Introduction
Myocarditis is cardiac inflammation that can be
caused by viruses, bacteria or autoimmune diseases (1). The development of myocarditis can be
divided into two stages. The first stage involves humoral immunity
and pathogen-mediated cellular immune activation, inflammatory
cytokine production and pathway activation, leading to apparent
cardiomyocyte necrosis. The second stage is characterized by
chronic inflammation of the myocardium, which causes myocardial
remodeling and fibrosis, ultimately progressing into dilated
cardiomyopathy (DCM) (2).
Autoimmunity one of the main pathological causes of the development
of myocarditis. Myocardial inflammatory injury promotes myocardial
fibrosis, which is critical to the progression of myocarditis into
DCM (3,4). Since the different stages of
myocarditis exhibit different pathological characteristics,
distinct therapeutic approaches may be required. Inhibiting the
inflammatory response during the first stage of myocarditis and
myocardial fibrosis during the second stage may serve important
implications for disease outcomes (2). Experimental autoimmune myocarditis
(EAM) mouse models have immunological features paralleling and
resembling the myocarditis in humans, making it suitable for
pursuing pathophysiological insights into myocarditis (5). In this model, autoimmune myocarditis
can be induced by the subcutaneous inoculation of susceptible mice
with cardiac myosin and complete Freund's adjuvant (6). The first 21 days after inoculation
can be considered to correspond to the first stage of myocarditis
in humans (6,7). At this stage, antibody deposition in
cardiomyocytes leads to apoptosis, which in turn activates cellular
and humoral immunity. A large number of natural killer, T and other
immune cell types can infiltrate the myocardial tissue (7). This process leads to the release of
large quantities of IL-1β, TNF, IL-6 and other inflammatory
cytokines, exacerbating myocardial injury (7-11).
Subsequently, the inflammation would normally begin to subside,
whilst the secretion of TGF-β and collagen in the myocardium is
also increased (12). This is a
characteristic of the progressive accumulation of fibrotic tissues
in the myocardium, which dilates the ventricles and impair cardiac
function (12), in a manner that
is equivalent to the second stage of myocarditis. This EAM mouse
model is frequently used to study the different stages of
myocarditis pathogenesis (13).
Intervention at different stages of myocarditis development in
animal models would be beneficial for studying the effects of
potential therapeutic agents at different time points of
administration.
Supportive therapies and immunosuppression remain to
be the main treatment methods for myocarditis (1). However, side effects, such as weight
gain, reduced immunity and hypertension, often occur after
treatment (14-16).
Therefore, it is important to identify novel therapeutic agents
that may be beneficial for patients with myocarditis. According to
the theory of traditional Chinese medicine (TCM), radix
Sophora may treat inflammatory cardiomyopathy, while
Panax quinquefolium is effective in Dilated cardiomyopathy
(17). Pharmacological studies
involving rheumatoid arthritis models have previously shown that
Sophora flavescens alkaloids can inhibit NF-κB signaling and
reduce the levels of TNF-α and IL-6(18). By contrast, Panax
quinquefolium saponins have been shown to serve a protective
role in cardiac injury by attenuating cardiac fibrosis and
remodeling in rats with heart failure through the TGF-β1/Smad
pathway (19,20). In addition, Panax
quinquefolium saponins were found to ameliorate cardiac
remodeling and myocardial fibrosis in rat models of chronic
thromboembolic pulmonary hypertension (17,21).
However, to the best of our knowledge, all previous studies mainly
assessed the efficacy of single drugs or compounds at a certain
stage of myocarditis. Therefore, the previous findings
aforementioned may not completely reflect the therapeutic
characteristics and advantages of combined treatment with TCM
(22,23).
Alkaloids and saponins are the main
pharmacologically active components of Sophora flavescens
and Panax quinquefolium, respectively (24,25).
In the present study, an autoimmune myocarditis animal model was
used to observe the effects of Sophora flavescens alkaloids
(KuShen) and Panax quinquefolium saponins [XiYangShen;
combination of KuShen and XiYangShen (KX)] at different stages of
myocarditis. The mechanisms of action of KX were also explored to
provide an experimental basis for the future clinical application
of this formulation. To the best of our knowledge, the present
study is the first to investigate the effects of KX on different
stages of autoimmune myocarditis.
Materials and methods
Animals
A total of 96 male BALB/c mice aged 6-8 weeks
(weight, 18-20 g) were purchased from Changchun Changsheng Gene
Pharmaceutical Co., Ltd. [license number: SCXK (Liao)-2020-0001;
eligibility nos. 2107262101007676 and 21072621030386012]. Mice were
housed in a barrier system at a temperature of 23±2˚C, humidity of
60±10% and a 12/12-h light/dark cycle. All animals had free access
to food and water. The present study was approved by the animal
Ethics Committee of the Jilin Academy of TCM (approval no.
JLSZKYDWLL2021-003; Changchun, China).
Composition, ratio and concentration
selection of KX
The Sophora flavescens alkaloids were
composed by mixing matrine
(C15H24N2O) and oxymatrine
(C15H24N2O2) in a 1:1
ratio. The purity of the Panax quinquefolium saponins,
consisting of ginsenoside Rg1
(C42H72O14), ginsenoside Re
(C48H82O18) and ginsenoside rb1
(C54H92O23), was determined to be
≥70% through high-performance liquid chromatography. All these
compounds were provided by the New Drug Center of the Jilin Academy
of TCM.
Optimization of the compound formula was performed
using the uniform design model (a mathematical method) to reduce
the experiment times, the uniform design and data analysis were
performed using DPS v. 6.55 software. (26). Subsequently, EAM rat model was used
to determine the optimal group ratio of Sophora flavescens
alkaloids to Panax quinquefolium saponins, which was 1.1:1.
At that ratio, it significantly alleviated myocardial injury
(27).
EAM induction and KX treatment
After 1 week of adaptive feeding, BALB/c mice were
randomly divided into the following groups based on body weight (24
animals per group): Control; EAM; KX-High (KX dose, 275 mg/kg); and
KX-Low (KX dose, 138 mg/kg). All mice, except for those in the
control group, received subcutaneous (back, inguinal on both sides)
injections of 0.2 ml emulsion I on days 0, 7, 21 and 42 at the
beginning of the test. Porcine cardiac myosin (MilliporeSigma) was
dissolved in potassium phosphate buffer (pH=6.8) and mixed
thoroughly 1:1 with complete Freund's adjuvant (MilliporeSigma),
yielding a solution with 200 µg porcine cardiac myosin in 0.2 ml of
the emulsion (6).
In the control group, each animal was injected with
0.2 ml emulsion II (potassium phosphate buffer with complete
Freund's adjuvant at a 1:1 ratio) following the same protocol. High
and Low KX solutions were prepared by dissolving 275 or 138 mg KX,
respectively, in 10 ml distilled water. Mice in the KX-high and
KX-low groups were intragastrically fed by oral gavage (10 ml/kg)
with the experimental drug daily from day 0 until sacrifice (day 21
or 60). Mice in the control and EAM groups received an equivalent
volume of distilled water. All animals were anesthetized by an
intraperitoneal injection of sodium pentobarbital (50 mg/kg) and
then euthanized through cervical dislocation. In addition, mice
were anesthetized and sacrificed before the experimental endpoints
in case of persistent self-injurious behavior, non-healing wounds
and loss of appetite.
Histopathology
Mice were sacrificed after 21 or 60 days, before
their bodyweight and heart weight were measured. The heart tissues
were fixed in 15% formalin at a temperature of 23±2˚C for not less
than 48 h, embedded in paraffin and sectioned into transverse
sections (5-µm thickness) for H&E staining (at a temperature of
23±2˚C for 5-20 min). For the H&E staining, myocardial tissue
was examined under a light microscope at a magnification of x400,
the macroscopic score was determined using a five-point scale: 0,
no inflammation; 1, limited discoloration; 2, numerous small
lesions; 3, diffused discoloration not exceeding one-third of the
heart surface; and 4, diffused discoloration exceeding one-third of
the heart surface (28). After
processing the myocardial tissues in the same manner, Masson's
trichrome staining (at a temperature of 23±2˚C for 5-10 min) was
also performed. Myocardial tissue was examined under a light
microscope at a magnification of x400 and scored using ImageJ
(version 1.5.1; National Institutes of Health).
Immunohistochemical analysis. After
deparaffinization (the tissue sections were immersed in xylene for
10 min and re-soaked in xylene for another 10 min at a temperature
of 23±2˚C) and rehydration (descending ethanol hydration 100, 95,
90 and 80%) of the paraffin sections, tissues sections were
immersed in citrate buffer (pH 6.0; Wuhan Boster Biological
Technology, Ltd.; cat. no. AR0024) for 15 min at room temperature
for antigen retrieval. All sections were incubated in
H2O2 (3%)to block endogenous peroxidase
activity. This step was followed by incubation in 5%BSA (Wuhan
Boster Biological Technology, Ltd.; cat. no. AR0004) for 10 min at
room temperature to block nonspecific antigen-binding sites.
Subsequently, the sections were incubated with primary antibodies
(Santa Cruz Biotechnology, Inc.) against NF-κB (cat. no. sc-8008;
1:200 dilution) and TGF-β1 (cat. no. sc-130348; 1:200 dilution)
overnight at 4˚C, followed by incubation with HRP-conjugated
secondary antibodies (ProteinTech Group, Inc; cat. no. SA00001-2;
1:2,000 dilution) for 60 min at 4˚C. Then, sections were
counter-stained with DAB and hematoxylin for 3-10 min to
distinguish between cytoplasm and nucleus at room temperature.
After dehydration in ddH2O at room temperature, 90% ethanol for 5
min, 95% ethanol for 5 min, twice in 100% ethanol for 5 min and
n-butanol for 5 min, and permeabilization in xylene for 5 min,
sections were mounted using neutral gum. Positive staining was
observed under a light microscope (Olympus BX51; Olympus
Corporation). A total of five fields of view were randomly selected
for each tissue section and observed at a magnification of
x100.
ELISA analysis
The serum levels of CK-myocardial band (CK-MB), LDH
and the cardiac tissue levels of cardiac troponin I (cTn-I),
collagen type I (Col I), collagen type III (Col III) and the
inflammatory markers IL-1β, IL-6, TNF-α and TGF-β1 were detected
using mouse ELISA kits (R&D Systems China Co., Ltd.: CK-MB,
cat. no. 202109; LDH, cat. no. 202108; cTn-I, cat. no. 202108; Col
I, cat. no. 202110; IL-1β, cat. no. 202109; IL-6, cat. no. 202108;
TNF-α cat. no. 202107 and TGF-β1 cat. no. 202109). Cardiac
homogenate (10%) was prepared by grinding 100 mg of heart tissue in
1 ml of saline. In microplates, standards (50 µl) were added into
predefined wells, samples (10 µl serum or cardiac homogenate) and
sample diluent (40 µl) were added into testing sample wells, while
blank wells were left empty. In the wells for standards and
samples, horseradish peroxidase-labelled conjugates (100 µl) were
added before sealing the plates for incubation at 37˚C for 60 min.
After washing the plates 5 times, substrates A (50 µl) and B (50
µl) were added into each well. After incubation at 37˚C for 15 min,
stop solution (50 µl) was added to each well, and the absorbance of
each well was measured at 450 nm within 15 min (cat. no. ELx800;
BioTek Instruments, Inc.).
Western blotting
Protein extracts from cardiac tissue were prepared
in Radio-Immunoprecipitation Assay (RIPA) lysis buffer (CoWin
Biosciences; cat. no. 21821) containing protease and phosphatase
inhibitors. Proteins were separated through 10% SDS-PAGE and
transferred onto nitrocellulose membranes. After blocking in 5%
non-fat milk diluted in TBS-Tween 20 (0.1% Tween; CoWin
Biosciences) in the dark at 4˚C overnight, the membranes were
incubated with primary antibodies (Santa Cruz Biotechnology, Inc.)
against activated kinase 1 (TAK1)-binding protein 1 (TAB1, cat. no.
sc-166138; 1:100 dilution), NF-κB (cat. no. sc-8008; 1:200
dilution), phosphorylated (p)-NF-κB (cat. no. sc-136548; 1:200
dilution), IκB (cat. no. sc-74451; 1:100 dilution), p-IκB (cat. no.
sc-8404; 1:200 dilution), IKKα (cat. no. sc-7606; 1:200 dilution),
TGF-β1 (cat. no. sc-130348; 1:200 dilution), Smad2 (cat. no.
sc-101153; 1:200 dilution), Smad4(cat. no. sc-7966; 1:200
dilution), Col Ⅰ (cat. no. sc-376350; 1:100 dilution) or GAPDH
(cat. no. sc-365062; 1:100 dilution) for 2 h at 37˚C. Subsequently,
the membranes were washed by TBS-Tween-20 and incubated with a
secondary HRP-conjugated antibody (m-IgGκ, cat. no. sc-516102;
1:1,000 dilution or m-IgG Fc, cat. no. sc-525409; 1:1,000 dilution;
Santa Cruz Biotechnology, Inc.) for 1.5 h at 37˚C. Next, the
incubated membranes were washed again and visualized using an
enhanced chemiluminescence detection kit (CoWin Biosciences). The
levels of target proteins were normalized to those of GAPDH with
Gel imaging system (ChemiDoc-It 510 Imager, Ultra-Violet Products
Ltd).
Statistical analysis
GraphPad Prism software (version 9.0; GraphPad
Software Inc.) was used for all statistical analyses. Statistical
analysis was performed using two-way analysis of variance (ANOVA)
with Bonferroni post hoc tests for comparisons between different
time points and groups and one-way ANOVA with Tukey's post hoc test
or Kruskal-Wallis followed by Dunn's post hoc test for the
comparison among multiple groups. The macroscopic scores are
presented as the median + interquartile range, whilst all other
values are presented as the mean ± standard deviation. P<0.05
was considered to indicate a statistically significant
difference.
Results
Effects of KX on the cardiac indices
of mice
After 21 days, mice in the EAM group weighed
significantly less compared with those in the control group
(P<0.01; Fig. 1A), whilst those
in the KX-high group gained weight significantly more efficiently
compared with those in the EAM group (P<0.05; Fig. 1A). However, no significant changes
in heart weight was observed (P>0.05; Fig. 1B). The HW/BW was significantly
higher in the EAM group compared with that in the control group
(P<0.01; Fig. 1C), whereas it
was significantly decreased in the KX-high group compared with that
in the EAM group (P<0.05; Fig.
1C). However, there were no significant changes in KX-low group
(P>0.05; Fig. 1C).
After 60 days, the mice in the EAM group weighed
significantly less compared with those in the control group
(P<0.01; Fig. 1A), whilst those
in the KX group were significantly heavier compared with those
recorded in the EAM group (P≤0.05; Fig. 1A). The heart weight was
significantly higher in the EAM group compared with that in the
control group, which was reduced following intervention with KX
(P<0.01; Fig. 1B). The HW/BW
was also significantly higher in the EAM group compared with that
in the control group, which was in turn significantly reduced
following intervention with KX (P<0.01; Fig. 1C).
In the control group, no changes were observed in
the body weight of mice sacrificed after 21 and 60 days. In the EAM
group, the HW/BW was significantly increased (P<0.01; Fig. 1) after 60 days compared with that
after 21 days.
Effects of KX on myocardial injury in
mice
After 21 days, the serum levels of CK-MB, LDH and
cTn-I were significantly increased in the EAM group compared with
those in the control group (P<0.01; Fig. 2A-C). Compared with those in the EAM
group, the aforementioned indices were significantly decreased
after treatment with KX (P<0.01; Fig. 2A-C).
After 60 days, the serum levels of CK-MB, LDH and
cTn-I were significantly increased in the EAM group compared with
those in the control group (P<0.01; Fig. 2A-C). Compared with those in the EAM
group, the aforementioned indices were significantly decreased in
the KX-high group (P<0.01 or P<0.05), but in a dose-dependent
manner relationship (Fig. 2A-C).
H&E staining of the myocardial tissues showed that, compared
with that in the control group, the EAM group exhibited
inflammatory cell infiltration and myocardial tissue damage after
both 21 and 60 days (P<0.01). However, this inflammatory damage
was significantly alleviated following treatment with high-dose KX
(Fig. 2D; P<0.05).
In control mice, there were no changes observed
between 21 and 60 days. However, the levels of CK-MB, LDH and cTn-I
were significantly lower after 60 days compared with those after 21
days (P<0.01; Fig. 2A-C).
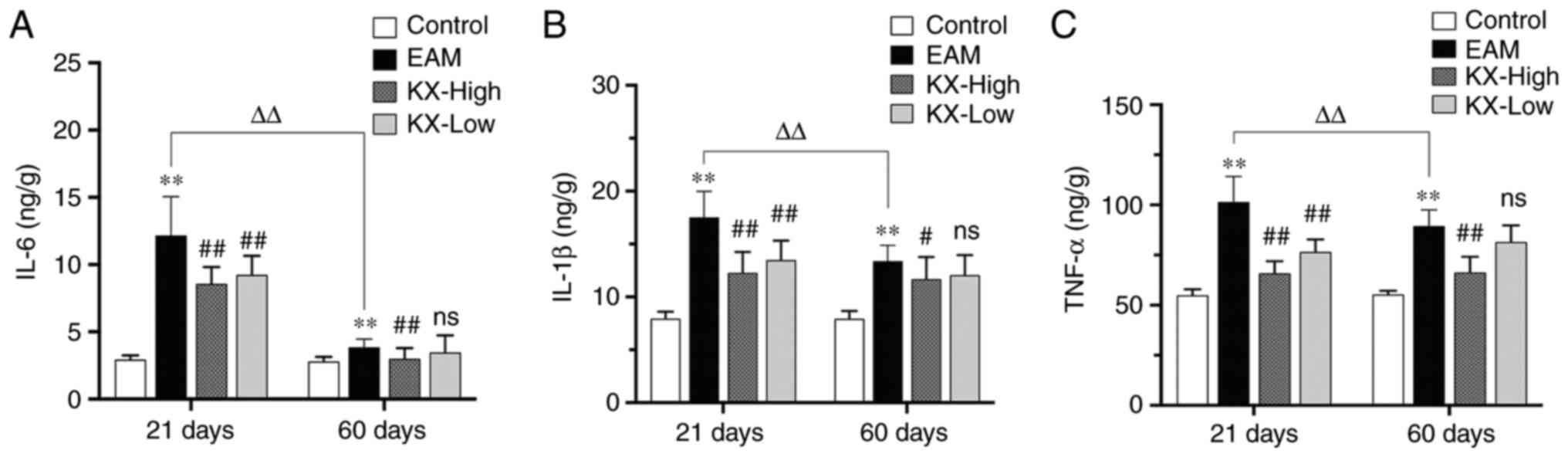
Effects of KX on inflammatory factors
in the mouse myocardium
After 21 days, the levels of IL-6, IL-1β and TNF-α
were significantly increased in the EAM group compared with those
in the control group (P<0.01; Fig.
3A-C). Compared with those in the EAM group, these levels were
significantly reduced after intervention with KX (P<0.01;
Fig. 3A-C).
After 60 days, the levels of IL-6, IL-1β and TNF-α
were significantly higher in the EAM group compared with those in
the control group (P<0.01; Fig.
3A-C). Compared with those in the EAM group, these levels were
significantly lower after treatment with high-dose KX (P<0.01 or
P<0.05; Fig. 3A-C), but in a
dose-dependent manner.
There were no changes observed in terms of the
inflammatory cytokines measured in the present study in the control
mice between 21 and 60 days. However, in the EAM group the levels
of IL-6, IL-1β and TNF-α were significantly lower in the myocardium
of mice after 60 days compared with those in mice after 21 days
(P<0.01; Fig. 3).
Effects of KX on TAB1 and NF-κB
signaling in the mouse myocardium
After 21 days, the expression levels of TAB1, IKKα,
p-IκB/IκB, p-NF-κB/NF-κB and TGF-β1 were significantly increased in
EAM mice compared with those in the control group (P<0.01;
Fig. 4A and B). These levels were significantly
decreased (P<0.01 or P<0.05; Fig. 4A and B) after intervention with KX. TAB1
appeared as two bands, which may be a consequence of the expression
of the TAB isoforms (Fig. 4A).
The expression of NF-κB and TGF-β1 in the myocardium
was next examined using immunohistochemistry. The results showed
that their levels were significantly increased (P<0.01) in the
EAM group compared with those in the control group. However, these
were significantly reversed (P<0.01 or P<0.05) after
intervention with KX (Fig. 4C and
D).
Effects of KX on cardiac fibrosis in
mice
After 21 days, the levels of TGF-β1 were
significantly higher in the myocardium of mice in the EAM group
compared with those in the control group (P<0.01). By contrast,
the levels of TGF-β1 was significantly lower in the KX-high group
compared with those in the EAM group, in a dose-dependent manner
(Fig. 5A). However, no significant
change was observed in the levels of Col I and Col III (Fig. 5B and C).
After 60 days, the levels of TGF-β1, Col I and Col
III were significantly increased in the myocardium of mice in the
EAM group compared with those in the control group (P<0.01).
Compared with those in the EAM group, these levels were
significantly lower in mice after intervention with KX (P<0.01
or P<0.05; Fig. 5A-C). The
results of cardiac Masson's Trichrome staining revealed that
myocardial fibrosis was significantly worse in EAM mice compared
with that in the control group (P<0.01). Compared with that in
the EAM group, myocardial fibrosis was significantly ameliorated
after intervention with KX (P<0.01; Fig. 5D). In the EAM group, the levels of
TGF-β1, Col I and Col III were significantly increased in the
myocardium of mice after 60 days compared with those after 21 days
(P<0.01; Fig. 5A-C).
Effects of KX on TGF-β1 and Smad2
expression in the mouse myocardium
After 60 days, the levels of TGF-β1, Smad2, Smad4,
Col I and NF-κB in the myocardium were significantly increased in
EAM mice compared with those in the control group (P<0.01).
These increases were significantly reversed in mice after treatment
with KX (P<0.01 or P<0.05; Fig.
6A and B).
NF-κB and TGF-β1 expression in mouse myocardium was
subsequently assessed using immunohistochemistry. These results
showed that the expression of NF-κB and TGF-β1 was significantly
increased in EAM mice compared with that in the control group
(P<0.01), which was in turn significantly reversed after
treatment with KX (P<0.01; Fig.
6C and D).
Discussion
Myocarditis can manifest with a variety of symptoms,
such as palpitations, chest pain and arrhythmia (29). Based on the dialectical treatments
described in TCM, Radix Sophorae flavescentis and Panax
quinquefolium can be combined to treat myocarditis, thereby
providing the superior synergistic effects of these Chinese herbs
(30). In our unpublished studies,
in vitro tissue culture and in vivo viral myocarditis
animal models were used to perform antiviral pharmacodynamic
experiments using different concentrations of KX. The results of
these studies showed that at 0.781 and 0.391 g/l, KX was efficient
against HeLa cell cytopathy induced by various enteroviruses, such
as coxsackievirus B3 (CVB3), CVB4, CVA16 and enterovirus E71. In
addition, the results of these studies showed that at 275 and 138
mg/kg, KX exerted significant therapeutic effects on BALB/c mice
with viral myocarditis by reducing aspartate aminotransferase,
creatine kinase (CK) and lactate dehydrogenase (LDH) levels in
serum. Based on results from a previous study (27), the main active components of
Sophora flavescens and Panax quinquefolium, namely
Sophora flavescens alkaloids and Panax quinquefolium
saponins, respectively, were extracted and applied in EAM mouse
models. The purpose of this approach was to identify the effective
components of these compounds and control their quality and
quantity.
An animal model of EAM was previously produced
through the injection of cardiac myosin into susceptible rodents,
which lack the immune tolerance mechanisms, to recreate the
inflammatory and fibrotic stages of myocarditis (31). Inflammation and fibrosis of the
myocardium are two important etiological causes of myocarditis
(32). Following an autoimmune
attack, a large number of immune cells infiltrate the myocardium,
leading to myocardial damage, myocardial structural disintegration
and the production of proinflammatory cytokines, such as IL-6,
IL-1β and TNF-α (33). This
process further exacerbates myocardial damage. After ~21 days,
myocarditis advances into the fibrotic stage, where the levels of
proinflammatory cytokines decrease but those of profibrotic factors
(such as TGF-β1) increase (34).
These changes interfere with the regulation of the extracellular
matrix architecture and promote the development of fibrosis,
thereby replacing necrotic myocardial tissues with collagen protein
(35).
In the present study, histopathological analysis of
the myocardium revealed marked inflammatory cell infiltration and
the presence of edematous and necrotic tissues in EAM mice
sacrificed after 21 days. These observations may be attributed to
the activation of the TAB1/NF-κB pathway and the promotion of
inflammatory factor expression (IL-6, IL-1β and TNF-α). There were
no changes observed in the control group between 21 and 60 days.
However, the EAM mice showed reduced inflammatory cell infiltration
after 60 days. In addition, increased myocardial tissue damage and
fibrosis was observed after 60 days compared with those after 21
days. This may be due to reduced secretion of inflammatory factors
in the myocardium, increased activation of the TGF-β1/Smad2 pathway
and an increase in the expression of the profibrotic factors
TGF-β1, Col I and Col III. These findings suggest that damage may
have changed from excessive inflammation at 21 days to myocardial
fibrotic damage at 60 days. These are consistent with the results
reported in previous studies (36,37).
TNF-α is mainly secreted by mononuclear macrophages
and is an important inflammatory and immunomodulatory factor. It is
responsible for the immunopathological damage of myocardial cells
(38). By contrast, IL-6 is mainly
secreted by macrophages and dendritic cells, which contributes to
host defenses against infection and tissue injury (39). However, excessive IL-6 synthesis
exacerbates the pathological response and promotes collagen
production by cardiac fibroblasts to exacerbate myocardial fibrosis
(40). This finding was previously
confirmed by the limited development of autoimmune myocarditis in
IL-6 knockout mice (41). IL-1β is
a potent proinflammatory cytokine that activates T cells and
induces cytokine production (39).
TAB1 is a protein activator of TAK1. The TAB1-TAK1 complex is
involved in NF-κB activation (32,42).
Overexpression of IL-1β, TNF-α and IL-6 can all activate NF-κB
through the TAB1 pathway (43).
This activated NF-κB then translocates to the nucleus as a
transcription factor to activate the expression of inflammatory
cytokines (44). This process
leads to a cascade amplification response mediated among
inflammatory cytokines to sustain chronic cardiomyocyte necrosis
and infiltration of inflammatory cells (45). It is the activation of this vicious
cycle that exacerbates myocardial injury (45). Therefore, TAB1 serves a key role in
the regulation of the immune-inflammatory response. Importantly,
inhibition of TAB1/NF-κB signaling and secretion of TNF-α, IL-6 and
IL-1β can effectively prevent myocardial inflammation and
remodeling (46-49).
The present study showed that treatment of EAM mice
with KX ameliorated myocardial injury in a dose-dependent manner,
in addition to inhibiting the secretion of TNF-α, IL-6, IL-1β and
NF-κB signaling proteins. Therefore, KX may reduce inflammation
through the TAB1/NF-κB pathway to reduce myocardial injury.
Numerous modern pharmacological studies have previously shown that
the Sophora flavescens family of TCM can reduce the levels
of TNF-α and IL-1β in ethanol-induced acute gastric ulcer rat model
and lipopolysaccharide-induced inflammation in zebrafish through
the NF-κB signaling pathway (18,50,51).
Combined with the findings of the present study, it was therefore
hypothesized that Sophora flavescens alkaloids in KX may
serve a therapeutic role in the first stage of EAM by inhibiting
inflammatory responses. Nevertheless, further investigation is
warranted to validate this finding.
TGF-β1 is an anti-inflammatory cytokine secreted by
macrophages that are activated by regulatory T-cells (52). Members of the TGF-β family bind to
type II receptors and recruit type I receptors, leading to the
phosphorylation and activation of type I receptors (53). In turn, type I receptors
phosphorylate the Smad downstream effector molecules, including
Smad1, Smad2, Smad3, Smad5 and Smad8, which translocate to the
nucleus to promote the expression of fibrotic genes, such as
effector connective tissue growth factor (CTGF), Col I and Col III
(54). TGF-β1 can also convert
fibroblasts into myofibroblasts, which synthesize and secrete
fibrillar Col I and Col III to promote the development of
myocardial fibrosis (55).
Previous studies have shown that inhibition of TGF-β1 can reduce
the production of Col I and Col III to prevent myocardial fibrosis
(56,57). Hao et al (58) reported that the mRNA expression and
promotion of Col I synthesis by TGF-β1 was eliminated by blocking
Smad expression in the myocardial infarct rat model, suggesting
that TGF-β1 and Smad are involved in collagen synthesis. Therefore,
inhibition of the TGF-β1 pathway may be key to the treatment of
myocardial fibrosis (12). In the
present study, the levels of TGF-β1, Col I and Col III, in addition
to the degree of myocardial fibrosis, were all significantly
reduced in EAM mice treated with KX after 60 days in a
dose-dependent manner. This treatment also significantly reduced
the expression of TGF-β1/Smad2 pathway signaling components in the
myocardial tissue. It is suggested that KX may delay the
development of myocardial fibrosis and irreversible pathological
changes to the myocardium through the TGF-β1/Smad2 pathway in mice
with inflammatory myocardial injury. A number of pharmacological
studies have shown that treatment with American ginseng (Panax
quinquefolium) can delay myocardial fibrosis and cardiac
remodeling in animal models through the TGF-β1/Smad signaling
pathway (17,19-21).
Collectively, the previous and present results indicate that
Panax quinquefolium saponins contained in KX can exert a
therapeutic effect on the second stage of EAM by delaying the
development of myocardial fibrosis.
Based on the present findings, KX may be efficacious
for the clinical treatment of myocarditis by attenuating the
inflammatory response whilst alleviating the clinical symptoms in
acute myocarditis. KX may be effective in delaying progression even
in patients in which the treatment of myocarditis is delayed. This
provides an important reference value for the clinical application
of KX.
However, the present study remain associated with a
number of limitations. There was lack of a positive control group.
Astragalus membranaceus would be the ideal positive control
group for this study. However, the present study was rather focused
on the effects of KX on different stages of autoimmune myocarditis
and made comparisons among different stages. The present study also
did not measure oxidative stress parameters, which can provide
evidence of cell damage. There was a lack of echocardiography test
results, which are critical for cardiac function assessment in
mice. Analysis of cardiac function by echocardiography, along with
the effect of KX on the differentiation of CD4+ T cells,
would be of potential interest for future studies.
Results of the present study suggested that KX may
reduce the inflammatory response to autoimmune myocarditis and
attenuate pathological injury during the first stage of EAM through
the TAB1/NF-κB pathway. Subsequently, KX may also delay the
progression of autoimmune myocarditis onto myocardial fibrosis
through the TGF-β1/Smad2 pathway during the second stage of EAM.
This reflects the multifaceted, multi-target and multi-pathway
action of KX in the treatment of myocarditis, in addition to the
synergy among Chinese herbal therapies. However, in the present
study, there is insufficient evidence to state that Sophora
flavescens alkaloids and Panax quinquefolium saponins
inhibit the first and second phases of EAM, respectively.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Jilin Science
and Technology Development Plan Project (grant no.
20200403128SF).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL and TD made substantial contributions to the
conception and design of the study. ML and YL were responsible for
the experimental procedures, data acquisition, analysis and
interpretation and confirm the authenticity of all the raw data. ML
performed the drafting of the article and critically revised it for
important intellectual content. HX was responsible for the review
of data and experiments. All authors read and approved the final
manuscript. All authors agree to be accountable for all aspects of
the work in ensuring that questions related to the accuracy or
integrity of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
All experiments and animal care procedures were
approved by the animal ethics committee of Jilin Academy of
Traditional Chinese Medicine(approval no. JLSZKYDWLL2021-003).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tschöpe C, Ammirati E, Bozkurt B, Caforio
ALP, Cooper LT, Felix SB, Hare JM, Heidecker B, Heymans S, Hübner
N, et al: Myocarditis and inflammatory cardiomyopathy: Current
evidence and future directions. Nat Rev Cardiol. 18:169–193.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Canter CE and Simpson KE: Diagnosis and
treatment of myocarditis in children in the current era.
Circulation. 129:115–128. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jaén RI, Fernández-Velasco M, Terrón V,
Sánchez-García S, Zaragoza C, Canales-Bueno N, Val-Blasco A,
Vallejo-Cremades MT, Boscá L and Prieto P: BML-111 treatment
prevents cardiac apoptosis and oxidative stress in a mouse model of
autoimmune myocarditis. FASEB J. 34:10531–10546. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fairweather D, Kaya Z, Shellam GR, Lawson
CM and Rose NR: From infection to autoimmunity. J Autoimmun.
16:175–186. 2001.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Błyszczuk P: Myocarditis in humans and in
experimental animal models. Front Cardiovasc Med.
6(64)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ciháková D, Sharma RB, Fairweather D,
Afanasyeva M and Rose NR: Animal models for autoimmune myocarditis
and autoimmune thyroiditis. Methods Mol Med. 102:175–193.
2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pollack A, Kontorovich AR, Fuster V and
Dec GW: Viral myocarditis-diagnosis, treatment options, and current
controversies. Nat Rev Cardiol. 12:670–680. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Neu N, Rose NR, Beisel KW, Herskowitz A,
Gurri-Glass G and Craig SW: Cardiac myosin induces myocarditis in
genetically predisposed mice. J Immunol. 139:3630–3636.
1987.PubMed/NCBI
|
|
9
|
Blyszczuk P, Behnke S, Lüscher TF,
Eriksson U and Kania G: GM-CSF promotes inflammatory dendritic cell
formation but does not contribute to disease progression in
experimental autoimmune myocarditis. Biochim Biophys Acta.
1833:934–944. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
van Heeswijk RB, De Blois J, Kania G,
Gonzales C, Blyszczuk P, Stuber M, Eriksson U and Schwitter J:
Selective in vivo visualization of immune-cell infiltration in a
mouse model of autoimmune myocarditis by fluorine-19 cardiac
magnetic resonance. Circ Cardiovasc Imaging. 6:277–284.
2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li Y, Heuser JS, Cunningham LC, Kosanke SD
and Cunningham MW: Mimicry and antibody-mediated cell signaling in
autoimmune myocarditis. J Immunol. 177:8234–8240. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Blyszczuk P, Müller-Edenborn B, Valenta T,
Osto E, Stellato M, Behnke S, Glatz K, Basler K, Lüscher TF,
Distler O, et al: Transforming growth factor-β-dependent Wnt
secretion controls myofibroblast formation and myocardial fibrosis
progression in experimental autoimmune myocarditis. Eur Heart J.
38:1413–1425. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tajiri K, Imanaka-Yoshida K, Tsujimura Y,
Matsuo K, Hiroe M, Aonuma K, Ieda M and Yasutomi Y: A new mouse
model of chronic myocarditis induced by recombinant bacille
calmette-guèrin expressing a t-cell epitope of cardiac myosin heavy
chain-α. Int J Mol Sci. 22(794)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kleinert S, Weintraub RG, Wilkinson JL and
Chow CW: Myocarditis in children with dilated cardiomyopathy:
Incidence and outcome after dual therapy immunosuppression. J Heart
Lung Transplant. 16:1248–1254. 1997.PubMed/NCBI
|
|
15
|
Felix SB, Staudt A, Dörffel WV, Stangl V,
Merkel K, Pohl M, Döcke WD, Morgera S, Neumayer HH, Wernecke KD, et
al: Hemodynamic effects of immunoadsorption and subsequent
immunoglobulin substitution in dilated cardiomyopathy: Three-month
results from a randomized study. J Am Coll Cardiol. 35:1590–1598.
2000.PubMed/NCBI View Article : Google Scholar
|
|
16
|
De Luca G, Campochiaro C, Sartorelli S,
Peretto G, Sala S, Palmisano A, Esposito A, Candela C, Basso C,
Rizzo S, et al: Efficacy and safety of mycophenolate mofetil in
patients with virus-negative lymphocytic myocarditis: A prospective
cohort study. J Autoimmun. 106(102330)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang YJ, Zhang XL, Li MH, Iqbal J,
Bourantas CV, Li JJ, Su XY, Muramatsu T, Tian NL and Chen SL: The
ginsenoside Rg1 prevents transverse aortic constriction-induced
left ventricular hypertrophy and cardiac dysfunction by inhibiting
fibrosis and enhancing angiogenesis. J Cardiovasc Pharmacol.
62:50–57. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Niu Y, Dong Q and Li R: Matrine regulates
Th1/Th2 cytokine responses in rheumatoid arthritis by attenuating
the NF-κB signaling. Cell Biol Int. 41:611–621. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zheng X, Wang S, Zou X, Jing Y, Yang R, Li
S and Wang F: Ginsenoside Rb1 improves cardiac function and
remodeling in heart failure. Exp Anim. 66:217–228. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang QW, Yu XF, Xu HL, Zhao XZ and Sui DY:
Ginsenoside re improves isoproterenol-induced myocardial fibrosis
and heart failure in rats. Evid Based Complement Alternat Med.
2019(3714508)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li CY, Deng W, Liao XQ, Deng J, Zhang YK
and Wang DX: The effects and mechanism of ginsenoside Rg1 on
myocardial remodeling in an animal model of chronic thromboembolic
pulmonary hypertension. Eur J Med Res. 18(16)2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yin HJ, Zhang Y and Jiang YR: Effect of
folium panax quinquefolium saponins on apoptosis of cardiac muscle
cells and apoptosis-related gene expression in rats with acute
myocardial infarction. Zhongguo Zhong Xi Yi Jie He Za Zhi.
25:232–235. 2005.PubMed/NCBI(In Chinese).
|
|
23
|
Wei N, Zhang C, He H, Wang T, Liu Z, Liu
G, Sun Z, Zhou Z, Bai C and Yuan D: Protective effect of saponins
extract from Panax japonicus on myocardial infarction: Involvement
of NF-κB, Sirt1 and mitogen-activated protein kinase signalling
pathways and inhibition of inflammation. J Pharm Pharmacol.
66:1641–1651. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Szczuka D, Nowak A, Zakłos-Szyda M, Kochan
E, Szymańska G, Motyl I and Blasiak J: American ginseng (Panax
quinquefolium L.) as a source of bioactive phytochemicals with
pro-health properties. Nutrients. 11(1041)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
He X, Fang J, Huang L, Wang J and Huang X:
Sophora flavescens Ait: Traditional usage, phytochemistry
and pharmacology of an important traditional Chinese medicine. J
Ethnopharmacol. 172:10–29. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang G, Tang Y, Shang J, Wang Z, Yu H, Du
W and Fu Q: Flow-injection chemiluminescence method to detect a β2
adrenergic agonist. Luminescence. 30:102–109. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ding T, Liu B, Wang X, Wen FC, Ji FL, Song
LL and Xu HB: Effects of KX composition and single ingredient on
the model of autoimmunity myocarditis. Chi J Tradit Chin Med Pharm.
32:3710–3712. 2017.(In Chinese).
|
|
28
|
Aretz HT: Myocarditis: The dallas
criteria. Hum Pathol. 18:619–624. 1987.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sinagra G, Anzini M, Pereira NL, Bussani
R, Finocchiaro G, Bartunek J and Merlo M: Myocarditis in clinical
practice. Mayo Clin Proc. 91:1256–1266. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li Y, Yu P, Fu W, Cai L, Yu Y, Feng Z,
Wang Y, Zhang F, Yu X, Xu H and Sui D:
Ginseng-Astragalus-oxymatrine injection ameliorates
cyclophosphamide-induced immunosuppression in mice and enhances the
immune activity of RAW264.7 cells. J Ethnopharmacol.
279(114387)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang S, Liu X, Sun C, Yang J, Wang L, Liu
J, Gong L and Jing Y: Apigenin attenuates experimental autoimmune
myocarditis by modulating Th1/Th2 cytokine balance in mice.
Inflammation. 39:678–686. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sagar S, Liu PP and Cooper LT Jr:
Myocarditis. Lancet. 379:738–747. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang J, Liu T, Chen X, Jin Q, Chen Y,
Zhang L, Han Z, Chen D, Li Y, Lv Q and Xie M: Bazedoxifene
regulates Th17 immune response to ameliorate experimental
autoimmune myocarditis via inhibition of STAT3 activation. Front
Pharmacol. 11(613160)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Muller AM, Fischer A, Katus HA and Kaya Z:
Mouse models of autoimmune diseases-autoimmune myocarditis. Curr
Pharm Des. 21:2498–2512. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Li J, Schwimmbeck PL, Tschope C, Leschka
S, Husmann L, Rutschow S, Reichenbach F, Noutsias M, Kobalz U,
Poller W, et al: Collagen degradation in a murine myocarditis
model: Relevance of matrix metalloproteinase in association with
inflammatory induction. Cardiovascular Res. 56:235–247.
2002.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Izumi T, Takehana H, Matsuda C, Yokoyama
H, Kohno K, Suzuki K and Inomata T: Experimental autoimmune
myocarditis and its pathomechanism. Herz. 25:274–278.
2000.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Esfandiarei M and McManus BM: Molecular
biology and pathogenesis of viral myocarditis. Annu Rev Pathol.
3:127–155. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Liu T, Yang F, Liu J, Zhang M, Sun J, Xiao
Y, Xiao Z, Niu H, Ma R, Wang Y, et al: Astragaloside IV reduces
cardiomyocyte apoptosis in a murine model of coxsackievirus
B3-induced viral myocarditis. Exp Anim. 68:549–558. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Camporeale A and Poli V: IL-6, IL-17 and
STAT3: A holy trinity in auto-immunity? Front Biosci (Landmark Ed).
17:2306–2326. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
40
|
Meléndez GC, McLarty JL, Levick SP, Du Y,
Janicki JS and Brower GL: Interleukin 6 mediates myocardial
fibrosis, concentric hypertrophy, and diastolic dysfunction in
rats. Hypertension. 56:225–231. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Eriksson U, Kurrer MO, Schmitz N, Marsch
SC, Fontana A, Eugster HP and Kopf M: Interleukin-6-deficient mice
resist development of autoimmune myocarditis associated with
impaired upregulation of complement C3. Circulation. 107:320–325.
2003.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Shim JH, Xiao C, Paschal AE, Bailey ST,
Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, et al:
TAK1, but not TAB1 or TAB2, plays an essential role in multiple
signaling pathways in vivo. Genes Dev. 19:2668–2681.
2005.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yang C, Yang C, Zhang J, Guo Y, Chen N,
Yin B, Zhou Q, Zhang T, Guo S and Deng G: MicroRNA-211 regulates
the expression of TAB1 and inhibits the NF-κB signaling pathway in
lipopolysaccharide-induced endometritis. Int Immunopharmacol.
96(107668)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Cheng Z, Taylor B, Ourthiague DR and
Hoffmann A: Distinct single-cell signaling characteristics are
conferred by the MyD88 and TRIF pathways during TLR4 activation.
Sci Signal. 8(ra69)2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Libby P, Nahrendorf M and Swirski FK:
Leukocytes link local and systemic inflammation in ischemic
cardiovascular disease: An expanded ‘Cardiovascular Continuum’. J
Am Coll Cardiol. 67:1091–1103. 2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Liu T, Zhang M, Niu H, Liu J, Ruilian M,
Wang Y, Xiao Y, Xiao Z, Sun J, Dong Y and Liu X: Astragalus
polysaccharide from astragalus melittin ameliorates inflammation
via suppressing the activation of TLR-4/NF-κB p65 signal pathway
and protects mice from CVB3-induced virus myocarditis. Int J Biol
Macromol. 126:179–186. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Xue YL, Zhang SX, Zheng CF, Li YF, Zhang
LH, Hao YF, Wang S and Li XW: Silencing of STAT4 protects against
autoimmune myocarditis by regulating Th1/Th2 immune response via
inactivation of the NF-κB pathway in rats. Inflammation.
42:1179–1189. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Pan A, Tan Y, Wang Z and Xu G: STAT4
silencing underlies a novel inhibitory role of microRNA-141-3p in
inflammation response of mice with experimental autoimmune
myocarditis. Am J Physiol Heart Circ Physiol. 317:H531–H540.
2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zhu Z, Xueying L, Chunlin L, Wen X,
Rongrong Z, Jing H, Meilan J, Yuwei X and Zili W: Effect of
berberine on LPS-induced expression of NF-κB/MAPK signalling
pathway and related inflammatory cytokines in porcine intestinal
epithelial cells. Innate Immun. 26:627–634. 2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Hu J, Luo J, Zhang M, Wu J, Zhang Y, Kong
H, Qu H, Cheng G and Zhao Y: Protective effects of radix sophorae
flavescentis carbonisata-based carbon dots against ethanol-induced
acute gastric ulcer in rats: Anti-Inflammatory and antioxidant
activities. Int J Nanomedicine. 16:2461–2475. 2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Hwang SJ, Song YS and Lee HJ: Phaseolin
attenuates lipopolysaccharide-induced inflammation in RAW 264.7
cells and zebrafish. Biomedicines. 9(420)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Noack M and Miossec P: Th17 and regulatory
T cell balance in autoimmune and inflammatory diseases. Autoimmun
Rev. 13:668–677. 2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Morikawa M, Derynck R and Miyazono K:
TGF-β and the TGF-β Family: Context-dependent roles in cell and
tissue physiology. Cold Spring Harb Perspect Biol.
8(a021873)2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Biernacka A, Dobaczewski M and
Frangogiannis NG: TGF-β signaling in fibrosis. Growth Factors.
29:196–202. 2011.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Cowling RT, Kupsky D, Kahn AM, Daniels LB
and Greenberg BH: Mechanisms of cardiac collagen deposition in
experimental models and human disease. Transl Res. 209:138–155.
2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Zhang Y, Wang J, Li H, Yuan L, Wang L, Wu
B and Ge J: Hydrogen sulfide suppresses transforming growth
factor-β1-induced differentiation of human cardiac fibroblasts into
myofibroblasts. Sci China Life Sci. 58:1126–1134. 2015.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Johnston EF and Gillis TE: Transforming
growth factor beta-1 (TGF-β1) stimulates collagen synthesis in
cultured rainbow trout cardiac fibroblasts. J Exp Biol.
220:2645–2653. 2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Hao J, Ju H, Zhao S, Junaid A, Scammell-La
Fleur T and Dixon IM: Elevation of expression of Smads 2, 3, and 4,
decorin and TGF-beta in the chronic phase of myocardial infarct
scar healing. J Mol Cell Cardiol. 31:667–678. 1999.PubMed/NCBI View Article : Google Scholar
|