Introduction
Multidisciplinary team meetings (MDTMs) are defined
as meetings of a group of medical experts in different fields who
gather at a specific time to discuss and determine the most
appropriate treatment based on objective evidence (1). At present, MDTMs are widely used in
both Europe and the United States of America, and play an
increasingly important role in the treatment of cancer and other
diseases (1). The present case
report highlights the importance of multidisciplinary collaboration
in the treatment of complex hepatic solitary fibrous tumors
(HSFTs).
Solitary fibrous tumors (SFTs) are also known as
hemangiopericytomas (2), which
originate from the mesenchymal tissue and feature pericytic,
fibroblastic and myofibroblastic differentiation (3). SFTs were initially described by
Klemperer and Rabin in 1931(4).
The incidence rate of SFTs remains at 1 case/million
individuals/year (5). Notably, the
majority of SFTs occur in the thoracic cavity; however, previous
studies have demonstrated the occurrence of SFTs throughout
extrathoracic sites, such as the retroperitoneal space (6), meninges (7), orbit (8), breast (9), thyroid gland (10), pericardium (11), parotid gland (12), spine (13), pelvic cavity (14), omentum (15), perineum (16), bladder (17), prostate (18), external auditory canal (19), pancreas (20) and, less often, in the liver
(21).
Clinical manifestations of SFTs depend on the size
and location of the tumor (22).
HSFTs are usually asymptomatic; however, they may lead to
corresponding non-specific clinical symptoms, such as abdominal
pain, bloating, indigestion, weight loss, nausea and vomiting, and
these symptoms often occur as the tumor size increases (22). Notably, only a small number of
patients develop paraneoplastic syndromes, such as hypertrophic
osteoarthropathy and hypoglycemia (5). Hypertrophic osteoarthropathy is
attributed to the overexpression of vascular endothelial growth
factor (5), and hypoglycemia is
caused by the overexpression of insulin-like growth factor
2(23). A previous study
demonstrated that the vast majority of SFTs are benign, and seldom
recur or metastasize (24).
Notably, TP53, PDGFRB and TERT promoter regions may be involved in
the malignant transformation (25,26).
Imaging is often non-specific, meaning that using
radiography to distinguish SFTs from other tumors, such as
hepatocellular carcinoma, leiomyoma, sarcoma, sclerosed hemangioma
and inflammatory pseudotumors, may be challenging (22). The current diagnosis of SFT is
based on the histopathological and immunohistochemical features
(27). Histopathological
characteristics of an SFT include spindle cells and collagen fibers
(27). Immunohistochemical
analysis of an SFT demonstrates the expression of vimentin, CD34,
STAT6 and CD99, and in some cases, Bcl-2 and β-catenin (22-24,27-32).
In addition, previous studies have demonstrated that STAT6 nuclear
protein and the NAB2-STAT6 fusion gene are regarded as more precise
tools for SFT diagnosis (5).
At present, the preferred treatment option for SFTs
is the complete surgical removal of the tumor (27). However, metastasis and recurrence
may still occur, despite the ongoing development of this
therapeutic approach (27). To
date, the clinical benefits of adjuvant chemotherapy and radiation
for the treatment of SFTs remain unclear (33). The present study reports a case in
which a multidisciplinary collaboration approach was used for the
treatment of an HSFT appearing as a dumbbell-shaped growth through
the diaphragm into the right thoracic cavity. The corresponding
literature was also reviewed.
Case report
A 59-year-old female patient visited The Second
Affiliated Hospital of Zunyi Medical University (Zunyi, China) on
June 3, 2020, for abdominal distension that had persisted for 1
month. The patient presented with no prior history of viral
hepatitis, chronic alcohol consumption or other chronic liver
diseases. A physical examination demonstrated abdominal distension
and a solid mass (volume, 5x6 cm) that was palpable in the right
upper quadrant. Laboratory examination demonstrated that
biochemical indices, including routine blood tests, coagulation
tests, and liver function and tumor marker analyses (including
carcinoembryonic antigen, cancer antigen 19-9, α-fetoprotein,
squamous cell carcinoma antigen, progastrin-releasing peptide,
neuron-specific enolase and cytokeratin fragment 19), were within
healthy ranges.
Magnetic resonance imaging (MRI; Fig. 1) and computed tomography (CT;
Fig. 2) of the chest and upper
abdomen demonstrated that the tumor was dumbbell-shaped with clear
boundaries; notably, the tumor was mainly located in the right lobe
of the liver and the remaining section was present in the thoracic
cavity. The blood supply to the tumor originated from the hepatic
artery. There was a partial defect in the diaphragm and the tumor
passed into the thoracic cavity. Additionally, atelectasis in the
right lower lobe of the lung was present. It was concluded that the
tumor originated from the liver, and was closely associated with
the diaphragm and thoracic cavity.
For a definitive diagnosis, a fine-needle biopsy of
the tumor was performed. Histopathology (Fig. 3) demonstrated that the tumor cells
were spindle-shaped, with scant cytoplasm, and homogeneous staining
of the oval nuclei. Moreover, the tumor cells were surrounded by
abundant collagen fibers, and neither cellular atypia nor mitotic
figures were present. Immunohistochemical analysis (Fig. 3) demonstrated that the tumor cells
were positive for vimentin, CD34 and STAT6, and negative for
desmin, cytokeratin (CK), smooth muscle actin (SMA), S100, myoblast
determination protein 1 (MyoD1), anaplastic lymphoma kinase (ALK),
epithelial membrane antigen (EMA), CD31, CD68, hepatocyte paraffin
1 (HepPar1) and CD117. The results of the fine-needle biopsy
supported the diagnosis of an HSFT.
Due to the large tumor size and complex anatomy, an
MDTM was subsequently performed to discuss the management of the
tumor. The meeting members included seven chief physicians from the
Departments of Oncology, Interventional Radiology, Thoracic
Surgery, Hepatobiliary Surgery, Pathology, Imaging and
Anesthesiology (The Second Affiliated Hospital of Zunyi Medical
University). In the MDTM, meeting members discussed the requirement
for arterial embolization due to the abundant blood supply and
large size of the tumor. This can result in the tumor becoming
ischemic and necrotic, or the tumor may shrink (34). However, the therapeutic effects of
hepatic artery embolization alone remain limited, and tumor cells
may produce a variety of vascular growth factors under hypoxia to
stimulate angiogenesis (35).
Therefore, we hypothesized that hepatic artery embolization
combined with radical surgery may be an optimal treatment
option.
Moreover, the possibility of metastasis to the
diaphragm and the right lower lobe of the lung was discussed.
Notably, the primary tumor and metastatic lesions may require
resection simultaneously; however, this could not be performed by
single-discipline surgeons. Moreover, a broad range of anatomical
variations or extensive adhesions of the tumor may be present,
which may cause tumor rupture during surgery, as well as tumor
dissemination. Therefore, resection following the collaboration of
experienced hepatobiliary and thoracic surgeons may improve the
safety of the surgery. In addition, the possibility of adjuvant
radiotherapy and chemotherapy were rejected in the meeting due to
their unclear roles in SFTs (33).
Following the MDTM, a consensus was established and
a personalized treatment protocol was developed. This included
transhepatic arterial embolization to block the major arterial
blood supply, and a full resection of the tumor, using the
collaboration of experienced hepatobiliary and thoracic surgeons.
The treatment protocol was fully discussed with the patient and
written informed consent was obtained. Angiography showed the
staining of the tumor with contrast agents, with multiple branches
of the hepatic artery participating in the blood supply to the
tumor. Subsequently, an interventional radiologist used self-made
gelatin sponge particles as embolic agents, to embolize three
branches of the hepatic artery. Re-examination of the angiography
demonstrated that the staining of the tumor was significantly
reduced. There were no complications associated with the hepatic
artery embolization.
On day 1 post-hepatic artery embolization, a
laparotomy was performed using an anti-L-shaped incision in the
epigastrium. Intraoperative abdominal exploration demonstrated that
a large tumor of the right liver lobe (volume, 8x7x6 cm), with a
hard texture, clear boundaries and an intact envelope, protruded
through the surface of the right liver lobe and passed through the
diaphragm into the thoracic cavity. Intraoperative thoracic
exploration demonstrated that the thoracic tumor (volume, 10x7x7
cm) was intimately connected with the tumor of the right liver lobe
and exhibited a strong adhesion to the right lower lobe of the
lung, which may have been invaded by the tumor. No metastasis was
noted within the remaining thoracic and abdominal cavities.
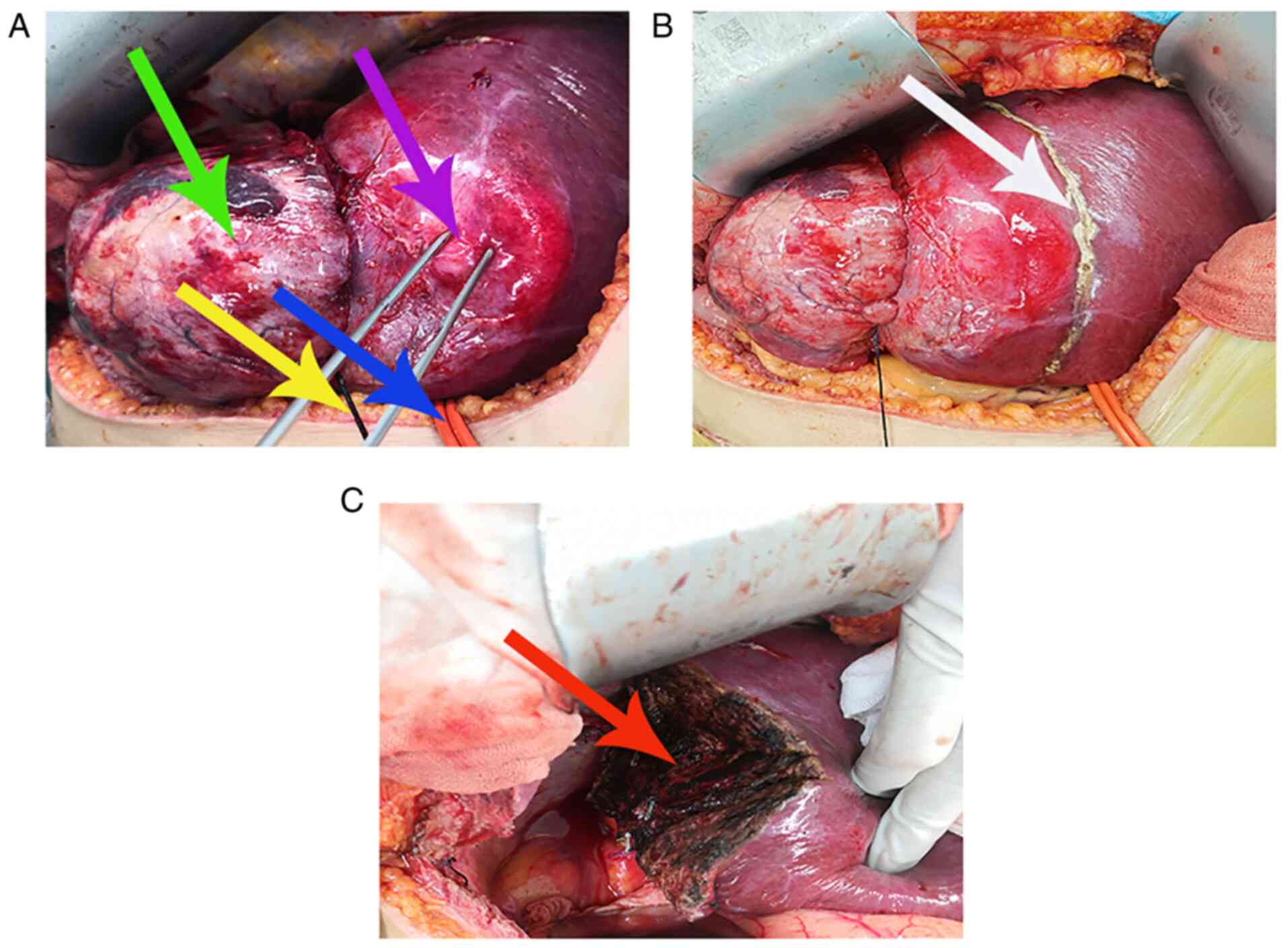
A self-made red urinary catheter (Fig. 4) was used for the first porta
hepatis occlusion, to fully expose the liver and the tumor.
Subsequently, a pre-cut liver line (Fig. 4) was marked using an electrocautery
knife at a distance of 2 cm from the tumor. The tumor was fully
resected. Meanwhile, resection and repair of the diaphragm, and
wedge resection of the right lower lobe of the lung were performed.
During surgery, the first porta hepatis was intermittently occluded
three times. The first time was for 10 min, the second time was for
15 min and the third time was for 5 min. Notably, the
intraoperative hemorrhage volume was ~200 ml, and no blood
transfusion was performed.
The gross specimen of the tumor (Fig. 5A) demonstrated a dumbbell shape
(volume, 14x8x7 cm) with an intact envelope. The cut surface of the
tumor (Fig. 5B) demonstrated a
grey-white fibrous appearance, with a hard texture and clear
boundaries.
Postoperative histopathological analysis
demonstrated a negative resection margin (Fig. 6), and the remaining
histopathological features were the same as those observed
following fine-needle biopsy. In addition, no tumor invasion was
observed in the resected lung tissues or diaphragm. Postoperative
immunohistochemical analysis (Fig.
6) demonstrated that the tumor cells were positive for
vimentin, CD34, and STAT6, and also for CD99, Bcl2 and β-catenin.
The remaining immunohistochemical features were the same as those
observed following fine-needle biopsy of the tumor.
In the course of the histopathological examinations,
the tissue sections were stained with hematoxylin and eosin for 6
min at room temperature. In the process of the immunohistochemical
examinations, the tumor tissues were fixed in 10% neutral buffered
formalin for 24 h at room temperature. Paraffin sections (slice
thickness, 4 µm) were produced using paraffin-embedded tissues.
Antigen repair was performed using a pressure cooker to heat tissue
sections in ethylene diamine tetraacetic acid buffer (pH 9.0) for
20 min. Endogenous peroxidase was blocked using incubation with 3%
H2O2 for 15 min at room temperature. Sections
were incubated overnight with primary antibodies at 4˚C. Following
primary incubation, the sections were incubated with secondary
antibodies for 30 min at room temperature. The primary antibodies
used in the present study were as follows: Monoclonal mouse
anti-human vimentin (ready-to-use; cat. no. GM088702), monoclonal
mouse anti-human CD34 (ready-to-use; cat. no. GM716502), monoclonal
mouse anti-human CD99 (ready-to-use; cat. no. GT212302), monoclonal
mouse anti-human bcl-2 (ready-to-use; cat. no. GM088702),
monoclonal rabbit anti-human β-catenin (ready-to-use; cat. no.
GT211902), monoclonal mouse anti-human desmin (ready-to-use; cat.
no. GT225202), monoclonal mouse anti-human CK (ready-to-use; cat.
no. GT207902), monoclonal mouse anti-human SMA (ready-to-use; cat.
no. GM085102), monoclonal mouse anti-human S100 (ready-to-use; cat.
no. GT224902), monoclonal rabbit anti-human MyoD1 (ready-to-use;
cat. no. GT218802), monoclonal mouse anti-human ALK (ready-to-use;
cat. no. GT231102), monoclonal mouse anti-human EMA (ready-to-use;
cat. no. GM061302), monoclonal mouse anti-human CD31 (ready-to-use;
cat. no. GT232102), monoclonal mouse anti-human CD68 (ready-to-use;
cat. no. GM087602), monoclonal mouse anti-human HepPar1
(ready-to-use; cat. no. GM715802) and monoclonal rabbit anti-human
CD117 (ready-to-use; cat. no. GT224802), all purchased from Gene
Tech Biotechnology, Co., Ltd. Polyclonal rabbit anti-human STAT6
(ready-to-use; cat. no. CSR-0281) was purchased from Celnovte
Biotechnology Co., Ltd. The secondary antibody sheep
anti-mouse/rabbit IgG (ready-to-use; cat. no. GK600705A), purchased
from Gene Tech Biotechnology, Co., Ltd., was labeled with
horseradish peroxidase. In addition, the nuclei were stained using
hematoxylin for 6 min at room temperature. The stained sections
were analyzed using an Olympus BX46 light microscope (Olympus
Corporation, Tokyo, Japan). Immunohistochemical analysis was
performed using Image J (version 1.46a; National Institutes of
Health).
The postoperative course was uneventful and no
abdominal distention occurred. However, the nutritional status of
the patient was poor, and recovery time was prolonged.
Subsequently, the patient was discharged from the hospital 12 days
after the surgery. The patient had not received any postoperative
adjuvant chemotherapy. Following discharge, patient follow-up was
performed at 1, 2, 3, 6 and 12 months. Examinations during
follow-up consultations included MRI and CT scans of the chest and
upper abdomen, and tumor marker analysis. At the end of the 1-year
follow-up, the patient remained healthy and demonstrated no signs
of recurrence.
Discussion
An SFT is a rare tumor of mesenchymal origin, with
prominent histological characteristics of a hemangiopericytoma-like
branching vascular pattern (2).
The prevalence of SFTs does not differ between men and women
(2), and the age of onset is 20-70
years (5).
The majority of patients with SFTs are clinically
asymptomatic; however, the current study presented the case of a
patient with abdominal distension that had persisted for 1 month
due to the tumor oppressing neighboring anatomical structures.
SFTs often present with typical features during
imaging, including single, clear boundaries, inhomogeneous
enhancement and high vascularization (36). The results of a previous study
demonstrated inhomogeneous enhancement, which may have been due to
the differential enhancement of the admixed cellular and
collagenous components (26).
However, not all SFT imaging is typical (37). Imaging examinations, including MRI,
CT and abdominal ultrasound scans, may be used to reveal liver
tumors. Among them, MRI is considered the gold-standard imaging
modality for SFTs (38). The
results of a previous study demonstrated masses of predominantly
low or intermediate signal intensities on both T1 and T2-weighted
images, which may reflect the high content of fibrous collagenous
tissue, hypocellularity and the relatively small number of mobile
protons (37). Moreover,
hyperintensity on T2-weighted images may be associated with
necrosis, cystic or myxoid degeneration, prominent vascular
structures and hypercellular areas (37).
Laboratory examination results often present within
healthy ranges; however, a few patients have presented with liver
dysfunction or increased levels of thrombocyte or C-reactive
protein, which were not specific and did not directly correspond to
the diagnosis of an SFT (39,40).
In the current case, the patient presented with no
abnormalities.
The diagnosis of an SFT depends on the pathological
examination, due to inaccuracies in laboratory and imaging
examinations. Therefore, a fine-needle biopsy was performed in the
present study in order to obtain pathological results and a
definitive diagnosis, and this provided a basis for determining
whether adjuvant therapy was required. However, the use of a
fine-needle biopsy for diagnosis remains controversial. On one
hand, previous research has demonstrated that a fine-needle biopsy
may help obtain tumor tissues, and that these may be useful for the
pathological and differential diagnosis of the tumor (21). On the other hand, research has
demonstrated that the results of fine-needle biopsy may cause tumor
dissemination, or at the least may be misleading or unclear. This
is due to the fact that benign and malignant tumors may exist in
the same lesion at the same time, and the punctured tissue may not
contain malignant components (22,28).
Common histological and immunohistochemical features
are useful to determine a definitive diagnosis. In the present
case, pathological examinations of fine-needle biopsy and surgery
specimens supported the diagnosis of an HSFT. The histopathology
results demonstrated a diffuse proliferation of spindle cells
surrounded by collagen fibers. Tissue sections were stained with
H&E for pathological examination, and the results demonstrated
no malignant transformation. Immunohistochemical analysis
demonstrated that the tumor cells were positive for vimentin, CD34,
STAT6, CD99, Bcl2 and β-catenin; however, they were negative for
desmin, CK, SMA, S100, MyoD1, ALK, EMA, CD31, CD68, HepPar1 and
CD117 which is indicative of an HSFT (24,33,39).
The majority of SFTs are benign, but malignant
features must be considered when the following evidence is
presented: Infiltrative margins, high cellularity, prominent
cellular atypia, tumor necrosis and increased mitotic activity
(>4 mitoses/10 high-power fields) (3). In the present case, the tumor
originated from the liver and grew through the diaphragm into the
right thoracic cavity. Thus, this was considered as possessing
malignant characteristics. However, it was morphologically benign
(no infiltrative margin, low cellularity, no cellular atypia, no
significant tumor necrosis and a low mitotic rate). In addition, no
tumor invasion was observed in the resected lung tissues and
diaphragm. The aforementioned results indicated that there was no
specific association between the behavioral and morphological
features, and this is comparable to the results of previous studies
(2,24).
Radical surgery is the preferred treatment for an
HSFT. The resection margin must be at least 1 cm away from the
tumor to avoid tumor residue. Intraoperative frozen sections must
also be routinely performed. Notably, if infiltrative margins are
found, further resection must be considered (41). To date, the treatment methods of
HSFTs are increasingly diversified and complicated, and knowledge
of a single discipline is insufficient to deal with complex cases.
MDTMs are interdisciplinary, centralized, individualized and
precise, and play an important role in the treatment of complex
tumors (42). In the present case,
surgery was the most optimal treatment method for the tumor.
However, the tumor involved multiple organs and the surgical risk
was high; therefore, removal of the tumor was determined to be
difficult for doctors of a single discipline. Based on the
aforementioned assessment, MDTMs were organized to develop a
personalized treatment strategy from a multidisciplinary
perspective, in order to successfully treat the tumor according to
the specific situation of the patient.
In the present case, the etiology of the diaphragm
defect may have been either congenital or acquired. A congenital
diaphragm defect may be caused by dysplasia of the diaphragm. The
potential mechanism underlying an acquired diaphragm defect is as
follows: As the tumor volume increases, the abdominal pressure
increases, leading to an increase in the pressure difference
between the thoracic cavity and the abdominal cavity. This
ultimately causes the tumor to break through the weak area of the
diaphragm. In addition, the results of the present case report
demonstrated no association between the diaphragm defect and tumor
aggressiveness, and this may be due to a clear boundary, the intact
envelope of the tumor and a lack of tumor invasion of the
diaphragm. To the best of our knowledge, an HSFT exhibiting a
dumbbell-shaped appearance has not been previously reported. The
dumbbell-shaped appearance of the tumor may be caused by the growth
of the tumor through the narrow diaphragm defect. Moreover, the
atelectasis in the right lower lobe may have been caused by
intrathoracic tumor compression.
Transarterial chemoembolization (TACE) is an
important yet variably effective treatment for the management of
hepatic malignancies. The arterial blood supply is blocked by
chemical embolization, which may result in ischemic necrosis of the
tumor (33). In the literature,
only three previous cases involving the treatment of an HSFT using
TACE have been previously reported (33,43,44).
The results of on of these cases demonstrated that the tumor was
located in the center of the liver, and invaded the left and right
lobes. This was therefore unresectable and TACE was performed on
the patient three times (33). The
second case investigated a tumor that was located in the right lobe
of the liver with right parietal metastasis; notably, TACE was
performed, followed by subtotal resection of the right liver and
craniectomy in the patient (43).
The final study demonstrated that the tumor was located in hepatic
segments IV, V, VI and VIII. Subsequently, a right portal vein
embolization was performed followed by TACE, and a right
hepatectomy was also performed (44). In the present case, transhepatic
arterial embolization was performed before surgery. Following
transhepatic arterial embolization, the tumor blood supply was
significantly reduced, which greatly decreased the difficulty of
the surgery.
The MDTM held during the present study included
discussions of serious complications that may occur following
hepatic artery embolization, and debate over whether adjuvant
chemotherapy and radiation should be performed.
Results of a previous study revealed that few
patients suffered from ectopic embolism-related complications, such
as tumor rupture, cholecystitis, splenic infarction, liver
abscesses, and cerebral and pulmonary embolism (45). These complications were rare
(45), and were associated with
non-selective embolization, the number of procedures and the volume
of embolic material (46). Results
of previous studies also demonstrated that the large tumor size may
impact the risk of rupture (47)
and liver failure (48).
To avoid the occurrence of serious complications,
selective catheterization and slow infusion of the embolic material
were performed in the present case. In addition, the results of a
previous study demonstrated that post-embolization syndrome is
closely associated with the side effects of chemotherapy drugs
(49). Thus, self-made gelatin
sponges were used in the present study to replace the chemotherapy
drugs and result in fewer side effects.
Adjuvant therapy is not always necessary and is
reserved for when a resection is incomplete, or when pathological
examination reveals the features of malignancy or postoperative
recurrence with metastasis (25).
The role of chemotherapy and radiotherapy in the aforementioned
tumor types is ambiguous (33).
Doxorubicin has been used as a first-line therapy in advanced
soft-tissue sarcoma for >40 years (5). The mechanism of action of doxorubicin
involves the insertion of DNA, which disrupts DNA damage repair
through topoisomerase II, thus generating free radicals and leading
to ulterior cell membrane damage (5). As previously reported,
de-differentiated SFT (DD-SFT) with a high malignancy (significant
genomic instability, and substantial cytogenetic losses and gains)
is the subtype of SFT with the fastest growth (5). Therefore, doxorubicin may be a
valuable option for patients suffering from DD-SFT (5). However, doxorubicin administration in
patients with non-DD-SFT may be detrimental, adding genomic
instability through its direct genotoxic action, or by
doxorubicin-mediated oxidative stress production (5). In the present case, histopathology
and immunohistochemistry revealed a well-differentiated tumor.
Thus, it was determined that doxorubicin was not suitable for
chemotherapy. In addition, the use of gemcitabine, as a pyrimidine
antitumor agent, has only been reported sporadically in cases of
SFT and exhibits poor treatment effectiveness (50,51).
A small retrospective study involving 14 patients
with recurrent intracranial SFT demonstrated that the use of
external radiation therapy extended overall survival time compared
with surgery alone (10.3 vs. 5.3 years) (38). However, a large retrospective study
consisting of 549 cases of SFT, 428 (78%) of which underwent
surgery and 121 (22%) of which underwent surgery plus postoperative
radiotherapy, demonstrated that there was no significant difference
in patient overall survival time between the two groups (52). Therefore, radiotherapy was
determined to be unsuitable for the present case.
The prognosis of SFTs is often associated with
resectability (28). The results
of a previous study demonstrated that the 5-year survival rate of
patients who underwent curative resection was significantly
increased compared with that of patients who underwent non-curative
resection [partial excision (79%) or biopsy (50%)] (53). Results of a previous study
demonstrated that the 5-year survival rate of patients who
underwent curative resection was 100% (54). Similarly, 10-year survival rates of
54% have been observed when complete curative resection is an
option (53). It has also been
suggested that patients with malignant histological features are
more susceptible to recurrence and metastasis (27).
In conclusion, although the majority of HSFTs are
benign, they exhibit the potential for malignant transformation.
Thus, patients require long-term follow-up. HSFT is a rare
mesenchymal tumor, and there is limited previous evidence detailing
the diagnosis and treatment of this tumor type. Treatment options
require multidisciplinary collaboration when complex tumor
structures arise.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL conceived and designed the present case report.
JL and JW collected the clinical data and conducted the follow-up
visit. ZC and JW performed the histopathological analysis and
provided the associated images. SH and JW performed the imaging
analysis and provided the imaging data. JL wrote the initial draft
of the report. The present article has been revised by SH, JW and
ZC. ZC performed the surgery and provided care for the patient. JL
and ZC confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang YR, Wang H, Zhou N, Zhang YD, Lin Y,
Wu LY, Wei SF, Ma YY and Wang CX: A multidisciplinary team approach
to the treatment of liver cirrhosis. J Inflamm Res. 14:5443–5450.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Feng LH, Dong H, Zhu YY and Cong WM: An
update on primary hepatic solitary fibrous tumor: An examination of
the clinical and pathological features of four case studies and a
literature review. Pathol Res Pract. 211:911–917. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Buccauw K, Sciot R, Wolter P, Aerts R and
Claus F: Delayed liver metastasis of a meningeal solitary fibrous
tumor. Acta Gastroenterol Belg. 74:567–569. 2011.PubMed/NCBI
|
|
4
|
Klemperer P and Coleman BR: Primary
neoplasms of the pleura. A report of five cases. Am J Ind Med.
22:1–31. 1992.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Martin-Broto J, Mondaza-Hernandez JL,
Moura DS and Hindi N: A comprehensive review on solitary fibrous
tumor: New insights for new horizons. Cancers (Basel).
13(2913)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sun PP, Du XM, Gao Y, Zhao HY, Wang LL,
Zhang Y and Li Y: Clinicopathologic features of retroperitoneal
malignant solitary fibrous tumors. Crit Rev Eukaryot Gene Expr.
31:21–33. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cheng L, Ni H and Dai Y: Intracranial
solitary fibrous tumor mimicking meningioma: A case report.
Medicine (Baltimore). 99(e23504)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jackson CH, Hunt BC and Harris GJ: Fate
and management of incompletely excised solitary fibrous tumor of
the orbit: A case series and literature review. Ophthalmic Plast
Reconstr Surg. 37:108–117. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nitta T, Kimura K, Tominaga T, Ikari A,
Takashima Y, Hirata A, Takeshita A, Ishibashi T and Iwamoto M:
Malignant solitary fibrous tumor of the breast. Breast J.
27:391–393. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ghasemi-Rad M, Wang KY, Jain S and Lincoln
CM: Solitary fibrous tumor of thyroid: A case report with review of
literature. Clin Imaging. 53:105–107. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sheikhy A, Fallahzadeh A, Ahmadi-Tafti SH,
Hosseini K, Mohseni-Badalabadi R, Shahbazi N, Ghorashi SM and
Tajdini M: Intrapericardial solitary fibrous tumor: A case report
and review of literature. Echocardiography. 38:1052–1056.
2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Romano N, Ferrari A, Moroni M, Dessanti P,
Bardine A, D'Amato M and Stefanini T: Solitary fibrous tumor of the
deep parotid gland. Ear Nose Throat J: Oct 14, 2020 (Epub ahead of
print).
|
|
13
|
Su HY, Tsai TH, Yang SF and Lee JY:
Dumbbell-shaped solitary fibrous tumor of thoracic spine. Kaohsiung
J Med Sci. 35:517–518. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Qin J, Zhu Y, Kong M, Wang P, Xia D and
Wang S: Robot-assisted laparoscopic resection of a pelvic solitary
fibrous tumor. J Int Med Res: Feb 2, 2021 (Epub ahead of
print).
|
|
15
|
Guo YC, Yao LY, Tian ZS, Shi B, Liu Y and
Wang YY: Malignant solitary fibrous tumor of the greater omentum: A
case report and review of literature. World J Clin Cases.
9:445–456. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tandon N: Solitary Fibrous tumor of the
vulva: Case report of a rare entity and review of literature. Int J
Gynecol Pathol. 40:234–239. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Krishnamurthy S, Menon M, Parameswaran A
and Sivaraman A: A case of solitary fibrous tumor of urinary
bladder. Indian J Pathol Microbiol. 64:847–849. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Takeuchi Y, Kato D, Nakane K, Kawase K,
Takai M, Iinuma K, Saigo C, Miyazaki T and Koie T: Solitary fibrous
tumor of the prostate: A case report and literature review.
Medicina (Kaunas). 57(1152)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Masmoudi M, Hasnaoui M, Dgani I, Thabet W,
Ben Abdeljalil N, Ch C, Mighri K and Driss N: Solitary fibrous
tumor of the external auditory canal. Ear Nose Throat J: Mar 8,
2021 (Epub ahead of print).
|
|
20
|
Addeo P, Averous G and Bachellier P:
Solitary fibrous tumor of the pancreas. J Gastrointest Surg.
25:569–570. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sun Z, Ding Y, Jiang Y, Zhang Q, Li Z,
Xiang J, Duan J, Yan S and Wang W: Ex situ hepatectomy and liver
autotransplantation for a treating giant solitary fibrous tumor: A
case report. Oncol Lett. 17:1042–1052. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Debs T, Kassir R, Amor IB, Martini F,
Iannelli A and Gugenheim J: Solitary fibrous tumor of the liver:
Report of two cases and review of the literature. Int J Surg.
12:1291–1294. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Andaluz Garcia I, Tavecchia M and Olveira
Martin A: A solitary fibrous tumor: An entity to consider in the
diagnosis of liver masses. Rev Esp Enferm Dig.
111(969)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sun K, Lu JJ, Teng XD, Ying LX and Wei JF:
Solitary fibrous tumor of the liver: A case report. World J Surg
Oncol. 9(37)2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Vennarecci G, Ettorre GM, Giovannelli L,
Del Nonno F, Perracchio L, Visca P, Corazza V, Vidiri A, Visco G
and Santoro E: Solitary fibrous tumor of the liver. J Hepatobiliary
Pancreat Surg. 12:341–344. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fuksbrumer MS, Klimstra D and Panicek DM:
Solitary fibrous tumor of the liver: Imaging findings. AJR Am J
Roentgenol. 175:1683–1687. 2000.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mao M, Zhou L, Huang C, Yan X, Hu S, Yin
H, Zhao Q and Song D: Case report: A malignant liver and thoracic
solitary fibrous tumor: A 10-year journey from the brain to the
liver and the spine. Front Surg. 7(570582)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Korkolis DP, Apostolaki K, Aggeli C,
Plataniotis G, Gontikakis E, Volanaki D, Sebastiadou M,
Dimitroulopoulos D, Xinopoulos D, Zografos GN and Vassilopoulos PP:
Solitary fibrous tumor of the liver expressing CD34 and vimentin: A
case report. World J Gastroenterol. 14:6261–6264. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Changku J, Shaohua S, Zhicheng Z and
Shusen Z: Solitary fibrous tumor of the liver: Retrospective study
of reported cases. Cancer Invest. 24:132–135. 2006.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rouy M, Guilbaud T and Birnbaum DJ: Liver
solitary fibrous tumor: A rare incidentaloma. J Gastrointest Surg.
25:852–853. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nam HC, Sung PS, Jung ES and Yoon SK:
Solitary fibrous tumor of the liver mimicking malignancy. Korean J
Intern Med. 35:734–735. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Alonso Batanero S, Garrosa Munoz S,
Iglesias Iglesias MJ and Munoz-Bellvis L: A giant solitary fibrous
tumor arising from the hepatic round ligament. Dig Liver Dis.
53:379–380. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
El-Khouli RH, Geschwind JF, Bluemke DA and
Kamel IR: Solitary fibrous tumor of the liver: Magnetic resonance
imaging evaluation and treatment with transarterial
chemoembolization. J Comput Assist Tomogr. 32:769–771.
2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Nishikawa H, Osaki Y, Iguchi E, Takeda H,
Nakajima J, Matsuda F, Sakamoto A, Henmi S, Hatamaru K, Saito S, et
al: Comparison of the efficacy of transcatheter arterial
chemoembolization and sorafenib for advanced hepatocellular
carcinoma. Exp Ther Med. 4:381–386. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Li X, Liu M, Zhang H, Liu H and Chen J:
Clinical study of apatinib combined with EGFR-TKI in the treatment
of chronic progression after EGFR-TKI treatment in non-small cell
lung cancer (ChiCTR1800019185). Thorac Cancer. 11:819–826.
2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nath DS, Rutzick AD and Sielaff TD:
Solitary fibrous tumor of the liver. AJR Am J Roentgenol.
187:W187–W190. 2006.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Obuz F, Secil M, Sagol O, Karademir S and
Topalak O: Ultrasonography and magnetic resonance imaging findings
of solitary fibrous tumor of the liver. Tumori. 93:100–102.
2007.PubMed/NCBI
|
|
38
|
Bokshan SL, Doyle M, Becker N, Nalbantoglu
I and Chapman WC: Hepatic hemangiopericytoma/solitary fibrous
tumor: A review of our current understanding and case study. J
Gastrointest Surg. 16:2170–2176. 2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Silvanto A, Karanjia ND and Bagwan IN:
Primary hepatic solitary fibrous tumor with histologically benign
and malignant areas. Hepatobiliary Pancreat Dis Int. 14:665–668.
2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Jakob M, Schneider M, Hoeller I, Laffer U
and Kaderli R: Malignant solitary fibrous tumor involving the
liver. World J Gastroenterol. 19:3354–3357. 2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Perini MV, Herman P, D'Albuquerque LA and
Saad WA: Solitary fibrous tumor of the liver: Report of a rare case
and review of the literature. Int J Surg. 6:396–399.
2008.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wang C, Song D, Xu Z and Wang J: Clinical
application of multidisciplinary teams in tumor therapy. Chin J
Cancer Res. 29:168–170. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Peng L, Liu Y, Ai Y, Liu Z, He Y and Liu
Q: Skull base metastases from a malignant solitary fibrous tumor of
the liver. A case report and literature review. Diagn Pathol.
6(127)2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Bejarano-Gonzalez N, Garcia-Borobia FJ,
Romaguera-Monzonis A, García-Monforte N, Falcó-Fagés J, Bella-Cueto
MR and Navarro-Soto S: Solitary fibrous tumor of the liver. Case
report and review of the literature. Rev Esp Enferm Dig.
107:633–639. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Marelli L, Stigliano R, Triantos C,
Senzolo M, Cholongitas E, Davies N, Tibballs J, Meyer T, Patch DW
and Burroughs AK: Transarterial therapy for hepatocellular
carcinoma: Which technique is more effective? A systematic review
of cohort and randomized studies. Cardiovasc Intervent Radiol.
30:6–25. 2007.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Bae SI, Yeon JE, Lee JM, Kim JH, Lee HJ,
Lee SJ, Suh SJ, Yoon EL, Kim HR, Byun KS and Seo TS: A case of
necrotizing pancreatitis subsequent to transcatheter arterial
chemoembolization in a patient with hepatocellular carcinoma. Clin
Mol Hepatol. 18:321–325. 2012.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Nishida K, Lefor AK and Funabiki T:
Rupture of hepatocellular carcinoma after transarterial
chemoembolization followed by massive gastric bleeding. Case
Reports Hepatol. 2018(4576276)2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hickey R, Mouli S, Kulik L, Desai K,
Thornburg B, Ganger D, Baker T, Abecassis M, Ralph Kallini J, Gabr
A, et al: Independent analysis of albumin-bilirubin grade in a
765-patient cohort treated with transarterial locoregional therapy
for hepatocellular carcinoma. J Vasc Interv Radiol. 27:795–802.
2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Szemitko M, Golubinska-Szemitko E,
Wilk-Milczarek E and Falkowski A: Side effect/complication risk
related to injection branch level of chemoembolization in treatment
of metastatic liver lesions from colorectal cancer. J Clin Med.
10(121)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Park MS, Ravi V, Conley A, Patel SR, Trent
JC, Lev DC, Lazar AJ, Wang WL, Benjamin RS and Araujo DM: The role
of chemotherapy in advanced solitary fibrous tumors: A
retrospective analysis. Clin Sarcoma Res. 3(7)2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Outani H, Kobayashi E, Wasa J, Saito M,
Takenaka S, Hayakawa K, Endo M, Takeuchi A, Kobayashi H, Kito M, et
al: Clinical outcomes of patients with metastatic solitary fibrous
tumors: A Japanese Musculoskeletal Oncology Group (JMOG)
multiinstitutional study. Ann Surg Oncol. 28:3893–3901.
2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Haas RL, Walraven I, Lecointe-Artzner E,
van Houdt WJ, Strauss D, Schrage Y, Hayes AJ, Raut CP, Fairweather
M, Baldini EH, et al: Extrameningeal solitary fibrous
tumors-surgery alone or surgery plus perioperative radiotherapy: A
retrospective study from the global solitary fibrous tumor
initiative in collaboration with the Sarcoma Patients EuroNet.
Cancer. 126:3002–3012. 2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Spitz FR, Bouvet M, Pisters PW, Pollock RE
and Feig BW: Hemangiopericytoma: A 20-year single-institution
experience. Ann Surg Oncol. 5:350–355. 1998.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Espat NJ, Lewis JJ, Leung D, Woodruff JM,
Antonescu CR, Shia J and Brennan MF: Conventional
hemangiopericytoma: Modern analysis of outcome. Cancer.
95:1746–1751. 2002.PubMed/NCBI View Article : Google Scholar
|