Introduction
Chemotherapy is the primary approach in the clinic
for cancer treatment (1).
Doxorubicin (Dox) is a quinone-containing anthracycline antibiotic
widely used in clinics, frequently used for the treatment of
multiple types of cancer, including breast cancer, lung cancer,
sarcomas, as well as leukemia. It is one component in the CHOP
regimen for lymphoma. However, the consequent dose-limiting
cardiotoxicity is a significant obstacle to the application of Dox
and a chief limiting factor in delivering optimal chemotherapy to
cancer patients (2). Hence,
investigating novel approaches to prevent Dox-induced
cardiotoxicity is urgently needed.
It has been identified that several molecular
pathogenesis processes are involved in Dox-induced cardiotoxicity
(3), including accumulation of
reactive oxygen species (ROS), mitochondrial damage, mitochondrial
iron accumulation, transcription alterations, as well as DNA damage
repair (4). Moreover, it has been
widely illustrated that autophagy reduction in cardiomyocytes may
be responsible for the cardiotoxicity of Dox (5). Hence, autophagy acts as a potential
target for preventing Dox-induced cardiotoxicity.
Thymoquinone (2-isopropyl-5-methylbenzo-1,
4-quinone) (TQ), the most abundant constituent of the volatile oil
also present in the fixed oil, is the biologically active compound
of Nigella sativa seeds (6). Several studies have suggested the
capacity of TQ on immunomodulatory and anti-inflammatory effects
(7). Earlier researh demonstrated
that TQ showed significant protective effects against Dox-induced
cardiotoxicity in rats (8). More
recently, a series of studies suggested that the inhibition of
oxidative stress levels may be responsible for TQ action on the
protective effects of cardiotoxicity (9,10).
However, the potential mechanism of TQ on the protection of
Dox-induced cardiotoxicity is still not fully understood. In the
present study, novel mechanisms of action involved in the
TQ-induced protective effects of cardiotoxicity were found to be
related to an increased autophagy level and decreased apoptosis.
Further mechanistic study suggested that TQ significantly increased
AMP-activated protein kinase (AMPK) pathway activity. Our study may
provide novel approaches to avoid cardiotoxicity when administering
Dox in cancer patients.
Materials and methods
Cell culture
The H9c2 (rat heart-derived embryonic myoblast) cell
line was purchased from the American Type Culture Collection
(ATCC). The H9c2 cells were passaged 10 to 25 times before
conducting experiments. Cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.),
referred to as a high-glucose medium, supplemented with 10% fetal
bovine serum (FBS) and 100 U/ml of penicillin/streptomycin within
cultured dishes incubated at 37˚C in a humidified atmosphere of 5%
CO2. After application of 0.5% FBS-supplemented DMEM for
24 h, H9c2 cardiomyocytes were subjected to treatment with Dox
(Energy Chemical) for 24 h or pretreatment with TQ (Energy
Chemical) for 30 min before Dox. To analyze the consequence of
whether autophagy exerts apoptosis, cells were pretreated with
bafilomycin A1 (Energy Chemical) for 30 min before exposure to TQ or
Dox for 24 h.
CCK-8 and lactate dehydrogenase (LDH)
assays for H9c2 cells
The CCK-8 (Sigma-Adrich; Merck KGaA) and LDH
(Promega Corp.) assays were conducted to evaluate H9c2 cell
proliferation. The H9c2 cells were harvested and plated into
96-well plates at a final concentration of 5x105 cells
per well. The cells were pretreated with TQ (5 and 10 µM) 30 min
before administration of Dox (5 µM). After another 24-h incubation
at 37˚C, CCK-8 solution (10 µl) was added to each well. After
incubation for 4 h, the optical density (OD) values were detected
at 450 nm. For the LDH detection assay, LDH reagent was added to
the collected culture medium incubated for 30 min at room
temperature (RT), protected from light. Then, the OD value was
quantified by a spectrophotometer at 490 nm. The cell survival rates
(%) of the different groups were determined by dividing the OD
value of the experimental group by the control group in the CCK-8
assays. The relative cell death rate (%) was calculated by dividing
the OD value of the experimental group by the control group in the
LDH assays.
Apoptosis detection in the H9c2
cells
The apoptosis of H9c2 cells was conducted using an
Annexin V-FITC Apoptosis Detection Kit (Solarbio) according to the
manufacturer's instructions. Cells were fixed with cold 70% ethanol
for 1 h and then centrifuged and washed twice using cold PBS. Then
the cells were incubated with Annexin V (1X) and propidium iodide
(PI) (1X) for 30 min in the dark. The cells were washed twice again
with PBS and re-suspended with detection buffer. An Accuri C6 flow
cytometer (BD Biosciences) was used to detect apoptotic cells. The
quantification was analyzed by Flowjo 7.6.5 software
(Becton-Dickinson and Company).
Immunofluorescence
The H9c2 cells were fixed with 4% paraformaldehyde
for 15 min at RT. Subsequently, the cells were washed with NaCl/Pi,
permeabilized with 0.2% Triton X-100, and then were incubated with
the LC3B primary rabbit antibody (cat: 3868; 1:100) at 4˚C
overnight. Cells were then incubated with Alexa 594-conjugated
goat-anti-rabbit secondary antibody (cat: 8889, 1:200) for 1 h at
37˚C. The images were captured by DMi8 fluorescence microscope
(x400 magnification) (Leica Microsystems GmbH). The LC3 puncta were
measured by ImageJ software (v1.8.0) (National Institutes of
Health, Bethesda, MD, USA).
Western blot analysis
Homogenates of H9c2 cells were used for western blot
analysis. Protein (30 µg per lane) was separated on a 10% gel by
SDS-PAGE and transferred to a PVDF membrane. The membranes were
blocked with 5% skim milk in 1X Tris-buffer containing 0.1%
Tween-20 for 1 h. The blots were incubated overnight with primary
antibodies, including apoptosis, autophagic protein markers, and
key molecules in the AMPK pathway. Rabbit-cleaved caspase-3 (cat:
9664), LC3B (cat: 3868), phosphorylated (p-)LKB1 (cat: 3050), LKB1
(cat: 3055), AMPKα (cat: 5832), p-AMPKα (Thr172) (cat: 50081),
p-ULK1 (Ser555) (cat: 5869), p-ULK1 (Ser317) (cat: 37762), ULK1
(cat: 8054), mTor (cat: 2972), p-mTOR (cat: 5536), and β-actin
(cat: 3700) (dilution 1:1,000, Cell Signaling Technology, Inc.),
were incubated with the membranes at 4˚C overnight, which were
further incubated with anti-rabbit horseradish peroxidase
(HRP)-conjugated secondary antibody (cat: 7074) (dilution 1:2,000;
Cell Signaling Technology, Inc.) for 1 h at RT. The blots were
developed with an ECL chemiluminescence detection reagent (Bio-Rad
Laboratories, Inc.). The protein bands were visualized by a
ChemiDoc XRS+ Imager (Bio-Rad Laboratories, Inc.). ImageJ software
(v1.8.0) was used to analyze the relative intensity.
Statistics
The data were analyzed by GraphPad 8.00 software
(GraphPad Software, Inc.). The data are expressed as the mean ± SD,
of three independent experiments. Unpaired Student's t-test was
used to carry out the significance comparing 2 groups. Multigroup
comparisons of the means were carried out by one-way analysis of
variance (ANOVA) test with post hoc contrasts by
Student-Newman-Keuls test when comparing groups ≤3. For groups
>3, Tukey's test was conducted as a post hoc test. Student's
t-test was used for two group comparisons. Statistical significance
for all tests was set at P<0.05 (*).
Results
TQ reverses Dox-induced H9c2 cell
death
Firstly, to choose the non-toxic concentration of
TQ, the CCK-8 assay was conducted. As shown in Fig. 1A, no significant toxicity of TQ on
H9c2 cells was noted under 10 µM after a 24-h incubation.
Subsequently, as described in ‘Materials and methods’ section,
cells were pre-treated with TQ and Dox was administrated for
subsequent CCK-8 assay of cell viability. As illustrated in
Fig. 1B, administration of Dox (5
µM) significantly decreased the viability of H9c2 cells, while TQ
significantly reversed the inhibition of Dox-induced H9c2 cell
viability. LDH assay was further conducted to evaluate cell death
after co-administrated of Dox and TQ. As shown in Fig. 1C, although Dox significantly
induced H9c2 cell death, TQ significantly decreased the cell death
of H9ce cells in a dose-dependent manner. As shown in Fig. 1B and 1C, dexrazoxane (DEX) was used as a
positive control, which is the only clinically approved drug for
anthracycline-induced myocardial damage. These results
preliminarily demonstrated that TQ is a candidate cardio-protective
molecule, for which its mechanisms should be studied further.
TQ antagonizes Dox-induced apoptosis
of H9c2 cells
Flow cytometry was conducted to evaluate the effects
of the co-administration of Dox and TQ on H9c2 cell apoptosis. As
shown in Fig. 2A, Dox (5 µM)
significantly induced H9c2 cell apoptosis. The apoptotic cells
under Dox treatment were approximately 20%. Pre-treatment with TQ
significantly and dose-dependently decreased the Dox-induced
apoptosis. These results further verified that TQ can recover H9c2
cell proliferation under Dox treatment.
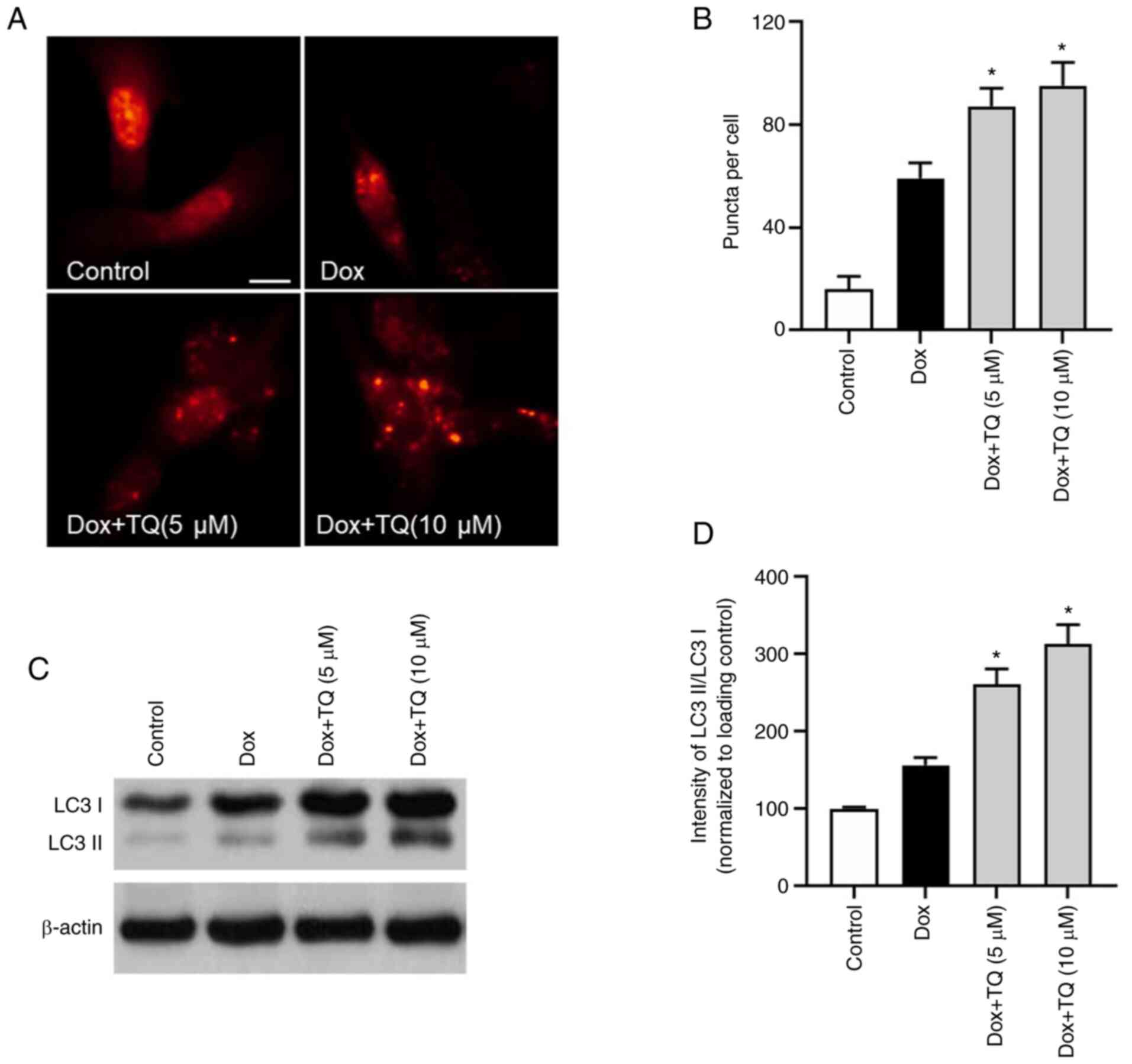
TQ regulates autophagy in H9c2
cells
Autophagy protein LC3 was visualized by
immunofluorescence. As shown in Fig.
3A and B, the percentage of
LC3 puncta formation in Dox-treatment H9c2 cells was upregulated.
However, in the TQ co-treatment groups, the rate of LC3 puncta
formation was significantly and potently increased in a
dose-dependent manner, which indicated that TQ treatment
upregulated the autophagy level in the H9c2 cells. Furthermore, as
shown in Fig. 3C and D, western blot analysis of LC3 protein
showed that, after co-treatment with TQ, the LC3II/LC3I ratio was
significantly upregulated, suggesting that TQ regulated autophagy
in the H9c2 cells.
TQ-induced autophagy regulates
Dox-induced apoptosis in H9c2 cells
To assess the role of TQ-induced autophagy in
Dox-mediated apoptosis, H9c2 cells were pretreated with bafilomycin
A1, an inhibitor of autophagic flux by disruption of vacuolar-type
H+-ATPase. Bafilomycin (Baf) pretreatment restored the
decreased percentage of apoptotic cells (Fig. 4A and C). In addition, the TQ-induced increase
in the ratio of LC3II to LC3I was enhanced with bafilomycin
(Fig. 4B and D). Moreover, bafilomycin pretreatment also
increased active caspase-3 expression (Fig. 4B and E). These results indicated that the
activation of autophagy may be responsible for reversing apoptosis
in H9c2 cells induced by TQ.
TQ decreases Dox-mediated H9c2 cell
cytotoxicity by activating the LKB1/AMPK/ULK1 axis
Western blot analysis was conducted to evaluate the
effect of TQ on AMPK-related molecules. As shown in Fig. 5A-C, the expression levels of
phosphorylated (p-)LKB1, p-AMPKα, and p-ULK1 at Ser317 were
significantly increased after TQ treatment, indicating that
activation of LKB1/AMPK signaling is responsible for the mechanisms
of action of TQ. Moreover, the expression levels of p-mTOR and
p-ULK1 at Ser575 were significantly decreased after TQ treatment,
which further verified that the activation of AMPK signaling is a
crucial mechanism of TQ-mediated reversal of the apoptosis induced
by Dox in H9c2 cells.
 | Figure 5TQ activates the LKB1/AMPK/ULK1
pathway. (A) Western blot analysis shows the expression of p-LKB1,
LKB1, p-AMPKα, AMPKα, p-ULK1 (ser317), p-mTOR, and p-ULK1 (ser575)
after Dox or Dox in combination use with TQ. (B) Relative intensity
(% of loading control) of p-LKB1, LKB1, p-AMPKα, AMPKα, p-ULK1
(ser317), p-mTOR, and p-ULK1 (ser575) after Dox or Dox in
combination use with TQ which was determined by Image J software.
(C) Relative intensity (% of total subtype) of p-LKB1, LKB1,
p-AMPKα, AMPKα, p-ULK1 (ser317), p-mTOR, and p-ULK1 (ser575) after
Dox or Dox in combination use with TQ which was determined by Image
J software. Data are presented as the mean ± SD of three
independent experiments. A one-way ANOVA test with post hoc
Student-Newman-Keuls test was used for evaluating the difference.
Significance was indicated at P<0.05: *P<0.05, Dox
vs. the Dox+TQ group. TQ, thymoquinone; Dox, doxorubicin; LKB1,
liver kinase B1; AMPK, AMP-activated protein kinase; ULK1,
unc-51-like kinase 1; mTOR, mammalian target of rapamycin; p-,
phosphorylated. |
Discussion
Chemotherapeutic drug-induced cardiomyopathy is a
complication associated with high morbidity during chemotherapy
(11). Doxorubicin (Dox) is an
anthracycline drugs which has been utilized for anti-neoplastic
therapy for several years. Nevertheless, cardiac toxicity induced
by Dox greatly limits its further application, and is always
difficult to predict, remaining the most prevalent side effect
threatening the survival of patients (12,13).
Although adequate treatment strategies may decrease the risk of
cardiotoxicity of Dox, the prognosis of Dox-induced heart failure
remains uncertain or uncontrollable (14). Hence, the investigation of novel
strategies for preventing myocardial cells from Dox-induced
toxicity is still urgently needed.
Thymoquinone (TQ) is one of the most abundant
constituents of the volatile oil (also present in the fixed oil)
and is a biologically active compound of Nigella sativa
seeds. TQ has been documented as exhibiting a series of
bioactivities on anti-inflammatory, antioxidant, anti-cancer, as
well as antimicrobial properties (15-18).
Previously, TQ has shown promising roles against oxidative
damage-induced free radical agents and Dox, which induce
cardiotoxicity. It exerts suppressive activity on carcinogenesis,
eicosanoid production, and membrane lipid peroxidation. Moreover,
TQ works as an effective chemo-protective phytochemical, and
destroys Fe-NTA-induced oxidative stress (19-21).
In the present study, we reported the novel protective effects of
TQ on Dox-induced cardiotoxicity in an H9c2 cell in vitro
model. TQ showed significant protective effects against Dox-induced
H9c2 cell death. This result was in accordance with recent
documented studies (9,22). Based on this finding, we further
evaluated the potential mechanisms of action of the TQ effects on
protecting Dox-induced cardiotoxicity. The apoptotic rates in H9c2
cells were significantly decreased by TQ administration, indicating
the definite protective effects of TQ on Dox-induced
cardiotoxicity. Dox-induced cardiotoxicity is involved in a series
of molecular mechanisms and signaling pathways, including oxidative
stress, autophagy, iron metabolism, and inflammation (23). More recently, it has been indicated
that dysregulation of autophagy plays a vital role in Dox-induced
cardiotoxicity (24), and a series
of natural products were reported to activate the protective
effects on cardiotoxicity by inducing autophagy (25-27).
Hence, the potential autophagy influenced by TQ was evaluated in
H9c2 cells after Dox treatment. As expected, the autophagy level
was significantly upregulated after administration of TQ.
Therefore, we hypothesized that the mechanisms of action of TQ were
involved in inducing protective autophagy in Dox-treated H9c2 cells
and preventing apoptosis, thereby preventing cardiotoxicity.
The autophagy inhibitor bafilomycin A1 was used to
verify the results. After co-treatment with bafilomycin A1, the
TQ-induced myocardial cell protection effect was diminished.
Moreover, western blot assays showed that after bafilomycin A1
co-administration, autophagy- and apoptosis-related proteins came
back to the level of the Dox-treated group only. These findings
demonstrated that TQ prevents Dox-induced H9c2 cell apoptosis
through induction of autophagy.
Autophagy is able to be mediated by several
pathways. For example, the kinase mTOR is a critical regulator of
autophagy induction, with activated mTOR (Akt and MAPK signaling)
suppressing autophagy and negative regulation of mTOR (AMPK and p53
signaling) promoting it (28). In
the present study, after screening a series of signaling pathways,
we found that the activation of LKB1/AMPK and downregulation of
mTOR activity were involved in the mechanisms of action of TQ. In
the aforementioned western blot assays, TQ was able to
significantly upregulate the level of phosphorylated (p-)LKB1 and
p-AMPK in the TQ+Dox group, compared with that in the Dox group.
Moreover, the level of p-mTOR and its downstream molecule, p-ULK1
(ser575) was downregulated in the TQ+Dox group. In addition, the
expression level of neither AMPK nor mTOR were significantly
altered. These results indicated that the activation of the
LKB1/AMPK pathway is responsible for the protective effects of TQ
in the H9c2 cell model.
Candidate drugs for treating degenerative diseases
are responsible for producing toxicity (29). Although in our evaluation, TQ
showed less toxicity in H9c2 cells, it is necessary to understand
the potential toxic effects and safety and risk for possible human
use. One of the positive characteristics of bioactive compounds of
medicinal plants is their low toxicity, which demonstrates
promising and prominent effects in health management (30,31).
For TQ, it is documented that no toxic effects were found toward
fibroblast-like synoviocytes at less than 10 µM. In addition, TQ
supplementation did not show any toxic effect on the liver and
reduced the aspartate aminotransferase (AST) and alanine
aminotransferase (ALT) levels in treated rats (32). Moreover, two studies indicated that
an acceptable dose of TQ (0.6 mg/kg/day) is suitable for humans
(33,34). The above information preliminarily
confirms that TQ is a safe bioactive agent for humans.
TQ has also been subjected to intensive exploration
in regards to its toxicity, appropriate dose, and upper tolerable
limit through various animal-based efficacy trials. The outcomes of
most of the trials confirmed that TQ does not display any toxic
effect within the range of 10-100 mg/kg body both in subchronic and
subacute doses (35). Moreover,
Gali-Muhtasib et al (36)
found that TQ exhibited its anti-oncogenic perspective at minimum
doses of 5-20 mg/kg rather than its reported LD50 value.
Several investigations revealed no side effect of TQ prolonged
consumption at 10-100 mg/kg for up to 20 weeks (36). Hence, these sufficient shreds of
evidence indicate that TQ is a safe agent for humans, and
well-designed clinical trials in humans are required to confirm
these effects for Dox-induced cardiotoxicity patients.
While this study was being conducted, it was
documented that TQ could attenuate Dox-induced cardiotoxicity in
rats, which further verify our positive results (9). In addition, our study further
indicated the potential and novel mechanisms of action of TQ on
protective effects of cardiomyocytes, which has not yet been
reported. Hence, based on multiple evidence, TQ is a potential
active molecule and provides insight for further evaluation on
antagonizing anthracycline-induced cardiotoxicity.
In conclusion, TQ has been preliminarily confirmed
to be a potential candidate for protecting Dox-induced
cardiotoxicity. The mechanisms of action involved in the
TQ-mediated cardioprotective effects consist in the upregulation of
autophagy in cardiomyocytes. However, well-designed clinical trials
in humans are required to confirm these effects for Dox-induced
cardiotoxicity in these patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DL and LZ were involved in conducting the
experiments, and collecting and analyzing the data. DL and LZ
confirm the authenticity of all the raw data. DL contributed to the
research methodology and writing of the original draft. LZ was
responsible for writing, review of the manuscript and editing. All
authors read and approved the final manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gotwals P, Cameron S, Cipolletta D,
Cremasco V, Crystal A, Hewes B, Mueller B, Quaratino S,
Sabatos-Peyton C, Petruzzelli L, et al: Prospects for combining
targeted and conventional cancer therapy with immunotherapy. Nat
Rev Cancer. 17:286–301. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tan TC, Neilan TG, Francis S, Plana JC and
Scherrer-Crosbie M: Anthracycline-induced cardiomyopathy in adults.
Compr Physiol. 5:1517–1540. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Octavia Y, Tocchetti CG, Gabrielson KL,
Janssens S, Crijns HJ and Moens AL: Doxorubicin-induced
cardiomyopathy: From molecular mechanisms to therapeutic
strategies. J Mol Cell Cardiol. 52:1213–1225. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu
YL, Liu LF and Yeh ET: Identification of the molecular basis of
doxorubicin-induced cardiotoxicity. Nat Med. 18:1639–1642.
2012.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Li DL, Wang ZV, Ding G, Tan W, Luo X,
Criollo A, Xie M, Jiang N, May H, Kyrychenko V, et al: Doxorubicin
blocks cardiomyocyte autophagic flux by inhibiting lysosome
acidification. Circulation. 133:1668–1687. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ali BH and Blunden G: Pharmacological and
toxicological properties of Nigella sativa. Phytother Res.
17:299–305. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Shaterzadeh-Yazdi H, Noorbakhsh MF, Hayati
F, Samarghandian S and Farkhondeh T: Immunomodulatory and
anti-inflammatory effects of thymoquinone. Cardiovasc Hematol
Disord Drug Targets. 18:52–60. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nagi MN and Mansour MA: Protective effect
of thymoquinone against doxorubicin-induced cardiotoxicity in rats:
A possible mechanism of protection. Pharmacol Res. 41:283–289.
2000.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Karabulut D, Ozturk E, Kaymak E, Akin AT
and Yakan B: Thymoquinone attenuates doxorubicin-cardiotoxicity in
rats. J Biochem Mol Toxicol. 35(e22618)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pehlivan DY, Durdagi G, Oyar EO, Akyol S
and Ozbek M: The effects of melatonin and thymoquinone on
doxorubicin-induced cardiotoxicity in rats. Bratisl Lek Listy.
121:753–759. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Curigliano G, Cardinale D, Suter T,
Plataniotis G, de Azambuja E, Sandri MT, Criscitiello C, Goldhirsch
A, Cipolla C and Roila F: ESMO Guidelines Working Group.
Cardiovascular toxicity induced by chemotherapy, targeted agents
and radiotherapy: ESMO clinical practice guidelines. Ann Oncol. 23
(Suppl 7):vii155–166. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Robertson ID, Naprasnik A and Morrow D:
The sources of pesticide contamination in Queensland livestock.
Aust Vet J. 67:152–153. 1990.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wenningmann N, Knapp M, Ande A, Vaidya TR
and Ait-Oudhia S: Insights into doxorubicin-induced cardiotoxicity:
Molecular mechanisms, preventive strategies, and early monitoring.
Mol Pharmacol. 96:219–232. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Al-Malky HS, Al Harthi SE and Osman AM:
Major obstacles to doxorubicin therapy: Cardiotoxicity and drug
resistance. J Oncol Pharm Pract. 26:434–444. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ali MY, Akter Z, Mei Z, Zheng M, Tania M
and Khan MA: Thymoquinone in autoimmune diseases: Therapeutic
potential and molecular mechanisms. Biomed Pharmacother.
134(111157)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gholamnezhad Z, Havakhah S and Boskabady
MH: Preclinical and clinical effects of Nigella sativa and
its constituent, thymoquinone: A review. J Ethnopharmacol.
190:372–386. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Imran M, Rauf A, Khan IA, Shahbaz M,
Qaisrani TB, Fatmawati S, Abu-Izneid T, Imran A, Rahman KU and
Gondal TA: Thymoquinone: A novel strategy to combat cancer: A
review. Biomed Pharmacother. 106:390–402. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Isaev NK, Chetverikov NS, Stelmashook EV,
Genrikhs EE, Khaspekov LG and Illarioshkin SN: Thymoquinone as a
potential neuroprotector in acute and chronic forms of cerebral
pathology. Biochemistry (Mosc). 85:167–176. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Abdel-Daim MM, Abo El-Ela FI, Alshahrani
FK, Bin-Jumah M, Al-Zharani M, Almutairi B, Alyousif MS, Bungau S,
Aleya L and Alkahtani S: Protective effects of thymoquinone against
acrylamide-induced liver, kidney and brain oxidative damage in
rats. Environ Sci Pollut Res Int. 27:37709–37717. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nili-Ahmadabadi A, Alibolandi P, Ranjbar
A, Mousavi L, Nili-Ahmadabadi H, Larki-Harchegani A,
Ahmadimoghaddam D and Omidifar N: Thymoquinone attenuates
hepatotoxicity and oxidative damage caused by diazinon: An in vivo
study. Res Pharm Sci. 13:500–508. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Alam MF, Khan G, Safhi MM, Alshahrani S,
Siddiqui R, Sivagurunathan Moni S and Anwer T: Thymoquinone
ameliorates doxorubicin-induced cardiotoxicity in swiss albino mice
by modulating oxidative damage and cellular inflammation. Cardiol
Res Pract. 2018(1483041)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Öztürk E, Kaymak E, Akin A, Karabulut D,
Ünsal HM and Yakan B: Thymoquinone is a protective agent that
reduces the negative effects of doxorubicin in rat testis. Hum Exp
Toxicol. 39:1364–1373. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Timm KN and Tyler DJ: The role of AMPK
activation for cardioprotection in doxorubicin-induced
cardiotoxicity. Cardiovasc Drugs Ther. 34:255–269. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Dirks-Naylor AJ: The role of autophagy in
doxorubicin-induced cardiotoxicity. Life Sci. 93:913–916.
2013.PubMed/NCBI
|
|
25
|
Shabalala S, Muller CJF, Louw J and
Johnson R: Polyphenols, autophagy and doxorubicin-induced
cardiotoxicity. Life Sci. 180:160–170. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ma Y, Yang L, Ma J, Lu L, Wang X, Ren J
and Yang J: Rutin attenuates doxorubicin-induced cardiotoxicity via
regulating autophagy and apoptosis. Biochim Biophys Acta Mol Basis
Dis. 1863:1904–1911. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xu ZM, Li CB, Liu QL, Li P and Yang H:
Ginsenoside Rg1 prevents doxorubicin-induced cardiotoxicity through
the inhibition of autophagy and endoplasmic reticulum stress in
mice. Int J Mol Sci. 19(3658)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Codogno P, Mehrpour M and Proikas-Cezanne
T: Canonical and non-canonical autophagy: Variations on a common
theme of self-eating? Nat Rev Mol Cell Biol. 13:7–12.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Khan N and Sultana S: Inhibition of two
stage renal carcinogenesis, oxidative damage and hyperproliferative
response by Nigella sativa. Eur J Cancer Prev. 14:159–168.
2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Vance SH, Benghuzzi H, Wilson-Simpson F
and Tucci M: Thymoquinone supplementation and its effect on kidney
tubule epithelial cells in vitro. Biomed Sci Instrum. 44:477–482.
2008.PubMed/NCBI
|
|
31
|
Mu GG, Zhang LL, Li HY, Liao Y and Yu HG:
Thymoquinone pretreatment overcomes the insensitivity and
potentiates the antitumor effect of gemcitabine through abrogation
of notch1, PI3K/Akt/mTOR regulated signaling pathways in pancreatic
cancer. Dig Dis Sci. 60:1067–1080. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Qadri SM, Mahmud H, Föller M and Lang F:
Thymoquinone-induced suicidal erythrocyte death. Food Chem Toxicol.
47:1545–1549. 2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Khader M, Bresgen N and Eckl P: In vitro
toxicological properties of thymoquinone. Food Chem Toxicol.
47:129–133. 2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Dollah MA, Parhizkar S, Latiff LA and Bin
Hassan MH: Toxicity effect of Nigella sativa on the liver
function of rats. Adv Pharm Bull. 3:97–102. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Abukhader M: The effect of route of
administration in thymoquinone toxicity in male and female rats.
Indian J Pharm Sci. 74(195)2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gali-Muhtasib H, Kuester D, Mawrin C,
Bajbouj K, Diestel A, Ocker M, Habold C, Foltzer-Jourdainne C,
Schoenfeld P, Peters B, et al: Thymoquinone triggers inactivation
of the stress response pathway sensor CHEK1 and contributes to
apoptosis in colorectal cancer cells. Cancer Res. 68:5609–5618.
2008.PubMed/NCBI View Article : Google Scholar
|