotoxicity and increases
transplanted Schwann cell survival in a cervical hemicontusion
spinal cord injury model. J Neurochem. 115:864–872. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li X, Zhan J, Hou Y, Hou Y, Chen S, Luo D,
Luan J, Wang L and Lin D: Coenzyme Q10 regulation of apoptosis and
oxidative stress in H2O2 induced BMSC death
by modulating the Nrf-2/NQO-1 signaling pathway and its application
in a model of spinal cord injury. Oxid Med Cell Longev.
2019(6493081)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Talati C, Sallman D and List A:
Lenalidomide: Myelodysplastic syndromes with del(5q) and beyond.
Semin Hematol. 54:159–166. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Flowers CR, Leonard JP and Fowler NH:
Lenalidomide in follicular lymphoma. Blood. 135:2133–2136.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jia Z, Zhu H, Li J, Wang X, Misra H and Li
Y: Oxidative stress in spinal cord injury and antioxidant-based
intervention. Spinal Cord. 50:264–274. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hall ED: Antioxidant therapies for acute
spinal cord injury. Neurotherapeutics. 8:152–167. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhu X, Jiang S, Hu N, Luo F, Dong H, Kang
YM, Jones KR, Zou Y, Xiong L and Ren J: Tumour necrosis
factor-alpha inhibition with lenalidomide alleviates tissue
oxidative injury and apoptosis in ob/ob obese mice. Clin Exp
Pharmacol Physiol. 41:489–501. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li L, Hua Y, Dong M, Li Q, Smith DT, Yuan
M, Jones KR and Ren J: Short-term lenalidomide (Revlimid)
administration ameliorates cardiomyocyte contractile dysfunction in
ob/ob obese mice. Obesity (Silver Spring). 20:2174–2185.
2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Eitan E, Hutchison ER, Greig NH, Tweedie
D, Celik H, Ghosh S, Fishbein KW, Spencer RG, Sasaki CY, Ghosh P,
et al: Combination therapy with lenalidomide and nanoceria
ameliorates CNS autoimmunity. Exp Neurol. 273:151–160.
2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang N, Yin Y, Xu SJ, Wu YP and Chen WS:
Inflammation & apoptosis in spinal cord injury. Indian J Med
Res. 135:287–296. 2012.PubMed/NCBI
|
|
29
|
Keane RW, Davis AR and Dietrich WD:
Inflammatory and apoptotic signaling after spinal cord injury. J
Neurotrauma. 23:335–344. 2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Qu X, Mei J, Yu Z, Zhai Z, Qiao H and Dai
K: Lenalidomide regulates osteocytes fate and related
osteoclastogenesis via IL-1β/NF-kappaB/RANKL signaling. Biochem
Biophys Res Commun. 501:547–555. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cai Z, Zhao B, Deng Y, Shangguan S, Zhou
F, Zhou W, Li X, Li Y and Chen G: Notch signaling in
cerebrovascular diseases (Review). Mol Med Rep. 14:2883–2898.
2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wei H, Huang L, Wei F, Li G, Huang B, Li J
and Cao C: 2020. Up-regulation of miR-139-5p protects diabetic mice
from liver tissue damage and oxidative stress through inhibiting
notch signaling pathway. Acta Biochim Biophys Sin (Shanghai).
52:390–400. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang S, Botchway BOA, Zhang Y and Liu X:
Resveratrol can inhibit notch signaling pathway to improve spinal
cord injury. Ann Anat. 223:100–107. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhang Y, Gao T, Li X, Wen CC, Yan XT, Peng
C and Xiao Y: Circ_0005075 targeting miR-151a-3p promotes
neuropathic pain in CCI rats via inducing NOTCH2
expression. Gene. 767(145079)2021.PubMed/NCBI View Article : Google Scholar
|