Introduction
Intracerebral hemorrhage (ICH) is a type of stroke
that is relatively common but lacks clear treatment strategy
(1). ICH accounts for 10-15% of
all cases of stroke in the US (2).
In Globally, ICH is one of the most deleterious types of all
strokes; it has an incidence of 10-30 per 100,000 patients
annually, with a morbidity rate of 75% and a mortality rate of
30-50% January 1980, and November 2008(3). ICH is diagnosed more frequently in
the elderly (>55 years old) and in males, with predilection
observed in patients of African and Asian ethnicity (4,5).
Hematoma following ICH destroys the surrounding vascular system,
which induces hemorrhage and potentiates growth of hematoma, which
leads to neuronal deficit (6).
Furthermore, ICH induces inflammation to promote edema formation,
which aggravates the hydrostatic pressure around the hematoma,
resulting in cell death (7,8).
Neurological deficit, which is typically caused by
neuroinflammation, is associated with the release of endogenous
ligands that primarily activate Toll-like receptors (TLRs)
(9). Accumulating evidence
suggests that TLR4-mediated neuroinflammation is one of the key
causes of secondary brain injury following ICH (10,11).
Upregulated TLR4 expression levels are associated poorer outcomes
in patients with ICH (12). By
contrast, treatment with antagonists of TLR4 attenuates brain
injury following ICH (13).
microRNAs (miRNAs or miRs) belong to a family of
small non-coding RNAs that are ~20 nucleotides in length (14). They regulate expression of target
mRNAs, in turn regulating a number of human diseases (14-17).
Numerous miRNAs have been documented as potential molecular markers
for diagnosis, treatment and development of ICH (15), including miR-183-5p (16) and miR-331-3p (17). According to results from a miRNA
PCR array, miR-30e-5p expression is downregulated in peripheral
blood samples from patients with ICH (18). However, little is known about the
role of miR-30e-5p in ICH.
A number of studies have reported the effect of
miR-30e-5p in human disease, such as squamous cell carcinoma of the
head and neck, where it inhibits metastasis and angiogenesis by
targeting astrocyte elevated gene-1 expression (19,20).
Furthermore, miR-30e-5p has been found to reverse hypoxia-induced
apoptosis of human stem cell-derived cardiomyocytes by targeting
Bim expression (21). A previous
study observed that miR-30e-5p suppresses inflammation and protects
against cardiac dysfunction following myocardial infarction by
targeting PTEN expression (22).
Nevertheless, the potential association between miR-30e-5p and
TLR4, in addition to their physiological roles in ICH remain poorly
understood. Therefore, the present study investigated the effect of
miR-30e-5p overexpression on neuronal function and apoptosis and
inflammation, in addition to TLR4 signaling, in rats with ICH.
Materials and methods
Animals
Healthy male adult Sprague-Dawley rats (n=72; age,
10-12 weeks; weight, 250-300 g; Model Animal Research Center of
Nanjing University) were maintained at 22-25˚C with 12/12-h
day/night cycle, humidity of 50-60% and free access to food and
water at specific-pathogen-free grade. The animal experiments were
approved by the Institutional Animal Care and Use Committee of
Jinan First People's Hospital (approval no. JNFPH2019-03; Ji'nan,
China). The total duration of the experiment was 10 days from the
establishment of animal models to complete assessment of the
neurological function. After collecting blood in tail vein, rats
were anesthetized with intraperitoneal injection of 3%
pentobarbital sodium (30 mg/kg) and sacrificed by cervical
dislocation. Death was confirmed by cessation of breathing and
faded eye color.
Groups
miR-negative control (NC) mimic, miR-30e-5p mimic,
pcDNA3.1, and pcDNA3.1-TLR4 were purchased from Shanghai GenePharma
Co., Ltd. Rat is an ideal pathophysiological disease model that is
widely used (23,24). The ICH model in rats was
established by IC injection of collagenase type IV (0.23 U in 1 µl
sterile saline; Beijing Solarbio Science & Technology Co.,
Ltd.) into the right striatum, as previously described (25). For the rats in the ICH group
(n=11), miR-NC mimic + pcDNA3.1 was injected into the right lateral
ventricle of rats 3 days prior to the establishment of ICH
model.
Rats in the sham group (n=11) were injected with 1
µl sterile saline. For rats in the remaining groups (ICH +
miR-30e-5p mimic + pcDNA3.1 and ICH + miR-30e-5p mimic +
pcDNA3.1-TLR4; both n=11), miR-30e-5p mimic + pcDNA3.1 or
miR-30e-5p mimic + pcDNA3.1-TLR4 was injected into the right
lateral ventricle of rats 3 days prior to establishment of ICH
model (26).
Neurological function
A 24-point neurological scoring system was used to
test neurological deficit, including Longa, beam balance test
(BBT), Bederson and foot fault asymmetry in rats. Neurological
function was assessed on day 7 after the establishment of ICH.
Longa standards (27) were as
follows: 0, no neurological impairment; 1, impaired stretching of
contralateral limbs; 2, circling around; 3, tilting to the side of
hemiplegia upon waking and 4, impaired walking or
unconsciousness.
The rats underwent BBT, in which they were allowed
to walk on a balance beam (square stick; length, 80.0 cm; width,
2.5 cm; 10.0 cm above ground) to walk on. The following six-grade
standard was applied (28): 0,
jump on the balance beam and walk without falling; 1, jump on the
balance beam with time of falling ≤50%; 2, jump on the balance beam
with time of falling >50%; 3, jump on the balance beam with the
healthy lateral hind limb with no movement of paralyzed hind limb;
4, sit on the balance beam without walking and 5, fall from the
balance beam.
For Bederson score which is used for disability
assessment (29), the rats were
lifted 10 cm above the desk. After seizing the tail by an
experimenter, healthy rats should straighten the foreleg. The
standards used for scoring this were as follows (29): 0, no neurological impairment; 1,
lateral wrist and elbow joint flexion and shoulder adduction and
flexion; 2, lateral wrist and elbow joint flexion, shoulder
adduction and flexion and decreased thrust to the paralytic side
and 3, chasing tail with circling to the paralytic side.
For foot fault asymmetry score, rats were placed in
a wet box (2.3x2.3 cm mesh) and the number of times the forelegs
touched the mesh was counted. Score was calculated as follows
(30): (no. of missteps of
forelegs opposite to the lesion-no. of missteps of forelegs on the
same side as the lesion)/total steps. A positive score indicates
impaired function toward the lesion, while a negative score
indicates impairment of function on the same side as the
lesion.
ELISA
Following the measurement of neurological deficit in
rats, 1 ml blood from the tail vein was collected and centrifuged
at 1,500 x g for 10 min at 4˚C. Serum samples were stored at -20˚C.
The concentration of IL-6 (cat. no. ERA31RBX10), TNF-α (cat. no.
ERA56RB) and IL-1β (cat. no.# BMS630TEN) was evaluated using ELISA
kits (all Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocols. Optical density was measured at 450 nm
using a BioTek Epoch microplate reader.
Reverse transcription-quantitative
(RT-q)PCR
TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract total RNA from rat brain
tissue. RNA (1 µg) was subjected to RT using avian myeloblastosis
virus reverse transcriptase (Takara Biotechnology Co., Ltd.)
following manufacturer's protocol. The quantification of cDNA was
performed using SYBR Premix Ex Taq™ (Takara
Biotechnology Co., Ltd.) and LightCycler 480 system (Roche
Diagnostics). The thermocycling conditions were as follows: Initial
denaturation at 95˚C for 10 min, followed by 40 cycles of 95˚C for
10 sec, 60˚C for 20 sec and 72˚C for 10 sec. U6 was used as the
internal reference for miR-30e-5p, while GAPDH was used as the
internal reference for TLR4. The primer sequences are listed in
Table I. The gene expression was
analyzed by the 2-ΔΔCq method (31).
 | Table IPrimer sequences for reverse
transcription-quantitative PCR. |
Table I
Primer sequences for reverse
transcription-quantitative PCR.
| Name | Forward | Reverse |
|---|
| GAPDH |
5'-CCATAAAGGGCATCCTGGGCT-3' |
5'-TTACTCCTTGGAGGCCATGTA-3' |
| U6 |
5'-CTCGCTTCGGCAGCACA-3' |
5'-AACGCTTCACGAATTTGCGT-3' |
| miR-30e-5p |
5'-TGTAAACATCCTTGACTGGAAG-3' |
5'-CTTCCAGTCAAGGATGTTTACA-3' |
| TLR4 |
5'-CGCCTAAAACCCATTATGTTTACA-3' |
5'-TGTAAACATAATGGGTTTTAGGCG-3' |
Western blotting
Total protein from brain tissue of rats was
extracted using RIPA buffer (Roche Diagnostics). Protein
concentration was using BCA kit (Boster Biological Technology). In
total, 30 µg protein sample was loaded into each lane, followed by
separation by 8% SDS-PAGE. Next, proteins were transferred onto
PVDF membranes, followed by blocking with 5% BSA (Thermo Fisher
Scientific, Inc.) for 1 h at room temperature and incubation with
primary antibodies against TLR4 (1:1,000; cat. no. 19811-1-AP;
Proteintech Group, Inc.), GAPDH (1:2,500; cat. no. ab9485; Abcam),
myeloid differentiation factor 88 (MyD88; 1:1,000; cat. no. ab2064;
Abcam) and TIR-domain-containing adapter-inducing IFN-β (TRIF;
1:1,000; cat. no. ab13810; Abcam) overnight at 4˚C. The membranes
were rinsed using 0.1% TBST three times for 5 min each, before
being incubated with horseradish peroxidase-conjugated anti-rabbit
IgG secondary antibody (1:5,000; cat. no. ab288151; Abcam) for 1 h
at room temperature. The membranes were rinsed using 0.1% TBST
three times for 5 min each and developed using Pierce enhanced
chemiluminescence system (Thermo Fisher Scientific, Inc.). GAPDH
was used as the internal reference. Image J (Version 1.8.0,
National Institutes of Health) was used for the analysis of the
gray value.
Cell culture
PC12 cells were purchased from the American Type
Culture Collection and maintained in DMEM supplemented with 10% FBS
and 1% penicillin-streptomycin (all Invitrogen; Thermo Fisher
Scientific, Inc.) at 37˚C with 5% CO2.
Dual luciferase reporter assay
The potential interaction between TLR4 and
miR-30e-5p was predicted using MicroRNA Target Prediction Database
(mirdb.org). Rat wild-type TLR4 3'-untranslated region
(UTR), which contained potential binding sites for miR-30e-5p, was
cloned into the pmirGLO vector (Promega Corporation). Mutant TLR4
3'-UTR was obtained using Q5 Site-Directed Mutagenesis kit (cat.
no. E0554S; New England BioLabs, Inc.) according to the
manufacturer's protocol.
PC12 cells (2x105) were seeded in 6-well
plates and co-transfected with wild-type or mutant TLR4 luciferase
construct alongside mimic negative control (NC)
(5'-UUCUCCGAACGUGUCACGU-3') or miR-30e-5p mimic
(5'-UGUAAACAUCCUUGACUGGAAG-3') for 48 h at 37˚C using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. At 48 h
after transfection, luciferase activity was evaluated using the
Dual-Luciferase Reporter Assay System (Promega Corporation) and
normalized to that of the Renilla construct.
RNA immunoprecipitation (RIP)
assay
Firstly, nuclei were extracted using NP-40 lysis
buffer (240 µl, Beyotime Institute of Biotechnology) from PC12
cells (5x106) by centrifugation at 15,000 x g and 4˚C
for 10 min, lysed and incubated with primary antibodies against
argonaute2 (Ago2; 1: 30, 8 µl; cat. no. ab186733; Abcam) and
negative control IgG (1:30 (8 µl); cat. no. ab205718; Abcam) at 4˚C
overnight. RNA immunoprecipitated with Ago2 was isolated following
addition of 25 µl protein A agarose (cat. no. ab169993; Abcam) and
protein G agarose beads (cat. no. ab174816; Abcam) following
manufacturer's protocol. Completes were isolated by centrifugation
at 1,000 x g for 5 min at 4˚C and boiling for 3 min. After washing
with ice cold low salt wash buffer, RNA was purified and
reverse-transcribed into cDNA. Gene expression was assessed by
RT-qPCR, as aforementioned.
Statistical analysis
Data analysis was performed using SPSS 21.0 (IBM
Corp.). All experiments were repeated three times. All data are
presented as mean ± standard deviation. Unpaired t-test was used
for comparisons between two groups, while one-way analysis of
variance followed by Tukey's post hoc test was used for comparisons
between >2 groups. The correlation between miR-30e-5p and TLR4
was assessed by Pearson's correlation analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Verification of establishment of ICH
in rats
First, successful establishment of ICH in rats was
determined. The neurological function scores of rats in the ICH
group, including those of Longa (Fig.
1A), BBT (Fig. 1B), Bederson
(Fig. 1C) and foot fault asymmetry
(Fig. 1D), were significantly
higher compared with those in the sham group, indicating successful
establishment of ICH in rats.
miR-30e-5p expression is decreased and
TLR4 expression is increased following ICH
TLR4 mRNA expression levels were significantly
upregulated in the perihematomal striatum of rats at 24 h following
ICH compared with the sham group (Fig.
2A). To identify the potential role of miR-30e-5p following
ICH, miR-30e-5p expression was measured in the perihematomal
striatum from rats with ICH. miR-30e-5p expression was lower in the
perihematomal striatum of rats at 24 h following ICH compared with
the sham group (Fig. 2B). In
addition, there was an inverse correlation between TLR4 and
miR-30e-5p expression in the perihematomal striatum of rats at 24 h
following ICH (Fig. 2C). Moreover,
TLR4 protein expression levels (Fig.
2D and E) were upregulated in
the perihematomal striatum of rats at 24 h following ICH compared
with those in the sham group. The findings indicated the opposite
roles of miR-30e-5p and TLR4 in ICH.
TLR4 is a direct target of miR-30e-5p
in PC12 cells
The potential association between miR-30e-5p and
TLR4 was predicted by sequence complementarity according to miRDB
(Fig. 3A). To verify if miR-30e-5p
directly targets the 3'-UTR of TLR4, dual luciferase reporter assay
was performed in PC12 cells. miR-30e-5p mimic was effectively
transfected into PC12 cells, as evidenced by upregulated expression
levels of miR-30e-5p in the miR-30e-5p mimic compared with miR-NC
mimic group (Fig. 3B). In
addition, compared with miR-NC mimic, miR-30e-5p mimic inhibited
luciferase activity of wild-type TLR4 3'-UTR construct but not that
of the mutant construct (Fig. 3C).
The RIP assay showed that IgG did not enrich miR-30e-5p or Ago2
compared with input, indicating the success of RIP assay. RIP assay
revealed that both miR-30e-5p and TLR4 were significantly enriched
in the Ago2 group (Fig. 3D),
suggesting that miR-30e-5p directly targets the 3'UTR of TLR4 in
PC12 cells.
 | Figure 3TLR4 is a direct target of miR-30e-5p
in PC12 cells. (A) Association between miR-30e-5p and TLR4 was
predicted by MicroRNA Target Prediction Database. (B) Reverse
transcription-quantitative PCR analysis was used to detect
miR-30e-5p levels in miR-NC and miR-30e-5p mimic groups. (C) Dual
luciferase reporter assay was used to test luciferase activity.
**P<0.01, ***P<0.001 vs. miR-NC mimic.
(D) RIP assay was used to detect miR-30e-5p and TLR4 enrichment in
Anti-IgG, Input, and Anti-Ago2 group. n=3. **P<0.01
vs. Anti-IgG. miR, microRNA; TLR, Toll-like receptor; NC, negative
control; Ago2, argonaute2; UTR, untranslated region; WT, wild-type;
MUT, mutant. |
miR-30e-5p overexpression attenuates
neurological deficit and inflammation by targeting TLR4 following
ICH
To clarify the association between miR-30e-5p and
TLR4 in ICH, miR-30e-5p and TLR4 was overexpressed by IC
ventricular injection of miR-30e-5p mimic and pcDNA3.1-TLR4 3 days
prior to establishment of ICH in rats. miR-30e-5p was successfully
overexpressed in the perihematomal striatum of rats, which was
reflected by significantly increased miR-30e-5p expression levels
compared with those injected with miR-NC mimic (Fig. 4A). pcDNA3.1-TLR4 was successfully
transfected into the perihematomal striatum of rats, which was
shown by increased TLR4 expression compared with that in the
pcDNA3.1 group (Fig. 4B). The
neurological deficit of rats with ICH, including Longa (Fig. 4C), BBT (Fig. 4D), Bederson (Fig. 4E) and foot fault asymmetry
(Fig. 4F) was alleviated by
overexpression of miR-30e-5p; this effect was reversed by
co-overexpression of TLR4. Additionally, serum levels of
proinflammatory cytokines TNF-α, IL-1β, and IL-6 in rats were
decreased by overexpression of miR-30e-5p; this was reversed by
co-overexpression of TLR4 (Fig.
5A-C). The findings indicated the opposite roles of miR-30e-5p
and TLR4 in neurological deficit and inflammation.
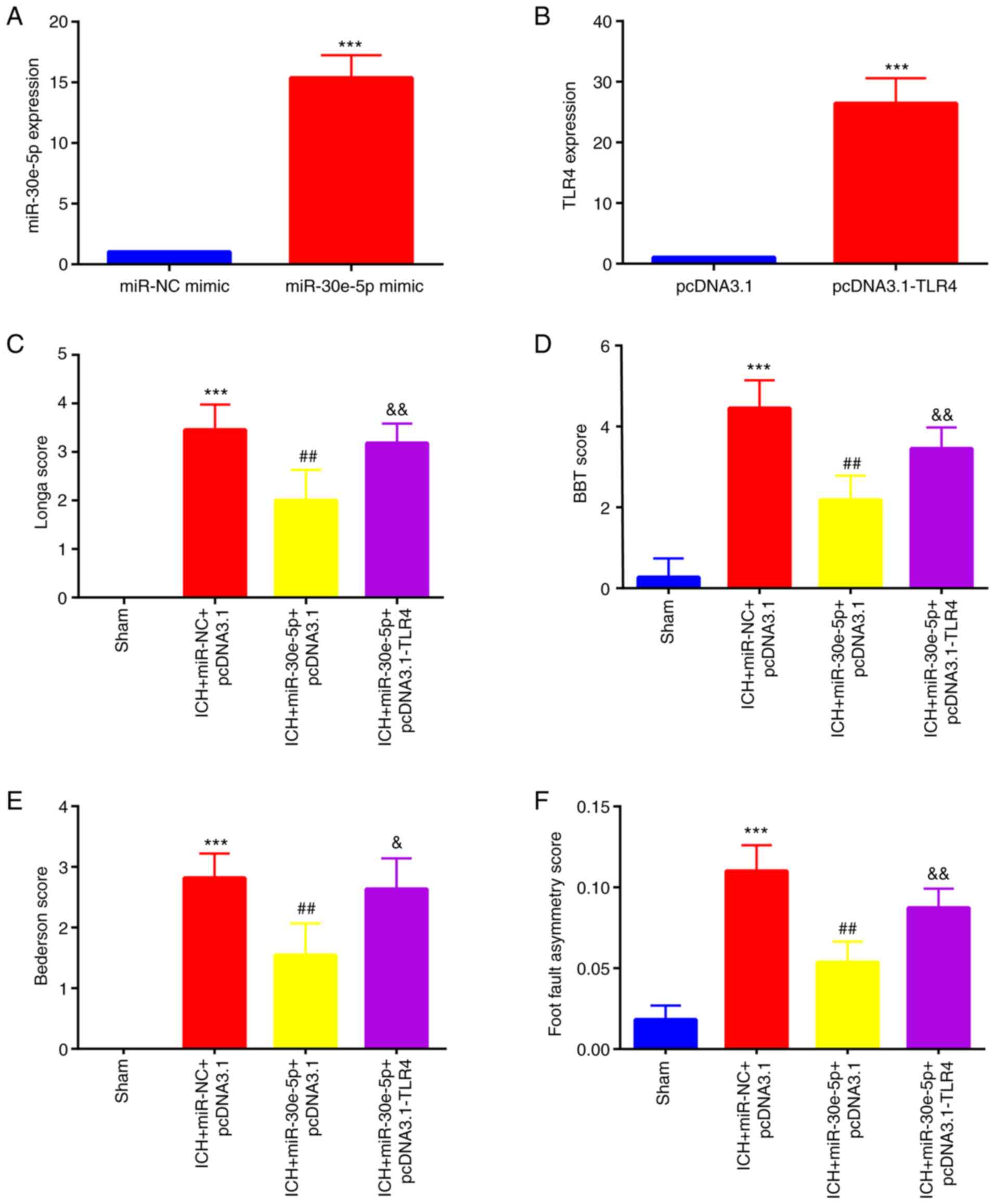 | Figure 4miR-30e-5p attenuates brain injury
following ICH by targeting TLR4. Reverse transcription-quantitative
PCR was used to detect (A) miR-30e-5p levels in miR-NC and
miR-30e-5p mimic (***P<0.001 vs. miR-NC mimic) and
(B) TLR4 level in pcDNA3.1 and pcDNA3.1-TLR4 group. n=3.
***P<0.001 vs. pcDNA3.1. A 24-point neurological
scoring system was used to test neurological deficit, including (C)
Longa, (D) BBT, (E) Bederson and (F) foot fault asymmetry (n=11) in
rats in sham, ICH + miR-NC + pcDNA3.1, ICH + miR-30e-5p + pcDNA3.1
and ICH + miR-30e-5p + pcDNA3.1-TLR4 groups.
***P<0.001 vs. sham; ##P<0.01 vs. ICH +
miR-NC + pcDNA3.1; &P<0.01,
&&P<0.01 vs. ICH + miR-30e-5p + pcDNA3.1.
ICH, intracerebral hemorrhage; BBT, beam balance test; miR,
microRNA; TLR, Toll-like receptor; NC, negative control. |
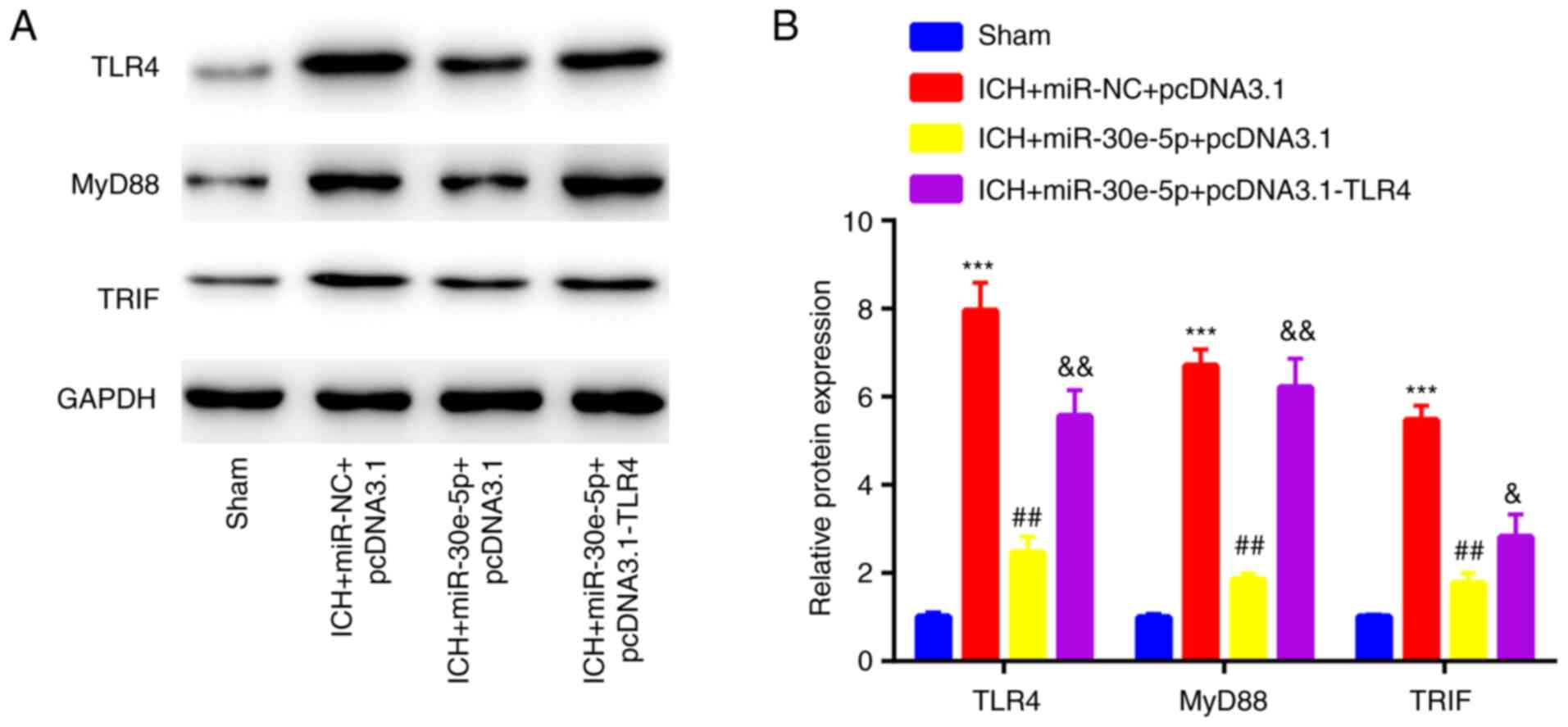
miR-30e-5p overexpression suppresses
TLR4 expression and inactivates downstream MyD88/TRIF signaling
following ICH
To determine whether the neuroprotective role of
miR-30e-5p in rats with ICH was associated with regulation of TLR4
and MyD88/TRIF signaling, the expression profile of associated
proteins was assessed in the perihematomal striatum of rats.
miR-30e-5p overexpression decreased TLR4 expression levels as well
as those of MyD88 and TRIF, which were originally induced by ICH.
However, the inhibitory effects of miR-30e-5p overexpression were
partially reversed by co-overexpression of TLR4 (Fig. 6A and B). The findings indicated the opposite
roles of miR-30e-5p and TLR4 in MyD88/TRIF signaling.
Discussion
ICH is a lethal form of stroke for which there is no
effective long-term treatment option (1). As a pathogen recognition receptor
(32), TLR4 is involved in
regulation of neuroinflammation during ICH (9). However, the role TLR4 in the
molecular pathological network of ICH is poorly understood. In the
present study, upregulation of TLR4 expression was observed in rats
with ICH, which suggested a role for TLR4 in ICH. In addition,
miR-30e-5p expression in rats with ICH demonstrated an inverse
correlation with TLR4 expression.
miR-30e-5p is associated with a number of diseases
(33-35).
p53-induced miR-30e-5p expression suppresses metastasis of
colorectal cancer by targeting integrin subunit α6 and β1
expression (33). In addition,
miR-30e-5p levels are decreased in the plasma of patients with
moderate and severe diabetic kidney disease compared with patients
with type 1 diabetes mellitus, where miR-30e-5p regulates
expression of genes associated with cell apoptosis,
differentiation, oxidative stress, angiogenesis and hypoxia
(34). In another study,
overexpression of miR-30e-5p was found to inhibit
endothelial-mesenchymal transition of lipopolysaccharide-treated
human brain microvascular endothelial cells by targeting neuronal
growth regulator 1 expression (35). However, little is known regarding
the association between miR-30e-5p and ICH or the potential
interaction between miR-30e-5p and TLR4 in ICH.
ICH induces inflammation to promote edema formation,
which triggers detrimental effects in the area surrounding the
hematoma, including cell death (7). Increased expression of apoptosis
marker cleaved caspase-3 and inflammatory cytokines, including
TNF-α, IL-1β and IL-6, has been previously reported in rats with
ICH (36). In the present study,
miR-30e-5p mimic inhibited TLR4 expression by targeting its 3'UTR.
In addition, miR-30e-5p mimic prevented neurological injury,
apoptosis and inflammation, as evidenced by rescued neurological
deficit score and TNF-α, IL-1β and IL-6 levels in rats with ICH.
The neuroprotective effect of miR-30e-5p mimic on rats with ICH was
partially reversed by overexpression of TLR4. However, the
underlying molecular mechanism of this remains to be
elucidated.
It has previously been documented that TLR4
regulates inflammation in ICH by regulating downstream MyD88/TRIF
signaling (37). TLR4 interacts
with MyD88 and TRIF to regulate gene expression of inflammatory
mediators, such as cytokines TNF-α, IL-1β and IL-6 (38,39).
The present study also investigated the effects of miR-30e-5p on
TLR4/MyD88/TRIF signaling. Overexpression of miR-30e-5p decreased
ICH-induced activation of TLR4/MyD88/TRIF signaling. Furthermore,
the effect of miR-30e-5p mimic on TLR4/MyD88/TRIF signaling was
partially reversed by overexpression of TLR4 in rats with ICH,
which demonstrated that the effect of miR-30e-5p mimic on
inactivation of TLR4 signaling may be due to its ability to inhibit
TLR4 expression. miR-30e-5p decreased luciferase activity of TLR4
construct in PC12 cells. miR-30e-5p was inversely correlated with
TLR4 expression and miR-30e-5p overexpression decreased TLR4
expression in rat brain following ICH.
PiggyBac, which is frequently used for gene
overexpression, can be inserted into genome to make overexpression
more durable (40); PiggyBac
should be used in future study to improve the stability of
miR-30e-5p overexpression. Additionally, the present study focused
on changes in behavioral performance; pathological images of brain
and neurons in sham and ICH groups will be obtained by another
ongoing study.
Collectively, the present results indicated that
miR-30e-5p overexpression decreased TLR4 expression in the brain
tissue of rats with ICH, resulting in inactivation of MyD88/TRIF
signaling and protection against neurological deficit, apoptosis
and inflammation. The present study provided mechanistic insight
into how miR-30e-5p exerts neurological protection in rats with
ICH.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HS and NX performed experiments and data analysis.
SJ designed and supervised the study and wrote and revised the
manuscript. All authors have read and approved the final
manuscript. HS and SJ confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The animal experiments were approved by the
Institutional Animal Care and Use Committee of Jinan First People's
Hospital (approval no. JNFPH2019-03; Ji'nan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xi G, Strahle J, Hua Y and Keep RF:
Progress in translational research on intracerebral hemorrhage: Is
there an end in sight? Prog Neurobiol. 115:45–63. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ziai WC and Carhuapoma JR: Intracerebral
hemorrhage. Continuum (Minneap Minn). 24:1603–1622. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
van Asch CJ, Luitse MJ, Rinkel GJ, van der
Tweel I, Algra A and Klijn CJ: Incidence, case fatality, and
functional outcome of intracerebral haemorrhage over time,
according to age, sex, and ethnic origin: A systematic review and
meta-analysis. Lancet Neurol. 9:167–176. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Qureshi AI, Tuhrim S, Broderick JP, Batjer
HH, Hondo H and Hanley DF: Spontaneous intracerebral hemorrhage. N
Engl J Med. 344:1450–1460. 2001.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rymer MM: Hemorrhagic stroke:
Intracerebral hemorrhage. Mo Med. 108:50–54. 2011.PubMed/NCBI
|
|
6
|
Rodríguez JA, Sobrino T, López-Arias E,
Ugarte A, Sánchez-Arias JA, Vieites-Prado A, de Miguel I, Oyarzabal
J, Páramo JA, Campos F, et al: cM352 reduces brain damage and
improves functional recovery in a rat model of intracerebral
hemorrhage. J Am Heart Assoc. 6(e006042)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sheth KN and Rosand J: Targeting the
immune system in intracerebral hemorrhage. JAMA Neurol.
71:1083–1084. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhou Y, Wang Y, Wang J, Anne Stetler R and
Yang QW: Inflammation in intracerebral hemorrhage: From mechanisms
to clinical translation. Prog Neurobiol. 115:25–44. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rivest S: Regulation of innate immune
responses in the brain. Nat Rev Immunol. 9:429–439. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Fang H, Wang PF, Zhou Y, Wang YC and Yang
QW: Toll-like receptor 4 signaling in intracerebral
hemorrhage-induced inflammation and injury. J Neuroinflammation.
10(27)2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang D, Shen X, Pang K, Yang Z and Yu A:
VSIG4 alleviates intracerebral hemorrhage induced brain injury by
suppressing TLR4-regulated inflammatory response. Brain Res Bull.
176:67–75. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sansing LH, Harris TH, Welsh FA, Kasner
SE, Hunter CA and Kariko K: Toll-like receptor 4 contributes to
poor outcome after intracerebral hemorrhage. Annal Neurol.
70:646–656. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang YC, Wang PF, Fang H, Chen J, Xiong XY
and Yang QW: Toll-like receptor 4 antagonist attenuates
intracerebral hemorrhage-induced brain injury. Stroke.
44:2545–2552. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu DZ, Tian Y, Ander BP, Xu H, Stamova
BS, Zhan X, Turner RJ, Jickling G and Sharp FR: Brain and blood
microRNA expression profiling of ischemic stroke, intracerebral
hemorrhage, and kainate seizures. J Cereb Blood Flow Metab.
30:92–101. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang Y, Song Y, Pang Y, Yu Z, Hua W, Gu Y,
Qi J and Wu H: miR-183-5p alleviates early injury after
intracerebral hemorrhage by inhibiting heme oxygenase-1 expression.
Aging (Albany NY). 12:12869–12895. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nie H, Hu Y, Guo W, Wang W, Yang Q, Dong
Q, Tang Y, Li Q and Tang Z: miR-331-3p inhibits inflammatory
response after intracerebral hemorrhage by directly targeting
NLRP6. Biomed Res Int. 2020(6182464)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang J, Zhu Y, Jin F, Tang L and He Z and
He Z: Differential expression of circulating microRNAs in blood and
haematoma samples from patients with intracerebral haemorrhage. J
Int Med Res. 44:419–432. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang S, Li G, Liu C, Lu S, Jing Q, Chen
X, Zheng H, Ma H, Zhang D, Ren S, et al: miR-30e-5p represses
angiogenesis and metastasis by directly targeting AEG-1 in squamous
cell carcinoma of the head and neck. Cancer Sci. 111:356–368.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang Z, Qin H, Jiang B, Chen W, Cao W,
Zhao X, Yuan H, Qi W, Zhuo D and Guo H: miR-30e-5p suppresses cell
proliferation and migration in bladder cancer through regulating
metadherin. J Cell Biochem. 120:15924–15932. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mo B, Wu X, Wang X, Xie J, Ye Z and Li L:
miR-30e-5p mitigates hypoxia-induced apoptosis in human stem
cell-derived cardiomyocytes by suppressing Bim. Int J Biol Sci.
15:1042–1051. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen Y, Yin Y and Jiang H: miR-30e-5p
alleviates inflammation and cardiac dysfunction after myocardial
infarction through targeting PTEN. Inflammation. 44:769–779.
2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li W, Li X, Li T, Jiang MG, Wan H, Luo GZ,
Feng C, Cui X, Teng F, Yuan Y, et al: Genetic modification and
screening in rat using haploid embryonic stem cells. Cell Stem
Cell. 14:404–414. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li T, Shuai L, Mao J, Wang X, Wang M,
Zhang X, Wang L, Li Y, Li W and Zhou Q: Efficient production of
fluorescent transgenic rats using the piggyBac Transposon. Sci Rep.
6(33225)2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jung KH, Chu K, Jeong SW, Han SY, Lee ST,
Kim JY, Kim M and Roh JK: HMG-CoA reductase inhibitor,
atorvastatin, promotes sensorimotor recovery, suppressing acute
inflammatory reaction after experimental intracerebral hemorrhage.
Stroke. 35:1744–1749. 2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ge XT, Lei P, Wang HC, Zhang AL, Han ZL,
Chen X, Li SH, Jiang RC, Kang CS and Zhang JN: miR-21 improves the
neurological outcome after traumatic brain injury in rats. Sci Rep.
4(6718)2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bachour SP, Hevesi M, Bachour O, Sweis BM,
Mahmoudi J, Brekke JA and Divani AA: Comparisons between Garcia,
Modo, and Longa rodent stroke scales: Optimizing resource
allocation in rat models of focal middle cerebral artery occlusion.
J Neurol Sci. 364:136–140. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yue X, Liu L, Yan H, Gui Y, Zhao J and
Zhang P: Intracerebral hemorrhage induced brain injury is mediated
by the interleukin-12 receptor in rats. Neuropsychiatr Dis Treat.
16:891–900. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liu H, Shen H, Harvey BK, Castillo P, Lu
H, Yang Y and Wang Y: Post-treatment with amphetamine enhances
reinnervation of the ipsilateral side cortex in stroke rats.
NeuroImage. 56:280–289. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Han J, Zhang J, Zhong Z, Li Z, Pang W, Hu
J and Chen L: Gualou Guizhi decoction promotes neurological
functional recovery and neurogenesis following focal cerebral
ischemia/reperfusion. Neural Regen Res. 13:1408–1416.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Molteni M, Gemma S and Rossetti C: The
role of toll-like receptor 4 in infectious and noninfectious
inflammation. Mediators Inflamm. 2016(6978936)2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Laudato S, Patil N, Abba ML, Leupold JH,
Benner A, Gaiser T, Marx A and Allgayer H: P53-induced miR-30e-5p
inhibits colorectal cancer invasion and metastasis by targeting
ITGA6 and ITGB1. Int J Cancer. 141:1879–1890. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Dieter C, Assmann TS, Costa AR, Canani LH,
de Souza BM, Bauer AC and Crispim D: MiR-30e-5p and MiR-15a-5p
expressions in plasma and urine of type 1 diabetic patients with
diabetic kidney disease. Front Genet. 10(563)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Dong Y, Fan X, Wang Z, Zhang L and Guo S:
Circ_HECW2 functions as a miR-30e-5p sponge to regulate LPS-induced
endothelial-mesenchymal transition by mediating NEGR1 expression.
Brain Res. 1748(147114)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang SD, Cui YJ, Xu JQ and Gao H:
miR-140-5p attenuates neuroinflammation and brain injury in rats
following intracerebral hemorrhage by targeting TLR4. Inflammation.
42:1869–1877. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lin S, Yin Q, Zhong Q, Lv FL, Zhou Y, Li
JQ, Wang JZ, Su BY and Yang QW: Heme activates TLR4-mediated
inflammatory injury via MyD88/TRIF signaling pathway in
intracerebral hemorrhage. J Neuroinflamm. 9(46)2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Miyake K: Endotoxin recognition molecules
MD-2 and toll-like receptor 4 as potential targets for therapeutic
intervention of endotoxin shock. Curr Drug Targets Inflamm Allergy.
3:291–297. 2004.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Takeda K and Akira S: TLR signaling
pathways. Semin Immunol. 16:3–9. 2004.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhang W, Tian Y, Gao Q, Li X, Li Y, Zhang
J, Yao C, Wang Y, Wang H, Zhao Y, et al: Inhibition of apoptosis
reduces diploidization of haploid mouse embryonic stem cells during
differentiation. Stem Cell Reports. 15:185–197. 2020.PubMed/NCBI View Article : Google Scholar
|