Introduction
Subarachnoid hemorrhage (SAH) caused by ruptured
aneurysm is associated with high mortality and disability rates
(1,2). Cerebral vasospasm (CV) is a
representative factor for worsening of prognosis in patients with
aneurysmal subarachnoid hemorrhage (aSAH) (3). CV refers to a transient, self-limited
narrowing of the intracranial arteries, which typically occurs
between 4 and 14 days after an aSAH (4). CV can affect up to 30~40% of patients
with aSAH, and it is associated with delayed cerebral ischemia
(DCI) in 20~30% of cases (5,6).
Although the exact pathophysiology of CV remains unclear,
inflammatory reaction might play an important role in the
development of CV and DCI (7).
Leukocyte infiltration can occur due to aSAH, resulting in the
release of inflammatory cytokines, including interleukin (IL)-1,
IL-6, and tumor necrosis factor (TNF)-α (4). Subsequently, activation of microglia
and macrophage can lead to a widespread inflammatory cascade
(8,9). In prior studies with aSAH, WBC
derives and high-sensitivity C-reactive protein (hsCRP) have been
investigated as blood markers reflecting systemic inflammation
(10-12).
It is known that elevated leukocyte count is associated with a
higher risk of symptomatic vasospasm and that increased hsCRP is a
predictor of secondary deterioration in patients with good-grade
aSAH (13,14). Procalcitonin (PCT) is a blood
marker that can reflect the status of systemic inflammation
(15,16).
PCT is an amino-acid precursor of calcitonin. It is
synthesized in C cells of the thyroid (17). It can also be produced in various
parenchymal tissues and differentiated cells, especially during a
pathologic inflammatory state, such as a bacterial infection or
sepsis (18). Furthermore, the
level of PCT is valuable in predicting outcome both for systemic
infection, and for the development of systemic inflammatory
response syndrome (SIRS) after trauma, burn, or stroke (17,19-21).
SIRS has also been verified as an independent risk factor of poor
prognosis in aSAH (22). As an
inflammatory response marker, the level of PCT maybe associated
with the occurrence of CV which is caused by the inflammatory
response after aSAH. PCT might be an interesting target to examine
in patients with aSAH. However, the predictive value of PCT in CV
after aSAH has not yet been established. Thus, the purpose of our
study was to investigate the predictive value of PCT at early phase
to distinguish CV from systemic infection in patients with
aSAH.
Materials and methods
Study population
This multi-center retrospective study was performed
with prospectively collected data. Local Institutional Review Board
approval was obtained from Soonchunhyang University Bucheon
Hospital, St. Vincent's Hospital, and Hangang Sacred Heart
Hospital. Medical records of patients with aSAH between January
2013 and December 2021 were reviewed. Treatment modality for aSAH
was determined based on our policies and each neurosurgeon's
preference. Inclusion criteria were as follows: 1) Age of 18 to 90
year, 2) patients who underwent endovascular treatment for SAH
caused by ruptured aneurysm which was confirmed by computed
tomography angiography or magnetic resonance angiography, and 3)
who received treatment for ruptured aneurysm within 72 h of ictus.
Exclusion criteria were: 1) SAH caused by factors other than
aneurysm, such as trauma and other cerebrovascular diseases, 2)
patients who had not received treatment for ruptured aneurysm
itself, 3) history of infection or stroke within four weeks before
aSAH, 4) underlying cancer, severe kidney or liver dysfunction, 5)
history of auto-immune or hematologic disease, and 6) patients with
inappropriate laboratory data or loss of follow-up within 90
days.
Baseline characteristics and
laboratory data
Demographic data of age, gender, and past medical
history were obtained. Systemic infections, such as pneumonia,
urinary tract infection (UTI), central nervous system (CNS)
infection, catheter-related infection, and sepsis were defined by
the department of infectious disease, according to medical criteria
(4,23). Initial Hunt-Hess (H-H) grade (a
high H-H grade: IV-V), a modified Fischer grade, size and location
of aneurysm were assessed by four neurosurgeons. Laboratory data of
detailed peripheral blood count, erythrocyte sedimentation rate
(ESR), high-sensitivity C-reactive protein (hsCRP), and PCT were
recorded with routinely collected peripheral blood at 5 to 7 days
after neuro-intervention. Clinical outcome was evaluated with a
modified Rankin Scale (mRS) score at 90 days after aSAH. A
favorable clinical outcome was defined as mRS 0-2. Development of
CV or DCI was assessed according to prior multidisciplinary
research group, as follows: 1) Symptomatic CV was defined as
clinical deterioration deemed secondary to vasospasm, and 2) DCI
was defined as symptomatic vasospasm, with cerebral ischemia
attributable to vasospasm (24,25).
Statistical analysis
Continuous variables were analyzed using paired
Student's t-test and presented as mean ± standard deviation (SD) or
median with interquartile range [IQR]. Categorical variables were
analyzed with a χ2 test and expressed as frequency with
percentage. Receiver operating characteristic (ROC) analysis was
used to evaluate the predictable value of PCT for systemic
infection in all patients. To clarify the association between PCT
and CV, additional ROC analysis was performed in patients without
systemic infection. Subsequently, subgroup analysis was performed
for patients without systemic infection, after dichotomization
according to the identified cutoff value of PCT level for CV.
Univariate and multivariate logistic regression analyses were used
to investigate factors associated with CV, based on odds ratio (OR)
with 95% confidence interval (CI) as an estimate for each endpoint.
All data were analyzed using Stata Statistical Software, release 15
(Stata, College Station, TX, USA). Two-tailed P-value ≤0.05 was
considered statistically significant.
Results
Baseline characteristics
A total of 374 patients were divided to infection
group (164 patients, 43.9%) and infection-free group (210 patients,
56.1%), according to the presence or absence of systemic infection.
The infection group (164 patients) contained with pneumonia (72
patients, 43.9%), UTI (41 patients, 25.0%), CNS infection (14
patients, 8.5%), catheter-related infection (12 patients, 7.3%),
and sepsis (25 patients, 15.2%). Clinical presentations of systemic
infection were as follows: pneumonia in 72 patients, UTI in 41
patients, CNS infection in 14 patients, sepsis in 25 patients, and
others in 12 patients. There were no significant differences in
age, gender, or past history of hypertension between the infection
group and the infection-free group (age: 57.1±13.5 vs. 59.2±14.4,
P=0.411; female gender 68.9% vs. 63.8%, P=0.197; and hypertension
59.1% vs. 60.9%, P=0.539). The infection group showed higher median
H-H grade and higher rate of high H-H grade (H-H grades IV-V) than
the infection-free group [4 (2-5) vs. 3 (1-4), P=0.002; 29.9% vs.
18.1%, P=0.006]. Mean intensive care unit stay days was
significantly longer in the infection group at (13.7±7.2) days than
in the infection-free group at (7.2±3.4) (P<0.001). In addition,
the infection group had higher rate of modified Fisher grade III-IV
than the infection-free group (44.5% vs. 31.4%, P=0.003). Between
groups, there were no significant differences in the size or
location of aneurysm, or CSF diversion. In laboratory findings,
mean levels of WBC, neutrophil, ESR, hsCRP, and PCT were
significantly higher in the infection group than in the
infection-free group (WBC: 13.91±6.53 vs. 9.33±4.38, P=0.016;
neutrophil 12.01±5.97 vs. 8.40±4.38, P=0.017; ESR: 34.44±21.27 vs.
22.73±17.88, P=0.022; hsCRP: 7.59±3.17 vs. 3.67±1.64, P=0.001; and
PCT: 0.31±0.22 vs. 0.08±0.07, P<0.001). Occurrence rates of CV
and DCI were significantly higher in the infection group than in
the infection-free group (34.2% vs. 18.0%, P=0.010; and 31.1% vs.
15.7%, P=0.009, respectively). The infection group showed higher
median value of 3 months mRS than the infection free group [3 (1-5)
vs. 2 (1-3), P=0.003]. The infection group had lower rate of
favorable clinical outcome, but higher mortality rate than the
infection free group (favorable outcome: 43.3% vs. 81.9%,
P<0.001; mortality 12.2% vs. 5.2%, P=0.002) (Table I).
 | Table IBaseline characteristics of whole
participants stratified by infection status in patients with
aneurysmal subarachnoid hemorrhage. |
Table I
Baseline characteristics of whole
participants stratified by infection status in patients with
aneurysmal subarachnoid hemorrhage.
| Variables | Infection (n=164,
43.9%) | Infection free
(n=210, 56.1%) | P-value |
|---|
| Details of infection,
n (%) | | | |
|
Pneumonia | 72 (43.9) | | |
|
Urinary
tract infection | 41 (25.0) | | |
|
CNS
infection | 14 (8.5) | | |
|
Catheter-related
infection | 12 (7.3) | | |
|
Sepsis | 25 (15.2) | | |
| Demographics | | | |
|
Age, mean ±
SD | 57.1±13.5 | 59.2±14.4 | 0.411 |
|
Female, n
(%) | 113 (68.9) | 134 (63.8) | 0.197 |
|
Hypertension,
n (%) | 97 (59.1) | 128 (60.9) | 0.529 |
|
H-H grade,
median (IQR) | 4 (2-5) | 3 (1-4) | 0.002a |
|
High H-H
grade (H-H grades IV-V), n (%) | 49 (29.9) | 38 (18.1) | 0.006b |
|
Days of stay
in ICU | 13.7±7.2 | 7.2±3.4 |
<0.001a |
| Radiological
characteristics | | | |
|
Modified
Fisher grade, median (IQR) | 3 (1-4) | 3 (1-4) | 0.108 |
|
Modified
Fisher grade III-IV, n (%) | 73 (44.5) | 66 (31.4) | 0.003b |
|
Aneurysm
size, mm, mean ± SD | 6.1±3.4 | 5.8±3.7 | 0.434 |
| Aneurysm
locations | | | |
|
Anterior
circulation, n (%) | 131 (79.9) | 175 (83.3) | 0.249 |
|
Posterior
circulation, n (%) | 33 (20.1) | 35 (16.7) | 0.218 |
| CSF diversion | | | |
|
Extra-ventricular
drainage, n (%) | 34 (20.7) | 35 (16.7) | 0.347 |
|
Lumbar
drainage, n (%) | 12 (7.3) | 14 (6.7) | 0.522 |
| Laboratory
findings, mean ± SD | | | |
|
Red blood
cells, x1012/l | 4.31±0.68 | 4.21±0.64 | 0.571 |
|
White blood
cells, x109/l | 13.91±6.53 | 9.33±4.38 | 0.016a |
|
Neutrophils,
x109/l | 12.01±5.97 | 8.40±4.38 | 0.017a |
|
Platelets,
x109/l | 222±108 | 247±114 | 0.178 |
|
Erythrocyte
sedimentation rate, mm/h | 34.44±21.27 | 22.73±17.88 | 0.022a |
|
High-sensitivity
C-reactive protein, mg/l | 7.59±3.17 | 3.67±1.64 | 0.001a |
|
Procalcitonin,
ng/ml | 0.31±0.22 | 0.08±0.07 |
<0.001a |
| Clinical
outcomes | | | |
|
Cerebral
vasospasm, n (%) | 56 (34.2) | 38 (18.1) | 0.010b |
|
Delayed
cerebral ischemia, n (%) | 51 (31.1) | 33 (15.7) | 0.009b |
|
mRS score at
3 months, median (IQR) | 3 (1-5) | 2 (1-3) | 0.003a |
|
Favorable
clinical outcome, n (%) | 71 (43.3) | 172 (81.9) |
<0.001b |
|
Mortality, n
(%) | 20 (12.2) | 11 (5.2) | 0.002b |
Predictive values of PCT for infection
and vasospasm after aSAH
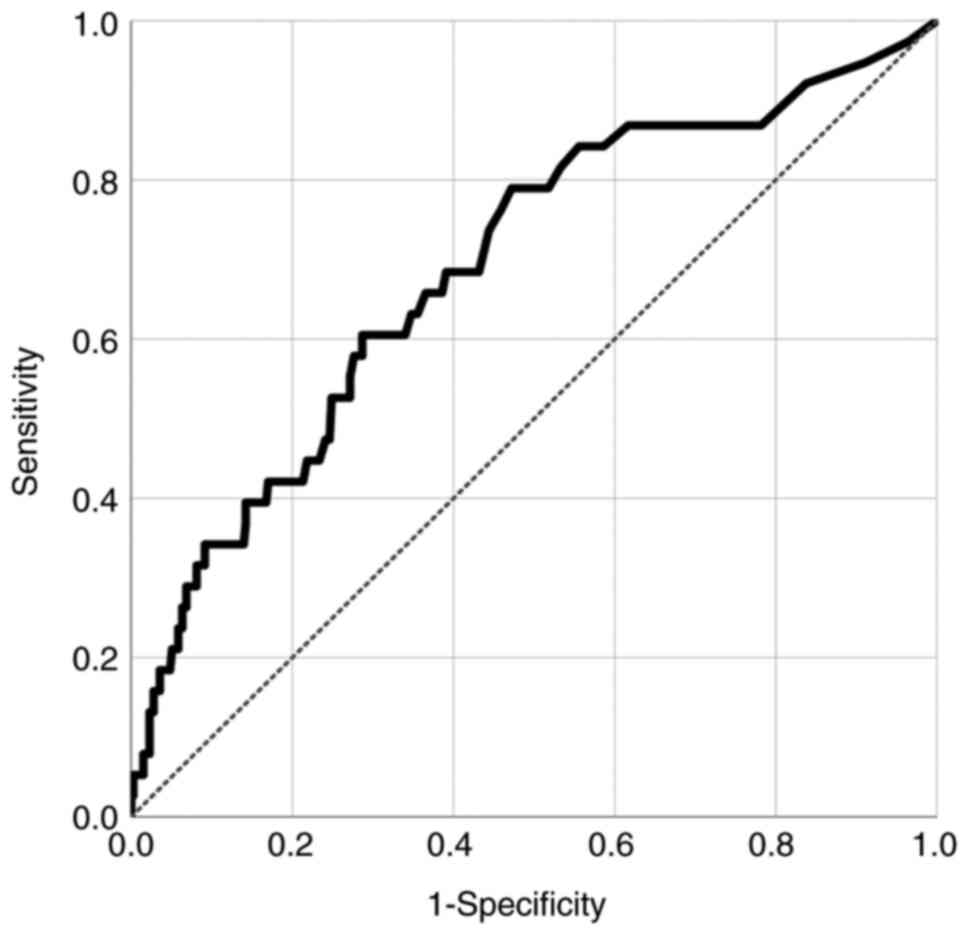
In ROC analysis, 0.21 ng/ml of PCT was determined as
an optimal cutoff value to predict systemic infection after
endovascular treatment for aSAH [area under the curve (AUC): 0.762,
standard error (SE): 0.030, 95% CI: 0.708-0.822; P<0.001]
(Fig. 1). To identify the
predictabilities of blood parameters in CV rather than systemic
infection, ROC analyses were performed for patients in the
infection-free group. The predictive value of PCT for the
infection-free group was higher than that of hsCRP for identifying
patients who could develop CV after aSAH [PCT: (AUC) 0.691, (SE)
0.047, (95% CI) 0.598-0.784, P<0.001 vs. hsCRP: (AUC) 0.602,
(SE) 0.064, (95% CI) 0.537-0.671, P=0.015]. Other blood parameters,
such as RBC, WBC, neutrophils, platelets, and ESR were not
statistically significant in predicting CV in aSAH patients without
systemic infection (Table II).
The optimal cutoff value of PCT level was 0.09 ng/ml as a predictor
of CV in patients without systemic infection after aSAH (AUC:
0.691, SE: 0.047, 95% CI: 0.598-0.784; P<0.001) (Fig. 2).
 | Table IIBlood parameters for predicting
cerebral vasospasm after aneurysmal subarachnoid hemorrhage in
patients without systemic infection. |
Table II
Blood parameters for predicting
cerebral vasospasm after aneurysmal subarachnoid hemorrhage in
patients without systemic infection.
| Variables | AUC | SE | 95% CI | P-value |
|---|
| Red blood
cells | 0.514 | 0.092 | 0.408-0.622 | 0.241 |
| White blood
cells | 0.564 | 0.073 | 0.473-0.697 | 0.108 |
| Neutrophils | 0.545 | 0.080 | 0.452-0.637 | 0.139 |
| Platelets | 0.522 | 0.088 | 0.434-0.619 | 0.187 |
| Erythrocyte
sedimentation rate | 0.583 | 0.071 | 0.476-0.646 | 0.204 |
| High-sensitivity
C-reactive protein | 0.602 | 0.064 | 0.537-0.671 | 0.015a |
| Procalcitonin | 0.691 | 0.047 | 0.598-0.784 |
<0.001a |
Subgroup analysis according to level
of PCT in patients without infection
Enrolled 210 patients in the infection-free group
were dichotomized according to the identified cutoff value of PCT
(0.09 ng/ml). A comparative analysis between high (≥0.09 ng/ml) and
low (<0.09 ng/ml) PCT groups was then conducted. The high PCT
group had higher rates of H-H grades IV-V and modified Fisher grade
III-IV than the low PCT group (23.0% vs. 11.87%, P=0.003 and 40.1%
vs. 18.3%, P=0.002, respectively). The mean level of hsCRP in the
high PCT group was significantly higher than that in the low PCT
group (5.17±4.21 vs. 1.78±1.44, P=0.001). Furthermore, the high PCT
group showed more occurrence of CV and unfavorable outcome than the
low PCT group (CV: 23.9% vs. 10.8%; P=0.003 and favorable outcome:
89.7% vs. 72.0%; P=0.008) (Table
III).
 | Table IIISubgroup analysis according to level
of PCT (0.09 ng/ml) in patients without systemic infection. |
Table III
Subgroup analysis according to level
of PCT (0.09 ng/ml) in patients without systemic infection.
| Variables | PCT <0.09 ng/ml
(n=93; 44.3%) | PCT ≥0.09 ng/ml
(n=117; 55.7%) | P-value |
|---|
| Age, mean ± SD | 58.2±14.7 | 59.9±15.9 | 0.507 |
| Female, n (%) | 65 (69.9) | 69 (58.9) | 0.253 |
| Hypertension, n
(%) | 61 (65.6) | 67 (57.3) | 0.447 |
| High H-H grade (H-H
grades IV-V), n (%) | 11 (11.8) | 27 (23.0) | 0.003a |
| Modified Fisher
grade III-IV, n (%) | 17 (18.3) | 47 (40.1) | 0.002a |
| Aneurysm size, mm,
mean ± SD | 5.6±3.3 | 5.9±3.1 | 0.327 |
| Aneurysm locations
(anterior/posterior) | 87/19 | 88/16 | 0.169 |
| Red blood cells
(x1012/l), mean ± SD | 4.28±0.77 | 4.15±0.69 | 0.307 |
| White blood cells
(x109/l), mean ± SD | 8.79±5.13 | 9.75±5.82 | 0.077 |
| Neutrophils
(x109/l), mean ± SD | 7.63±4.79 | 9.01±5.47 | 0.067 |
| Platelets
(x109/l), mean ± SD | 249±121 | 245±107 | 0.339 |
| ESR (mm/h) mean ±
SD | 21.41±20.77 | 23.77±19.25 | 0.188 |
| HsCRP (mg/l) mean ±
SD | 1.78±1.44 | 5.17±4.21 | 0.001b |
| Procalcitonin
(ng/ml), mean ± SD | 0.04±0.03 | 0.11±0.09 |
<0.001b |
| Cerebral vasospasm,
n (%) | 10 (10.8) | 28 (23.9) | 0.003a |
| Favorable clinical
outcome, n (%) | 67 (72.0) | 105 (89.7) | 0.008a |
Predicting factors for cerebral
vasospasm in aSAH patients without infection
Univariate and multivariate logistic regression
analyses were performed to identify predicting factors for CV after
aSAH in patients without infection. High H-H grade (IV-V) (OR:
2.84; 95% CI: 1.47-4.97; P=0.001) and modified Fisher grade III-IV
(OR: 3.62; 95% CI: 1.54-6.26; P<0.001) could independently
predict CV. Among blood parameters, elevated hsCRP (≥3.1 mg/l) (OR:
1.62; 95% CI: 1.36-2.42; P=0.033) and elevated PCT (≥0.09 ng/ml)
(OR: 1.82; 95% CI: 1.42-2.96; P=0.015) were independently
associated with the occurrence of CV (Table IV).
 | Table IVUnivariate and multivariate logistic
regression analysis of risk factors associated with cerebral
vasospasm after aneurysmal subarachnoid hemorrhage in patients
without systemic infection. |
Table IV
Univariate and multivariate logistic
regression analysis of risk factors associated with cerebral
vasospasm after aneurysmal subarachnoid hemorrhage in patients
without systemic infection.
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variables | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Age (≥70
years) | 1.88
(0.72-2.84) | 0.298 | | |
| Female | 1.44
(0.74-2.38) | 0.242 | | |
| Hypertension | 1.75
(0.81-3.17) | 0.337 | | |
| Hunt-Hess grades
IV-V | 3.21
(1.47-5.87) |
<0.001a | 2.84
(1.47-4.97) | 0.001a |
| Modified Fisher
grade III-IV | 3.62
(1.54-6.26) |
<0.001a | 3.04
(1.40-5.68) |
<0.001a |
| Red blood
cells | 1.69
(0.86-4.52) | 0.261 | | |
| White blood
cells | 1.57
(0.80-3.82) | 0.137 | 1.25
(0.68-2.98) | 0.207 |
| Neutrophils | 1.64
(0.68-4.54) | 0.228 | | |
| Platelets | 1.46
(0.64-2.78) | 0.306 | | |
| Erythrocyte
sedimentation rate | 1.51
(0.90-3.42) | 0.126 | 1.34
(0.88-3.22) | 0.196 |
| High-sensitivity
C-reactive protein | 1.71
(1.44-3.12) | 0.019a | 1.62
(1.36-2.42) | 0.033a |
| Procalcitonin | 1.94
(1.51-3.67) | 0.010a | 1.82
(1.42-2.96) | 0.015a |
Discussion
It is known that inflammation play a crucial role in
the prognosis of patients with aSAH (13,26).
The unfavorable outcome in patients with aSAH is facilitated by CV
and subsequent DCI. This process is promoted by inflammatory
processes (8). Previous studies
have analyzed the associations of various inflammatory markers with
the occurrence of CV or DCI, and prognosis of patients with aSAH
(9,12,27).
Blood parameters, such as inflammation-based scores, WBC derives,
and hsCRP have been investigated as inflammatory markers in aSAH
(8,27). However, reliable inflammatory
markers that can accurately predict the prognosis of aSAH have not
yet been established. In our study, the roles of PCT as a predictor
of systemic infection and CV were investigated in patients with
aSAH. PCT elevation is observed in various conditions, such as
trauma and burn, as well as infectious disease (18,19,21).
Karlsson et al have shown that a substantial concentration
decrease of PCT was more important for favorable outcome in sepsis
than the absolute value of PCT (28). Likewise, PCT may reflect the
inflammation status in various conditions. Therefore, we
investigated whether PCT level in patients with aSAH could predict
the occurrence of CV which is facilitated by inflammatory
response.
To the best of our knowledge, research on PCT in
patients with aSAH or other cerebrovascular disease is limited.
Oconnor et al have examined PCT level as a sepsis marker in
patients with head trauma and aSAH (29). However, they did not clarify the
relationship between PCT levels and sepsis in patients with aSAH.
Another prospective study has reported that PCT of 0.2 ng/ml or
greater is very specific for sepsis in patients with aSAH (30). Shi et al have shown that PCT
is a reliable prognostic biomarker of pneumonia, and that the
optimal cut-off value of serum PCT levels to predict pneumonia is
0.22 ng/ml (AUC: 0.796, 95% CI: 0.716-0.876; P<0.001), in
patients with acute ischemic stroke (17). Even with differences in details and
disease category, our study showed similar results. In our cohort
with patients who underwent endovascular treatment for aSAH, PCT
level in patients with systemic infection (0.31 ng/ml) was
significantly higher than that in patients of the systemic
infection-free group (0.08 ng/ml). The optimal cutoff value of PCT
to discriminate systemic infection, such as pneumonia, UTI, CNS
infection, and sepsis was 0.21 ng/ml (AUC: 0.762, 95% CI:
0.708-0.822; P<0.001). Therefore, PCT has predictive value of
systemic infection in patients with aSAH.
The main purpose of our analysis was to assess the
association between early PCT levels and the development of CV
after aSAH. To minimize the influence of systemic infections on
PCT, patients without systemic infection after aSAH were isolated
from this series of consecutive patients. The PCT level of 0.09
ng/ml was an optimal cutoff value to predict CV in patients without
systemic infection after aSAH (AUC: 0.691, 95% CI: 0.598-0.784;
P<0.001). Furthermore, the predictable value of PCT was higher
than those of other blood parameters, such as hsCRP, RBC, WBC,
neutrophils, platelets, and ESR. After extracting the
infection-free group according to the cutoff value (0.09 ng/ml) of
PCT, patients with PCT of 0.09 ng/ml or greater had higher rate of
CV than patients with lower PCT (<0.09 ng/ml). Elevated PCT
(≥0.09 ng/ml) was an independent predictor for CV after aSAH in our
cohort. Veldeman et al have performed a prospective
observational study to validate the association of PCT and DCI in
patients who have undergone surgical clipping or endovascular
treatment for aSAH, and analyzed PCT levels at multiple time points
with repetitive measurement (4).
Early PCT levels at 3 days after aSAH had a predictive value for
the development of DCI (AUC: 0.661, P=0.003) and unfavorable
clinical outcome (AUC: 0.674, P=0.003). In a subgroup analysis with
infection-free patients (n=72), PCT levels were higher in patients
with DCI than in patients without DCI (P=0.001). In addition, they
revealed that PCT concentrations increased gradually after DCI but
decreased with successful treatment. Guresir et al have
reported the predictive value of PCT for the prognosis of aSAH.
Multivariate regression analysis revealed that elevated baseline
PCT within 24 h was associated with unfavorable clinical outcome in
patients with World Federation of Neurological Surgeons (WFNS)
scale I-II SAH (OR: 26.0; 95% CI: 2.9-235.5; P=0.004) (12). These previous studies demonstrated
similar results to our study. PCT levels in patients with aSAH have
a predictive value for the occurrence of CV. Subsequently CV could
induce DCI and unfavorable clinical outcome. The activated
inflammatory response status may facilitate the development of CV,
and PCT can reflect this inflammatory response status.
Our study contained patients with relatively good
grade SAH, such as low H-H and modified Fisher grade. Because
patients who had undergone surgical clipping were primarily
excluded, a subgroup analysis was performed after excluding
patients with systemic infection. However, these selections of
patients were performed to minimized the influence of systemic or
post-operative infection. The enrolled patients of our study could
not be considered as a good representation of entire aSAH patients.
However, our study showed that PCT had a predictive value for the
development of CV as well as systemic infection after aSAH. The
exact mechanism for an association between PCT in the early phase
of aSAH and the development of CV remains unclear. Nevertheless,
initially obtained laboratory values have the potential to allow
very early identification of patients with a higher risk of further
deterioration during the course of treatment. Physicians should pay
attention to early phase PCT level which has a predictability for
systemic infection and CV after aSAH.
The present study has some limitations. First, the
retrospective nature of the study design with a small sample size
might have induced various biases. Second, our study did not
include other inflammatory markers or possible confounding
variables, such as IL-1, IL-6, or (TNF)-α. Third, dynamic change
with repeated measurement of PCT level was not analyzed. Fourth, we
did not evaluate PCT levels in CSF that might not respond to
systemic bacterial infections, making it a potentially more
sensitive target for CV. Nevertheless, the results of the present
study should be considered with a view to further validation in
future studies.
In conclusion, our study showed a predictable value
of PCT in patients with aSAH. During the early period of aSAH after
endovascular treatment, PCT can provides information for systemic
infection. It can be used as a predictor of CV. aSAH patients with
PCT levels ≥0.21 ng/ml are more likely to have a systemic
infection. A PCT level of 0.09 ng/ml or higher in aSAH patients
without systemic infection suggests a high probability of
developing CV. Further studies are needed to clarify the role of
PCT in aSAH.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the National Research
Foundation of Korea (grant no. NRF-2021R1G1A1094797).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HJY designed the study. HJY and JHK performed data
analysis, interpretation of the data and drafting of the
manuscript. HJY carried out statistical analysis. HJY and JHK
confirm the authenticity of all the raw data. HJY supervised the
study. HJY, JHK, DS and BK performed data acquisition, and checked
the integrity of the data and accuracy of the data analysis. All
authors read and approved the final manuscript.
Ethical approval and consent to
participate
The present study was approved by the ethics
committee of the Soonchunhyang University Bucheon Hospital
(approval no. 2022-01-016), St. Vincent's Hospital (approval no.
VC18RESI0027) and Hangang Sacred Heart Hospital (approval no.
2022-021). Informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Authors' information
Professor Ho Jun Yi: ORCID: 0000-0003-3061-0689.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rinkel GJ and Algra A: Long-term outcomes
of patients with aneurysmal subarachnoid haemorrhage. Lancet
Neurol. 10:349–356. 2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dijkland SA, Jaja BNR, van der Jagt M,
Roozenbeek B, Vergouwen MDI, Suarez JI, Torner JC, Todd MM, van den
Bergh WM, Saposnik G, et al: Between-center and between-country
differences in outcome after aneurysmal subarachnoid hemorrhage in
the subarachnoid hemorrhage international trialists (SAHIT)
repository. J Neurosurg. 1–9. 2019.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
3
|
Li K, Barras CD, Chandra RV, Kok HK,
Maingard JT, Carter NS, Russell JH, Lai L, Brooks M and Asadi H: A
review of the management of cerebral vasospasm after aneurysmal
subarachnoid hemorrhage. World Neurosurg. 126:513–527.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Veldeman M, Lepore D, Hollig A, Clusmann
H, Stoppe C, Schubert GA and Albanna W: Procalcitonin in the
context of delayed cerebral ischemia after aneurysmal subarachnoid
hemorrhage. J Neurosurg. 1-9:2020.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
5
|
Connolly ES Jr, Rabinstein AA, Carhuapoma
JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech
AM, Ogilvy CS, et al: Guidelines for the management of aneurysmal
subarachnoid hemorrhage: A guideline for healthcare professionals
from the American heart association/american stroke association.
Stroke. 43:1711–1737. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Committee for Guidelines for Management of
Aneurysmal Subarachnoid Hemorrhage, Japanese Society on Surgery for
Cerebral Stroke. Evidence-based guidelines for the management of
aneurysmal subarachnoid hemorrhage. English edition. Neurol Med
Chir (Tokyo). 52:355–429. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rass V and Helbok R: Early brain injury
after poor-grade subarachnoid hemorrhage. Curr Neurol Neurosci Rep.
19(78)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yun S, Yi HJ, Lee DH and Sung JH: Systemic
inflammation response index and systemic immune-inflammation index
for predicting the prognosis of patients with aneurysmal
subarachnoid hemorrhage. J Stroke Cerebrovasc Dis.
30(105861)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
McBride DW, Blackburn SL, Peeyush KT,
Matsumura K and Zhang JH: The role of thromboinflammation in
delayed cerebral ischemia after subarachnoid hemorrhage. Front
Neurol. 8(555)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kasius KM, Frijns CJ, Algra A and Rinkel
GJ: Association of platelet and leukocyte counts with delayed
cerebral ischemia in aneurysmal subarachnoid hemorrhage.
Cerebrovasc Dis. 29:576–583. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ogden M, Bakar B, Karagedik MI, Bulut IU,
Cetin C, Aydin G, Kisa U and Ozveren MF: Analysis of biochemical
laboratory values to determine etiology and prognosis in patients
with subarachnoid hemorrhage: A clinical study. Neurol Res.
41:156–167. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Güresir E, Coch C, Fimmers R, Ilic I,
Hadjiathanasiou A, Kern T, Brandecker S, Güresir Á, Velten M,
Vatter H and Schuss P: Initial inflammatory response is an
independent predictor of unfavorable outcome in patients with
good-grade aneurysmal subarachnoid hemorrhage. J Crit Care.
60:45–49. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chamling B, Gross S, Stoffel-Wagner B,
Schubert GA, Clusmann H, Coburn M and Höllig A: Early diagnosis of
delayed cerebral ischemia: possible relevance for inflammatory
biomarkers in routine clinical practice? World Neurosurg.
104:152–157. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Turner CL, Budohoski K, Smith C,
Hutchinson PJ, Kirkpatrick PJ and Murray GD: STASH collaborators.
Elevated baseline C-reactive protein as a predictor of outcome
after aneurysmal subarachnoid hemorrhage: Data from the simvastatin
in aneurysmal subarachnoid hemorrhage (STASH) trial. Neurosurgery.
77:786–793. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li Q and Gong X: Clinical significance of
the detection of procalcitonin and C-reactive protein in the
intensive care unit. Exp Ther Med. 15:4265–4270. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li Y, Chen L, Fang W and Chen H:
Application value of procalcitonin, C-reactive protein and
interleukin-6 in the evaluation of traumatic shock. Exp Ther Med.
17:4586–4592. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shi G, Li M, Zhou R, Wang X, Xu W, Yang F
and Xue S: Procalcitonin related to stroke-associated pneumonia and
clinical outcomes of acute ischemic stroke after IV rt-PA
treatment. Cell Mol Neurobiol. 42:1419–1427. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Schuetz P, Birkhahn R, Sherwin R, Jones
AE, Singer A, Kline JA, Runyon MS, Self WH, Courtney DM, Nowak RM,
et al: Serial procalcitonin predicts mortality in severe sepsis
patients: Results from the multicenter procalcitonin monitoring
SEpsis (MOSES) study. Crit Care Med. 45:781–789. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang R, He M, Ou XF, Xie XQ and Kang Y:
Serum procalcitonin level predicts acute kidney injury after
traumatic brain injury. World Neurosurg. 141:e112–e117.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sakran JV, Michetti CP, Sheridan MJ,
Richmond R, Waked T, Aldaghlas T, Rizzo A, Griffen M and Fakhry SM:
The utility of procalcitonin in critically ill trauma patients. J
Trauma Acute Care Surg. 73:413–418. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cabral L, Afreixo V, Meireles R, Vaz M,
Chaves C, Caetano M, Almeida L and Paiva JA: Checking procalcitonin
suitability for prognosis and antimicrobial therapy monitoring in
burn patients. Burns Trauma. 6(10)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
van Gijn J, Kerr RS and Rinkel GJ:
Subarachnoid haemorrhage. Lancet. 369:306–318. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Thompson K, Venkatesh B and Finfer S:
Sepsis and septic shock: Current approaches to management. Intern
Med J. 49:160–170. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Frontera JA, Fernandez A, Schmidt JM,
Claassen J, Wartenberg KE, Badjatia N, Connolly ES and Mayer SA:
Defining vasospasm after subarachnoid hemorrhage: What is the most
clinically relevant definition? Stroke. 40:1963–1968.
2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Vergouwen MD, Vermeulen M, van Gijn J,
Rinkel GJ, Wijdicks EF, Muizelaar JP, Mendelow AD, Juvela S, Yonas
H, Terbrugge KG, et al: Definition of delayed cerebral ischemia
after aneurysmal subarachnoid hemorrhage as an outcome event in
clinical trials and observational studies: Proposal of a
multidisciplinary research group. Stroke. 41:2391–2395.
2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Höllig A, Remmel D, Stoffel-Wagner B,
Schubert GA, Coburn M and Clusmann H: Association of early
inflammatory parameters after subarachnoid hemorrhage with
functional outcome: A prospective cohort study. Clin Neurol
Neurosurg. 138:177–183. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Morga R, Dziedzic T, Moskala M, Slowik A
and Pera J: Clinical relevance of changes in peripheral blood cells
after intracranial aneurysm rupture. J Stroke Cerebrovasc Dis.
29(105293)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Karlsson S, Heikkinen M, Pettila V, Alila
S, Väisänen S, Pulkki K, Kolho E and Ruokonen E: Finnsepsis Study
G. Predictive value of procalcitonin decrease in patients with
severe sepsis: A prospective observational study. Crit Care.
14(R205)2010.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Oconnor E, Venkatesh B, Mashongonyika C,
Lipman J, Hall J and Thomas P: Serum procalcitonin and C-reactive
protein as markers of sepsis and outcome in patients with
neurotrauma and subarachnoid haemorrhage. Anaesth Intensive Care.
32:465–470. 2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Festic E, Siegel J, Stritt M and Freeman
WD: The utility of serum procalcitonin in distinguishing systemic
inflammatory response syndrome from infection after aneurysmal
subarachnoid hemorrhage. Neurocrit Care. 20:375–381.
2014.PubMed/NCBI View Article : Google Scholar
|