Introduction
There is an increase in incidences of pancreatic
cancers worldwide (1). For
periampullary malignancies, the mortality rate is 17-50% (2). Pancreaticoduodenectomy is the primary
treatment strategy for periampullary malignancies including
pancreatic adenocarcinoma and the other benign situations (1). However, pancreaticoduodenectomy is
the cornerstone treatment for periampullary malignancies (2). The operative procedure is complex.
Patents require a long recovery time and are often exposed to
peri-operative complications (3).
Perioperative hemodynamic optimization therapy is
used to improve cardiac function to meet the increased demand
during the perioperative period and to reduce hypervolemia or
hypovolemia, tissue hypoperfusion and other postoperative
complications (4).
Chloride-liberal fluid administration is common practice after
pancreaticoduodenectomy (5).
Inappropriate perioperative resuscitation during major abdominal
surgeries and tissue hypoperfusion and/ or edema for
pancreaticoduodenectomy is associated with the development of
postoperative complications (2,3,5,6).
Enhanced recoveries after surgical procedures for patients
undergoing pancreaticoduodenectomy have advocated a more equitable
use of intravenous fluid administration (7,8). The
management of carcinoma of the head of the pancreas by
pancreatoduodenectomy, also known as Whipple procedure, as well as
some current guidelines (9), such
as the enhanced recovery after surgery recommendations, are focused
on hypothermia, wound catheters, antimicrobial treatment and
thromboprophylaxis therapies (9).
Enhanced recovery after surgery procedures decreases the length of
hospital stay for patients who underwent pancreaticoduodenectomy
without adverse effects on readmission and perioperative morbidity
and mortality (9). By contrast,
this protocol has no recommendations for fluid administration
optimization (5,6). A multicenter randomized controlled
trial (3) reported that
perioperative precise fluid administration during
pancreaticoduodenectomy can reduce the rate of complications after
surgeries and length of hospital stay. However, this study was
performed on a small sample size. Also, the optimal nutritional
therapy is debatable for pancreaticoduodenectomy (10).
The present single center retrospective study aimed
to compare perioperative hemodynamic optimization therapy and usual
protocols in terms of perioperative cardiac function for 252
patients who underwent elective pancreaticoduodenectomy.
Materials and methods
Inclusion criteria
Patients (age >18 years old) with periampullary
malignancies including pancreatic adenocarcinoma and the other
benign situations who underwent elective pancreaticoduodenectomy
were included in the analysis.
Exclusion criteria
Patients with preoperative coagulopathy, female
patients with pregnancies, renal impairment (creatinine >250
µM/l), American Society Anesthesiology physical status >IV and
chronic liver disease (as per Child-Pugh classification), patients
who underwent distal, central, or total pancreatectomy and
pancreatic enucleation were excluded from the study.
Cohorts
A total of 142 patients underwent elective
pancreaticoduodenectomy under usual protocol of enhanced recovery
after surgery procedures (UC cohort) and 110 patients underwent
elective pancreaticoduodenectomy under protocols of enhanced
recovery after surgical procedures with intraoperative fluid
optimization (FO cohort).
Usual protocols of enhanced recovery
after surgery procedures
Patients took no solids in the 6 h prior to surgery
and no liquids in the 2 h prior to surgery . No intravenous fluid
was administrated before anesthesia. Intrathecal 300-400 µg
morphine (Duramorph; Hikma Pharmaceuticals USA, Inc.) analgesia was
inserted at a lumbar spinal level before anesthesia. In cases of
morphine allergies, patients received epidural analgesia inserted
at a T8/9 or
T9/10 level. A total of 3 mg/kg
intravenous propofol (Diprivan®; Fresenius Kabi USA
LLC), 1-3 µg/kg intravenous fentanyl (Novaplus; Hospira, Inc.) and
1 mg/kg intravenous bolus succinylcholine (Anectine; Sandoz, Inc.)
injection were administered to induce anesthesia. Intraoperatively
patients received 8 mg intravenous dexamethasone (APP
Pharmaceuticals, LLC), 40 mg subcutaneous enoxaparin
(Lovenox®; Sanofi Aventis) and 1 g intravenous
paracetamol (B. Braun). In addition, 1 g intravenous ceftriaxone
(Rocephin; Pfizer, Inc.), 1 g intravenous ampicillin (Pfizer Inc.)
and 500 mg intravenous metronidazole (Pfizer Inc.) were
administered as prophylaxis. Continuous electrocardiography,
capnography, pulse oximetry, central venous pressure, blood
pressure, pulse pressure variations, body temperature and urine
output were recorded intraoperatively. Sevoflurane (Piramal
Critical Care, Inc.) with air and oxygen (50:50) mixture was used
to maintain bispectral index 40-60. A total of 0.1-0.3 µg/kg/min
intravenous remifentanil (Ultiva®; Mylan Institutional
LLC) was started after administration of anesthesia and
discontinued after the closure of the wound. Epidural analgesia was
given with 10 ml 0.2% ropivacaine (Novartis International AG) using
an epidural catheter 30 min prior to wound closure. This epidural
anesthesia was followed by 10 ml/h epidural infusion. Subsequently,
patients received 3 µg/kg of intravenous fentanyl (3). All interventions were administered by
anesthesiologists (minimum 3-years' experience) at the
institutes.
Intraoperative fluid and vasoactive
medication optimization
Blood pressure was measured using an arterial line
catheter (20G; Vygon (UK), Ltd.) before anesthesia. A central
venous catheter [Vygon (UK), Ltd.] was used to record central
venous pressure. The FloTrac™ pulse contour device (Vigileo™,
Edwards Lifesciences) was connected to a hemodynamic monitor
(Philips Healthcare) and arterial line catheter. The arterial line
pressure bag using the sensor stopcock was maintained at 300 mmHg.
Patients of the UG cohort underwent usual cardiovascular monitoring
by anesthesiologists for intraoperative fluid and vasoactive
medication optimization. Patients of the FO cohort followed
protocol (Fig. 1) for
intraoperative fluid and vasoactive medication optimization. In the
FO cohort, fluid interventions were given if stroke volume
variations were >20% during and at the end of surgeries
(3).
High cardiac index
Cardiac index >2 l/min/m2 was
considered a high cardiac index.
Outcomes measures
Postsurgical management. After operations,
all patients were admitted to postoperative intensive care units
(PICU) for 1 day and then transfer to the ward. All patients
received 0.05-0.1 mg/kg/h of ketamine (Ketalar®; Pfizer
Inc.) infusion in PICU for 1 day. A total of 20 µg twice a day of
bolus fentanyl (5 min lockout) was given to patients until they
could take a solid diet. After initiation of solid intake, patients
received 10-20 mg of oral oxycodone (OxyContin; Purdue Pharma L.P.)
every 4 h. Epidural anesthesia was locked after 2-3 days from the
operation. All patients received 1 g intravenous paracetamol four
times a day for 2 days and 40 mg intravenous pantoprazole
(Protonix®, Pfizer Inc.) once a day for 1 week after
surgeries. Rescue analgesia included 30 mg/8 h intravenous
ketorolac (Athemex Pharmaceuticals) or 50-100 mg/6 h intravenous
tramadol (Ultram; ProCare). Prophylaxis antibiotics were continued
for 1 day after the operation and physiotherapies were delivered
twice daily after operations. Nasogastric tubes (Roche Diagnostics)
were removed after the operation if less than 300 ml of drainage
was reported within 6 h. After surgery, a liquid diet was started
and a solid diet was started 2 days after surgery. The surgical
drains were removed if there were no pancreatic or biliary leakage.
Pancreatic enzyme supplements (Doctor' Best, Inc.) were given when
the soft diet was started. Strict serum glucose control (target:
6-10 mM/l) was maintained using an insulin sliding scale. A urinary
catheter was removed 3 days after the surgeries. A total of 200
mg/12 h docusate sodium (Perrigo Co.) was given 4 days after
surgeries for regular bowel motions (3). After surgeries, patients were managed
by surgeons (who performed surgeries; minimum 3-years of
experience) and physicians (minimum 3-years of experience; unaware
of intraoperative fluid and vasoactive medication optimization
protocol) of the institutes in PICU and ward.
Preoperative demographical characters and clinical
conditions of patients, operative details and postoperative details
were retrospectively collected from patients' records of
institutes. Fluid balance was calculated as per Eq. 1(3):
(1) Fluid balance =
Total input fluid - Total output fluid
Were Total input fluid = Intravenous fluid
administration + parenteral medication + water from feeding + oral
water intake. Total output fluid = Blood loss + urine output +
fluid loss from drains + vomiting.
Length of hospital stay
Patients that showed unassisted mobilization, normal
eating and drinking without nausea, defecation, satisfactory oral
analgesia, no evidence of complications or particularly
infection(s) were discharged from the hospital (3). The length of hospital stay was
defined as discharge from operation theater to discharge from the
ward.
Amount of fluid and vasoactive
administrations
Data regarding fluid administrations and vasoactive
medications were retrospectively collected from medical records of
patients of institutes.
Complications
The unexpected events that occurred after discharge
from the operation theater to discharge from the ward were
considered complications. The Clavien-Dindo Classification
(11) was used to grade
complications. The Clavien-Dindo Classification was graded as: i)
I, deviation from the normal postoperative condition that did not
require pharmacological or surgical treatment; ii) II, deviation
required pharmacological treatment; iii) IIIa, deviation required
endoscopic, radiation, or surgical interventions and did not
require general anesthesia; iv) IIIb, deviation required
endoscopic, radiation, or surgical interventions and required
general anesthesia; v) IVa, single organ life-threatening
dysfunctions; vi) IVb, multiple organ life-threatening
dysfunctions; and vii) V, death (12). The International Study Group of
Pancreatic Surgery was used to grade and classify pancreatic leaks
and delayed gastric emptying (13). According to requirements for
repairing, the postoperative pancreatic fistula was graded as: i)
A, required minor adjustments; ii) B, required significant changes
from the normal clinical route; and iii) C, required major invasive
procedures (13). A statement from
the European Society of Anesthesiology and the European Society of
Intensive Care Medicine joint task force for the European
Perioperative Clinical Outcome was used to define the other
perioperative complications (14).
Statistical analysis
The study assumed that the length of the hospital
stay of patients would have been 16±4 days (3). The sample size was calculated based
on the length of the hospital stay of patients, 80% power
calculation (β=0.2) and two-sided type-I error of 0.05% (α=0.05) at
a 95% of confidence level. The sample size (minimum number of
patients required in each cohort) was 105. InStat 3.01 (GraphPad
Software, Inc.) was used for statistical analysis. Categorial and
ordinal variables are presented as frequency (percentages) and
continuous variables are presented as mean ± standard deviation.
Fisher exact test or the Chi-square test (χ2-test) of
independence was used for categorical and ordinal variables.
Distribution of continuous data checked whether they are distrusted
normal or not normal visually through frequency distribution. For
the normal distribution of data of continuous variables, an
unpaired t-test was used for statistical analysis. For the not
normal distribution of data of continuous variables, the
Mann-Whitney test was used for statistical analysis. The variance
homogeneity of normally distributed continuous data has been
checked using the Brown- Forsythe test. If population variance is
not equal unpaired t-test with Welch correction was used for
continuous data and if population variance is equal unpaired t-test
was used for continuous data. All results were considered
significant at a 95% confidence interval of the difference
(Cl).
Results
Study population
From 15 May 2018 to 21 May 2020, a total of 270
patients underwent elective pancreaticoduodenectomy at the
department of gastroenterology of the parent hospital and the
referring hospitals. Among them, two patients have preoperative
coagulopathy, one female patient had a pregnancy, two patients had
renal impairment, three patients had the American Society
Anesthesiology physical status >IV, one patient had chronic
liver disease and seven patients underwent distal, central, or
total pancreatectomy and two patients had pancreatic enucleation.
Therefore, data of these patients (n=18) were excluded from the
analysis. Data of preoperative demographical characteristics and
clinical conditions, operative and postoperative details, as well
as length of hospital stay, complications, amount of fluid and
vasoactive administrations of a total of 252 patients were
retrospectively collected from hospital records after having
received written approval from authorities and analyzed. The flow
chart of the study is reported in Fig.
2.
Demographical characters,
comorbidities, blood chemistry and operative variables
Cancer was the most common reason for
pancreaticoduodenectomy. There were no significant differences
between preoperative demographical characters, ethnicity,
preoperative comorbidities, preoperative blood chemistry,
preoperative glomerular filtration rate and operative variables of
the enrolled patients (Table I;
Fisher's exact test/χ2-test and parametric/
nonparametric test; P>0.05 for all parameters).
 | Table IPreoperative demographical
characters, preoperative clinical conditions and operative
variables of the enrolled patients. |
Table I
Preoperative demographical
characters, preoperative clinical conditions and operative
variables of the enrolled patients.
| | Cohorts | |
|---|
| Parameters | UC | FO | Comparisons between
cohorts, P-value |
|---|
| Numbers of patients
who underwent pancreaticoduodenectomy | 142 | 110 | |
| Age, years | | | 0.303 |
|
Range | 52-74 | 52-75 | |
|
Mean ±
SD | 62.15±7.15 | 63.21±9.15 | |
| Sex | | | 0.701 |
|
Male | 85(60) | 63(57) | |
|
Female | 57(40) | 47(43) | |
| Body mass index,
kg/m2 | | | 0.101 |
|
Range | 21-29 | 21-29 | |
|
Mean ±
SD | 24.95±1.85 | 25.33±1.77 | |
| Ethnicity | | | 0.935 |
|
Han
Chinese | 130(92) | 101(92) | |
|
Mongolian | 10(7) | 8(7) | |
|
Tibetan | 2(1) | 1(1) | |
| Comorbidities | | | 0.602 |
|
Diabetes | 45(32) | 39(35) | |
|
Chronic
obstructive pulmonary diseases | 8(6) | 2(2) | |
|
Hypertension | 21(15) | 20(18) | |
|
Peripheral
vascular disease | 2(1) | 1(1) | |
|
Malignancy | 111(78) | 101(92) | |
|
Ischemic
heart disease | 4(3) | 2(2) | |
| Blood
chemistry | | | |
|
Hemoglobin,
mg/dl | | | 0.106 |
|
Range | 129-147 | 126-143 | |
|
Mean
± SD | 13.35±0.75 | 13.51±0.81 | |
|
White blood
cell count, x109/l | | | 0.063 |
|
Range | 5.65-8.55 | 5.75-9.05 | |
|
Mean
± SD | 7.15±0.14 | 7.19±0.20 | |
|
Platelets,
x109/l | | | 0.071 |
|
Range | 180-290 | 178-289 | |
|
Mean
± SD | 230±13 | 227±13 | |
|
Albumin,
g/l | | | 0.088 |
|
Range | 36-46 | 32-42 | |
|
Mean
± SD | 39±5 | 38±4 | |
|
Creatinine,
µM/l | | | 0.115 |
|
Range | 58-87 | 60-95 | |
|
Mean
± SD | 68±4 | 69±6 | |
|
Bilirubin,
µM/l | | | 0.075 |
|
Range | 7-14 | 8-18 | |
|
Mean
± SD | 9±3 | 8.4±2.1 | |
|
Urea,
µM/l | | | 0.064 |
|
Range | 4.2-6.8 | 4.6-7.7 | |
|
Mean
± SD | 5.85±0.85 | 6.08±1.11 | |
| Glomerular
filtration rate, ml/min/1.73 m2 | | | 0.079 |
|
Range | 81-91 | 78-92 | |
|
Mean ±
SD | 85±4 | 84±5 | |
| American Society
Anesthesiology physical status | | | 0.852 |
|
I-II | 45(32) | 31(28) | |
|
III-IV | 97(68) | 79(72) | |
| Anesthesia
method | | | 0.224 |
|
Morphine | 115(81) | 82(75) | |
|
Epidural
anesthesia | 27(19) | 28(25) | |
| Duration of
surgery, h | | | 0.103 |
|
Range | 7.21-9.65 | 7.11-9.55 | |
|
Mean ±
SD | 8.55±0.42 | 8.45±0.55 | |
Length of hospital stays
The length of hospital stay for patients in the FO
cohort was shorter than that of patients in the UC cohort
[11.02±2.07 days (range, 7-10 days) vs. 14.95±3.97 days (range:
7-23 days); P<0.0001; degree of freedom (df): 250; 95%
CI: -4.752 to -3.113; t-test; Fig.
3).
Fluid administration during hospital
stays
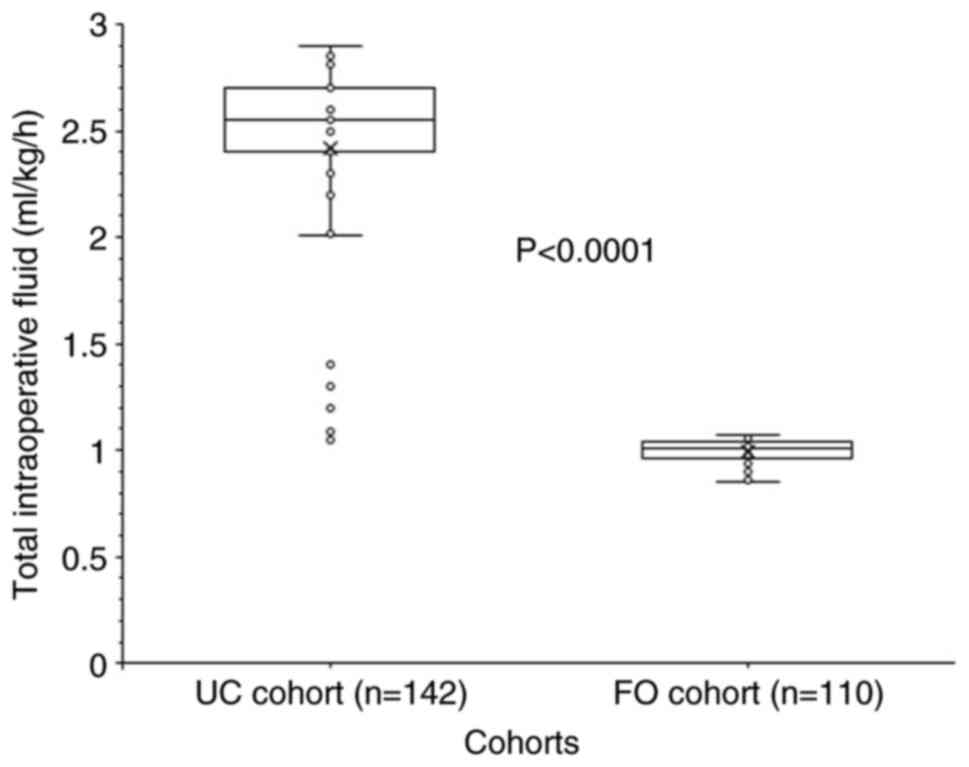
Total intraoperative fluid was given at rate of
0.99±0.05 ml/kg/h (range, 0.85-1.01 ml/kg/h) for patients in the FO
cohort and at rate of 2.42±0.4 ml/kg/h (range, 1.05-2.9 ml/kg/h)
for patients in the UC cohort. Intraoperative fluid administration
rate was higher for patients of the UC cohort than the FO cohort
(P<0.0001, df: 147; 95% CI: -1.492 to -1.359; t-test with Welch
correction; Fig. 4).
Administration of blood products in the UC cohort was also higher
than in the FO cohort (P=0.033). This result may be related to
intraoperative bleeding and surgery time. Intraoperative bleeding
was higher in the UC cohort than that in the FO cohort (P=0.036).
The surgery time was higher in the UC cohort compared with that in
the FO cohort; however, this was not statistically significant.
The amount of crystalloid fluid (P<0.0001) and
blood products (P=0.033) administered were higher in the UC cohort
than those in the FO cohort. Also, urine output was higher in the
UC cohort than that in the FO cohort (P<0.0001). The
postoperative input fluid was the similar in both cohorts
(P>0.05 for all types of administrations). Total input fluid in
the UC cohort was 1.17 times higher than that in the FO cohort.
However, total output fluid in the UC cohort was only 1.06 times
higher than that in the FO cohort. The details of fluid
administration during hospital stays are reported in Table II. Fluid balances in the UC cohort
were higher than those in the FO cohort (6,101±695 ml vs. 4,623±358
ml; P<0.0001; df: 220; 95% CI: -1611.6 to -1345.4; t-test with
Welch correction; Fig. 5).
 | Table IIFluid administration during hospital
stays. |
Table II
Fluid administration during hospital
stays.
| | Cohorts | |
|---|
| Parameters | UC | FO | Comparisons between
cohorts, P-value |
|---|
| Numbers of patients
who underwent pancreaticoduodenectomy | 142 | 110 | |
| Intravenous fluid
administration, ml | | | |
|
Crystalloid | | | <0.0001 |
|
Range | 6,000-8,000 | 4,000-7,600 | |
|
Mean
± SD |
8,218±964a | 6,297±846 | |
|
Colloid | | | <0.0001 |
|
Range | 0-550 | 0-550 | |
|
Mean
± SD |
146±128a | 279±169 | |
|
Blood
products | | | 0.033 |
|
Range | 0-250 | 0-250 | |
|
Mean
± SD | 40±92a | 18±65 | |
| Parenteral
medication, ml | | | 0.445 |
|
Range | 950-1,100 | 950-1,100 | |
|
Mean ±
SD | 1,009±42 | 1,005±43 | |
| Water from feeding
+ oral water intake, ml | | | 0.071 |
|
Range | 2,800-3,100 | 2,800-3,100 | |
|
Mean ±
SD | 2,969±86 | 2,950±74 | |
| Total input fluid,
ml | | | <0.0001 |
|
Range | 10,050-14,400 | 8,050-11,850 | |
|
Mean ±
SD |
12,382±945a | 10,549±868 | |
| Urine output,
ml | | | <0.0001 |
|
Range | 5,000-6,700 | 3,950-6,500 | |
|
Mean ±
SD |
5,620±226a | 5,345±601 | |
| Blood loss, ml | | | 0.036 |
|
Range | 100-550 | 50-300 | |
|
Mean ±
SD |
206±114a | 233±79 | |
| Fluid loss from
drains, ml | | | <0.0001 |
|
Range | 250-600 | 250-400 | |
|
Mean ±
SD | 446±85a | 336±59 | |
| Vomiting, ml | | | 0.221 |
|
Range | 0-100 | 0-100 | |
|
Mean ±
SD | 8±24 | 12±30 | |
| Total output fluid,
ml | | | <0.0001 |
|
Range | 5,350-7,000 | 4,250-7,200 | |
|
Mean ±
SD |
6,281±355a | 5,926±676 | |
Intraoperative vasoactive
medication
The number of patients in the UC cohort requiring
intraoperatively metaraminol was higher than that in the FO cohort
(P<0.0001; Fisher's test). However, the number of patients
administered intraoperatively with noradrenaline (P<0.0001;
Fisher's test) and dopamine/dobutamine (P<0.0001; Fisher's test)
was higher in the FO cohort than that in the UC cohort. The details
of intraoperative vasoactive medication are reported in Table III.
 | Table IIIIntraoperative vasoactive
medication. |
Table III
Intraoperative vasoactive
medication.
| | Cohorts | |
|---|
| Parameters | UC | FO | Comparisons between
cohorts, P-value |
|---|
| Numbers of patients
who underwent pancreaticoduodenectomy | 142 | 110 | |
| Any vasoactive
drug | 135(95) | 109(99) | 0.072 |
| Noradrenaline | 45(32) | 98(89)b | <0.0001 |
| Ephedrine | 40(28) | 41(37) | 0.126 |
| Metaraminol |
110(77)a | 15(14) | <0.0001 |
|
Dopamine/Dobutamine | 7(5) | 40(36)b | <0.0001 |
| β-blockers | 22(15) | 22(20) | 0.352 |
Complications
A total of 116 (82%) from the UC cohort and 81 (74%)
of patients from the FO cohort reported complications (P=0.128;
Fisher's test). The higher numbers of patients of the UC cohort
were reported for grade A pancreatic fistula (P=0.011; Fisher's
test), grade C pancreatic fistula (P=0.037; Fisher's test), all
grades of pancreatic fistula (P=0.0001; Fisher's test), arrhythmia
(P=0.026; Fisher's test), postoperative delirium (P=0.001; Fisher's
test), electrolyte disturbances (P=0.028; Fisher's test),
hyponatremia (P=0.046; Fisher's test), refractory analgesia
(P=0.014; Fisher's test) and blood products requirements (P=0.035;
Fisher's test) than those of the FO cohort. The number of patients
that reported drug reaction was greater in the FO cohort than that
in the UC cohort (P=0.012; Fisher's test). The details of
perioperative and postoperative complications are reported in
Table IV.
 | Table IVPerioperative and postoperative
complications. |
Table IV
Perioperative and postoperative
complications.
| | Cohorts | |
|---|
| Parameters | UC | FO | Comparisons between
cohorts, P-value |
|---|
| Numbers of patients
who underwent pancreaticoduodenectomy | 142 | 110 | |
| Clavien-Dindo
Classification | | | |
|
I | 18(13) | 12(11) | 0.699 |
|
II | 25(18) | 15(14) | 0.487 |
|
IIIa | 1(1) | 5(4) | 0.089 |
|
IIIb | 1(1) | 5(4) | 0.089 |
|
Iva | 4(3) | 1(1) | 0.391 |
|
IVb | 1(1) | 1(1) | 0.999 |
|
V | 0 (0) | 0 (0) | N/A |
| Total | 50(35) | 39(35) | 0.999 |
| Wound
infection | 23(16) | 15(14) | 0.599 |
| Superficial
surgical site infection | 21(15) | 15(14) | 0.857 |
| Deep surgical site
infection | 11(8) | 5(4) | 0.436 |
| Sepsis | 17(12) | 9(8) | 0.406 |
| Pancreatic
fistula | | | |
|
Grade A | 21(15)a | 5(4) | 0.011 |
|
Grade B | 11(8) | 4(4) | 0.192 |
|
Grade C | 6(4)a | 0 (0) | 0.037 |
|
Total | 38(27)a | 9(8) | 0.0001 |
| Delayed gastric
emptying | 21(15) | 10(9) | 0.183 |
| Bile leak. the
presence of bile in the drainage fluid that persisted on
postoperative day 4 | 6(4) | 1(1) | 0.141 |
| Cardiorespiratory
complications | 37(26) | 20(18) | 0.172 |
| Acute respiratory
distress syndrome | 5(4) | 1(1) | 0.236 |
| Pneumonia | 7(5) | 5(4) | 0.998 |
| Pulmonary
atelectasis | 11(8) | 4(4) | 0.192 |
| Pulmonary
congestion | 11(8) | 8(7) | 0.998 |
| Cardiogenic
pulmonary edema | 3(2) | 1(1) | 0.634 |
| Arrhythmia | 12(8)a | 2(2) | 0.026 |
| Acute pancreatitis,
serum lipase >50 U/dl | 1(1) | 5(4) | 0.089 |
| Gastrointestinal
bleeding | 7(5) | 1(1) | 0.143 |
| Acute kidney
injury | 9(6) | 2(2) | 0.119 |
| Postoperative
delirium | 21(15)a | 3(3) | 0.001 |
| Ischemic
hepatitis | 7(5) | 1(1) | 0.143 |
| Nausea | 23(16) | 21(19) | 0.617 |
| Vomiting | 16(11) | 18(16) | 0.267 |
| Electrolyte
disturbances | 31(22)a | 12(11) | 0.028 |
| Hypokalemia | 18(13) | 9(8) | 0.307 |
| Hyponatremia | 9(6)a | 1(1) | 0.046 |
| Hypomagnesemia | 7(5) | 5(4) | 0.998 |
|
Hypophosphatemia | 7(5) | 1(1) | 0.143 |
| Hyperkalemia | 4(3) | 1(1) | 0.391 |
| Hypernatremia | 2(2) | 1(1) | 0.999 |
| Endocrine
abnormalities | 3(2) | 2(2) | 0.634 |
| Drug reaction | 1(1) | 8(7)b | 0.012 |
| Refractory
analgesia | 22(15)a | 5(4) | 0.014 |
| Urinary tract
infection | 2(2) | 1(1) | 0.999 |
| Fluid overload | 3(2) | 1(1) | 0.634 |
| Blood products
requirements | 23(16)a | 8(7) | 0.035 |
| Return to operation
theatre from postoperative intensive care units and/or ward | 22(15) | 17(15) | 0.998 |
| Return to
postoperative intensive care units from the ward | 13(9)a | 1(1) | 0.004 |
| Number of
complications | 522 | 287 | N/A |
| Patients with
complications | 116(82) | 81(74) | 0.128 |
| Complications per
patients | 3.68 | 2.61 | N/A |
The UC cohort reported 3.68 complications/patient,
while the FO cohort reported 2.61 complications/patient. The
patients who underwent pancreaticoduodenectomy under usual protocol
reported higher numbers of perioperative and postoperative
complications than those who underwent pancreaticoduodenectomy
under usual protocol with intraoperative fluid optimization
(P=0.024; df: 8, χ2-test; Fig. 6). The number of patients with >4
complications in FO cohort was lower than that in the UC cohort [25
(23%) vs. 62 (44%); P=0.0005; Fisher's exact test].
Discussion
The present study indicated that patients who
underwent pancreaticoduodenectomy under the usual protocol of
enhanced recovery after surgical procedures with intraoperative
fluid optimization had shorter hospital stay, lower intraoperative
fluid rate, fewer fluid balance during hospital stay and fewer
perioperative and postoperative complications than those who
underwent pancreaticoduodenectomy under the usual protocol of
enhanced recovery after surgical procedures. The results of the
current study are in agreement with the results of a multicenter
randomized controlled trial (3),
retrospective analyses (2,5,6,15),
whereas it is only partially in agreement with the results from a
retrospective analysis (16) and a
randomized trial (17). A clinical
trial performed on the English population (3), retrospective analyses on the North
American population (2,15) and the Australian population
(5) have small sample sizes;
notably, a small sample size may increase the risk of a type-I
error. In addition, several retrospective studies (2,5,6,15)
used old records of patients for chart reviews and data analyses.
At the time of those trials several complications were not clearly
understood, like the pancreatic leak which was not clearly
understood until 2005(6). Enhanced
recovery after surgery procedures protocol (8) is implemented with specific guidelines
(18,19). Pancreaticoduodenectomy under usual
protocol with intraoperative fluid optimization may have
perioperative and postoperative benefits.
The range of hospital stay of all patients was 7-23
days even though enhanced recovery after surgery procedures
protocol (8) is implemented in the
study. Total input fluid in the UC cohort was 1.17 times higher
than that in the FO cohort. However, total output fluid in the UC
cohort was only 1.06 times higher than that in the FO cohort.
Postoperative input fluid was similar in the two cohorts. Thus,
intraoperative fluid administration was higher in the UC cohort
than in the FO cohort. Goal-directed fluid administration reduces
hospital stays (20). The results
of hospital stay, fluid administration and fluid output of the
current study were in agreement with the results from a multicenter
randomized controlled trial (3)
and retrospective study (21).
Pancreaticoduodenectomy under usual protocol with intraoperative
fluid optimization improves the outcomes.
In the present study reported that the number of
patients that suffered from pancreatic fistula, arrhythmia,
postoperative delirium, electrolyte disturbances, hyponatremia,
refractory analgesia and blood product requirements was higher in
the UC cohort than in the FO cohort. The results of perioperative
and postoperative complications of the current study concur with
the study on elective cardiac surgery (22). Fluid liberal therapies can have
requirements for the administration of blood products, such as
blood and plasma transfusions (23), and administration of these blood
products to patients can reduce survival after surgery (24). Perioperative fluid choice and
therapies should be individualized (25) because body weight and body mass
index are associated with perioperative and postoperative
complications (10). A fluid
liberal regimen is responsible for perioperative and postoperative
complications.
The data of the current study also showed a lower
percentage of patients with >4 complications in the FO cohort
than in the UC cohort. The results of complications of the current
study were not consistent with those of a multicenter randomized
controlled trial (3). The small
sample size of a multicenter randomized controlled trial (3) may be responsible for the
contradictory results. The present results also demonstrated the
advantage of a new practice of intraoperative fluid optimization in
decreasing the number of complications.
A greater number of patients received noradrenaline
and dopamine/dobutamine during pancreaticoduodenectomy under the
usual protocol with intraoperative fluid optimization than during
pancreaticoduodenectomy under the usual protocol. Noradrenaline and
dopamine/dobutamine preserved plasma volume and compensated for the
requirements for fluid administration (3). To the best of our knowledge, the
effects of fluid administrations combined with adrenergic and/or
vasoactive therapies have not been formally evaluated in human
clinical trials. However, the experimental study reported that α1
agonists with vasoactive drugs can accelerate the distribution and
the elimination of fluid, while β1 agonists can delay the
distribution and the elimination of fluid (26). The low dose of phenylephrine
administration can slow down the distribution of fluid from the
plasma to the interstitial fluid space and decrease the risk of
hypovolemia (27). However, volume
expansions of fluid in conjugations with vasoactive medications are
difficult to understand because of the confounding effects of
anesthesia, surgery and clinical and pathological characteristics
of patients (3). In addition to
parenteral fluid administrations, the current study provides
recommendations for intraoperative vasoactive medications.
In the current study, the patients in the FO cohort
received fluid administration if stroke volume variations were
>20% during and at the end of surgeries. The cut-off value of
fluid interventions in the current study was the same as that of a
multicenter randomized controlled trial (3) and a retrospective study (28). The stroke volume variations are
inconclusive between 9 and 13% limits for 25% of patients under
general anesthesia (29,30). However, the previous randomized
trials (31-33)
on conventional fluid administration optimization protocols are
less fluid restrictive than what was used in the surgeries of the
current studies. The current study justified the cut-off value of
fluid interventions.
The present study had several limitations. For
example, it did not report data on the size of the pancreatic duct,
the texture of the pancreas, the number of lymph nodes retrieved
and the complexity of surgeries. However, the present study was
focused on non-surgical factors and patient's outcomes. The sample
size was calculated based on the hospital stay of patients, however
it was not calculated for complications. The protocols are designed
for pancreaticoduodenectomy and cannot be extrapolated to other
cardiac, emergency and orthopedic surgeries. The present study is a
non-randomized, single-center study and lacks dynamic study.
In conclusion, the findings of the current study
indicated that patients who underwent pancreaticoduodenectomy under
usual protocol with intraoperative fluid optimization had shorter
hospital stay, fewer fluid balance during hospital stays, fewer
perioperative and postoperative complications, as well as fewer
requirements for intraoperative blood products than those who
underwent pancreaticoduodenectomy under the usual protocol without
intraoperative fluid optimization. Intraoperative fluid
administration optimization with proper intraoperative vasoactive
medications during pancreaticoduodenectomy decreases the risk of
perioperative and postoperative complications.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LL was the project administrator and contributed to
supervision, validation, methodology and the literature review of
the study. YW contributed to the investigation, methodology,
conceptualization, software and literature review of the study. XN
contributed to formal analysis, data curation, resources and the
literature review of the study and drafted and edited the
manuscript for intellectual content. All authors agree to be
accountable for all aspects of work ensuring integrity and
accuracy. LL and YW confirm the authenticity of all the raw data.
All authors read and approved the final version of manuscript.
Ethics approval and consent to
participate
The designed protocol (approval no. DMUCL1520, dated
10 December 2020) was approved by the Dalian Medical University
review board and the Chinese Society of Critical Care Medicine. The
study reporting adheres to the law of China, the STROBE (the
strengthening the reporting of observational studies in
epidemiology) guidelines and the V2008 Declarations of Helsinki.
Consent for participation in the present study was not required due
to the retrospective nature of the study. Being a retrospective
study the registration in the Chinese Clinical Trial Registry was
waived by the institutional review board.
Patient consent for publication
An informed consent form was signed by the relatives
of the patients regarding publication of the anonymized information
of the patients in the form of an article before surgeries.
Competing interests
The authors declared that they have no competing
interests.
Authors' information
Lian Lian, y554158@163.com, ORCID iD:
0000-0002-4699-7032. Yi Wang, u996537@163.com, ORCID iD:
0000-0003-3042-7390. Xueqin Ning, xueqin.ning@gmail.com, ORCID iD:
0000-0001-9889-1785.
References
|
1
|
Rahib L, Smith BD, Aizenberg R, Rosenzweig
AB, Fleshman JM and Matrisian LM: Projecting cancer incidence and
deaths to 2030: The unexpected burden of thyroid, liver and
pancreas cancers in the United States. Cancer Res. 74:2913–2921.
2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wright GP, Koehler TJ, Davis AT and Chung
MH: The drowning whipple: Perioperative fluid balance and outcomes
following pancreaticoduodenectomy. J Surg Oncol. 110:407–411.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Weinberg L, Ianno D, Churilov L, Chao I,
Scurrah N, Rachbuch C, Banting J, Muralidharan V, Story D, Bellomo
R, et al: Restrictive intraoperative fluid optimisation algorithm
improves outcomes in patients undergoing pancreaticoduodenectomy: A
prospective multicentre randomized controlled trial. PLoS One.
12(e0183313)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fellahi JL, Futier E, Vaisse C, Collange
O, Huet O, Loriau J, Gayat E, Tavernier B, Biais M, Asehnoune K, et
al: Perioperative hemodynamic optimization: From guidelines to
implementation-an experts' opinion paper. Ann Intensive Care.
11(58)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Weinberg L, Wong D, Karalapillai D, Pearce
B, Tan CO, Tay S, Christophi C, McNicol L and Nikfarjam M: The
impact of fluid intervention on complications and length of
hospital stay after pancreaticoduodenectomy (Whipple's procedure).
BMC Anesthesiol. 14:1–8. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Behman R, Hanna S, Coburn N, Law C, Cyr
DP, Truong J, Lam-McCulloch J, McHardy P, Sawyer J, Idestrup C and
Karanicolas PJ: Impact of fluid resuscitation on major adverse
events following pancreaticoduodenectomy. Am J Surg. 210:896–903.
2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lassen K, Soop M, Nygren J, Cox PB, Hendry
PO, Spies C, von Meyenfeldt MF, Fearon KC, Revhaug A, Norderval S,
et al: Consensus review of optimal perioperative care in colorectal
surgery: Enhanced recovery after surgery (ERAS) group
recommendations. Arch Surg. 144:961–969. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kagedan DJ, Ahmed M, Devitt KS and Wei AC:
Enhanced recovery after pancreatic surgery: A systematic review of
the evidence. HPB. 17:11–16. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Melloul E, Lassen K, Roulin D, Grass F,
Perinel J, Adham M, Wellge EB, Kunzler F, Besselink MG, Asbun H, et
al: Guidelines for perioperative care for pancreatoduodenectomy:
Enhanced recovery after surgery (ERAS) recommendations 2019. World
J Surg. 44:2056–2084. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gianotti L, Besselink MG, Sandini M,
Hackert T, Conlon K, Gerritsen A, Griffin O, Fingerhut A, Probst P,
Abu Hilal M, et al: Nutritional support and therapy in pancreatic
surgery: A position paper of the international study group on
pancreatic surgery (ISGPS). Surgery. 164:1035–1048. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang HB, Xiong GB, Zhu F, Wang M, Zhang H,
Feng YC, Yu S, Jin JK and Qin RY: Clavien-Dindo classification and
influencing factors analysis of complications after laparoscopic
pancreaticoduodenectomy. Zhonghua Wai Ke Za Zhi. 56:828–832.
2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
12
|
Liver and Gastrointestinal Disease
Foundation: The Clavien-Dindo classification. https://www.assessurgery.com/clavien-dindo-classification/.
Accessed on 10 December 2020.
|
|
13
|
Dusch N, Lietzmann A, Barthels F,
Niedergethmann M, Rückert F and Wilhelm TJ: International study
group of pancreatic surgery definitions for postpancreatectomy
complications: Applicability at a high-volume center. Scand J Surg.
106:216–223. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jammer I, Wickboldt N, Sander M, Smith A,
Schultz MJ, Pelosi P, Leva B, Rhodes A, Hoeft A, Walder B, et al:
Standards for definitions and use of outcome measures for clinical
effectiveness research in perioperative medicine: European
perioperative clinical outcome (EPCO) definitions: a statement from
the ESA-ESICM joint taskforce on perioperative outcome measures.
Eur J Anaesthesiol. 32:88–105. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Melis M, Marcon F, Masi A, Sarpel U,
Miller G, Moore H, Cohen S, Berman R, Pachter HL and Newman E:
Effect of intra-operative fluid volume on peri-operative outcomes
after pancreaticoduodenectomy for pancreatic adenocarcinoma. J Surg
Oncol. 105:81–84. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Eng OS, Goswami J, Moore D, Chen C, Gannon
CJ, August DA and Carpizo DR: Intraoperative fluid administration
is associated with perioperative outcomes in
pancreaticoduodenectomy: A single center retrospective analysis. J
Surg Oncol. 108:242–247. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fischer M, Matsuo K, Gonen M, Grant F,
Dematteo RP, D'Angelica MI, Mascarenhas J, Brennan MF, Allen PJ,
Blumgart LH and Jarnagin WR: Relationship between intraoperative
fluid administration and perioperative outcome after
pancreaticoduodenectomy: Results of a prospective randomized trial
of acute normovolemic hemodilution compared with standard
intraoperative management. Ann Surg. 252:952–958. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lassen K, Coolsen MM, Slim K, Carli F, de
Aguilar-Nascimento JE, Schäfer M, Parks RW, Fearon KC, Lobo DN,
Demartines N, et al: Guidelines for perioperative care for
pancreaticoduodenectomy: Enhanced recovery after surgery
(ERAS®) society recommendations. Clin Nutr. 31:817–830.
2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lassen K, Coolsen MM, Slim K, Carli F, de
Aguilar-Nascimento JE, Schäfer M, Parks RW, Fearon KC, Lobo DN,
Demartines N, et al: Guidelines for perioperative care for
pancreaticoduodenectomy: Enhanced recovery after surgery
(ERAS®) society recommendations. World J Surg.
37:240–258. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pearse RM, Harrison DA, MacDonald N,
Gillies MA, Blunt M, Ackland G, Grocott MP, Ahern A, Griggs K,
Scott R, et al: Effect of a perioperative, cardiac output-guided
hemodynamic therapy algorithm on outcomes following major
gastrointestinal surgery: A randomized clinical trial and
systematic review. JAMA. 311:2181–2190. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nikfarjam M, Weinberg L, Low N, Fink MA,
Muralidharan V, Houli N, Starkey G, Jones R and Christophi C: A
fast track recovery program significantly reduces hospital length
of stay following uncomplicated pancreaticoduodenectomy. JOP.
14:63–70. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Vretzakis G, Kleitsaki A, Stamoulis K,
Bareka M, Georgopoulou S, Karanikolas M and Giannoukas A:
Intra-operative intravenous fluid restriction reduces perioperative
red blood cell transfusion in elective cardiac surgery, especially
in transfusion-prone patients: A prospective, randomized controlled
trial. J Cardiothorac Surg. 5:1–10. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cumberworth A and Cumberworth J:
Intraoperative fluids and postoperative haemoglobin. Br J Anaesth.
116(723)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sutton JM, Kooby DA, Wilson GC, Squires MH
III, Hanseman DJ, Maithel SK, Bentrem DJ, Weber SM, Cho CS, Winslow
ER, et al: Perioperative blood transfusion is associated with
decreased survival in patients undergoing pancreaticoduodenectomy
for pancreatic adenocarcinoma: A multi-institutional study. J
Gastrointest Surg. 18:1575–1587. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Navarro LH, Bloomstone JA, Auler JO Jr,
Cannesson M, Rocca GD, Gan TJ, Kinsky M, Magder S, Miller TE,
Mythen M, et al: Perioperative fluid therapy: A statement from the
international fluid optimization group. Perioper Med. 4:1–20.
2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li Y, Xiaozhu Z, Guomei R, Qiannan D and
Hahn RG: Effects of vasoactive drugs on crystalloid fluid kinetics
in septic sheep. PLoS One. 12(e0172361)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li YH, Zhu HB, Zheng X, Chen HJ, Shao L
and Hahn RG: Low doses of esmolol and phenylephrine act as
diuretics during intravenous anesthesia. Crit Care.
16(R18)2012.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Weinberg L, Banting J, Churilov L, McLeod
RL, Fernandes K, Chao I, Ho T, Ianno D, Liang V, Muralidharan V, et
al: The effect of a surgery-specific cardiac output-guided
haemodynamic algorithm on outcomes in patients undergoing
pancreaticoduodenectomy in a high-volume centre: A retrospective
comparative study. Anaesth Intensive Care. 45:569–580.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Vos JJ, Poterman M, Salm PP, Van Amsterdam
K, Struys MM, Scheeren TW and Kalmar AF: Noninvasive pulse pressure
variation and stroke volume variation to predict fluid
responsiveness at multiple thresholds: A prospective observational
study. Can J Anaesth. 62:1153–1160. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cannesson M, Le Manach Y, Hofer CK, Goarin
JP, Lehot JJ, Vallet B and Tavernier B: Assessing the diagnostic
accuracy of pulse pressure variations for the prediction of fluid
responsiveness: A ‘gray zone’ approach. Anesthesiology.
115:231–241. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ramsingh DS, Sanghvi C, Gamboa J,
Cannesson M and Applegate RL II: Outcome impact of goal directed
fluid therapy during high risk abdominal surgery in low to moderate
risk patients: A randomized controlled trial. J Clin Monit Comput.
27:249–257. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jammer I, Tuovila M and Ulvik A: Stroke
volume variation to guide fluid therapy: Is it suitable for
high-risk surgical patients? A terminated randomized controlled
trial. Perioper Med (Lond). 4(6)2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Scheeren TW, Wiesenack C, Gerlach H and
Marx G: Goal-directed intraoperative fluid therapy guided by stroke
volume and its variation in high-risk surgical patients: A
prospective randomized multicentre study. J Clin Monit Comput.
27:225–233. 2013.PubMed/NCBI View Article : Google Scholar
|