Introduction
Type 1 diabetes mellitus (T1DM) has been considered
to be a risk factor for inducing stroke, Alzheimer's disease (AD),
vascular dementia and other types of dementia (1-3).
Moreover, it has been reported that in T1DM mice and rats
impairments in cognitive function increase brain cell apoptosis,
tau protein expression and oxidative stress (4,5). It
has also been demonstrated that insulin, the effective drug in the
treatment of T1DM, improves cognitive function in comorbid patients
with diabetes and AD (6).
Glucagon-like peptide 1 (GLP-1) is a growth factor
and endogenous incretin hormone and its analogs, such as
liraglutide and exenatide, are currently used in the treatment of
type 2 diabetes mellitus (DM) (7,8).
Furthermore, in addition to improving glycemic control, liraglutide
has been demonstrated to cross the blood-brain barrier and bind to
the GLP-1 receptor in the brain, which exerts neuroprotective
effects in several neurological disorders, such as stroke, AD and
Parkinson's disease (9,10). Liraglutide administered
peripherally attenuates impairments in cognition and synaptic
plasticity, promotes neurogenesis and reduces cell apoptosis in the
streptozotocin (STZ)-induced T1DM mouse model (11,12).
Furthermore, the Wnt/β-catenin signaling pathway is
an essential pathway for regulating cell proliferation, migration
and differentiation (13). In the
brain, the Wnt/β-catenin signaling pathway regulates neuronal
survival, differentiation and synaptogenesis and serves an
important role in the pathogenesis of AD (14,15).
Moreover, the Wnt/β-catenin signaling pathway also mediates
post-stroke angiogenesis and neurogenesis (16,17).
Therefore, the present study aimed to investigate the effects of
liraglutide, insulin and their co-treatment on neuronal apoptosis
in STZ-induced diabetic mice, and if the activation of the
Wnt/β-catenin signaling pathway was associated with the underlying
mechanism.
Materials and methods
Animals and study design
In total 40 female C57BL/6J mice (age, 10-11 weeks;
weight, 19.1-21.5 g) were purchased from Beijing Huafukang
Biotechnology Co., Ltd. and were allowed to acclimate for 1 week
before the experiments. Subsequently, the T1DM model was
established via a single intraperitoneal injection of STZ (150
mg/kg) and control mice, referred to as the normal glucose
tolerance (NGT; n=8) group, were injected with citrate buffer (100
mM citrate; pH 4.2-4.5).
After 2 weeks, mice injected with STZ and confirmed
to have diabetes (random blood glucose, ≥250 mg/dl), were randomly
assigned to the following four treatment groups for 8 weeks
(n=8/group): i) DM model group (STZ), treated with subcutaneous
injection of normal saline; ii) insulin group (INS), treated with
subcutaneous injection of insulin (10 units/kg body weight/day
insulin determir; Levemir®; Novo Nordisk A/S); iii)
liraglutide group (LRG), treated with subcutaneous injection of
liraglutide (0.6 mg/kg/day; Novo Nordisk A/S); and iv) combined
insulin and liraglutide group (LRG + INS), subcutaneous injection
of insulin (10 units/kg/day) and liraglutide (0.6 mg/kg/day).
Furthermore, although it was not expected, a rapid decrease in body
weight of >15-20% was defined as a potential humane endpoint for
the study.
All mice were housed under standard laboratory
conditions from the start of acclimatization in an air-conditioned
atmosphere with a 12-h light/dark cycle, a humidity of 40-60% and a
temperature of 22˚C. Mice were provided with ad libitum
access to water and food for 11 weeks. Body weight and pedal dorsal
vein blood glucose, which was assessed using the Accu-Chek compact
glucometer (Roche Diagnostics), were recorded once a week. At the
end of the experiment, mice were sacrificed using an overdose of
isoflurane (5%) and were subsequently decapitated. Trunk blood
(0.8-1 ml) was collected from the severed neck. One hemisphere of
the brain was quickly dissected and stored at -80˚C until RNA and
protein extraction (n=4/group) was performed. Meanwhile the other
hemisphere was fixed with 4% paraformaldehyde overnight at room
temperature for histopathological and immunohistochemical assays
(n=4/group). All animal procedures were approved by the
Institutional Animal Care and Use Committee of the Institute of
Laboratory Animals Science of the Chinese Academy of Medical
Sciences and Peking Union Medical College (Beijing, China).
Experiments were performed according to the Laboratory Animal
Management Regulations in China (18) and also adhered to the Guide for the
Care and Use of Laboratory Animals published by the National
Institutes of Health (19).
Moreover, all animal studies were performed in accordance with the
Animal Research: Reporting of In Vivo Experiments guidelines
(20). During the course of this
study, none of the animals exhibited a weight loss of >20%, one
mouse in each of the STZ, INS, and INS + LRG groups were sacrificed
because of significantly elevated blood glucose, obvious signs of
dehydration and weakness. In addition, two more mice in the INS +
LRG group died at week 14 and 15, respectively, within a few hours
after injection of the treatment drug, probably due to hypoglycemia
(Fig. S1). Data from these mice
were excluded from the analysis.
H&E staining
The cerebral hemispheres fixed in 4%
paraformaldehyde overnight were embedded in paraffin, and sections
5-µm thick were prepared. The tissues were then stained with
hematoxylin solution (cat. no. G1004-100ML; Wuhan Servicebio
Technology Co., Ltd.) for 5 min at room temperature, followed by
immersion in 1% acid alcohol differentiation solution for 5 min to
be de-stained. Subsequently, the tissues were rinsed in distilled
water, stained again with eosin dye (cat. no. G1001-100ML; Wuhan
Servicebio Technology Co., Ltd.) for 5 min at room temperature,
dehydrated with anhydrous ethanol and xylene and then mounted with
neutral gum. Morphological changes in hippocampal and cortical
neurons were observed using light microscopy. In total five fields
were randomly selected from the hippocampus and cortex
(magnification, x400) and the number of neurons were counted using
image analysis software (ImageJ; version 1.46a; National Institutes
of Health).
TUNEL staining
Dewaxed tissue sections were dewaxed with anhydrous
ethanol and xylene for 45 min at room temperature, followed by
fixation in proteinase K working solution (cat. no. G1205; Wuhan
Servicebio Technology Co., Ltd.) at 37˚C for 30 min and then rinsed
using phosphate-buffered saline (PBS) solution. Subsequently,
sections were soaked in 0.2% Triton X-100 solution for 5 min at
room temperature to enhance permeability and incubated with the
TUNEL reaction mixture (cat. no. G1501; Wuhan Servicebio Technology
Co., Ltd.) for 60 min at 37˚C. The samples were washed with PBS and
then incubated with DAPI solution (cat. no. G1012; Wuhan Servicebio
Technology Co., Ltd.) for 10 min at room temperature, followed by
washing with PBS, and then mounted with anti-fade mounting medium.
Samples were imaged using a fluorescence microscope. In total five
fields of the cortex were randomly selected from each section and
the number of apoptotic cells was quantified as apoptotic rate
(%)=(number of apoptosis-positive cells/total cells) x100.
Immunohistochemistry for Ki67
First, 30-µm thick sections were deparaffinized in
xylene for 2 min at room temperature and then rehydrated in
descending grades of ethanol (100, 95 and 70% ethanol) for another
5 min at room temperature. The sections were washed with PBS at
room temperature and then incubated in 3%
H2O2 at 37˚C for 25 min to block endogenous
peroxidase activity. After blocking in 3% bovine serum albumin
(cat. no. GC305010-25G; Wuhan Servicebio Technology Co., Ltd.) for
30 min at room temperature, sections were incubated with primary
antibody against Ki67 (1:500; cat. no. GB111141; Wuhan Servicebio
Technology Co., Ltd.) overnight at 4˚C. Following the primary
incubation cells were incubated with goat anti-rabbit secondary
antibody conjugated to horseradish peroxidase (HRP; 1:1,000; cat.
no. 7074S; Cell Signaling Technology, Inc.) for 1 h at room
temperature. The peroxidase was visualized using the DAB detection
kit (cat. no. G1212-200T; Wuhan Servicebio Technology Co., Ltd.)
and counterstained with hematoxylin solution (cat. no. G1004-100ML;
Wuhan Servicebio Technology Co., Ltd.) for 10 min at room
temperature. Brain sections were imaged using light microscopy.
Western blotting
The isolated brain tissues were homogenized on ice
in RIPA lysis buffer (cat. no. P0013C; Beyotime Institute of
Biotechnology) and phenylmethanesulfonyl fluoride in the presence
of protease and phosphatase inhibitors. The homogenates were
centrifuged at 12,000 x g for 15 min at 4˚C and the supernatants
were extracted to quantify protein concentration using a BCA
Protein Assay Kit (cat. no. P0012S; Beyotime Institute of
Biotechnology). Equal amounts of protein (50 µg per lane) were
separated using SDS-PAGE on a 12% gel, transferred to
polyvinylidene difluoride membranes and blocked with 5% non-fat
milk for 1 h at room temperature. The membranes were incubated with
the following primary antibodies: rabbit anti-Wnt3a (1:1,000; cat.
no. 26744-1-AP; ProteinTech Group, Inc.), S33-phosphorylated
(p)β-catenin (1:5,000; cat. no. 80067-1-RR; ProteinTech Group,
Inc.), β-catenin (1:5,000; cat. no. 51067-2-AP; ProteinTech Group,
Inc.), GSK-3β (1:1,000; cat. no. 22104-1-AP; ProteinTech Group,
Inc.), Caspase-3 (1:1,000; cat. no. 9662S; Cell Signaling
Technology, Inc.), Bcl-2 (1:1,000; cat. no. 12789-1-AP; ProteinTech
Group, Inc.), Bax (1:5,000; cat. no. 50599-2-Ig; ProteinTech Group,
Inc.), mouse anti-S9-pGSK-3β (1:1,000; cat. no. 67558-1-Ig;
ProteinTech Group, Inc.), rabbit anti-β-actin (1:1,000; cat. no.
4970S; Cell Signaling Technology, Inc.) primary antibodies at 4˚C
overnight. After washed with TBST, the membranes were incubated
with goat anti-rabbit secondary antibody conjugated to HRP
(1:1,000; cat. no. 7074S; Cell Signaling Technology, Inc.) or horse
anti-mouse secondary antibody conjugated to HRP (1:1,000; cat. no.
7076S; Cell Signaling Technology, Inc.) at room temperature for 1
h. Proteins were visualized using an enhanced chemiluminescence
(ECL) reagent (cat. no. P06M31M; Gene-Protein Link). The results
were normalized to β-actin and the protein band densitometry was
semi-quantified using ImageJ software (version 1.46a; National
Institutes of Health) All protein bands in a given western blot
image were derived from the same membrane.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using Tissue Total RNA
Isolation Kit V2 (cat. no. RC112; Vazyme Biotech Co., Ltd.)
according to the manufacturer's protocol. Complementary DNA was
synthesized using the PrimeScript™ RT Reagent Kit with
Genomic DNA (cat. no. RR047A; Takara Biotechnology Co., Ltd.),
according to the manufacturer's instructions. qPCR primers
(Table I) were synthesized by
Beijing Nuosai Genome Research Center Co., Ltd. qPCR was performed
using TB Green® Premix Ex Taq™ II (cat. no.
RR82LR; Takara Biotechnology Co., Ltd.). The thermocycling
conditions were as follows: After the initial denaturation for 30
sec at 95˚C, 40 PCR cycles were performed (95˚C for 5 sec, 60˚C for
30 sec and 72˚C for 30 sec). The relative mRNA expression levels
were determined using the 2-ΔΔCq method (21) with the housekeeping gene β-actin as
an internal control.
 | Table ISequences of primers used for reverse
transcription-quantitative PCR. |
Table I
Sequences of primers used for reverse
transcription-quantitative PCR.
| Gene | Sequence
(5'-3') |
|---|
| Wnt3a |
TGGAGGAATGGTCTCTCGGG |
| |
GCACTTGAGGTGCATGTGAC |
| GSK-3β |
GTAGCCCAGGGAGGTCACTA |
| |
CAGCCTTCCTAAGCTGGCAT |
| β-catenin |
CTGGGACTCTGCACAACCTT |
| |
CAGTGTCGTGATGGCGTAGA |
| Bax |
TCTCCGGCGAATTGGAGATG |
| |
ACCCGGAAGAAGACCTCTCG |
| Bcl-2 |
GCAGCTTCTTTTCGGGGAAG |
| |
CTCCAGCATCCCACTCGTAG |
| Caspase-3 |
TGGCTTGCCAGAAGATACCG |
| |
ATGCTGCAAAGGGACTGGAT |
| β-actin |
CACTGTCGAGTCGCGTCCA |
| |
GTCATCCATGGCGAACTGGT |
Statistical analysis
All data analysis was performed using SPSS 25.0
software (IBM Corp.). All figures were created using GraphPad Prism
8.0 software (GraphPad Software, Inc.). Data from at least three
independent experiments are presented as the mean ± SD. One-way
ANOVA was used to make statistical comparisons among more than two
groups followed by Bonferroni's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of insulin, liraglutide and
combined drugs on metabolic parameters in DM mice
Compared with the NGT control, saline-treated DM
mice exhibited significant hyperglycemia and weight loss, which
indicated the successful establishment of the T1DM mouse model
(Fig. 1; Tables SI and SII) (22). However, compared with
saline-treated DM mice, once-daily insulin treatment failed to
control blood glucose levels or lower body weight. Furthermore,
liraglutide monotherapy had no effect on body weight compared with
the saline-treated DM group and the INS group but exhibited
significantly lower blood glucose levels after week 17 and
approached those of the control group after week 20. However, the
combined treatment (LRG + INS) group did not significantly improve
glycemic control and led to further weight loss compared with the
saline-treated DM group. These results suggested that liraglutide
monotherapy exerted the greatest efficacy in reducing metabolic
disturbances in DM mice.
Insulin and liraglutide attenuate
diabetes-induced neuronal damage in mice
The pathological damage of brain tissue in different
regions of the brain in each group of mice was assessed using
H&E staining (Fig. 2A).
Compared with the NGT group, neurons in the STZ group exhibited
marked pathological changes in the cortex and hippocampal cornu
ammonis-1 and dentate gyrus (DG) regions, as demonstrated by loose
cortical interstitium and neuronal degeneration, including
irregular neuronal arrangement, increased intercellular space,
nucleus condensation and significantly decreased neuronal density
(Fig. 2B). However, neurons in the
INS, LRG and INS + LRG groups were neatly arranged with clearly
visible nucleus and cytoplasm, displaying round vesicular nuclei
and prominent nucleoli (Fig. 2A),
and significantly increased neuronal density (Fig. 2B), similar to those exhibited by
the NGT group. These results suggested that either insulin,
liraglutide, or combined drugs prevented neuronal damage in DM
condition.
 | Figure 2Effect of insulin, liraglutide and
combined drugs on pathological changes in the cortex, hippocampal
CA1 and DG regions in diabetic mice. (A) H&E staining of
neurons in the cortex, hippocampal CA1 and DG regions. Scale bar,
100 µm. (B) Neuronal density in the cortex, hippocampus including
CA1 and DG regions. Data are presented as the mean ± SD
(n=4/group). *P<0.05 vs. NGT; #P<0.05
vs. STZ. CA1, cornu ammonis-1; DG, dentate gyrus; STZ, saline
treated type 1 diabetes group; INS, insulin treatment group; LRG,
liraglutide treatment group; INS + LRG, insulin and liraglutide
treatment group; NGT, normal glucose tolerance group. |
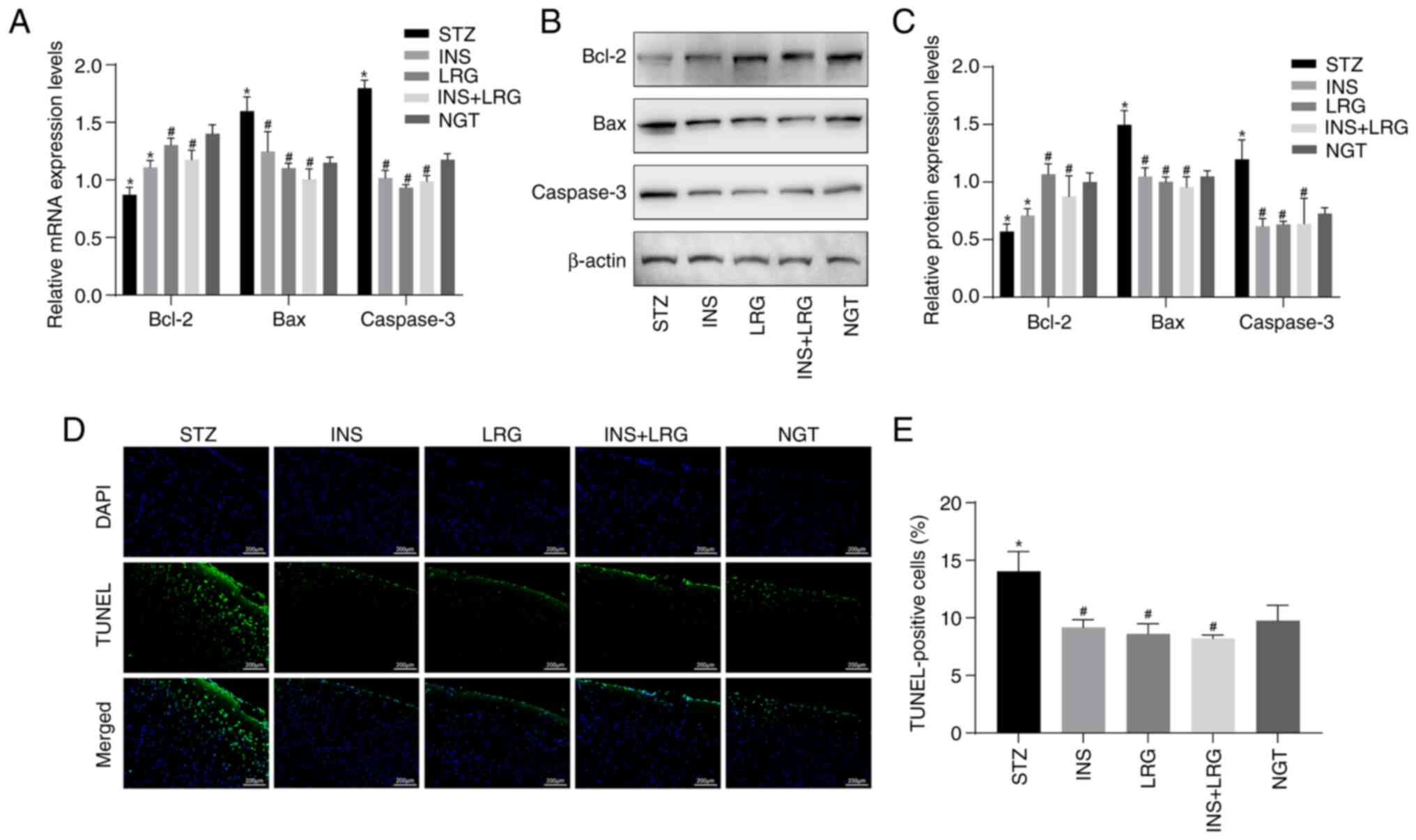
Insulin and liraglutide reduce the
apoptotic rate of neurons and regulate the expression levels of
related proteins in DM mice
The mRNA and protein expression levels of Bax, Bcl-2
and Caspase-3 in brain tissue were determined using RT-qPCR and
western blotting. The mRNA and protein expression levels of Bax and
Caspase-3 were significantly higher in neurons of the STZ group
compared with the NGT group, along with significantly lower
expression levels of Bcl-2 (Fig.
3A-C). Compared with the STZ group, the mRNA and protein
expression levels of Bax and Caspase-3 were significantly decreased
in the brain tissue of the INS, LRG and LRG + INS groups, whereas
Bcl-2 mRNA and protein expression levels were significantly
increased in the LRG and LRG + INS groups. Furthermore, Bcl-2 mRNA
and protein expression levels in the INS group was not
significantly different compared with the STZ group. Apoptosis of
neurons in the brain was detected using the TUNEL assay. The
results demonstrated that the mean percentage of TUNEL-positive
cells was significantly increased in the STZ group compared with
the NGT group (Fig. 3D and
E). However, among the INS, LRG
and LRG + INS groups, the mean percentage of apoptotic cells was
significantly lower compared with the STZ group and no significant
difference was observed when compared with the NGT group. These
results suggested that either liraglutide, insulin, or the
combination of these drugs may potentially inhibit neuronal
apoptosis in DM mice.
Insulin and liraglutide activate the
Wnt/β-catenin signaling pathway in DM mice
mRNA and protein expression levels of Wnt/β-catenin
signaling pathway-associated proteins in the brains of mice were
determined (Fig. 4). Compared with
the STZ group, the expression levels of Wnt3a and S9-pGSK-3β were
significantly increased, whereas the expression levels of GSK-3β
and S33-pβ-catenin were significantly decreased in the INS, LRG and
LRG + INS groups. In the LRG and LRG + INS groups, β-catenin mRNA
and protein expression levels were significantly upregulated
compared with the STZ and INS groups, while there was no
significant difference among the STZ, INS and NGT groups. These
results suggested that either liraglutide, insulin or the
combination drug therapy activated the Wnt/β-catenin signaling
pathway in the brain of DM mice.
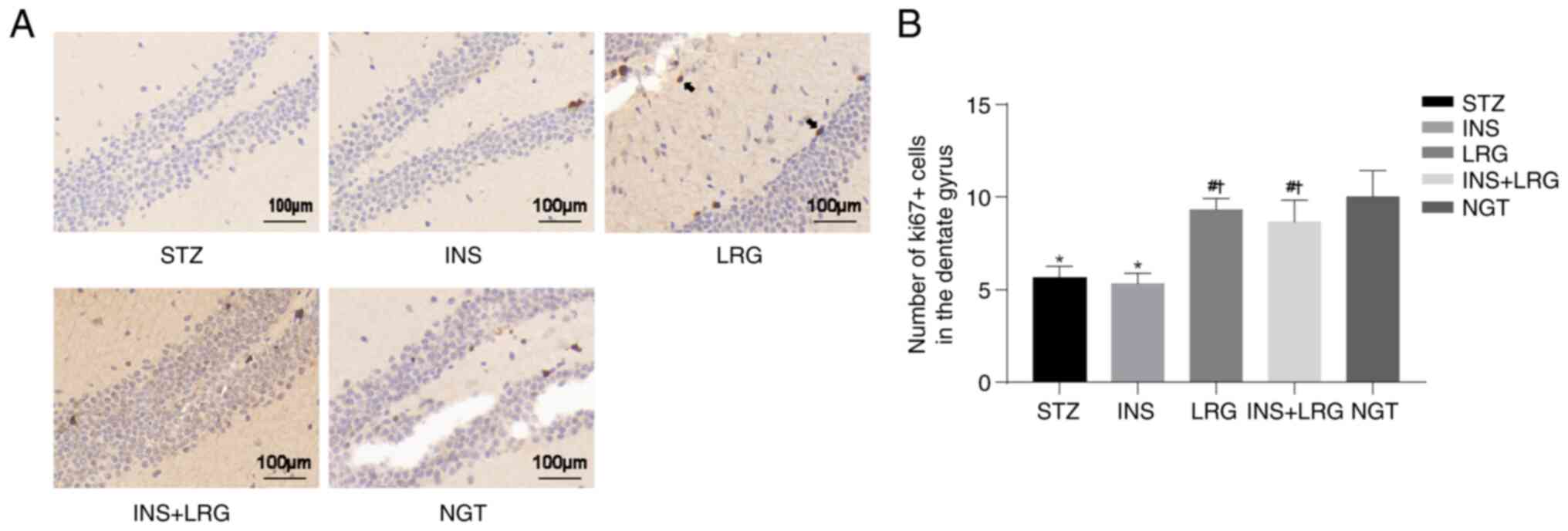
Liraglutide promotes neurogenesis in
DM mice
Neuronal proliferation was investigated using Ki67
immunostaining of the hippocampus. The results demonstrated that
compared with the NGT group the number of Ki67-positive neurons in
the DG region was significantly reduced in the STZ group, whereas
it was significantly increased in the LRG and LRG + INS groups
(Fig. 5). No significant
differences were observed between the STZ and INS groups.
Discussion
Multiple epidemiological studies have demonstrated
that numerous patients with T1DM are at an increased risk for
stroke, cognitive impairment, dementia and neurodegenerative
diseases (23-25).
However, there is a lack of effective clinical drug therapy due to
incomplete knowledge of the underlying disease process. Over the
last few years the roles of insulin and GLP-1 analogs in the
central nervous system of animal models with DM have been
increasingly investigated (6,10).
To the best of our knowledge, this is the first study to have
compared the effects of peripherally-administered insulin,
liraglutide and their combination, on brain pathological changes in
an STZ-induced mouse model of T1DM and to explore the underlying
mechanisms. The present study demonstrated that insulin,
liraglutide and the drugs combined equally significantly alleviated
DM-induced hippocampal and cortical neuronal injuries and loss.
Furthermore, treatment with liraglutide alone or in combination
with insulin administration significantly increased the
proliferation of newborn neurons (Ki67-positive neurons) in the
hippocampal DG region of DM mice. These protective effects may
involve the activation of the Wnt/β-catenin signaling pathway.
In the present study, the mortality rate in diabetic
mice was lower compared with previous studies in which the same
model was established but the mean blood glucose was higher
(26,27), and two mice in the combined
treatment group may have died due to hypoglycemia. Furthermore, the
results demonstrated that liraglutide, insulin and the combination
of both drugs had no significant effect on improving body weight,
but liraglutide significantly decreased blood glucose levels in DM
mice. Moreover, insulin monotherapy and the combination of the two
drugs failed to control blood glucose well. However, the mean blood
glucose level in the liraglutide treated group (16.41±6.36 mmol/l)
was much higher than the normal standard, which is consistent with
the results of previous studies (12,28).
It has also previously been reported that GLP-1 and its analogs
exert neuroprotective effects without significant improvement in
blood glucose levels in T1DM models (11,29).
It can therefore be hypothesized that liraglutide potentially
exerts direct neuroprotective effects independently from its
hypoglycemic effects.
Cell apoptosis is dependent on caspases, of which
Caspase-3 is central to the apoptotic signaling pathway (30). The proapoptotic factor Bax and the
antiapoptotic factor Bcl-2, members of the Bcl-2 family, control
the release of cytochrome c, which is involved in the
activation of Caspase-3(31).
Previous studies have reported that liraglutide alleviates neuronal
apoptosis in STZ-induced T1DM mouse models via modulating the mTOR
or PI3K/Akt signaling pathways (12,28).
Furthermore, insulin may prevent brain cell apoptosis by reducing
brain mitochondrial dysfunction and brain oxidative stress via its
antioxidant effects (32,33). In the present study it was
demonstrated that STZ-induced DM mice exhibited significantly
decreased mRNA and protein expression levels of Bcl-2 and
significantly increased mRNA and protein expression levels of Bax
and Caspase-3 compared with the NGT group. Moreover, the STZ group
exhibited increased apoptosis of cortical neurons. However,
insulin, liraglutide and the drugs combined equally significantly
inhibited the mRNA and protein expression of Bax and Caspase-3 and
had a significant inhibitory effect on apoptosis in cortical
neurons, and Bcl-2 expression was significantly upregulated in
either the liraglutide monotherapy and combined drug groups,
without significant changes after insulin treatment. These results
suggested that insulin and liraglutide potentially inhibited
Caspase-dependent apoptosis in STZ-induced DM mice.
Ki67 is a commonly used marker for assessing cell
proliferation (34). It has been
indicated that Ki67 immunoexpression is significantly reduced in
the DG of STZ-induced DM rats (35,36).
The results of the present study demonstrated that the number of
Ki67-positive cells was significantly decreased in the hippocampal
DG of mice in the STZ group and significantly increased after
liraglutide treatment, which suggested that liraglutide may have
alleviated diabetes-induced neurogenesis defects. A previous study
demonstrated that insulin-mediated protection of the hippocampus
did not involve neurogenesis (37), which is consistent with the results
of the present study. However, a limitation of the present study is
that Ki67 is simply a marker for proliferating cells, hence the use
of 5-bromo-2'-deoxyuridine as a marker for neurogenesis would be
more effective (38).
The Wnt/β-catenin signaling pathway serves an
important role in the regulation of numerous cellular events,
including the prevention of apoptosis as well as the enhancement of
cell proliferation (39). In the
brain, the Wnt/β-catenin signaling pathway has been shown to
alleviate the cognitive decline associated with AD by increasing
neurogenesis in DM rats (40).
Moreover, 10-O-(N N-dimethylaminoethyl)-ginkgolide B
methane-sulfonate alleviates cerebral ischemic injury induced by
middle cerebral artery occlusion/reperfusion surgery in mice via
activation of the Wnt/β-catenin signaling pathway to exert
antiapoptotic and neurogenetic activity (41). It has also previously been reported
that insulin and GLP-1 are direct activators of the Wnt/β-catenin
signaling pathway in multiple tissues and organs and that insulin
promotes the phosphorylation and inhibition of GSK-3β (42-45).
He et al (46) demonstrated
that liraglutide restores the viability, inhibits apoptosis and
protects the neuronal growth of cortical neurons under oxidative
stress possibly via activation of the Wnt/β-catenin signaling
pathway. Insulin contributes to the healing of diabetic corneal
epithelial wounds and recovery from nerve damage via Wnt/β-catenin
signaling (44). In the present
study, to the best of our knowledge, it was investigated for the
first time whether the application of insulin and liraglutide could
inhibit apoptosis in neurons of DM mice via the activation of the
Wnt/β-catenin signaling pathway. The results demonstrated that
either insulin, liraglutide or the combined drugs led to the
significantly decreased apoptosis of brain cells. This was
accompanied by a significant increase in the expression levels of
Wnt3a and S9-pGSK-3β and a significant decrease in GSK-3β and
S33-pβ-catenin protein expression levels in brain tissues.
In conclusion, the results of the present study
suggested that insulin, liraglutide and the combination of the two
drugs exerted similar neuroprotective effects on neuronal loss and
apoptosis in the hippocampus and cortex in an STZ-induced T1DM
mouse model. Moreover, these effects appeared to be associated with
the activation of the Wnt/β-catenin signaling pathway. These
results provide a theoretical basis for the potential of insulin
and liraglutide as a new drug with the capacity against
diabetes-induced cognitive impairments. A limitation of our present
study is the lack of Wnt3a-overexpression or knockdown experiments,
and more experimental data are required to explore the underlying
mechanisms in the future.
Supplementary Material
Kaplan-Meier survival curves for
different mouse groups. STZ, saline treated type 1 diabetes group;
INS, insulin treatment group; LRG, liraglutide treatment group; INS
+ LRG, insulin and liraglutide treatment group; NGT, normal glucose
tolerance group.
Changes in mouse body weight during
treatments.
Changes in mouse blood glucose levels
during treatments.
Acknowledgements
Not applicable.
Funding
Funding: This research was supported by the Chinese Academy of
Medical Sciences Innovation Fund for Medical Sciences (grant no.
CIFMS2021-I2M-1-002).
Availability of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ was responsible for data acquisition and drafting
of the manuscript. JY performed the animal experiments and data
acquisition. FP, LX and WL performed data acquisition and analyzed
and interpreted the data. HZ and YL were responsible for the study
concept and design, critical revision of the manuscript for
important intellectual content and study supervision. All authors
read and approved the manuscript for publication. HZ and YL confirm
the authenticity of all the raw data.
Ethics approval and consent to
participate
The animal experiments were reviewed and approved by
the Institutional Animal Care and Use Committee of the Institute of
Laboratory Animals Science, Chinese Academy of Medical Sciences and
Peking Union Medical College (approval no. XHDW-2018-00; Beijing,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Biessels GJ, Staekenborg S, Brunner E,
Brayne C and Scheltens P: Risk of dementia in diabetes mellitus: A
systematic review. Lancet Neurol. 5:64–74. 2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang C, Lv H, Li Q, Gong K, Yang LL, Wei
Z, Pan Y and Wang M: RNA sequencing of peripheral blood revealed
that the neurotropic TRK receptor signaling pathway shows apparent
correlation in recovery following spinal cord injury at small
cohort. J Mol Neurosci. 68:221–233. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Marini C, Baldassarre M, Russo T, De
Santis F, Sacco S, Ciancarelli I and Carolei A: Burden of
first-ever ischemic stroke in the oldest old: Evidence from a
population-based study. Neurology. 62:77–81. 2004.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ho N, Sommers MS and Lucki I: Effects of
diabetes on hippocampal neurogenesis: Links to cognition and
depression. Neurosci Biobehav Rev. 37:1346–1362. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Salem MA, Budzyńska B, Kowalczyk J, El
Sayed NS and Mansour SM: Tadalafil and bergapten mitigate
streptozotocin-induced sporadic Alzheimer's disease in mice via
modulating neuroinflammation, PI3K/Akt, Wnt/β-catenin, AMPK/mTOR
signaling pathways. Toxicol Appl Pharmacol.
429(115697)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dubey SK, Lakshmi KK, Krishna KV, Agrawal
M, Singhvi G, Saha RN, Saraf S, Saraf S, Shukla R and Alexander A:
Insulin mediated novel therapies for the treatment of Alzheimer's
disease. Life Sci. 249(117540)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Drucker DJ and Nauck MA: The incretin
system: Glucagon-like peptide-1 receptor agonists and dipeptidyl
peptidase-4 inhibitors in type 2 diabetes. Lancet. 368:1696–1705.
2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lovshin JA and Drucker DJ: Incretin-based
therapies for type 2 diabetes mellitus. Nat Rev Endocrinol.
5:262–269. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Darsalia V, Nathanson D, Nyström T, Klein
T, Sjöholm Å and Patrone C: GLP-1R activation for the treatment of
stroke: Updating and future perspectives. Rev Endocr Metab Disord.
15:233–242. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Batista AF, Bodart-Santos V, De Felice FG
and Ferreira ST: Neuroprotective actions of glucagon-like peptide-1
(GLP-1) analogues in Alzheimer's and Parkinson's diseases. CNS
Drugs. 33:209–223. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kong FJ, Wu JH, Sun SY, Ma LL and Zhou JQ:
Liraglutide ameliorates cognitive decline by promoting autophagy
via the AMP-activated protein kinase/mammalian target of rapamycin
pathway in a streptozotocin-induced mouse model of diabetes.
Neuropharmacology. 131:316–325. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yan W, Pang M, Yu Y, Gou X, Si P,
Zhawatibai A, Zhang Y, Zhang M, Guo T, Yi X and Chen L: The
neuroprotection of liraglutide on diabetic cognitive deficits is
associated with improved hippocampal synapses and inhibited
neuronal apoptosis. Life Sci. 231(116566)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lee JW, Lee YK, Yuk DY, Choi DY, Ban SB,
Oh KW and Hong JT: Neuro-inflammation induced by lipopolysaccharide
causes cognitive impairment through enhancement of beta-amyloid
generation. J Neuroinflammation. 5(37)2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jia L, Piña-Crespo J and Li Y: Restoring
Wnt/β-catenin signaling is a promising therapeutic strategy for
Alzheimer's disease. Mol Brain. 12(104)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xu D, Li F, Xue G, Hou K, Fang W and Li Y:
Effect of Wnt signaling pathway on neurogenesis after cerebral
ischemia and its therapeutic potential. Brain Res Bull. 164:1–13.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xu Y, Zhang G, Kang Z, Xu Y, Jiang W and
Zhang S: Cornin increases angiogenesis and improves functional
recovery after stroke via the Ang1/Tie2 axis and the Wnt/β-catenin
pathway. Arch Pharm Res. 39:133–142. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Commission SSaT: Regulations for the
administration of affairs concerning experimental animals. In:
Decree No. 2 of the State Science and Technology Commission China
Legal System Publishing House. State Science and Technology
Commission, China, 2011.
|
|
19
|
National Research Council (US): Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: The National Academies Collection: Reports funded by
National Institutes of Health. In: Guide for the Care and Use of
Laboratory Animals. 8th edition. National Academies Press (US).
National Academy of Sciences, Washington, DC, 2011.
|
|
20
|
Kilkenny C, Browne W, Cuthill IC, Emerson
M and Altman DG: National Centre for the Replacement, Refinement
and Reduction of Amimals in Research. Animal research: Reporting in
vivo experiments-the ARRIVE guidelines. J Cereb Blood Flow Metab.
31:991–993. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yu J, Shi YC, Ping F, Li W, Zhang HB, He
SL, Zhao Y, Xu LL and Li YX: Liraglutide inhibits
osteoclastogenesis and improves bone loss by downregulating Trem2
in female type 1 diabetic mice: Findings from transcriptomics.
Front Endocrinol (Lausanne). 12(763646)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shalimova A, Graff B, Gąsecki D, Wolf J,
Sabisz A, Szurowska E, Jodzio K and Narkiewicz K: Cognitive
dysfunction in type 1 diabetes mellitus. J Clin Endocrinol Metab.
104:2239–2249. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cameron FJ, Northam EA and Ryan CM: The
effect of type 1 diabetes on the developing brain. Lancet Child
Adolesc Health. 3:427–436. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Perkins BA, Lovblom LE, Lanctôt SO, Lamb K
and Cherney DZI: Discoveries from the study of longstanding type 1
diabetes. Diabetologia. 64:1189–1200. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Darwish MA, Abo-Youssef AM, Messiha BAS,
Abo-Saif AA and Abdel-Bakky MS: Resveratrol inhibits macrophage
infiltration of pancreatic islets in streptozotocin-induced type 1
diabetic mice via attenuation of the CXCL16/NF-κΒ p65 signaling
pathway. Life Sci. 272(119250)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Madrakhimov SB, Yang JY, Kim JH, Han JW
and Park TK: mTOR-dependent dysregulation of autophagy contributes
to the retinal ganglion cell loss in streptozotocin-induced
diabetic retinopathy. Cell Commun Signal. 19(29)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Palleria C, Leo A, Andreozzi F, Citraro R,
Iannone M, Spiga R, Sesti G, Constanti A, De Sarro G, Arturi F and
Russo E: Liraglutide prevents cognitive decline in a rat model of
streptozotocin-induced diabetes independently from its peripheral
metabolic effects. Behav Brain Res. 321:157–169. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hölscher C: The incretin hormones
glucagonlike peptide 1 and glucose-dependent insulinotropic
polypeptide are neuroprotective in mouse models of Alzheimer's
disease. Alzheimers Dement. 10 (Suppl 1):S47–S54. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Barman J, Kumar R, Saha G, Tiwari K and
Dubey VK: Apoptosis: Mediator molecules, interplay with other cell
death processes and therapeutic potentials. Curr Pharm Biotechnol.
19:644–663. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Singh R, Letai A and Sarosiek K:
Regulation of apoptosis in health and disease: The balancing act of
BCL-2 family proteins. Nat Rev Mol Cell Biol. 20:175–193.
2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pratchayasakul W, Thongnak LO,
Chattipakorn K, Lungaphin A, Pongchaidecha A, Satjaritanun P,
Jaiwongkam T, Kerdphoo S and Chattipakorn SC: Atorvastatin and
insulin equally mitigate brain pathology in diabetic rats. Toxicol
Appl Pharmacol. 342:79–85. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Malekiyan R, Abdanipour A, Sohrabi D and
Jafari Anarkooli I: Antioxidant and neuroprotective effects of
lycopene and insulin in the hippocampus of streptozotocin-induced
diabetic rats. Biomed Rep. 10:47–54. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Irfannuddin I, Sarahdeaz SFP, Murti K,
Santoso B and Koibuchi N: The effect of ketogenic diets on
neurogenesis and apoptosis in the dentate gyrus of the male rat
hippocampus. J Physiol Sci. 71(3)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
El-Akabawy G and El-Kholy W:
Neuroprotective effect of ginger in the brain of
streptozotocin-induced diabetic rats. Ann Anat. 196:119–128.
2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
ALmohaimeed HM, Mohammedsaleh ZM, Batawi
AH, Balgoon MJ, Ramadan OI, Baz HA, Al Jaouni S and Ayuob NN:
Synergistic anti-inflammatory and neuroprotective effects of
cinnamomum cassia and zingiber officinale alleviate
diabetes-induced hippocampal changes in male albino rats:
Structural and molecular evidence. Front Cell Dev Biol.
9(727049)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Haas CB, Kalinine E, Zimmer ER, Hansel G,
Brochier AW, Oses JP, Portela LV and Muller AP: Brain insulin
administration triggers distinct cognitive and neurotrophic
responses in young and aged rats. Mol Neurobiol. 53:5807–5817.
2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Fares J, Bou Diab Z, Nabha S and Fares Y:
Neurogenesis in the adult hippocampus: History, regulation, and
prospective roles. Int J Neurosci. 129:598–611. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Foulquier S, Daskalopoulos EP, Lluri G,
Hermans KCM, Deb A and Blankesteijn WM: WNT signaling in cardiac
and vascular disease. Pharmacol Rev. 70:68–141. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kim DY, Jung SY, Kim K and Kim CJ:
Treadmill exercise ameliorates Alzheimer disease-associated memory
loss through the Wnt signaling pathway in the
streptozotocin-induced diabetic rats. J Exerc Rehabil. 12:276–283.
2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Xu D, Hou K, Li F, Chen S, Fang W and Li
Y: XQ-1H alleviates cerebral ischemia in mice through inhibition of
apoptosis and promotion of neurogenesis in a Wnt/β-catenin
signaling dependent way. Life Sci. 235(116844)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhao C, Liang J, Yang Y, Yu M and Qu X:
The impact of glucagon-like peptide-1 on bone metabolism and its
possible mechanisms. Front Endocrinol (Lausanne).
8(98)2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Palsgaard J, Emanuelli B, Winnay JN,
Sumara G, Karsenty G and Kahn CR: Cross-talk between insulin and
Wnt signaling in preadipocytes: Role of Wnt co-receptor low density
lipoprotein receptor-related protein-5 (LRP5). J Biol Chem.
287:12016–12026. 2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Yang S, Zhang Y, Zhang Z, Dan J, Zhou Q,
Wang X, Li W, Zhou L, Yang L and Xie L: Insulin promotes corneal
nerve repair and wound healing in type 1 diabetic mice by enhancing
Wnt/β-catenin signaling. Am J Pathol. 190:2237–2250.
2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Doble BW and Woodgett JR: GSK-3: Tricks of
the trade for a multi-tasking kinase. J Cell Sci. 116:1175–1186.
2003.PubMed/NCBI View Article : Google Scholar
|
|
46
|
He W, Tian X, Lv M and Wang H: Liraglutide
protects neurite outgrowth of cortical neurons under oxidative
stress though activating the Wnt pathway. J Stroke Cerebrovasc Dis.
27:2696–2702. 2018.PubMed/NCBI View Article : Google Scholar
|