Introduction
Although the first description of subependymoma was
reported by Scheinker in 1945, the exact histogenesis remains
unknown (1,2). Subependymoma occurs more commonly in
men and those who are middle-aged or older (3,4). The
vast majority of subependymoma cases are asymptomatic and detected
only as incidental findings. Symptomatic subependymomas are
typically associated with cerebral spinal fluid (CSF) obstructions,
which lead to intracranial hypertension symptoms (such as headache,
vomiting, papilledema, and impaired consciousness) or symptoms
caused by the compression of nerve structures (such as convulsions,
motor paralysis, and sensory disturbances) (2,4).
Subependymomas commonly present as isolated lesions that arise in
the fourth ventricle or the frontal horn of the lateral ventricle
(3,5). To our knowledge, few studies have
reported the use of magnetic resonance perfusion (MRP) or magnetic
resonance spectroscopy (MRS) in the assessment of subependymoma. In
this article, we describe a case presenting with bilateral
subependymomas in the lateral ventricles, including the clinical
features, imaging results from conventional magnetic resonance
imaging (MRI), MRS, and MRP, histological outcomes, and the disease
management approach.
Case report
A 40-year-old Asian man presented at our institution
complaining of a bilateral parietal-occipital headache and
dizziness that lasted for 6 months. He denied experiencing vomiting
or nausea. Upon physical examination, the patient was completely
conscious, with a Glasgow Coma Scale score of 15. No symptoms of
motor paralysis, sensory disturbances, or visual problems were
documented, and laboratory tests were within normal limits.
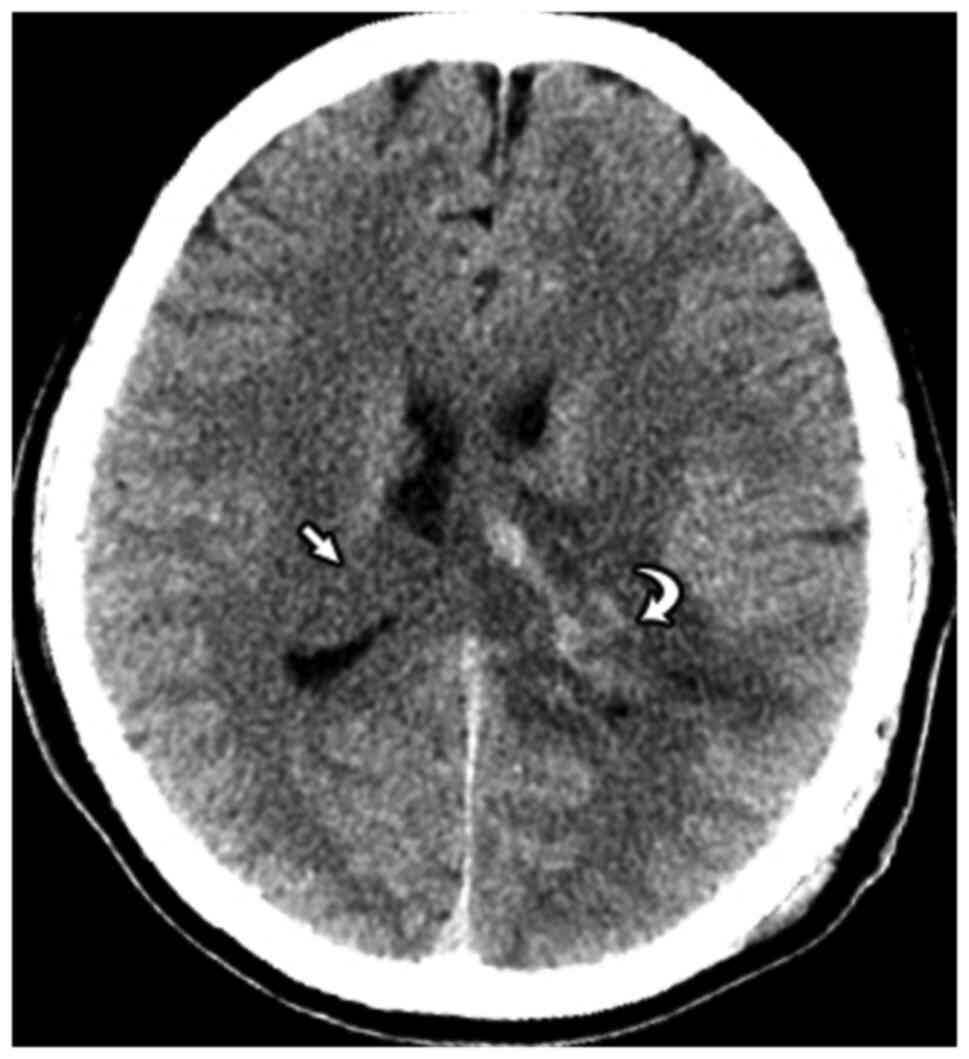
On MRI, we detected 2 large, well-defined, lobulated
masses located at the trigone and the occipital horn of the
bilateral lateral ventricles; the right lesion measured 42x18x19
mm, and the left lesion measured 43x21x25 mm. Both masses displayed
similar signal intensity as the normal gray matter on T1-weighted
(T1W) imaging, showed heterogeneous hyperintensity on T2-weighted
(T2W) imaging and fluid-attenuated inversion recovery (FLAIR)
imaging, displayed no evidence of restriction on diffusion-weighted
imaging (DWI), and were not enhanced by the use of a contrast
agent. Some small intramural cysts were observed, but no evidence
of calcification, hemorrhage, or hydrocephalus was noted. The
adjacent brain parenchyma showed no evidence of paraventricular
extension or infiltration. (Fig.
1A-F).
 | Figure 1Imaging results. Magnetic resonance
imaging (MRI) sequences, including (A) axial fluid-attenuated
inversion recovery (FLAIR), (B) coronal T2-weighted (T2W), (C)
axial diffusion-weighted imaging (DWI), (D) axial T2*, (E) axial
T1-weighted (T1W) non-contrast, and (F) axial T1W post-contrast,
show bilateral lateral ventricular masses (white arrows in A-F),
which appeared hyperintense on FLAIR and T2W sequences. The masses
were characterized by small regions of cystic degeneration, no
surrounding edema, no diffusion restriction, no intratumoral
hemorrhage, and no contrast enhancement. (G and H) Perfusion images
revealed some intratumoral areas of hyperperfusion (arrowheads). (I
and J) Magnetic resonance spectroscopy (MRS) multivoxel images show
an increase in choline (Cho) peaks and a decrease in N-acetyl
aspartate (NAA) peaks for both the right and left masses. |
MRP revealed high cerebral blood volume (CBV) in
some areas within these lesions. The relative CBV (rCBV) of the
right lesion was 1.97, and the rCBV of the left lesion was 1.3
(Fig. 1G and H). On multivoxel MRS, both lesions showed
high choline (Cho) and low N-acetyl aspartate (NAA) peaks, but the
Cho/NAA ratio of the left mass was 1.21, whereas the same ratio in
the right mass was 6.81. (Fig. 1I
and J).
Several days after admission, the patient underwent
partial resection surgery for the left ventricular tumor. A
postoperative computed tomography (CT) image demonstrated a large
isointense mass without evidence of calcification or hemorrhage in
the trigone and occipital horn of the right lateral ventricle, with
no signs of parenchymal invasion. A mixed-density mass was observed
in the trigone and occipital horn of the left lateral ventricle,
with hyperintense areas due to hemorrhage, and evidence of
postoperative paraventricular edema (Fig. 2).
Histopathologically, the macroscopic image showed a
soft tissue mass with pinkish surface color and no hemorrhage.
Microscopically, the tumor was composed of a cluster of epithelial
cells with round, basophilic nuclei and a few thick, hyalinized
vessels (Fig. 3A).
Immunohistochemical staining showed that the tumor cells were
positive for epithelial membrane antigen (EMA) and negative for
oligodendrocyte transcription factor 2 (OLIG2) and glial fibrillary
acidic protein (GFAP) (Fig. 3B-D).
The histopathological and immunohistochemical results were
consistent with a diagnosis of subependymoma.
Discussion
Subependymomas are uncommon benign tumors of the
central nervous system (WHO grade I) (6) and account for 0.2-0.7% of all
intracranial tumors (7).
Subependymomas tend to occur in older individuals, typically
presenting between the ages of 60 and 80 years, with a slight male
predominance (male to female ratio of 2.3 to 1) (3,8).
Typically, subependymomas (<2 cm), sharply demarcated nodules,
are identified as incidental findings associated with subtle to no
clinical symptoms. Unfortunately, during operation, there were no
macroscopic photographs obtained for this case to contribute to
medical literature. Symptomatic subependymomas are generally larger
lesions (3-5 cm) or small neoplasms that obstruct the flow of
cerebral spinal fluid (CSF), resulting in hydrocephalus (5,9).
Subependymomas are most commonly detected in the
fourth ventricle (50-60% of cases) or the lateral ventricle (30-40%
of cases), predominantly in the frontal horn (3,5).
Occasionally, subependymomas are detected in the third ventricle,
cerebellar pontine angle, or intraparenchymal and intraspinal
regions (7,10). Isolated subependymomas are the most
frequently detected type, whereas bilateral subependymomas are
uncommon. In our search of the literature, only 4 cases of
bilateral subependymomas have been reported (Table I) (4,9,11,12).
 | Table IReported cases of bilateral
subependymoma in the literature. |
Table I
Reported cases of bilateral
subependymoma in the literature.
| Number | Authors (Refs.) | Year of
publication | Sex of patients | Age of patient
(years) | Clinical
symptoms |
|---|
| 1 | Rath et al
(9) | 2005 | Male | 20 | Severe headache and
altered mental status |
| 2 | Kumar et al
(11) | 2012 | Male | 25 | No information
available |
| 3 | Miguel et al
(4) | 2015 | Female | 69 | Headache, ataxia,
apraxia, right-sided weakness, and neglect |
| 4 | Moinuddin et
al (12) | 2017 | Male | 48 | Seizure |
| 5 | Clinical case | 2022 | Male | 40 | Headache,
vertigo |
Upon CT, subependymomas typically present as
lobulated, well-defined masses that are hypointense or isointense
relative to the normal brain parenchyma, and extra-ventricular
invasion is uncommonly observed (9). Hydrocephalus presents in 85% of cases
(5). Cystic degeneration and
calcification are also common characteristics, particularly for
infratentorial neoplasms (3,13).
Upon magnetic resonance imaging (MRI), subependymomas are
characterized by hypointensity or isointensity on T1W (compared
with the normal white matter), hyperintensity on T2W, and no
evidence of restricted diffusion on DWI. Histological examination
often reveals that the heterogeneous signals observed on MRI are
due to necrosis, calcification, tiny areas of cystic degeneration,
and hemorrhage. Peritumoral edema tends to be absent. Following the
administration of a contrast agent (gadolinium), variable
enhancement patterns have been described for subependymomas.
Supratentorial subependymomas are often poorly enhanced or not
enhanced, whereas infratentorial masses display heterogeneous
enhancement (3,13). Our patient displayed similar
enhancing features to the patients described by Rath et al
and Kumar et al (9,11). Similar to reports by Miguel et
al and Moinuddin et al, our patient showed no evidence
of hemorrhage (4,12).
The performance of magnetic resonance perfusion
(MRP) or magnetic resonance spectroscopy (MRS) for the diagnosis of
subependymomas has not been frequently reported. A previous study
by Rumboldt concluded that subependymomas are typically
characterized by hypoperfusion (14). Hyperperfusion of subependymomas
reported in this case was in contrast to the common hypoperfusion
characteristic of subependymoma described by Rumboldt (14). In 2012, Abdel-Aal et al
(13) reported an intraventricular
subependymoma in the left lateral ventricle. The authors noticed
several areas characterized by hyperperfusion, with an rCBV value
of 3.91. Our findings were consistent with the results reported by
Abdel-Aal et al, as the rCBV value of the left tumor was 2,
and that of the right tumor was 1.3. Abdel-Aal et al
(13) speculate that
hyperperfusion in subependymomas may be caused by the presence of
thick hyalinized vessels rather than the neoangiogenesis observed
in typical high-grade tumors. MRS results in low-grade tumors are
generally characterized by normal choline (Cho) levels and slightly
reduced N-acetyl aspartate (NAA) levels (7). Several differences were observed
between the 2 neoplasms in our case. The right-sided mass showed
slightly high Cho and low NAA levels (Cho/NAA ratio of 1.21),
whereas the left-sided mass showed high Cho and low NAA levels
(Cho/NAA ratio of 6.81). Both tumors manifested appeared to present
high Cho values due to increased cellular activity.
Choroid plexus xanthogranuloma (CPX), choroid plexus
papillomas (CPP), choroid plexus carcinomas (CPC), and metastasis
are possible differential diagnoses for bilateral subependymomas.
CPP and CPC are less likely due to the patient's age, as these
entities are most often detected in children, and these entities
tend to display enhancing features (15). CPX is typically smaller than 1 cm,
appears strongly restricted on DWI, and presents with either
heterogeneous or rim-like enhancement on the post-contrast sequence
(16). Ventricular metastasis
typically presents with vivid enhancement and surrounding vasogenic
edema (3). Our patient was younger
than the typical age group associated with subependymoma
occurrence, and the trigone and occipital horn are not common
locations for subependymomas. However, based on the other imaging
characteristics and the lack of post-contrast enhancement,
subependymoma was our initial diagnosis.
The microscopic appearance of subependymomas is
characterized by multiple clusters of cells embedded in a dense
gliofibrillary matrix with cystic degeneration, and hyalinized
vessels are not rare findings (2,7,13).
Immunohistologically, subependymomas are commonly positive for
oligodendrocyte transcription factor 2 (OLIG2) and glial fibrillary
acidic protein (GFAP) and negative for epithelial membrane antigen
(EMA) (2,13). Although most of these typical
features were demonstrated in our case, GFAP and OLIG2 staining
were negative. Our results were similar to the findings obtained by
Belgian authors in 2015, in which 2 of 43 individuals were negative
for GFAP and 1 of 43 individuals were negative for OLIG2(10). By contrast, similar to our
findings, the report by Moinuddin et al described EMA
positivity (12). The origins of
subependymomas remain controversial. Several prior theories have
suggested that subependymomas arise from subependymal glia,
astrocytes of the subependymal plate, or ependymal cells. Due to
the lack of clear cellular origins, no immunohistological
classifications have been defined for the identification of
subependymoma (2). We believe that
the pathological findings for the left-sided mass removed from our
patient are most consistent with a diagnosis of typical
subependymoma.
Generally, the prognosis of subependymomas is good.
Subependymomas identified on incidental findings can be managed by
conservative treatment. Individuals with symptomatic subependymomas
are commonly treated by total surgical excision. However, when a
subependymoma appears in some critical areas, partial resection
could also be a favorable approach, and the maintenance of CSF flow
should be prioritized (2). To our
knowledge, radiotherapy and chemotherapy are unnecessary, even when
only a partial resection can be performed (8,9). The
recurrence rate of subependymoma is 7.9% (10). Varma et al (8) concluded that few patients exhibit any
recurrent masses following subependymoma resection on MRI imaging
after medium- to long-term follow-up, suggesting that shorter
follow-up times may be sufficient.
In conclusion, the classical appearance of
subependymoma is an isolated lesion arising from the fourth
ventricle and the frontal horn of the lateral ventricle. However,
atypical subependymomas presenting as bilateral intraventricular
neoplasms in the lateral ventricles can result in diagnostic
challenges. By focusing on the enhancing features, MRS and MRP can
be useful for providing accurate diagnoses and facilitating better
treatment planning.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
NDM and NDH contributed equally to this article as
co-first authors. NDM, NDH, and NMD were the patient's physicians,
reviewed the literature and contributed to acquisition, analysis
and interpretation of data and manuscript drafting. NDH and NMD
contributed to manuscript drafting and acquisition of data. DTG,
NQD, and PNH analyzed and interpreted the imaging findings. NDH and
NMD reviewed the literature, and contributed to conception and
design of the study, analysis and interpretation of data, and
drafted, reviewed and edited the manuscript. NDM and NDH confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent for patient information to
be published in this article was obtained.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Scheinker IM: Subependymoma: A newly
recognized tumor of subependymal derivation. J Neurosurg.
2:232–240. 1945.
|
|
2
|
Hernández-Durán S, Yeh-Hsieh TY and
Salazar-Araya C: Pedunculated intraventricular subependymoma:
Review of the literature and illustration of classical presentation
through a clinical case. Surg Neurol Int. 5(117)2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Smith AB, Smirniotopoulos JG and
Horkanyne-Szakaly I: From the radiologic pathology archives:
Intraventricular neoplasms: Radiologic-pathologic correlation.
Radiographics. 33:21–43. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Miguel Y, Jansen G and Alkherayf F: Unique
occurrence of a subependymoma presenting bilaterally with
hemorrhage: A case report. Open J Modern Neurosurg. 05:59–63.
2015.
|
|
5
|
Koeller KK and Sandberg GD: Armed Forces
Institute of Pathology. From the archives of the AFIP. Cerebral
intraventricular neoplasms: Radiologic-pathologic correlation.
Radiographics. 22:1473–1505. 2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ragel BT, Osborn AG, Whang K, Townsend JJ,
Jensen RL and Couldwell WT: Subependymomas: An analysis of clinical
and imaging features. Neurosurgery. 58:881–890; discussion 881-890.
2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Varma A, Giraldi D, Mills S, Brodbelt AR
and Jenkinson MD: Surgical management and long-term outcome of
intracranial subependymoma. Acta Neurochir (Wien). 160:1793–1799.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rath TJ, Sundgren PC, Brahma B, Lieberman
AP, Chandler WF and Gebarski SS: Massive symptomatic subependymoma
of the lateral ventricles: Case report and review of the
literature. Neuroradiology. 47:183–188. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bi Z, Ren X, Zhang J and Jia W: Clinical,
radiological, and pathological features in 43 cases of intracranial
subependymoma. J Neurosurg. 122:49–60. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kumar R, Sarkari A and Kakkar A: Mirror
image subependymoma. Neurol India. 60:684–685. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Moinuddin FM, Ikbar Khairunnisa N, Hirano
H, Hanada T, Hiraki T, Kirishima M, Kamimura K and Arita K:
Bilateral lateral ventricular subependymoma with extensive
multiplicity presenting with hemorrhage. Neuroradiol J. 31:27–31.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Abdel-Aal AK, Hamed MF, Al Naief NS,
Vattoth S and Bag A: Unusual appearance and presentation of
supratentorial subependymoma in an adult patient. J Radiol Case
Rep. 6:8–16. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rumboldt Z: Subependymoma. In: Brain
Imaging with MRI and CT: An Image Pattern Approach. Rumboldt Z,
Castillo M, Huang B and Rossi A (eds). Cambridge University Press,
Cambridge, pp353-354, 2012.
|
|
15
|
Jaiswal S, Vij M, Mehrotra A, Kumar B,
Nair A, Jaiswal AK, Behari S and Jain VK: Choroid plexus tumors: A
clinico-pathological and neuro-radiological study of 23 cases.
Asian J Neurosurg. 8:29–35. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yetkinel S and Bek S: Choroid plexus
xanthogranuloma: Is it an incidental finding? Turk J Neurol.
22:194–195. 2016.
|