Introduction
Gastric cancer is a common malignancy of the
digestive system that has a high incidence rate in East Asia
(1,2). In China, the incidence of gastric
cancer ranks as the 2nd highest among all malignant cancers
(3). Chemotherapy is a common
strategy used for the treatment of gastric cancer (4). However, whilst chemotherapy can
prolong disease-free survival and overall survival for certain
patients with this disease, several limitations exist in terms of
the efficacy of this therapy (5).
Over time, tumor cells become resistant to therapies initially used
for the treatment of the disease and to drugs with different
chemical structures and mechanisms of action (6,7). Both
primary and secondary resistance markedly limits the efficacy of
chemotherapy in patients with gastric cancer (6,7).
5-fluorouracil (5-FU) is the most commonly applied
chemotherapeutic agent for patients with gastric cancer that is
frequently used in combination with cisplatin, oxaliplatin and
calcium folinate as an adjuvant treatment (8,9).
Unfortunately, numerous patients with gastric cancer become
insensitive to 5-FU over time, which eventually diminishes the
efficacy of chemotherapy (10).
Therefore, studying gastric cancer cell resistance to 5-FU may aid
in predicting the effectiveness of chemotherapy in patients.
HIT000218960 is a recently discovered long noncoding RNA (lncRNA)
that is highly expressed in gastric (11) and papillary thyroid cancer (12). Furthermore, the Sun et al
(11) recently demonstrated that
HIT000218960 promotes gastric cancer cell proliferation and
migration through upregulation of the high mobility group A2
(HMGA2) protein.
The association between HIT000218960 and the effect
of chemotherapy on gastric cancer remains poorly understood.
Therefore, the present study analyzed the association between
HIT000218960 expression and the efficacy of 5-FU chemotherapy in
patients with gastric cancer. In addition, the molecular mechanism
of the effects of HIT000218960 on gastric cancer responses to 5-FU
was also explored in vitro.
Materials and methods
Patients
A total of 37 normal gastric mucosal tissues from
gastroscopy physical examination (17 females and 20 males; 38-69
years old; 60.3±5.8 years old; between January 2015 and June 2016
in The 970th Hospital of the PLA Joint Logistics Support Force,
Yantai, China) and 71 gastric cancer tissues from the surgical
removal of tumor, extracted during biopsy were used in the present
study. Patient data for the 71 gastric cancer cases (43 females and
28 males; 32-73 years old; 62.3±5.8 years old; between January 2015
and June 2016 in The 970th Hospital of the PLA Joint Logistics
Support Force) are provided in Table
I. TNM classification for gastric cancer was based on ‘Seventh
edition of TNM classification for gastric cancer’ (13).
 | Table IBasic clinical data of the 71
patients with gastric cancer. |
Table I
Basic clinical data of the 71
patients with gastric cancer.
| Variable | N (%) |
|---|
| Sex | |
|
Male | 43 (60.56) |
|
Female | 28 (39.44) |
| Age (years) | |
|
≥60 | 40 (56.34) |
|
<60 | 31 (43.66) |
| Tumor diameter
(cm) | |
|
≥3 | 48 (67.61) |
|
<3 | 23 (32.39) |
| TNM stage | |
|
I | 30 (42.25) |
|
II | 37 (52.11) |
|
III | 4 (5.64) |
| Histological
grade | |
|
I | 18 (25.35) |
|
II | 42 (59.15) |
|
III | 11 (15.50) |
| Lymph node
metastasis (n) | |
|
0 | 9 (12.68) |
|
1-2 | 14 (19.71) |
|
3-6 | 16 (22.54) |
|
≥7 | 32 (45.07) |
The inclusion criteria for patients with gastric
cancer included the following: i) Patients were pathologically
diagnosed with only gastric cancer; ii) Patients were aware of the
contents of this study; and iii) ≥18 years old. The inclusion
criteria for volunteers for normal mucosal tissue included the
following: i) Volunteers were determined to be free of any
malignant tumors and were healthy; ii) volunteers were aware of the
contents of this study; and iii) ≥18 years old.
Gastric patients exclusion criteria included the
following: i) Diagnosis with another malignant tumor, severe
cerebrocardiovascular disease or organ dysfunction; ii) lack of
basic and 3-year follow-up records; iii) death from other illnesses
or accident; iv) individuals who were pregnant, lactating or
diagnosed with chronic viral (human immunodeficiency virus or
Hepatitis B or C) or bacterial infections (M. tuberculosis);
v) individuals who underwent chemotherapy, immunotherapy or
targeted therapy prior to tissue acquisition; vi) individuals who
received chemotherapy that differed from specified methodology.
Exclusion criterion for individuals who donated 37 normal gastric
mucosal tissues was those who were diagnosed with any malignant
tumor or stomach disease.
The specified methodology consisted of 5 days of
treatment with an intravenous infusion of 425-750
mg/m2/day 5-fluorouracil (cat. no. H31020593; Shanghai
Xudonghaipu Pharmaceutical Co., Ltd.). On day 1 of treatment, an
intravenous infusion of 60-80 mg/m2cisplatin (cat. no.
H37021358; Qilu Pharmaceutical Co., Ltd.) was administered.
Treatment was given every 3 weeks for a total of 8 treatments.
After the chemotherapy treatment regimens ended, patient were
followed up for 3 years and were evaluated according to the
response evaluation criteria of solid tumors published in
2000(14). Complete response (CR)
is defined by the observation of the tumor disappearing completely.
Partial response (PR) is defined by the tumor receding by >50%,
osteolytic lesion abbreviation and partial calcification. No
response (NR) is defined by those who did not meet the evaluation
criteria of CR and PR.
All patients with gastric cancer were treated at The
970th Hospital of the PLA Joint Logistics Support Force (Yantai,
China) from January 2015 to June 2016. All patients were informed
and signed a consent form. All experiments involving human tissues
were approved and supervised by The Ethics Committee of The 970th
Hospital of the PLA Joint Logistics Support Force.
Reverse transcription-quantitative PCR
(RT-qPCR)
Liquid nitrogen was used to homogenize normal
gastric mucosal tissues and gastric cancer tissues prior to using
RNAiso plus (cat. no. 9108; Takara Bio, Inc.) to extract total RNA
according manufacturer's protocol. RNAiso plus was also used to
extract total RNA from cells (GES-1, SNU-5, SNU-1, SNU-16, AGS and
NCI-N87). cDNA was then synthesized using a PrimeScript™ RT reagent
kit containing the gDNA eraser (cat. no. RR047A; Takara Bio Inc.)
using the temperature protocol of 37˚C for 15 min and 85˚C for 5
sec. qPCR was then prepared using GoTaq® qPCR Master Mix
(cat. no. A6001; Promega Corporation), according to the
manufacturer's protocol. The thermocycling conditions were as
follows: Initial denaturation at 95˚C for 2 min, followed by 40
cycles of 95˚C for 15 sec and 60˚C for 30 sec. The sequences of the
qPCR primers used in this assay were from a previous study
(12): HIT000218960 forward,
5'-CGTGGAAAACTCCTAAATGTGT-3' and reverse,
5'-TCTATTACATGATGTTGCAGGC-3' and β-actin forward,
5'-AGCACAATGAAGATCAAGATCAT-3' and reverse,
5'-ACTCGTCATACTCCTGCTTGC-3'. The relative expression of
HIT000218960 was calculated using the 2-ΔΔCq method
(15) and β-actin was used as the
reference gene. The 71 gastric cancer tissues were subsequently
divided into the low (n=39) and high expression groups (n=32) in
accordance with the mean HIT000218960 values, before the 3-year
overall survival of the two groups of patients were compared.
Cell Culture
GES-1, SNU-5, SNU-1, SNU-16, AGS and NCI-N87 cell
lines were purchased from American Type Culture Collection and
cultured in DMEM (cat. no. 61870044; Thermo Fisher Scientific,
Inc.) containing 10% FBS (cat. no. 10437028; Thermo Fisher
Scientific, Inc.) at 37˚C with 5% CO2. For treatment, 50
µmol/l 5-FU (cat. no. 51-21-8; Sigma-Aldrich; Merck KGaA) were
added into the cell culture medium to stimulate cells for 24 h at
37˚C with 5% CO2.
Apoptosis
An Annexin V FITC/propidium iodide kit (Invitrogen;
Thermo Fisher Scientific, Inc.) was used to analyze apoptosis rate
in SNU-5, SNU-1, SNU-16, AGS and NCI-N87 cells after being
stimulated with 50 µmol/l 5-FU for 24 h at 37˚C. In each staining
reaction, 5 µl Annexin FITC and 10 µl PI staining solution were
used to stain 5x105 cells for 15 min at room
temperature. Beckman CytoFLEX flow cytometer (CytoFLEX; Beckman
Coulter, Inc.) was used for apoptosis analyses and data were
analyzed by FlowJo (X10.0.7; Stanford University). Early (Lower
right quadrant) + late (upper right quadrant) apoptotic cells were
analyzed.
Transfection
The SNU-5 and NCI-N87 cell lines overexpressing
HIT000218960 was established by Genomeditech Co. and was verified
using qPCR, with cells transfected with the empty plasmid (Lv-NC)
as the negative control. Briefly, the sequence of HIT000218960 was
obtained from the H-InvDB database (https://www.h-invitational.jp/). Jiman Biotechnology
(Shanghai) Co., Ltd. then prepared a lentivirus (MOI=20) for
overexpressing HIT000218960 according to these sequences. In
addition, siDirect version 2.0 (https://sidirect2.rnai.jp/) was used to design small
interfering (si-) RNA sequence based on the sequence of
HIT000218960 from the H-InvDB database. si-RNA sequence used for
verification included si-HIT000218960 guide RNA oligo sequences,
5'-ACCGAACUUUCGCCGAUUCUG-3' and passenger RNA oligo sequences,
5'-CAACCUAUCCAACUUCAAUUG-3'; and si-HMGA2 guide RNA oligo
sequences, 5'-UCUUUAGCUAUAUUUUCUGCA-3' and passenger RNA oligo
sequences, 5'-CAGAAAAUAUAGCUAAAGAGU-3' (Sangon Biotech, Co., Ltd.).
A negative control siRNA was used (Si-NC; guide RNA oligo
sequences, 5'-GUUCUCGAUUAUUCCGUUCA-3' and passenger RNA oligo
sequences, 5'-GAUCGCCAUAUGAUCAAGAGAU-3'). Gastric cancer cells
(2.5x106) were transfected with 50 nM siRNA using
Lipofectamine® 2000, according to the manufacturer's
protocol. Subsequent experiments were performed 72 h
post-transfection.
Western blotting
Total protein was extracted from SUN-5 and NCI-N87
cells using a lysis buffer (20 mmol/l Tris-Hcl; 2.5 mmol/L EDTA;
150 mmol/l NaCl; 0.5% NP-40; pH=8.0) (cat. no. P0013; Beyotime
Institute of Biotechnology) containing PMSF (cat. no. ST605;
Beyotime Institute of Biotechnology). After measuring total protein
concentrations using a bicinchoninic acid protein assay kit (cat.
no. P0012S; Beyotime Institute of Biotechnology), 40 µg total
protein/lane was separated using 10% SDS-PAGE. The protein was then
transferred to PVDF membranes. Membranes were blocked in 5% skimmed
milk at room temperature for 1 h prior to being incubated with
primary antibodies overnight at 4˚C. The membranes were then
incubated with secondary antibodies at room temperature for 2 h.
Following washing with PBS-Tween-20 (0.05%) three times, ECL
solution (cat. no. WBKLS0100; Beijing Xinjingke Biotechnologies
Co., Ltd.) was added to membranes to visualize the protein bands.
Image J software (v1.8.0; National Institutes of Health) was used
to analyze the protein band gray value. All antibodies used in the
present study were purchased from Abcam, and the information of
antibodies were showed as follows: Phosphorylated (p-) AKT (cat.
no. ab38449; 1:500), AKT (cat. no. ab8805; 1:1,000), p-mTOR (cat.
no. ab109268; 1:1,000), mTOR (cat. no. ab32028; 1:1,500), p-P70S6K
(cat. no. ab194521; 1:1,000), p70S6K (cat. no. ab32529; 1:2,000),
HMGA2 (cat. no. ab97276 1:1000), β-actin (cat. no. ab8226;
1:3,000), HRP-conjugated Goat Anti-Mouse IgG H&L (cat. no.
ab6789; 1:3,000) and HRP-conjugated Goat Anti-Rabbit IgG H&L
(cat. no. ab6721; 1:3,000).
Statistical analysis
Data are presented as mean ± standard deviation with
three independent repeats and analysis was conducted using the SPSS
software (version 22.0; IBM Corp.). Differences between two groups
were analyzed by unpaired t-test or χ2 tests. One-way
ANOVA followed by Tukey's test was used to compare the differences
between multiple groups. A Gehan-Breslow-Wilcoxon test was used to
compare the survival of patients that exhibit differential
expression levels of HIT000218960. Pearson correlation analysis was
used to analyze the correlation between the relative expression
levels of HIT000218960 and HMGA2 in the gastric cancer tissues.
P<0.05 was considered to indicate a statistically significant
difference.
Results
HIT000218960 expression levels
predicts the prognosis of patients with gastric cancer following
chemotherapy
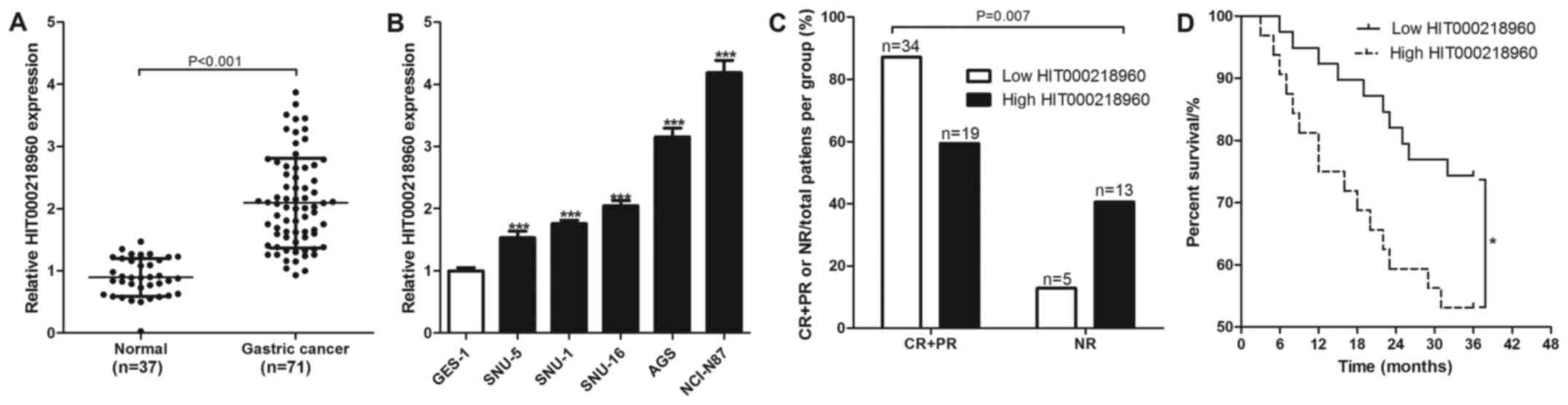
The expression levels of HIT000218960 were measured
using RT-qPCR in the 37 normal gastric mucosal and 71 gastric
cancer tissues collected. The relative expression level of
HIT000218960 in normal gastric mucosal tissues were found to be
significantly lower compared with that in the gastric cancer
tissues (Fig. 1A). Similarly, the
relative expression levels of HIT000218960 in normal gastric
mucosal epithelial cells (GES-1) were revealed to be significantly
lower compared with those in the gastric cancer cell lines SNU-5,
SNU-1, SNU-16, AGS and NCI-N87 (Fig.
1B).
Comparing the prognosis of chemotherapy-treated
patients with gastric cancer between the low and high HIT000218960
groups showed that 87.18% (34/39) patients in the low expression
group exhibited an effective response to chemotherapy, whilst only
59.38% (19/32) patients in the high expression group exhibited a
response to chemotherapy (Fig. 1C).
In addition, 12.82% (5/39) patients in the low HIT000218960
expression group exhibited no-response to chemotherapy, whilst only
40.62% (13/32) patients in the high HIT000218960 expression group
no-response to chemotherapy (Fig.
1C). The 71 patients with gastric cancer were also followed up
for 3 years and the results revealed that 74.36% (29/39) patients
with low HIT000218960 expression levels survived compared with
53.13% (17/32) in the high HIT000218960 expression group (Fig. 1D). HIT000218960 expression was found
to associate significantly with tumor diameter and TNM stage in
patients with gastric cancer (Table
II).
 | Table IIAssessment of the association between
HIT000218960 expression and the clinicopathological data of the 71
patients with gastric cancer. |
Table II
Assessment of the association between
HIT000218960 expression and the clinicopathological data of the 71
patients with gastric cancer.
| | HIT000218960
expression | |
|---|
| Variable | N | Low (n=39) | High (n=32) | χ2 | P-value |
|---|
| Sex | | | | | |
|
Male | 28 | 16 | 12 | 0.337 | 0.561 |
|
Female | 43 | 23 | 20 | | |
| Age (years) | | | | | |
|
≥60 | 40 | 20 | 20 | 0.899 | 0.343 |
|
<60 | 31 | 19 | 12 | | |
| Tumor diameter
(cm) | | | | | |
|
≥3 | 48 | 22 | 26 | 4.956 | 0.026 |
|
<3 | 23 | 17 | 6 | | |
| TNM stage | | | | | |
|
I | 30 | 20 | 10 | 6.736 | 0.034 |
|
II | 37 | 19 | 18 | | |
|
III | 4 | 0 | 4 | | |
| Histological
grade | | | | | |
|
I | 18 | 10 | 8 | 0.485 | 0.785 |
|
II | 42 | 24 | 18 | | |
|
III | 11 | 5 | 6 | | |
| Lymph node
metastasis (n) | | | | | |
|
0 | 9 | 7 | 2 | 3.388 | 0.336 |
|
1-2 | 14 | 9 | 5 | | |
|
3-6 | 16 | 8 | 8 | | |
|
≥7 | 32 | 15 | 17 | | |
HIT000218960 increases the resistance
of gastric cancer cells to 5-FU
Following treatment with 50 µmol/l 5-FU for 24 h,
gastric cancer cells exhibited a decreasing trend in the apoptosis
in cells with increasing HIT000218960 expression levels (Fig. 2A). SNU-5 cells overexpressing
HIT000218960 and NCI-N87 cells with HIT000218960 expression knocked
down were then established (Fig. 2B
and C), following which they were
treated with 50 µmol/l 5-FU for 24 h prior to apoptosis analysis.
HIT000218960 overexpression significantly reduced apoptosis in
SNU-5 cells whereas HIT000218960 knockdown increased apoptosis in
NCI-N87 cells after 5-FU treatment (Fig. 2D-F).
HIT000218960 upregulates the
expression of HMGA2 in gastric cancer tissues
HMGA2 protein expression were measured in normal
gastric mucosal and gastric cancer tissues. Relative HMGA2 protein
expression levels in normal gastric mucosal tissue were
demonstrated to be significantly lower compared with those in the
gastric cancer tissues (Fig. 3A).
The relative expression level of HIT000218960 was found to be
positively correlated with that of HMGA2 in the gastric cancer
tissues (Fig. 3B). Subsequently,
HIT000218960 overexpression resulted in significantly increased
HMGA2 protein expression in SNU-5 cells, whilst HMGA2 knockdown
significantly decreased HMGA2 levels in NCI-N87 and SNU-5 cells
(Fig. 3C). Following the
suppression of HMGA2 expression in both SNU-5 and NCI-N87 cells
(Fig. 3C), apoptosis was found to
be significantly increased following treatment with 5-FU (Fig. 3D and E). By contrast, HIT000218960
overexpression significantly inhibited 5-FU-induced apoptosis,
which was partially reversed by HMGA2 knockdown (Fig. 3F).
HIT000218960 activates the
AKT/mTOR/P70S6 kinase (P70S6K) via HMGA2 upregulation
The AKT/mTOR/P70S6K pathway has been previously
reported to regulate the apoptosis downstream of HMGA2 in mammalian
tumor cells (16-18).
Therefore, changes in the expression levels of the AKT/mTOR/P70S6K
signaling pathway components were analyzed in gastric cancer cells
after HMGA2 knockdown following treatment with 50 µmol/l 5-FU for
24 h. AKT, mTOR and P70S6K phosphorylation were revealed to be
significantly lower in the SNU-5 and NCI-N87 cells with HMGA2
expression knocked down compared with those of control cells
(Fig. 4). Taken together, these
data suggest that HIT000218960 enhanced resistance to
5-fluorouracil by promoting HMGA2 expression and by activating the
AKT/mTOR/P70S6K pathway in gastric cancer cells (Fig. 5).
Discussion
Although 75% of human genomic DNA is transcribed
into RNA, only 2% of the genome actually encodes proteins whereas
98% of transcripts are non-coding RNAs (19,20).
Single-stranded RNA molecules with a length of 20-24 nucleotides
are considered to be non-coding RNA molecules, whilst those with a
length of >200 nucleotides are categorized as lncRNAs (19,20).
lncRNAs were originally considered to be ‘junk’ RNA, but have been
discovered to serve important roles in dose compensation effects,
epigenetic regulation, cell cycle regulation and cell
differentiation regulation (21,22).
The abnormal expression of numerous lncRNAs have been demonstrated
in malignant tumor tissues, including urothelial carcinoembryonic
antigen 1 in lung cancer and colorectal neoplasia differentially
expressed in hepatocellular carcinoma (23,24),
where they can regulate tumor progression and are associated with
the efficacy and prognosis of chemotherapy (25). Additionally, lncRNAs are involved in
regulating cancer cell proliferation, invasion, migration and
sensitivity to chemotherapeutic agents in vitro (26,27).
The lncRNA HIT000218960 was originally reported to
be highly expressed in papillary thyroid cancer (12). Additionally, Sun et al
(11) previously demonstrated that
HIT000218960 was highly expressed in gastric cancer tissues
compared with that in normal tissues and found that HIT000218960
promoted the proliferation and migration of the gastric cancer
cells. The results of the present study also revealed that
HIT000218960 was highly expressed in gastric cancer tissues and
cell lines, consistent with the study by Sun et al (11). Furthermore, in the present study,
patients with gastric cancer who exhibit high HIT000218960 levels
did not respond as effectively to chemotherapy. To validate this
observation, gastric cancer cell lines overexpressing HIT000218960
and those with HIT000218960 expression knocked down were generated.
Higher levels of HIT000218960 expression were found to be
associated with increased resistance to 5-FU-induced apoptosis in
gastric cancer cells.
lncRNAs are non-coding RNAs that exert biological
functions by regulating the expression of other genes (25). The results of the present study
demonstrated that the relative expression levels of HIT000218960
were positively correlated with that of HMGA2 proteins in gastric
cancer tissues, where HIT000218960 positively regulated the
expression of HMGA2 in gastric cancer cells. HMGA2 has been
previously documented to promote cancer cell proliferation and
invasion, such as in breast cancer, colon cancer and lung cancer
(28). Additionally, HMGA2 protein
expression levels are positively associated with the degree of
deterioration of various malignant tumors, such as gastric cancer,
breast cancer and lung cancer and so on (28). As an oncogene, HMGA2 has been
discovered to protect cancer cells from the toxicity of
chemotherapy drugs (29,30). A previous study reported that HMGA2
exhibits dRP/AP lyase site cleavage activity and protects cancer
cells from DNA-damage-induced cytotoxicity during chemotherapy
(29). Furthermore, another
previous study demonstrated that three-dimensional collagen I
promotes gemcitabine resistance through HMGA2-dependent histone
acetyltransferase expression in pancreatic cancer cells (30). The present study revealed that HMGA2
knockdown increased 5-FU-induced apoptosis in gastric cancer cells.
Previous studies have shown that elevated HMGA2 expression in
gastric cancer tissues is significantly associated with patient
prognosis (31,32). In addition, HMGA2 has also been
shown to promote gastric cancer cell proliferation, invasion,
migration and resistance to chemotherapy (33,34).
The findings of the present study indicated that the enhanced
resistance of gastric cancer cells to 5-FU is mediated via the
promotion of HMGA2 expression. In addition, HIT000218960 could
positively regulate HMGA2 expression in gastric cancer.
The AKT/mTOR/P70S6K pathway is an important
signaling pathway that regulates cell survival and apoptosis
(35). It is also closely
associated with the mitochondria-mediated (36) and the Fas death receptor-mediated
cell death pathways (37,38). In the AKT/mTOR/P70S6K pathway,
phosphatidylinositol-dependent kinase 1 phosphorylates AKT, which
in turn activates mTOR and p70S6K, ultimately resulting in the
inhibition of apoptosis (39,40). A
previous study demonstrated that HMGA2 overexpression suppresses
cyclin dependent kinase inhibitor 2A levels in human umbilical cord
blood-derived stromal cells (hUCBSCs) to regulate aging and
proliferation of hUCBSCs through the Akt/mTOR/p70S6K pathway
(18). Furthermore, another study
revealed that microRNA-195 inhibits the proliferation and apoptosis
of esophageal carcinoma cells through the mTOR/p70S6K signaling
pathway by inhibiting HMGA2(41).
Therefore, the key components of the Akt/mTOR/p70S6K pathway were
analyzed in the present study, where the results demonstrated that
AKT, mTOR and P70S6K phosphorylation were significantly higher in
gastric cancer cells compared with those with HMGA2 expression
knocked down. These data indicated that HIT000218960 activated the
AKT/mTOR/p70S6K pathway by promoting HMGA2 levels.
The present study is associated with a number of
limitations. Although the association between HIT000218960
expression and the efficacy of chemotherapy in patients with
gastric cancer was elucidated, the limited number of patients
limited the reliability of clinical conclusions. Further analysis
with larger numbers of patients with gastric cancer will lead to a
more concrete clinical conclusion with regards to the ability of
HIT000218960 expression to predict the prognosis of patients with
gastric cancer following chemotherapy. The role of HIT000218960 has
been previously studied in gastric cancer (42). In this previous study, 103 gastric
cancer tissues and 62 normal gastric mucosa tissues were collected
before the expression of HIT000218960 in these tissues were
analyzed. In addition, their association with the prognosis of
patients with gastric cancer was assessed. HIT000218960 was also
found to be highly expressed in patients with gastric cancer, which
was associated with poor prognosis. However, it should be pointed
out that since these 103 patients with gastric cancer were not only
treated with chemotherapy, their clinical data could not be unified
with the present study. Nevertheless, this suggests that
HIT000218960, which is highly expressed in gastric cancer tissues,
may be associated with poorer prognosis by enhancing chemotherapy
resistance.
In conclusion, higher levels of HIT000218960
predicted poor prognosis for patients with gastric cancer after
treatment with chemotherapy. Mechanistically, this may be caused by
increased HMGA2 expression and activation of the AKT/mTOR/p70S6K
pathway, in turn inhibiting 5-FU-induced apoptosis of gastric
cancer cells (Fig. 5).
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
YC conceived and designed the current study. All
authors read and approved the final manuscript. LB and KD performed
the experiments, collected data, drafted the current study and
critically revised the manuscript for important intellectual
content. DT, XS and SW analyzed and interpreted the experimental
data. LB and KD confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was performed with the approval of
The Ethics Committee of The 970th Hospital of the PLA joint
Logistics Support Force, (Yantai, China). All patients were
informed and signed a consent form.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bertuccio P, Chatenoud L, Levi F, Praud D,
Ferlay J, Negri E, Malvezzi M and La Vecchia C: Recent patterns in
gastric cancer: A global overview. Int J Cancer. 125:666–673.
2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhuang Y, Peng LS, Zhao YL, Shi Y, Mao XH,
Guo G, Chen W, Liu XF, Zhang JY, Liu T, et al: Increased
intratumoral IL-22-producing CD4+T cells and Th22 cells correlate
with gastric cancer progression and predict poor patient survival.
Cancer Immunol Immunother. 61:1965–1975. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sastre J, Garcia-Saenz JA and Diaz-Rubio
E: Chemotherapy for gastric cancer. World J Gastroenterol.
12:204–213. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ge L, Hou L, Yang Q, Wu Y, Shi X, Li J and
Yang K: A systematic review and network meta-analysis protocol of
adjuvant chemotherapy regimens for resected gastric cancer.
Medicine (Baltimore). 98(e14478)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Janunger KG, Hafström L and Glimelius B:
Chemotherapy in gastric cancer: A review and updated meta-analysis.
Eur J Surg. 168:597–608. 2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ma J, Shen H, Kapesa L and Zeng S: Lauren
classification and individualized chemotherapy in gastric cancer.
Oncol Lett. 11:2959–2964. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nishina T, Boku N, Gotoh M, Shimada Y,
Hamamoto Y, Yasui H, Yamaguchi K, Kawai H, Nakayama N, Amagai K, et
al: Randomized phase II study of second-line chemotherapy with the
best available 5-fluorouracil regimen versus weekly administration
of paclitaxel in far advanced gastric cancer with severe peritoneal
metastases refractory to 5-fluorouracil-containing regimens
(JCOG0407). Gastric Cancer. 19:902–910. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sugarbaker PH: Twenty-year survival of
gastric cancer with peritoneal metastases using long-term
normothermic intraperitoneal 5-fluorouracil and systemic mitomycin
C: A case report. Int J Surg Case Rep. 61:302–304. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ajani J, Abramov M, Bondarenko I, Shparyk
Y, Gorbunova V, Hontsa A, Otchenash N, Alsina M, Lazarev S, Feliu
J, et al: A phase III trial comparing oral S-1/cisplatin and
intravenous 5-fluorouracil/cisplatin in patients with untreated
diffuse gastric cancer. Ann Oncol. 28:2142–2148. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sun L, Yu J, Wang P, Shen M and Ruan S:
HIT000218960 promotes gastric cancer cell proliferation and
migration through upregulation of HMGA2 expression. Oncol Lett.
17:4957–4963. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li T, Yang XD, Ye CX, Shen ZL, Yang Y,
Wang B, Guo P, Gao ZD, Ye YJ, Jiang KW and Wang S: Long noncoding
RNA HIT000218960 promotes papillary thyroid cancer oncogenesis and
tumor progression by upregulating the expression of high mobility
group AT-hook 2 (HMGA2) gene. Cell Cycle. 16:224–231.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Edge SB, Byrd DR, Compton CC (eds.), et
al: Stomach. In: AJCC Cancer Staging Manual. 7th edition. Springer,
New York, NY, pp117-126, 2010.
|
|
14
|
Kusaba H and Saijo N: A summary report of
response evaluation criteria in solid tumors (RECIST criteria). Gan
To Kagaku Ryoho. 27:1–5. 2000.PubMed/NCBI(In Japanese).
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta c(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang L, Wang H, Xu J, Zhu J and Ding K:
Inhibition of cathepsin S induces autophagy and apoptosis in human
glioblastoma cell lines through ROS-mediated PI3K/AKT/mTOR/p70S6K
and JNK signaling pathways. Toxicol Lett. 228:248–259.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ladu S, Calvisi DF, Conner EA, Factor VM
and Thorgeirsson SS: Co-expression of c-Myc and E2F1 in a mouse
model of liver cancer suppresses apoptosis through activation of
Akt/mTOR/p70S6K and COX-2 pathways: Relevance for human
hepatocellular carcinoma. Cancer Res,. 65
(9_Supplement)(258)2005.
|
|
18
|
Yu KR, Park SB, Jung JW, Seo MS, Hong IS,
Kim HS, Seo Y, Kang TW, Lee JY, Kurtz A and Kang KS: HMGA2
regulates the in vitro aging and proliferation of human umbilical
cord blood-derived stromal cells through the mTOR/p70S6K signaling
pathway. Stem Cell Res. 10:156–165. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yi Z, Hui L, Fang S, Kang Y, Wu W, Hao Y,
Li Z, Bu D, Sun N, Zhang MQ and Chen R: NONCODE 2016: An
informative and valuable data source of long non-coding RNAs.
Nucleic Acids Res. 44(D1):D203–D208. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ulitsky I: Evolution to the rescue: Using
comparative genomics to understand long non-coding RNAs. Nat Rev
Genet. 17:601–614. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Boon RA, Jaé N, Holdt L and Dimmeler S:
Long Noncoding RNAs: From clinical genetics to therapeutic targets?
J Am Coll Cardiol. 67:1214–1226. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Wang HM, Lu JH, Chen WY and Gu AQ:
Upregulated lncRNA-UCA1 contributes to progression of lung cancer
and is closely related to clinical diagnosis as a predictive
biomarker in plasma. Int J Clin Exp Med. 8:11824–11830.
2015.PubMed/NCBI
|
|
24
|
Tang D, Zhao L, Peng C, Ran K, Mu R and Ao
Y: LncRNA CRNDE promotes hepatocellular carcinoma progression by
upregulating SIX1 through modulating miR7. J Cell Biochem.
120:16128–16142. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kondo Y, Shinjo K and Katsushima K: Long
non-coding RNAs as an epigenetic regulator in human cancers. Cancer
Sci. 108:1927–1933. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shen XH, Qi P and Du X: Long non-coding
RNAs in cancer invasion and metastasis. Mod Pathol. 28:4–13.
2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang Z, Zhou C, Chang Y, Zhang Z, Hu Y,
Zhang F, Lu Y, Zheng L, Zhang W and Li X and Li X: Long non-coding
RNA CASC11 interacts with hnRNP-K and activates the WNT/β-catenin
pathway to promote growth and metastasis in colorectal cancer.
Cancer Lett. 376:62–73. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nie D, Zhang L, Guo Q and Mao X: High
mobility group protein A2 overexpression indicates poor prognosis
for cancer patients: A meta-analysis. Oncotarget. 9:1237–1247.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Summer H, Li O, Bao Q, Zhan L, Peter S,
Sathiyanathan P, Henderson D, Klonisch T, Goodman SD and Dröge P:
HMGA2 exhibits dRP/AP site cleavage activity and protects cancer
cells from DNA-damage-induced cytotoxicity during chemotherapy.
Nucleic Acids Res. 37:4371–4384. 2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dangi-Garimella S, Sahai V, Ebine K, Kumar
K and Munshi HG: Three-dimensional collagen I promotes gemcitabine
resistance in vitro in pancreatic cancer cells through
HMGA2-Dependent histone acetyltransferase expression. PLoS One.
8(e64566)2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhu J, Wang H, Xu S and Hao Y:
Clinicopathological and prognostic significance of HMGA2
overexpression in gastric cancer: A meta-analysis. Oncotarget.
8:100478–100489. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jun KH, Jung JH, Choi HJ, Shin EY and Chin
HM: HMGA1/HMGA2 protein expression and prognostic implications in
gastric cancer. Int J Surg. 24(Pt A):39–44. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang H, Jiang Z, Chen H, Wu X, Xiang J and
Peng J: MicroRNA-495 inhibits gastric cancer cell migration and
invasion possibly via targeting high mobility group AT-Hook 2
(HMGA2). Med Sci Monit. 23:640–648. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yang X, Zhao Q, Yin H, Lei X and Gan R:
MiR-33b-5p sensitizes gastric cancer cells to chemotherapy drugs
via inhibiting HMGA2 expression. J Drug Target. 25:653–660.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Franke TF, Hornik CP, Segev L, Shostak GA
and Sugimoto C: PI3K/Akt and apoptosis: Size matters. Oncogene.
22:8983–8998. 2003.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tsuruta F, Masuyama N and Gotoh Y: The
Phosphatidylinositol 3-Kinase (PI3K)-Akt Pathway Suppresses Bax
Translocation to Mitochondria. J Biol Chem. 277:14040–14047.
2002.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhu L, Derijard B, Chakrabandhu K, Wang
BS, Chen HZ and Hueber AO: Synergism of PI3K/Akt inhibition and Fas
activation on colon cancer cell death. Cancer Lett. 354:355–364.
2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Iyer AK, Azad N, Talbot S, Stehlik C, Lu
B, Wang L and Rojanasakul Y: Antioxidant c-FLIP inhibits Fas
ligand-induced NF-kappaB activation in a phosphatidylinositol
3-Kinase/Akt-dependent manner. J Immunol. 187:3256–3266.
2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhao P, Meng Q, Liu L-Z, You Y-P, Liu N
and Jiang B-H: Regulation of survivin by PI3K/Akt/p70S6K1 pathway.
Biochem Biophys Res Commun. 395:219–224. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chai X, Sun D, Han Q, Yi L, Wu Y and Liu
X: Hypoxia induces pulmonary arterial fibroblast proliferation,
migration, differentiation and vascular remodeling via the
PI3K/Akt/p70S6K signaling pathway. Int J Mol Med. 41:2461–2472.
2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Mo J, Li B, Zhou Y, Xu Y, Jiang H, Cheng
X, Wu X and Zhang Y: LINC00473 promotes hepatocellular carcinoma
progression via acting as a ceRNA for microRNA-195 and increasing
HMGA2 expression. Biomed Pharmacother. 120(109403)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Dong K, Bai L, Tong D, Shi X, Wei S and
Cai Y: Long non-coding RNA HIT000218960 is associated with poor
prognosis in patients with gastric cancer. Exp Ther Med.
22(694)2021.PubMed/NCBI View Article : Google Scholar
|