Introduction
Sepsis is a systemic inflammatory response syndrome
(SIRS) caused by infection, which could develop into septic shock
and multiple organ dysfunction syndrome (MODS), and has become one
of the leading causes of death in critically ill patients, with a
mortality rate of 25 to 40% (1,2).
Acute lung injury (ALI)/acute respiratory distress syndrome (ARDS)
is one of the most common complications of severe sepsis, involving
an inflammatory process (3,4).
Therefore, it is of great significance to explore the biomarkers
for the early diagnosis, treatment and prognosis evaluation of
sepsis-induced ALI. Pulmonary endothelial cell injury plays an
important role in the dysfunction of the alveolar-capillary
barrier, which is a characteristic feature of ALI (5,6).
Lipopolysaccharide (LPS), the bacterial endotoxin, is a primary
pathogenic mediator of pulmonary endothelial cell activation, which
triggers ALI, involving multiple inflammatory cells, and cytokines
(7,8). Alleviating the injury of pulmonary
endothelial cells is an important method to prevent sepsis-induced
ALI.
MicroRNAs (miRNAs or miRs) are a class of small
non-coding RNAs that have been discovered in recent years, which
can specifically recognize the 3'-UTR sites of target mRNAs to
inhibit protein translation or induce mRNA degradation, and thus
regulate gene expression at the post-transcriptional levels
(9). MiRNAs play crucial roles in
the regulation of multiple physiological and pathological processes
such as cell proliferation and apoptosis, differentiation, and
inflammatory response (10,11).
Previous research has revealed that several miRNAs are implicated
in the development of sepsis-induced ALI, such as miR-155 and
miR-146a (12). MiR-374a has been
demonstrated to inhibit the progression of lung adenocarcinoma and
non-small cell lung carcinoma (13-15).
MiR-374a was necessary for the inhibition of intestinal
inflammation and oxidative injury in rats with colitis (16). MiR-374a-5p was critical for the
protective effects on neonatal hypoxic-ischemic encephalopathy
(HIE) by inhibiting LPS-induced microglial neuroinflammation
(17). A previous study indicated
that miR-374a alleviated LPS-induced hyperpermeability of human
pulmonary artery endothelial cells (18). However, it remains unclear whether
miR-374a-5p is engaged in sepsis-induced ALI.
Zinc finger E-box binding homeobox 1 (ZEB1) is a
transcription factor which has critical roles in
epithelial-mesenchymal transition (EMT), senescence, and
angiogenesis (19-21).
It has been reported that ZEB1 induces inflammatory response in
periodontal disease and liver fibrosis (22,23).
In addition, the p38 MAPK signaling pathway is associated with
septic lung injury (24-26).
A previous study revealed that the p38 MAPK pathway could be
restricted by miR-200s by targeting ZEB1/2 in LPS-induced pulmonary
fibrosis (27).
In the present study, human pulmonary microvascular
endothelial cells (HPMVECs) induced by LPS were used to investigate
whether miR-374a-5p exerted a protective role in sepsis-induced ALI
through the p38 MAPK signaling pathway by targeting ZEB1.
Materials and methods
Patients and healthy subjects
In total, 25 patients with sepsis (49.5±10.3 years
old; 16 males and 9 females) following intensive care unit (ICU)
admission at General Hospital of Ningxia Medical University
(Yinchuan, China) from May 2017 to August 2018 were recruited in
the present study, according to diagnostic criteria proposed by The
Third International Consensus Definitions for Sepsis and Septic
Shock (Sepsis-3) (28). Exclusion
criteria were as follows: i) Patients younger than 18 years old;
ii) patients with chronic liver and renal insufficiency; iii)
patients with malignant tumors; iv) patients with haematological
diseases; v) patients with compromised immune systems; vi) pregnant
and lactating patients; and vii) patients with acute cardiovascular
and cerebrovascular diseases. In addition, 25 age- and sex-matched
healthy individuals (50.6±9.3 years old; 18 males and 7 females)
with no history of pulmonary infection or malignancies were
enrolled as the controls. Written informed consent was provided
from all participants, and the present study was approved by the
Ethics Committee of General Hospital of Ningxia Medical University
(approval no. IRB2017-GHNMU-38). Clinical characteristics of the
study subjects including sex, age, body mass index (BMI), acute
physiology and chronic health evaluation (APACHE) II score,
sequential organ failure assessment (SOFA) score, C-reactive
protein (CRP), procalcitonin (PTC), and blood gas analysis (lactic
acid, and PaO2 /FiO2) are presented in
Table I.
 | Table IDemographic and clinical
characteristics of patients with sepsis and healthy controls. |
Table I
Demographic and clinical
characteristics of patients with sepsis and healthy controls.
| Characteristic | Sepsis (n=25) | Healthy (n=25) | P-value |
|---|
| Sex,
male/female | 16/9 | 18/7 | 0.544 |
| Age, years | 49.5±10.3 | 50.6±9.3 | 0.694 |
| BMI,
kg/m2 | 20.5±1.9 | 20.5±1.8 | 0.999 |
| APACHE II
score | 15.9±3.2 | - | - |
| SOFA score | 6.1±1.3 | - | - |
| CRP, mg/l | 96.7±20.3 | 6.3±2.6 | <0.001 |
| PCT, ng/ml | 12.2±5.9 | 0.7±0.1 | <0.001 |
| Lactic acid,
mmol/l | 2. 8±1.8 | 1.0±0.4 | <0.001 |
| PaO2
/FiO2, mmHg | 187.7±38.1 | 378.3±43.6 | <0.001 |
Following admission, blood samples (5 ml) were
obtained on an empty stomach, and centrifuged at 3,000 x g for 10
min at 4˚C to completely remove cell debris. The collected serum
was transferred to RNase/DNase-free sterile tubes and stored at
-80˚C until further processing.
Cell culture and LPS treatment
HPMVECs, purchased from American Type Culture
Collection (ATCC no. PCS-100-022) were cultured in Dulbecco's
modified Eagle's medium (DMEM; Life Technologies; Thermo Fisher
Scientific, Inc.) plus 10% fetal bovine serum (FBS; Invitrogen;
Thermo Fisher Scientific, Inc.) and 100 µg/ml
penicillin/streptomycin (Life Technologies; Thermo Fisher
Scientific, Inc.) at 37˚C with 5% CO2. For the cellular
model of sepsis, HPMVECs were incubated with 1 µg/ml LPS
(Sigma-Aldrich; Merck KGaA) for 24 h at 37˚C. The ethics approval
for the use of primary cell lines was received.
Cell transfection
HPMVECs (1x104 cells/ml) cultured in
96-well plates were transfected with 50 nM miR-374a-5p mimics,
miRNA negative control (miR-NC), 4 µg pcDNA3.1 containing the open
reading frame of ZEB1 or pcDNA3.1 empty vector (Vector), chemically
synthesized by Shanghai GenePharma Co., Ltd. with Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) at 37˚C,
according to manufacturer's protocol. At 24 h after transfection,
HPMVECs were stimulated with 1 µg/ml LPS for 24 h. Sequences of the
oligonucleotides are shown in Table
II.
 | Table IISequences of the
oligonucleotides. |
Table II
Sequences of the
oligonucleotides.
|
Oligonucleotides | Sequences
(5'-3') |
|---|
| miR-374a-5p |
UUAUAAUACAACCUGAUAAGUG |
| mimics |
CUUAUCAGGUUGUAUUAUAAUU |
| miR-NC |
UUCUCCGAACGUGUCACGUTT |
| |
ACGUGACACGUUCGGAGAATT |
Luciferase reporter assay
The binding site between ZEB1 and miR-374a-5p was
predicted using StarBase v2.0 (https://starbase.sysu.edu.cn/). The 3'-UTR fragments
of ZEB1 containing the predicted wild-type (ZEB1-wt) or
corresponding mutated (ZEB1-mut) miR-374a-5p binding sites were
amplified by PCR and sub-cloned into a pmirGLO Dual-luciferase
miRNA Target Expression Vector (Promega Corporation). When cells
reached ~80% confluence, these luciferase reporter plasmids were
co-transfected with either miR-374a-5p mimic or miR-NC into HPMVECs
using Lipofectamine 2000. After 48 h of transfection, the cells
were subjected to further luciferase assays using a Bright-Glo™
LuciferaseAssay System (Promega Corporation). The luciferase
activity was normalized to Renilla luciferase activity
values.
Reverse transfection-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from serum and cultured
cells using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. RT
was conducted using a Taqman MicroRNA Reverse Transcription Kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.) for miRNA or a
Prime-Script™ One Step RT-qPCR kit (Takara Bio, Inc.) for mRNA
strictly according to the manufacturers' instructions. Real-time
PCR was conducted using SYBR Premix ExTagTM (Takara Bio,
Inc.) on a 7500 Fast Real-Time PCR System (Applied Biosystems;
Thermo Fisher Scientific, Inc.) with the following thermocycling
conditions: 95˚C for 10 min, followed by 40 cycles at 94˚C for 45
sec, 55˚C for 30 sec and 72˚C for 1 min. Relative expression levels
of miR-374a-5p and ZEB1 were calculated using the 2-ΔΔCq
method using U6 snRNA or GAPDH as internal controls (29). The primers used were as follows:
miR-374a-5p forward, 5'-GCGCGCTTATAATACAACCTGA-3' and reverse,
5'-AGAGCAGGGTCCGAGGT-3' (universal); ZEB1 forward,
5'-GATGATGAATGCGAGTCAGATGC-3' and reverse,
5'-ACAGCAGTGTCTTGTTGTTGT-3'; U6 forward,
5'-CAGCACATATACTAAAATTGGAACG-3' and reverse,
5'-ACGAATTTGCGTGTCATCC-3'; GAPDH forward,
5'-TGTGGGCATCAATGGATTTGG-3' and reverse,
5'-ACACCATGTATTCCGGGTCAAT-3'.
Cell Counting Kit-8 (CCK-8) assay
A CCK-8 Kit was purchased from Dojindo Laboratories,
Inc. to detect the cell viability according to the manufacturer's
instructions. In detail, LPS-treated or transfected HPMVECs were
seeded in a 96-well plate containing 5x103 cells/well
and cultured for 24 h at 37˚C. CCK-8 reagent was added to each well
and cells were further incubated at 37˚C for 4 h. The absorbance at
450 nm using a microplate reader (Bio-Rad Laboratories, Inc.) was
measured to assess the viability of cells. Each experiment was
repeated in triplicate.
Apoptosis detection using flow
cytometry
The apoptosis of HPMVECs were determined by flow
cytometry using an Annexin V-FITC/PI detection kit (Sigma-Aldrich;
Merck KGaA) in accordance with a previous study (30). Briefly, cells were washed with PBS,
re-suspended in binding buffer and stained with Annexin V/FITC and
PI solution for 30 min. The apoptotic cells were detected with a
flow cytometer (Beckman Coulter, Inc.) and Expo32 v1.2 software
(Beckman Coulter, Inc.).
Western blot analysis
Following lysis in RIPA buffer (Santa Cruz
Biotechnology, Inc.) and centrifugation at 12,000 x g for 20 min at
4˚C, proteins were extracted from serum or cells. Bradford method
was used to detect the concentration of the proteins using a
Bradford assay kit (Thermo Fisher Scientific, Inc.). Sodium dodecyl
sulfate polyacrylamide gel electrophoresis on 7.5-10% gels was used
to separate the proteins with equal amounts. The proteins (30 µg)
were then transferred onto PVDF membranes (MilliporeSigma). Samples
were blocked with 5% non-fat milk at room temperature for 1 h, and
incubated with the primary antibodies against Bax (product no.
5023), Bcl-2 (product no. 3498), ZEB1 (product no. 3396),
phosphorylated (p)-p38 (product no. 4511), p38 (product no. 8690),
p-JNK (product no. 4668), JNK (product no. 9252), p-ERK (product
no. 4370), ERK (product no. 4695) and GAPDH (product no. 5174) (all
1:1,000 dilution; Cell Signaling Technology, Inc.) overnight at
4˚C, and then the membrane was incubated with the secondary
antibodies (anti-rabbit IgG, HRP-conjugated; product no. 7074;
1:2,000 dilution; Cell Signaling Technology, Inc.) at room
temperature for 2 h. GAPDH was used as the internal control. A
Super Signal West Pico Chemiluminescent Substrate kit (Pierce;
Thermo Fisher Scientific, Inc.) was then used to scan the films
according to the manufacturer's protocol. Image-Pro Plus software
version 6.0 (Media Cybernetics, Inc.) was used to analyze the
relative protein expression.
Cytokine assessment
Enzyme-linked immunosorbent assay (ELISA) method was
performed to determine the concentration of interleukin (IL)-6
(cat. no. RAB0313), IL-1β (cat. no. RAB0273), and tumor necrosis
factor-α (TNF-α) (cat. no. RAB1089) in cell culture supernatant
using commercial ELISA kits (Sigma-Aldrich; Merck KGaA) according
to the manufacturer's instructions.
Statistical analysis
All analyses were performed using SPSS version 22.0
(IBM Corp.). Two-sided unpaired Student's t-test or analysis
of variance (ANOVA) with Tukey's post hoc test was used to compare
differences between groups. All results were presented as the mean
± standard deviation (SD) and a P-value <0.05 was considered to
indicate a statistically significant difference. Pearson's
correlation analysis was employed to analyze the association
between serum miR-374a-5p and ZEB1 mRNA levels.
Results
MiR-374a-5p is downregulated in
patients with sepsis and LPS-treated HPMVECs
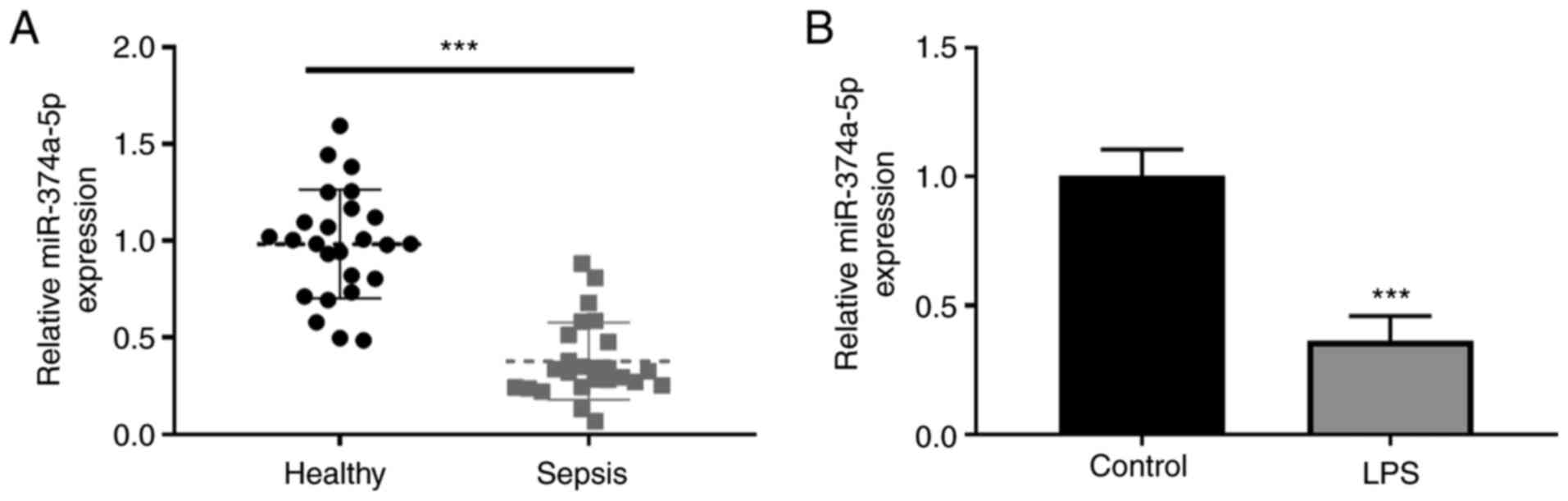
The serum expression levels of miR-374a-5p in
patients with sepsis and healthy controls were analyzed by RT-qPCR.
A low expression of serum miR-374a-5p was revealed in septic
patients compared with healthy controls (Fig. 1A). The expression level of
miR-374a-5p was further detected in sepsis-induced ALI in
vitro using LPS-treated HPMVECs. As revealed in Fig. 1B, miR-374a-5p was significantly
downregulated after LPS treatment in comparison with the untreated
cells.
Overexpression of miR-374a-5p
alleviates LPS-induced apoptosis and inflammation in HPMVECs
To further explore the effects of miR-374a-5p in
LPS-induced HPMVECs, the expression of miR-374a-5p was upregulated
in LPS-treated HPMVECs by transfection with miR-374a-5p mimic
(Fig. 2A). In addition,
upregulation of miR-374a-5p restored cell viability (Fig. 2B) which was decreased by LPS
treatment and attenuated the apoptotic rate which was induced by
LPS in HPMVECs (Fig. 2C and
D). Furthermore, LPS treatment
resulted in increased expression of Bax and a significant reduction
of Bcl-2 expression, while miR-374a-5p overexpression reversed
these effects of apoptosis-related proteins in LPS-treated HPMVECs
(Fig. 2E). In addition,
LPS-induced secretion of IL-6, IL-1β, and TNF-α in HPMVECs was
significantly impeded by miR-374a-5p overexpression (Fig. 2F).
 | Figure 2Overexpression of miR-374a-5p
alleviates LPS-induced apoptosis and inflammation in HPMVECs.
HPMVECs were transfected with miR-NC or miR-374a-5p mimic, and then
treated with 1 µg/ml LPS for 24 h. (A) RT-qPCR was used to confirm
the transfection efficiency of miR-374a-5p overexpression.
***P<0.001 vs. miR-NC. (B) Cell viability was
measured by CCK-8 assay. (C and D) The apoptotic ratio was
determined by flow cytometry. (E) The protein expression levels of
apoptosis-related genes Bax and Bcl-2 were detected by western
blotting. (F) ELISA assay was employed to examine the contents of
inflammatory cytokines IL-6, IL-1β and TNF-α in the medium.
**P<0.01. Results represent the means ± SD of 3
independent experiments. miR-374a-5p, microRNA-374a-5p; LPS,
lipopolysaccharide; HPMVECs, human pulmonary microvascular
endothelial cells; miR-NC, miRNA negative control; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; CCK-8, Cell
Counting Kit-8; ELISA, enzyme-linked immunosorbent assay; IL-,
interleukin; TNF-α, tumor necrosis factor-α. |
ZEB1 is directly targeted by
miR-374a-5p in HPMVECs
Subsequently, the downstream target of miR-374a-5p
was searched for using bioinformatics analysis. ZEB1 was predicted
as a direct target gene of miR-374a-5p with several putative
binding sites using StarBase v2.0 (https://starbase.sysu.edu.cn/) (Fig. 3A). Dual-luciferase reporter assay
demonstrated that the luciferase activity of ZEB1-wt was
significantly decreased in cells transfected with miR-374a-5p mimic
compared with miR-NC transfection (Fig. 3B). The mRNA and protein levels of
ZEB1 in miR-374a-5p-overexpressing HPMVECs were then assessed and
it was determined that the expression of ZEB1 at the mRNA and
protein levels was significantly downregulated by the miR-374a-5p
mimic (Fig. 3C and D). There was a higher expression of ZEB1
mRNA in the serum of patients with sepsis than that in healthy
controls (Fig. 3E). A negative
correlation between miR-374a-5p and ZEB1 mRNA expression was
revealed in patients with sepsis (Fig.
3F). As anticipated, LPS stimulation induced the upregulation
of ZEB1 mRNA and protein levels in HPMVECs (Fig. 3G and H).
MiR-374a-5p reduces LPS-induced HPMVEC
injury by targeting ZEB1
To verify whether miR-374a-5p was involved in
sepsis-induced ALI by targeting ZEB1, miR-374a-5p mimic was
co-transfected with ZEB1 into HPMVECs, and then the cells were
treated with LPS. The expression of ZEB1 in LPS-treated HPMVECs was
efficiently increased by transfection of ZEB1 overexpression vector
(Fig. 4A). Further results
revealed that upregulation of ZEB1 weakened the viability induced
by enforced expression of miR-374a-5p in LPS-treated cells
(Fig. 4B). As depicted in Fig. 4C-E, ZEB1 overexpression abolished
the inhibitory effects of miR-374a-5p on cell apoptosis, as
determined by the enhanced apoptotic ratio, the increased Bax level
and decreased Bcl-2 level. Introduction of ZEB1 counteracted the
suppressive effect of miR-374a-5p on IL-6, IL-1β, and TNF-α
production triggered by LPS in HPMVECs (Fig. 4F).
MiR-374a-5p exerts its function in
LPS-treated HPMVECs by regulating the ZEB1-mediated p38 MAPK
signaling pathway
To investigate the underlying mechanism of
miR-374a-5p in sepsis-induced ALI, the expression of the p38 MAPK
pathway was determined following overexpression of miR-374a-5p and
ZEB1. Western blotting confirmed that the phosphorylation levels of
p38, JNK, and ERK were significantly increased in LPS-treated
HPMVECs. When miR-374a-5p was overexpressed, the protein expression
of p38, JNK, and ERK phosphorylation levels was reduced, whereas
ectopic expression of ZEB1 antagonized the downregulation (Fig. 5).
 | Figure 5MiR-374a-5p exerts its function in
LPS-treated HPMVECs by regulating the ZEB1-mediated p38 MAPK
signaling pathway. Expression levels of p-p38 MAPK, p38 MAPK,
p-JNK, JNK, p-ERK, ERK were analyzed by western blotting. The
representative blots and quantitated results of p-p38/p38 ratio,
p-JNK/JNK ratio, and p-ERK/ERK ratio are shown in HPMVECs
transfected with miR-374a-5p mimic and ZEB1 after treatment of LPS.
Results represent the means ± SD of 3 independent experiments.
*P<0.05, **P<0.01 and
***P<0.001. MiR-374a-5p, microRNA-374a-5p; LPS,
lipopolysaccharide; HPMVECs, human pulmonary microvascular
endothelial cells; ZEB1, zinc finger E-box binding homeobox 1; p-,
phosphorylated. |
Discussion
Reportedly, the pathogenesis of sepsis-induced ALI
is associated with pulmonary endothelial dysfunction, leading to
abnormal apoptosis and inflammatory response (31,32).
In the present study, for the first time to the best of our
knowledge, it is reported that miR-374a-5p ameliorated the
progression of sepsis-induced ALI in vitro by targeting ZEB1
and thus inactivating the p38 MAPK signaling pathway. This
conclusion was supported by the following evidence. First,
miR-374a-5p expression was decreased in the serum of patients with
sepsis and in HPMVECs treated with LPS. Second, miR-374a-5p
overexpression alleviated LPS-induced apoptosis and inflammatory
response in HPMVECs. Third, ZEB1 was confirmed as a direct target
of miR-374a-5p, and ZEB1 upregulation could attenuate the
inhibition of miR-374a-5p on LPS-induced cell injury. Finally, the
p38 MAPK signaling axis was essential for LPS-induced injury, and
was also involved in the miR-374a-5p/ZEB1 network.
Increasing studies have shown that miR-374a-5p plays
its protective roles in the development of neurological dysfunction
(17,33) and myocardial injury (34,35).
In a study of metabolically healthy obese (MHO), serum miR-374a-5p
was highly expressed in MHO, and there was a positive association
between miR-374a-5p expression with the downregulation of
pro-inflammatory cytokines (36).
In the present study, serum miR-374a-5p expression was decreased in
septic patients when compared with healthy controls. Similarly, in
an in vitro cell model of LPS-induced ALI in HPMVECs,
miR-374a-5p expression was also downregulated when compared with
the untreated cells. MiR-374a-5p mimic was further used to
upregulate miR-374a-5p expression in HPMVECs, and it was
demonstrated that miR-374a-5p overexpression attenuated the
LPS-induced inhibition of cell viability. Bax and Bcl-2,
significant members of the Bcl-2 family, are vital regulators of
apoptotic cell death (37); and
the present research revealed that miR-374a-5p overexpression
decreased the proportion of apoptotic cells in LPS-treated HPMVECs,
as demonstrated by inhibition of Bax expression and upregulation of
Bcl-2. Pro-inflammatory cytokines such as IL-6, IL-1β and TNF-α and
inflammatory chemokines appear to trigger the inflammatory cascade,
leading to pathological changes (38). In the present study, stimulation of
LPS caused increased levels of IL-6, IL-1β and TNF-α in cell
supernatants. The gain-of-function assay revealed that miR-374a-5p
overexpression mitigated LPS-induced inflammatory response. These
results indicated the apoptosis and inflammatory inhibitory effects
of miR-374a-5p in HPMVECs.
ZEB1 may serve as a novel therapeutic target for
pulmonary inflammation (27),
neuroinflammation (39), and
inflammatory cancer (40). ZEB1
was confirmed as a direct downstream target of miR-374a-5p and
their expression was negatively correlated in the present study.
Moreover, ZEB1 overexpression was capable of reversing the
protective roles of miR-374a-5p in LPS-induced cell injury of
HPMVECs. Extensive evidence indicates that aberrant activation of
the p38 MAPK signaling pathway contributes to the pathogenesis of
sepsis-induced ALI (24-26).
In addition, ZEB1 is increased in LPS-induced pulmonary fibrosis,
which is associated with the p38 MAPK signaling pathway (27). JNK and ERK pathways, the other MAPK
pathways, also play vital roles in triggering the production of
pro-inflammatory cytokines in response to LPS stimulation (41). In the present study, it was
determined that p38, JNK, and ERK phosphorylation levels were
downregulated when miR-374a-5p was overexpressed in LPS-treated
HPMVECs, while ZEB1 overexpression led to the activation of p38
MAPK, JNK and ERK signaling pathways. Collectively, these findings
indicated that miR-374a-5p ameliorated sepsis-induced ALI through
the ZEB1-mediated p38 MAPK, JNK, and ERK pathways. However, there
were some limitations in the present study. First, further
validating investigations may have to be performed using a larger
cohort to compare cases of sepsis with healthy controls to provide
a greater statistical significance. Second, further investigation
is required to verify the in vivo role of miR-374a-5p in
sepsis-induced ALI using animal models. Third, the other signaling
pathways involved in the regulatory effect of miR-374a-5p on HPMVEC
cell behaviors were not further investigated.
Overall, the present study highlighted miR-374a-5p
as a novel target for sepsis therapy and demonstrated that
miR-374a-5p attenuated LPS-induced cell injury in HPMVECs, possibly
via the ZEB1-mediated p38 MAPK signaling pathway.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JS conducted most of the experiments and wrote the
manuscript. XM conducted the experiments and analyzed the data. JS
designed the study and revised the manuscript. All authors have
read and approved the final manuscript. JS and XM confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of General Hospital of Ningxia Medical University of
Science and Technology (Yinchuan, China; approval no.
IRB2017-GHNMU-38).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu KD and Matthay MA: Advances in
critical care for the nephrologist: Acute lung injury/ARDS. Clin J
Am Soc Nephrol. 3:578–586. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gotts JE and Matthay MA: Sepsis:
Pathophysiology and clinical management. BMJ.
353(i1585)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yang L, Zhang Z, Zhuo Y, Cui L, Li C, Li
D, Zhang S, Cui N, Wang X and Gao H: Resveratrol alleviates
sepsis-induced acute lung injury by suppressing inflammation and
apoptosis of alveolar macrophage cells. Am J Transl Res.
10:1961–1975. 2018.PubMed/NCBI
|
|
4
|
Wang RH, Xie YX, Qiu JW and Chen JY:
Influence of LincRNA-p21 on acute lung injury in sepsis. Eur Rev
Med Pharmacol Sci. 24:5618–5626. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Huang LS, Hong Z, Wu W, Xiong S, Zhong M,
Gao X, Rehman J and Malik AB: mtDNA activates cGAS signaling and
suppresses the YAP-mediated endothelial cell proliferation program
to promote inflammatory injury. Immunity. 52:475–486.e5.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cen M, Ouyang W, Zhang W, Yang L, Lin X,
Dai M, Hu H, Tang H, Liu H, Xia J and Xu F: MitoQ protects against
hyperpermeability of endothelium barrier in acute lung injury via a
Nrf2-dependent mechanism. Redox Biol. 41(101936)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nova Z, Skovierova H and Calkovska A:
Alveolar-capillary membrane-related pulmonary cells as a target in
endotoxin-induced acute lung injury. Int J Mol Sci.
20(831)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lu Q, Zhang D, Liu H and Xu H: miR-942-5p
prevents sepsis-induced acute lung injury via targeting TRIM37. Int
J Exp Pathol. 102:192–199. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Vishnoi A and Rani S: MiRNA biogenesis and
regulation of diseases: An overview. Methods Mol Biol. 1509:1–10.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chunlei H, Chang Z, Sheng L, Yanchun Z,
Lulin L and Daozhang C: Down-regulation of MiR-138-5p Protects
Chondrocytes ATDC5 and CHON-001 from IL-1 β-induced inflammation
via up-regulating SOX9. Curr Pharm Des. 25:4613–4621.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Qu SP, Li GW, Ma H and Xing Q:
MicroRNA-193a-3p participates in the progression of rheumatoid
arthritis by regulating proliferation and apoptosis of MH7A cells
through targeting IGFBP5. Eur Rev Med Pharmacol Sci. 23:4850–4857.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Han Y, Li Y and Jiang Y: The prognostic
value of plasma microRNA-155 and microRNA-146a level in severe
sepsis and sepsis-induced acute lung injury patients. Clin Lab.
62:2355–2360. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wu H, Liu Y, Shu XO and Cai Q: MiR-374a
suppresses lung adenocarcinoma cell proliferation and invasion by
targeting TGFA gene expression. Carcinogenesis. 37:567–575.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Xu Z, Xu J, Lu H, Lin B, Cai S, Guo J,
Zang F and Chen R: LARP1 is regulated by the XIST/miR-374a axis and
functions as an oncogene in non-small cell lung carcinoma. Oncol
Rep. 38:3659–3667. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhao M, Xu P, Liu Z, Zhen Y, Chen Y, Liu
Y, Fu Q, Deng X, Liang Z, Li Y, et al: Dual roles of miR-374a by
modulated c-Jun respectively targets CCND1-inducing PI3K/AKT signal
and PTEN-suppressing Wnt/β-catenin signaling in non-small-cell lung
cancer. Cell Death Dis. 9(78)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xiong Y, Qiu J, Li C, Qiu Y, Guo L, Liu Y,
Wan J, Li Y, Wu G, Wang L, et al: Fortunellin-induced modulation of
phosphatase and tensin homolog by microRNA-374a decreases
inflammation and maintains intestinal barrier function in colitis.
Front Immunol. 9(83)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen Z, Hu Y, Lu R, Ge M and Zhang L:
MicroRNA-374a-5p inhibits neuroinflammation in neonatal
hypoxic-ischemic encephalopathy via regulating NLRP3 inflammasome
targeted Smad6. Life Sci. 252(117664)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Adyshev DM, Moldobaeva N, Mapes B,
Elangovan V and Garcia JG: MicroRNA regulation of nonmuscle myosin
light chain kinase expression in human lung endothelium. Am J
Respir Cell Mol Biol. 49:58–66. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gao Y, Zhao Y, Zhang J, Lu Y, Liu X, Geng
P, Huang B, Zhang Y and Lu J: The dual function of PRMT1 in
modulating epithelial-mesenchymal transition and cellular
senescence in breast cancer cells through regulation of ZEB1. Sci
Rep. 6(19874)2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu Y, El-Naggar S, Darling DS, Higashi Y
and Dean DC: Zeb1 links epithelial-mesenchymal transition and
cellular senescence. Development. 135:579–588. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fu R, Lv WC, Xu Y, Gong MY, Chen XJ, Jiang
N, Xu Y, Yao QQ, Di L, Lu T, et al: Endothelial ZEB1 promotes
angiogenesis-dependent bone formation and reverses osteoporosis.
Nat Commun. 11(460)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Matsui S, Zhou L, Nakayama Y, Mezawa M,
Kato A, Suzuki N, Tanabe N, Nakayama T, Suzuki Y, Kamio N, et al:
MiR-200b attenuates IL-6 production through IKKβ and ZEB1 in human
gingival fibroblasts. Inflamm Res. 67:965–973. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yang J, Tao Q, Zhou Y, Chen Q, Li L, Hu S,
Liu Y, Zhang Y, Shu J, Zhang X, et al: MicroRNA-708 represses
hepatic stellate cells activation and proliferation by targeting
ZEB1 through Wnt/β-catenin pathway. Eur J Pharmacol.
871(172927)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lin LP, Niu GH and Zhang XQ: Influence of
lncRNA MALAT1 on septic lung injury in mice through p38 MAPK/p65
NF-κB pathway. Eur Rev Med Pharmacol Sci. 23:1296–1304.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hu XH, Situ HL, Chen JP and Yu RH: Lipoxin
A4 alleviates lung injury in sepsis rats through p38/MAPK signaling
pathway. J Biol Regul Homeost Agents. 34:807–814. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shao Y, Chen X, Liu Y, Chen X, Li C, Wang
L and Zhao W: Dexmedetomidine alleviates lung injury in sepsis mice
through regulating P38 MAPK signaling pathway. Panminerva Med.
63:563–564. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cao Y, Liu Y, Ping F, Yi L, Zeng Z and Li
Y: miR-200b/c attenuates lipopolysaccharide-induced early pulmonary
fibrosis by targeting ZEB1/2 via p38 MAPK and TGF-β/smad3 signaling
pathways. Lab Invest. 98:339–359. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (sepsis-3). JAMA.
315:801–810. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Crowley LC, Marfell BJ, Scott AP and
Waterhouse NJ: Quantitation of apoptosis and necrosis by annexin V
binding, propidium iodide uptake, and flow cytometry. Cold Spring
Harb Protoc. 2016:953–957. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mu S, Liu Y, Jiang J, Ding R, Li X, Li X
and Ma X: Unfractionated heparin ameliorates pulmonary
microvascular endothelial barrier dysfunction via microtubule
stabilization in acute lung injury. Respir Res.
19(220)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li X, Jamal M, Guo P, Jin Z, Zheng F, Song
X, Zhan J and Wu H: Irisin alleviates pulmonary epithelial barrier
dysfunction in sepsis-induced acute lung injury via activation of
AMPK/SIRT1 pathways. Biomed Pharmacother.
118(109363)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jiang F, Yang M, Wu C and Wang J:
Potential roles of miR-374a-5p in mediating neuroprotective effects
and related molecular mechanism. J Mol Neurosci. 69:123–132.
2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chen YQ, Yang X, Xu W, Yan Y, Chen XM and
Huang ZQ: Knockdown of lncRNA TTTY15 alleviates myocardial
ischemia-reperfusion injury through the miR-374a-5p/FOXO1 axis.
IUBMB Life. 73:273–285. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
35
|
Huang ZQ, Xu W, Wu JL, Lu X and Chen XM:
MicroRNA-374a protects against myocardial ischemia-reperfusion
injury in mice by targeting the MAPK6 pathway. Life Sci.
232(116619)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Doumatey AP, He WJ, Gaye A, Lei L, Zhou J,
Gibbons GH, Adeyemo A and Rotimi CN: Circulating MiR-374a-5p is a
potential modulator of the inflammatory process in obesity. Sci
Rep. 8(7680)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Voss AK and Strasser A: The essentials of
developmental apoptosis. F1000Res. 9(F1000)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wu X, Kong Q, Zhan L, Qiu Z, Huang Q and
Song X: TIPE2 ameliorates lipopolysaccharide-induced apoptosis and
inflammation in acute lung injury. Inflamm Res. 68:981–992.
2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Li D, Lang W, Zhou C, Wu C, Zhang F, Liu
Q, Yang S and Hao J: Upregulation of microglial ZEB1 ameliorates
brain damage after acute ischemic stroke. Cell Rep. 22:3574–3586.
2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
de Barrios O, Sanchez-Moral L, Cortés M,
Ninfali C, Profitós-Pelejà N, Martínez-Campanario MC, Siles L, Del
Campo R, Fernández-Aceñero MJ, Darling DS, et al: ZEB1 promotes
inflammation and progression towards inflammation-driven carcinoma
through repression of the DNA repair glycosylase MPG in epithelial
cells. Gut. 68:2129–2141. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Chen IC, Wang SC, Chen YT, Tseng HH, Liu
PL, Lin TC, Wu HE, Chen YR, Tseng YH, Hsu JH, et al: Corylin
ameliorates LPS-induced acute lung injury via suppressing the MAPKs
and IL-6/STAT3 signaling pathways. Pharmaceuticals (Basel).
14(1046)2021.PubMed/NCBI View Article : Google Scholar
|