Introduction
Since December, 2019, the new coronavirus disease
2019 (COVID-19) has become a worldwide hazard. The multi-organ
manifestations of COVID-19 have been well-established. The most
common manifestation is COVID-19-associated pneumonia. Although the
majority of cases of COVID-19 are mild, in more severe cases, acute
lung damage may be followed by interstitial lung disease, pulmonary
fibrosis and chronic lung function impairment (1,2).
Organizing pneumonia (OP) is a type of diffuse
interstitial lung disease, which is histopathologically
characterized by inflammatory debris in the distal airway
containing myofibroblasts, fibroblasts and inflammatory cells
embedded in a matrix of connective tissue, and interstitial
inflammation of the surrounding lung parenchyma (3). OP may develop in the context of
various clinical conditions, such as reactions to medication,
infections, connective tissue disorders and solid organ or
hematologic malignancies (4). The
term cryptogenic OP is used for the primary disease, in which no
cause is identified (5). Typical
radiological features of OP are peribronchovascular and peripheral
ground glass opacities (GGOs) or consolidations. These lesions may
be migratory and accompanied by nodules, masses and interstitial
opacities. Another radiological finding consistent with OP is the
reversed halo sign, a central GGO surrounded by a consolidation
halo. According to previous research, OP is considered to be in the
spectrum of manifestations of acute lung injury (6).
Research on viral-induced OP during severe acute
respiratory syndrome coronavirus (SARS), Middle East respiratory
syndrome (MERS) and H1N1 infection is extensive (7,8). Αn
increasing number of studies have revealed a link between
SARS-coronavirus 2 (CoV-2) infection and OP. COVID-19-associated OP
had been previously considered to be a result of COVID-19 infection
or a histological type of COVID-19-associated pneumonia (9). Of note, the most common findings on
chest computed tomography (CT) scans in patients with COVID-19 are
peripheral GGOs, consolidation or both, mostly in bilateral and
multifocal distributions that highly resemble a CT pattern of OP
(10). Moreover, postmortem lung
pathological examinations have demonstrated that the majority of
patients with COVID-19-associated pneumonia have secondary OP
(11).
Research concerning COVID-19-associated OP is still
limited. The present study reports two cases of patients with
COVID-19 with radiological evidence of OP following the initial
infection, who responded well to treatment with
corticosteroids.
Case report
Case 1
A 51-year-old male patient with no notable previous
medical history was admitted to the Emergency Department (ED) of
Laiko General Hospital complaining of fever, cough and dyspnea over
the last 7 days. The patient had been diagnosed with COVID-19 by
reverse transcription polymerase chain reaction (RT-PCR) of a
nasopharyngeal swab sample for SARS-CoV-2 4 days prior to his
admission. The patient was unvaccinated against SARS-CoV-2.
Upon admission, his body temperature was 37.4˚C, his
blood pressure was 120/70 mmHg, his heart rate was 94 beats per
minute, his respiratory rate was 32 breaths per minute, and his
oxygen saturation (SpO2) was 92% in room air. A chest
examination revealed crackles on auscultation in all lung fields.
Arterial blood gases analysis revealed a partial pressure of oxygen
(pO2) of 56 mmHg, partial pressure of carbon dioxide
(pCO2) 31 mmHg, pH 7.51, HCO3 24.7 mmol/l on
room air. A chest X-ray revealed diffuse infiltrates in all lung
fields (Fig. 1). The laboratory
findings included an increased white blood cell (WBC) count (11.22
k/µl; reference range, 4.5-11 k/µl) with neutrophilia (87.4%;
reference range, 40-74%) and lymphopenia (6.63%; reference range,
19-48%), elevated C-reactive protein (CRP) levels (151.77 mg/l;
reference range 0-5 mg/l), elevated lactate dehydrogenase (LDH)
levels (331 U/l; reference range, 135-225 U/l) and elevated
ferritin levels (1,200 ng/ml; reference range, 30-400 ng/ml).
Based on these findings, treatment with intravenous
remdesivir (200 mg on the first day, followed by 100 mg daily for
the following 4 days) and dexamethasone (6 mg/day) for 10 days was
commenced for SARS-CoV-2 infection. The patient also received
oxygen therapy with a Venturi mask, delivering an oxygen
concentration of 60%. The patient exhibited further respiratory
deterioration and required oxygen therapy with a high-flow nasal
cannula [oxygen flow rate, 60 l/min; fraction of inspired oxygen
(FiO2), 90%]. He also received a single dose of
intravenous tocilizumab (400 mg).
The patient's fever, cough, dyspnea and inflammatory
indices improved after the treatment was commenced, and on day 8 of
hospitalization (day 15 of illness), his SpO2 was 97%
while breathing through a high-flow nasal cannula with lower
settings (oxygen flow rate, 40 l/min; FiO2, 70%).
However, on day 14 of hospitalization (day 21 of illness), he
developed rapidly progressive respiratory failure, and his
SpO2 decreased to 90%; He thus required oxygen therapy
with a high-flow nasal cannula at maximum settings (oxygen flow
rate, 60 l/min; FiO2, 100%).
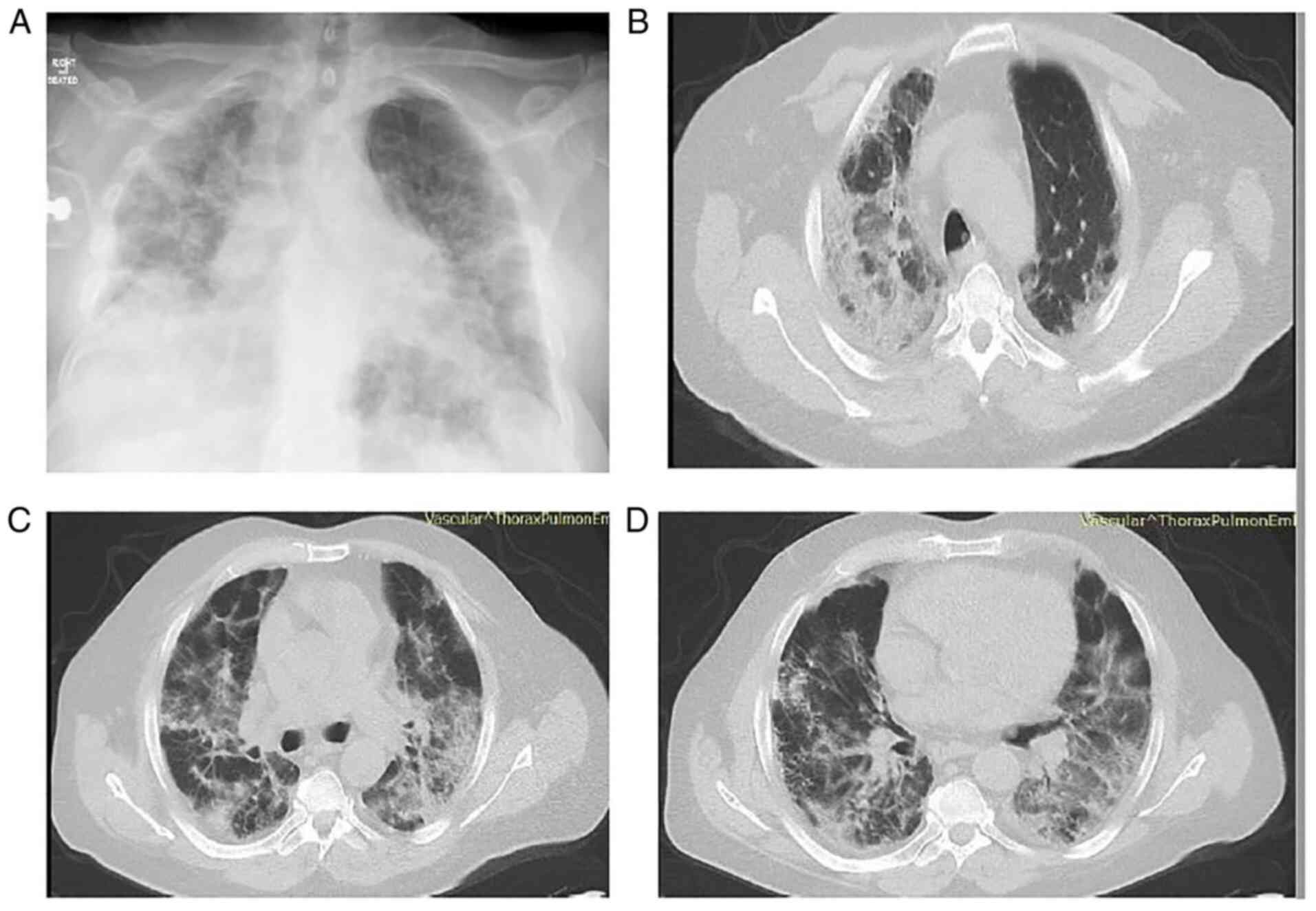
The patient underwent a new chest X-ray which
revealed worsening infiltrates in all lung fields (Fig. 2A). He also underwent a chest CT
scan and chest computed pulmonary angiogram (CTPA), which revealed
bilateral peripheral GGO infiltrates and consolidation in both
lower lung lobes, with areas of reversed halo sign (Fig. 2B-D). There were no findings
suggesting pulmonary embolism. Simultaneously with this
radiological and respiratory deterioration, the patient presented
with recurrent fever and significantly elevated CRP levels (279.31
mg/l; reference range, 0-5 mg/l). He received antimicrobial therapy
with intravenous piperacillin-tazobactam at 4.5 g four times daily
and intravenous linezolid 600 mg twice daily. Blood and sputum
culture did not reveal any infectious microorganisms. In addition,
serum procalcitonin levels were within the normal range. The
patient did not exhibit any improvement with antibiotics. Based on
clinical and radiological data, COVID-19-associated OP was
suspected, and systemic corticosteroid therapy (methylprednisolone
1 mg/kg/day) was initiated. On day 23 of hospitalization (day 30 of
illness), 3 days following the commencement of corticosteroid
therapy, his oxygenation level markedly improved. A chest X-ray and
CT imaging performed on day 14 following the commencement of
corticosteroid therapy (day 34 of illness) revealed an improvement
of lung infiltrates (Fig. 3). The
levels of CRP also returned to normal. The methylprednisolone
administration was decreased to 40 mg, and the patient was
discharged on day 39 following admission (day 46 of illness).
Following discharge, the methylprednisolone administration was
decreased to 32 mg for 10 days, 16 mg for 10 days and 8 mg for 10
days, and discontinued thereafter. The patient did not present with
a relapse on a follow-up at 3 months after discharge.
Case 2
A 71-year-old male patient presented to the ED of
Laiko General Hospital complaining of fever, cough and dyspnea over
the last 24 h. The patient had been diagnosed with COVID-19 by
RT-PCR testing of a nasopharyngeal swab sample for SARS-CoV-2 14
days prior to his admission. The patient reported low-grade fever
then for only 2 days without any other symptoms and had not
received any specific therapy for COVID-19.
The patient had a medical history of asthma,
arterial hypertension, dyslipidemia, appendectomy and inguinal
hernia surgery. His current medications included inhaled
formoterol/budesonide (160/4.5 µg twice daily),
olmesartan/hydrochlothiazide (40/12 mg once daily) and atorvastatin
(20 mg once daily). The patient was unvaccinated against
SARS-CoV-2.
Upon admission, his body temperature was 38.5˚C, his
blood pressure was 140/80 mmHg, his heart rate was 90 beats per
minute, his respiratory rate was 28 breaths per minute, and his
SpO2 was 87% in room air. A chest examination revealed
crackles on auscultation in all lung fields. Arterial blood gas
analysis revealed a pO2 of 52 mmHg, a pCO2 of
29 mmHg, pH 7.50 and HCO3 22.7 mmol/l in room air. A
chest X-ray revealed diffuse infiltrates in all lung fields with
consolidations in the right upper and middle lung field and in the
left middle lung field (Fig. 4).
The laboratory findings included an increased WBC count (13.56
k/µl; reference range, 4.5-11 k/µl) with neutrophilia (84.8%;
reference range, 40-74%) and lymphopenia (8.7%; reference range,
19-48%), elevated CRP levels (93 mg/l; reference range, 0-5 mg/l),
elevated LDH levels (500 U/l; reference range, 135-225 U/l) and
elevated ferritin levels (816 ng/ml; reference range, 30-400
ng/ml).
Based on these findings, treatment with intravenous
remdesivir (200 mg on the first day, followed by 100 mg daily for
the following 4 days) and dexamethasone (6 mg/day) was commenced
for SARS-CoV-2 infection for 10 days. The patient also received
oxygen therapy with a Venturi mask, delivering an oxygen
concentration of 60%. The patient exhibited further respiratory
deterioration with a further elevation of CRP levels (174 mg/l;
reference range, 0-5 mg/l) on the second day of hospitalization
(day 15 of illness) and required oxygen therapy with a high-flow
nasal cannula (oxygen flow rate, 60 l/min; FiO2, 90%).
He also received antimicrobial treatment with intravenous
ceftriaxone at 2 g once daily and linezolid 600 mg twice daily for
7 days.
The patient's clinical symptoms and inflammatory
indices (CRP, 17 mg/l; reference range, 0-5 mg/l) improved after
commencing the treatment, and on day 10 of hospitalization (day 23
of illness), his SpO2 was 97%, while breathing through a
Venturi mask delivering an oxygen concentration of 60%. The patient
did not exhibit any further improvement for the following 2 days.
On day 13 of hospitalization (day 26 of illness) the patient
developed recurrent low-grade fever and a concurrent new increase
in CRP levels (49 mg/l; reference range, 0-5 mg/l).
The patient underwent a new chest X-ray, which
revealed persistent infiltrates in all lung fields with
consolidations in the right upper and middle lung field, and in the
left middle lung field (Fig. 5A).
He also underwent a chest CT scan and CTPA, which revealed
bilateral consolidations with areas of reversed halo sign in all
lung fields and peripheral GGO infiltrates in both lower lung lobes
(Fig. 5B-D). There were no
findings suggesting pulmonary embolism. No infectious
microorganisms were isolated from blood and sputum cultures. Based
on the clinical and radiological data, COVID-19-associated OP was
suspected, and systemic corticosteroid therapy (methylprednisolone
at 1 mg/kg/day) was initiated on day 15 (day 28 of illness). On day
18, at 3 days following the commencement of corticosteroid therapy
(day 31 of illness), his oxygenation level improved considerably. A
chest X-ray and CT imaging performed on day 13 following the
commencement of corticosteroid therapy (day 28 of hospitalization,
day 41 of illness) revealed a notable improvement in previously
noted lung infiltrates (Fig. 6).
The levels of CRP also returned to normal. Methylprednisolone
administration was decreased to 40 mg, and the patient was
discharged on day 33 after admission (day 46 of illness). Following
discharge, methylprednisolone administration was decreased to 32 mg
for 7 days, 16 mg for 7 days and 8 mg for 7 days, and discontinued
thereafter. The patient did not present with a relapse and he had
improvement in chest X-ray at a follow-up 2 months after discharge
(Fig. 7).
Discussion
The present study describes the cases of two
individuals who had rapidly worsening respiratory symptoms after
the initial phase of COVID-19 infection. In numerous aspects, the
patients' symptoms are compatible with those of OP caused by
SARS-CoV-2. Following early treatment for their COVID-19 infection,
both patients had rapidly deteriorating respiratory symptoms that
were not responding to optimal therapy.
Of note, the patient in case 1 who was younger than
the patient in case 2 developed rapidly progressive respiratory
failure on the day 14, while the patient in case 2 recovered more
easily under the same treatment regimen. Genetic factors may be a
possible explanation for this event. Some patients have heritable,
single-gene mutations that influence their immune systems. Such
distinctive mutations have been detected in numerous cases of young
individuals who are healthy, yet suddenly develop a
life-threatening infection (12).
Furthermore, no substantial superimposed infection
was proven in the patients described herein. Moreover, their
condition markedly improved following the initiation of high-dose
corticosteroid treatment, suggesting that OP was the cause of their
decompensated respiratory symptoms.
Clinical and investigation data regarding
COVID-19-associated OP are limited. The diagnosis of OP was made in
some cases, by using only radiological data (13-19),
and in other cases, by performing transbronchial biopsy and a
histological examination of the obtained tissue (14,20-25).
The cases of COVID-19-associated organizing pneumonia reported in
the literature are summarized in Table
I.
 | Table ICases of COVID-19-associated
organizing pneumonia identified in the literature. |
Table I
Cases of COVID-19-associated
organizing pneumonia identified in the literature.
| Case no. | Author/(Refs.) | Age
(years)/sex | Diagnosis | Management | Outcome |
|---|
| 1 | Alsulami et
al (13) | 71/Μ | Radiological |
Corticosteroids | Recovery |
| 2 | Alsulami et
al (13) | 54/M | Radiological |
Corticosteroids | Recovery |
| 3 | Alsulami et
al (13) | 57/M | Radiological |
Corticosteroids | Recovery |
| 4 | Alsulami et
al (13) | 49/M | Radiological |
Corticosteroids | Recovery |
| 5 | Alsulami et
al (13) | 56/F | Radiological |
Corticosteroids | Recovery |
| 6 | Alsulami et
al (13) | 83/F | Radiological |
Corticosteroids | Recovery |
| 7 | Ng et al
(14) | 58/F | Radiological |
Corticosteroids | Recovery |
| 8 | de Oliveira Filho
et al (15) | 52/Μ | Radiological |
Corticosteroids | Recovery |
| 9 | de Oliveira Filho
et al (15) | 60/F | Radiological |
Corticosteroids | Recovery |
| 10 | de Oliveira Filho
et al (15) | 63/F | Radiological |
Corticosteroids | Recovery |
| 11 | Horii et al
(16) | 70/F | Radiological |
Corticosteroids | Recovery |
| 12 | Okamori et
al (17) | 60/M | Radiological |
Corticosteroids | Recovery |
| 13 | Okamori et
al (17) | 61/F | Radiological |
Corticosteroids | Recovery |
| 14 | Kim et al
(18) | 71/M | Radiological |
Corticosteroids | Recovery |
| 15 | Simões et al
(19) | 71/M | Radiological |
Corticosteroids | Recovery |
| 16 | Simões et al
(19) | 83/M | Radiological |
Corticosteroids | Recovery |
| 17 | Ng et al
(14) | 81/M | TBLB,
histopathological examination |
Corticosteroids | Recovery |
| 18 | Seo et al
(20) | 50/M | TBLB,
histopathological examination | Spontaneous
remission | Recovery |
| 19 | Funk et al
(21) | 49/M | TBLB,
histopathological examination | Spontaneous
remission | Recovery |
| 20 | Golbets et
al (22) | 36/M | TBLB,
histopathological examination |
Corticosteroids | Recovery |
| 21 | Kanaoka et
al (23) | 56/M | TBLB,
histopathological examination |
Corticosteroids | Recovery |
| 22 | Kanaoka et
al (23) | 84/F | TBLB,
histopathological examination |
Corticosteroids | Recovery |
| 23 | Cortés Colorado
et al (24) | 62/M | TBLB,
histopathological examination |
Corticosteroids | Recovery |
| 24 | Vadász et al
(25) | 57/M |
TBLB.histopathological examination |
Corticosteroids | Recovery |
| 25 | Vadász et al
(25) | 70/M |
TBLB,histopathological examination |
Corticosteroids | Recovery |
| 26 | Vadász et al
(25) | 76/M | TBLB,
histopathological examination |
Corticosteroids | Recovery |
A pathological investigation is essential for
definitive diagnosis of COVID-19-associated OP, as radiological
manifestations of bacterial co-infection and OP may be similar.
Therefore, it does not appear plausible to confirm the presence of
OP in patients with SARS-CoV-2-related pneumonia based solely on
radiological abnormalities (25).
However, in both cases present herein, the conduction of lung
biopsy was considered extremely dangerous and was thus not
performed, due to high hypoxic conditions.
Notably, not every case of OP is confirmed by
pathological data, as is well-recognized. The primary goal of a
biopsy in individuals with suspected OP is to rule out other
possible causes of comparable symptoms. As the histopathologic
characteristics of OP are similar to those of other interstitial
lung diseases, relying on transbronchial biopsies may increase the
risk of OP misdiagnosis or delay (13). Furthermore, the radiographic
characteristics of OP are distinct, and a chest CT scan has a good
diagnostic accuracy (79%) for OP (26).
A chest CT scan may be beneficial for ruling out
complications, such as pulmonary embolism, secondary pneumonia, or
OP in patients with SARS-CoV-2 infection who have clinical
deterioration despite optimal treatment and ventilatory support, or
who have worsening symptoms following an initial recovery. Both
patients in the present study waited an average of 21 days (20-22
days) from symptom initiation to undergo a chest CT scan, and their
average duration of stay after commencing treatment with steroids
was 10 days (5-15 days). In the case that additional imaging
analyses are required for such individuals, this time frame may be
an attractive approach.
From the beginning of the pandemic, some researchers
have expressed their concerns about potential widespread failure to
identify and treat COVID-19-associated OP (9). Radiological evidence of OP has been
documented during the later course of SARS-CoV-2 infection. In a
previous study, the observed prevalence of COVID-19-associated OP
was 12.5% (27). In another
observational study, the authors studied persistent lung changes
following COVID-19 infection and described ongoing symptoms in 39%
of participants. In that study, OP was observed in 4.8% of these
patients, with significant radiological and clinical improvement
following steroid administration (28). In addition, in a study on
respiratory intermediate care unit patients, 58% of patients that
underwent a chest CT scan had a radiological pattern consistent
with OP and a significantly decreased need for intubation and
in-hospital mortality compared to those with a GGO pattern
(29).
Of note, both patients described herein were
unvaccinated against SARS-CoV-2. A recent study demonstrated that
vaccination with at least two doses of COVID-19 vaccine was
associated with a significant decrease in reporting the most common
post-acute COVID-19 symptoms, such as fatigue, headache, weakness
and persistent muscle pain (30).
However, whether vaccination against SARS-CoV-2 exerts a protective
effect against the development of OP following COVID-19 remains to
be determined.
The role of corticosteroids in COVID-19-associated
pneumonia has been well-established. Dexamethasone therapy is
effective for COVID-19-associated pneumonia (31). Methylprednisolone has been reported
to increase the survival of hospitalized patients who experience
severe COVID-19 pneumonia (31). A
previous meta-analysis revealed a positive effect of
corticosteroids on short-term mortality and a decrease in the need
for mechanical ventilation in patients with COVID-19(32).
The cornerstone of OP treatment is corticosteroids.
Prednisone at a dosage of 0.75-1.5 mg/kg/day for 4 weeks, tapered
over a period of 3-6 months, is the current recommendation
(33). In COVID-19-associated OP,
case reports and case series, including ours, have documented
favorable outcomes with corticosteroid treatment, sometimes with
high doses and prolonged therapy, often weeks to months (13-19,
22-25). A single-center prospective observational study
discovered that almost a quarter of post-COVID-19 pneumonia
patients had recurrent dyspnea with radiological signs of OP, and
that restarting corticosteroids resulted in clinical and
radiological improvement in these patients (27). Of interest, the spontaneous
remission of OP without corticosteroid administration has been
described in two cases of histologically diagnosed
COVID-19-associated OP (20,21).
A previous study demonstrated that approximately one
third of OP cases exhibited a complete resolution of lesions at 1-2
months following disease onset, while those with greater areas of
consolidation and more extensive lung involvement were more likely
to develop fibrotic-like changes (34). There is currently no evidence to
indicate that corticosteroid therapy can prevent relapses of OP or
minimize the development of residual pulmonary fibrosis in
COVID-19-associated OP (35).
There has been no research to date, at least to the best of our
knowledge, on the impact of corticosteroids on the resolution of
COVID-19-related OP and long-term pulmonary outcomes. Large,
multicenter randomized control trials are required to determine the
optimal time to start, dosage, duration and benefit of
corticosteroid therapy in COVID-19-associated OP. Clinicians should
take the possible risks and advantages of corticosteroids into
account and utilize CT as an evaluation tool for the distinct
phases of CT patterns in patients with COVID-19.
In conclusion, the importance of detecting OP arises
from the fact that patients who receive treatment with
corticosteroids have a better prognosis and outcome. Increasing
awareness of this diagnosis may lead to more effective treatment
approaches for COVID-19 disease, further reduction of requirements
for ventilatory support, and improved survival. Patients with
COVID-19 that exhibit a minimal or no improvement despite optimal
therapy should be evaluated further with lung imaging, as they may
benefit from early diagnosis and targeted treatment. The optimal
time for corticosteroid administration, the dose and the duration
of therapy need to be addressed.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SM, SS and IE conceptualized the study. PP, PK, NM,
PS, NT and SC obtained medical images, and prepared the tables and
figures. VEG, CSi, CSt and AT advised on patient treatment and
wrote and prepared the draft of the manuscript. DAS and VEG
analyzed patient data and provided critical revisions. CSi, CSt,
AT, NM, PK, PP, SC, PS and NT made substantial contributions to
conception and design, and analysis and interpretation of data. VEG
and SM confirm the authenticity of all the data. All authors
contributed to manuscript revision and have read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed was obtained from the patients for
the publication of the data. A copy of the written consent is
available for review by the Editor-in-Chief of this journal on
request.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
Wang F, Kream RM and Stefano GB: Long-term
respiratory and neurological sequelae of COVID-19. Med Sci Monit.
26(e928996)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Vasarmidi E, Tsitoura E, Spandidos DA,
Tzanakis N and Antoniou KM: Pulmonary fibrosis in the aftermath of
the COVID-19 era (Review). Exp Ther Med. 20:2557–2560.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Roberton BJ and Hansell DM: Organizing
pneumonia: A kaleidoscope of concepts and morphologies. Eur Radiol.
21:2244–2254. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Oikonomou A and Hansell DM: Organizing
pneumonia: The many morphological faces. Eur Radiol. 12:1486–1496.
2002.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cottin V and Cordier JF: Cryptogenic
organizing pneumonia. Semin Respir Crit Care Med. 33:462–475.
2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mandal RV, Mark EJ and Kradin RL:
Organizing pneumonia and pulmonary lymphatic architecture in
diffuse alveolar damage. Hum Pathol. 39:1234–1238. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cornejo R, Llanos O, Fernández C, Carlos
Díaz J, Cardemil G, Salguero J, Luengo C, Tobar E, Romero C and
Gálvez LR: Organizing pneumonia in patients with severe respiratory
failure due to novel A (H1N1) influenza. BMJ Case Rep.
2010(bcr0220102708)2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tse GM, To KF, Chan PK, Lo AW, Ng KC, Wu
A, Lee N, Wong HC, Mak SM, Chan KF, et al: Pulmonary pathological
features in coronavirus associated severe acute respiratory
syndrome (SARS). J Clin Pathol. 57:260–265. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kory P and Kanne JP: SARS-CoV-2 organising
pneumonia: ‘Has there been a widespread failure to identify and
treat this prevalent condition in COVID-19?’. BMJ Open Respir Res.
7(e000724)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kanne JP, Little BP, Chung JH, Elicker BM
and Ketai LH: Essentials for radiologists on COVID-19: An
update-radiology scientific expert panel. Radiology. 296:E113–E114.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Copin MC, Parmentier E, Duburcq T, Poissy
J and Mathieu D: Lille COVID-19 ICU and Anatomopathology Group.
Time to consider histologic pattern of lung injury to treat
critically ill patients with COVID-19 infection. Intensive Care
Med. 46:1124–1126. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Andreakos E, Abel L, Vinh DC, Kaja E,
Drolet BA, Zhang Q, O'Farrelly C, Novelli G, Rodríguez-Gallego C,
Haerynck F, et al: A global effort to dissect the human genetic
basis of resistance to SARS-CoV-2 infection. Nat Immunol.
23:159–164. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Alsulami F, Dhaliwal I, Mrkobrada M and
Nicholson M: Post Covid-19 organizing pneumonia: Case series for 6
patients with post-COVID interstitial lung disease. J Lung Pulm
Respir Res. 8:108–111. 2021.
|
|
14
|
Ng BH, Ban AY, Nik Abeed NN and Faisal M:
Organising pneumonia manifesting as a late-phase complication of
COVID-19. BMJ Case Rep. 14(e246119)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
de Oliveira Filho CM, Vieceli T, de Fraga
Bassotto C, da Rosa Barbato JP, Garcia TS and Scheffel RS:
Organizing pneumonia: A late phase complication of COVID-19
responding dramatically to corticosteroids. Braz J Infect Dis.
25(101541)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Horii H, Kamada K, Nakakubo S, Yamashita
Y, Nakamura J, Nasuhara Y and Konno S: Rapidly progressive
organizing pneumonia associated with COVID-19. Respir Med Case Rep.
31(101295)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Okamori S, Lee H, Kondo Y, Akiyama Y,
Kabata H, Kaneko Y, Ishii M, Hasegawa N and Fukunaga K: Coronavirus
disease 2019-associated rapidly progressive organizing pneumonia
with fibrotic feature: Two case reports. Medicine (Baltimore).
99(e21804)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kim T, Son E, Jeon D, Lee SJ, Lim S and
Cho WH: Effectiveness of steroid treatment for SARS-CoV-2 pneumonia
with cryptogenic organizing pneumonia-like reaction: A case report.
Disaster Med Public Health Prep. 26:1–4. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Simões JP, Alves Ferreira AR, Almeida PM,
Trigueiros F, Braz A, Inácio JR, Medeiros FC, Braz S and Pais de
Lacerda A: Organizing pneumonia and COVID-19: A report of two
cases. Respir Med Case Rep. 32(101359)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Seo H, Jung J, Kim MJ, Jang SJ and Kim SH:
Radiologically suspected organizing pneumonia in a patient
recovering from COVID-19: A case report. Infect Chemother.
54:208–212. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Funk GC, Nell C, Pokieser W, Thaler B,
Rainer G and Valipour A: Organizing pneumonia following Covid19
pneumonia. Wien Klin Wochenschr. 133:979–982. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Golbets E, Kaplan A, Shafat T, Yagel Y,
Jotkowitz A, Awesat J and Barski L: Secondary organizing pneumonia
after recovery of mild COVID-19 infection. J Med Virol. 94:417–423.
2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kanaoka K, Minami S, Ihara S, Tanaka T,
Yasuoka H and Komuta K: Secondary organizing pneumonia after
coronavirus disease 2019: Two cases. Respir Med Case Rep.
32(101356)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cortés Colorado JM, Cardona Ardila LF,
Aguirre Vásquez N, Gómez Calderón KC, Lozano Álvarez SL and
Carrillo Bayona JA: Organizing pneumonia associated with SARS-CoV-2
infection. Radiol Case Rep. 16:2634–2639. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Vadász I, Husain-Syed F, Dorfmüller P,
Roller FC, Tello K, Hecker M, Morty RE, Gattenlöhner S, Walmrath
HD, Grimminger F, et al: Severe organising pneumonia following
COVID-19. Thorax. 76:201–204. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Johkoh T, Müller NL, Cartier Y, Kavanagh
PV, Hartman TE, Akira M, Ichikado K, Ando M and Nakamura H:
Idiopathic interstitial pneumonias: diagnostic accuracy of
thin-section CT in 129 patients. Radiology. 211:555–560.
1999.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shi H, Han X, Jiang N, Cao Y, Alwalid O,
Gu J, Fan Y and Zheng C: Radiological findings from 81 patients
with COVID-19 pneumonia in Wuhan, China: A descriptive study.
Lancet Infect Dis. 20:425–434. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Myall KJ, Mukherjee B, Castanheira AM, Lam
JL, Benedetti G, Mak SM, Preston R, Thillai M, Dewar A, Molyneaux
PL and West AG: Persistent post-COVID-19 interstitial lung disease.
An observational study of corticosteroid treatment. Ann Am Thorac
Soc. 18:799–806. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rocha AS, Meireles M, Vilaça H, Guimarães
TC, Martins MD, Santos LR, Castro A and Mesquita M: Outcomes of
Covid-19 organizing pneumonia in critically ill patients. J Infect.
83:496–522. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kuodi P, Gorelik Y, Zayyad H, Wertheim O,
Beiruti Wiegler K, Abu Jabal K, Dror AA, Nazzal S, Glikman D and
Edelstein M: Association between vaccination status and reported
incidence of post-acute COVID-19 symptoms in Israel: A
cross-sectional study of patients tested between March 2020 and
November 2021. medRxiv: Jan 17, 2022 (Epub ahead of print).
|
|
31
|
RECOVERY Collaborative Group. Horby P, Lim
WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N,
Brightling C, Ustianowski A, et al: Dexamethasone in hospitalized
patients with Covid-19. N Engl J Med. 384:693–704. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Edalatifard M, Akhtari M, Salehi M, Naderi
Z, Jamshidi A, Mostafaei S, Najafizadeh SR, Farhadi E, Jalili N,
Esfahani M, et al: Intravenous methylprednisolone pulse as a
treatment for hospitalised severe COVID-19 patients: Results from a
randomised controlled clinical trial. Eur Respir J.
56(2002808)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
van Paassen J, Vos JS, Hoekstra EM,
Neumann KMI, Boot PC and Arbous SM: Corticosteroid use in COVID-19
patients: A systematic review and meta-analysis on clinical
outcomes. Crit Care. 24(696)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cordier JF: Cryptogenic organising
pneumonia. Eur Respir J. 28:422–446. 2006.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang Y, Jin C, Wu CC, Zhao H, Liang T, Liu
Z, Jian Z, Li R, Wang Z, Li F, et al: Organizing pneumonia of
COVID-19: Time-dependent evolution and outcome in CT findings. PLoS
One. 15(e0240347)2020.PubMed/NCBI View Article : Google Scholar
|