Introduction
Surgical resection is the primary method for the
treatment of Merkel cell carcinoma (MCC). However, due to the rare
occurrence of this disease, high-quality clinical studies are
lacking and treatment recommendations for MCC are complex and
varied. Treatment approaches include wide local excision, Mohs
surgery, radiotherapy and immunotherapy, as well as different
combinations of these treatments (1). For wide local excision, the optimal
margin width for primary MCC remains to be fully determined.
Surgeons frequently trust their own judgment to try to maximize
outcomes while minimizing morbidity (2). Furthermore, recent advances in
immunotherapy for the treatment of metastatic melanoma have
prompted the application of immunotherapy in MCC. Therefore, the
optimal method of treatment for MCC remains to be determined. In
the present study, a case of MCC was treated with extended
resection and the desired efficacy was achieved. Combined with the
relevant literature, the present study may provide a reference for
the surgical treatment of MCC.
Case report
A 90-year-old female patient was admitted to
Guang'anmen Hospital (Beijing, China) in April 2019 with a mass on
the right thigh. At the time, a fingernail-sized mass was found in
the middle and lower parts of the right medial thigh but this was
not documented. Subsequently, the size of the mass gradually
increased, which was accompanied by itching, no tenderness and a
dark red color. The patient had a history of hypertension,
hyperthyroidism, glaucoma, cataract and a surgical history of
gallstones and hemorrhoids. The patient denied any history of local
trauma and had no family history of similar diseases. Physical
examination demonstrated no notable abnormalities, no enlargement
of the left popliteal fossa and the inguinal lymph nodes were
palpable. A specialist examination demonstrated that the lower
limbs of the patient were symmetrical in shape. The mass was on the
right pretibial thigh and was accompanied by a 3x3-cm dark red
nodule, which was ~8 cm above the knee with clear boundaries. The
skin had a smooth, fixed basal surface and was visibly dry with
flaking. There was no tenderness, the temperature of the skin was
normal and there was no swelling. Furthermore, there was no canker
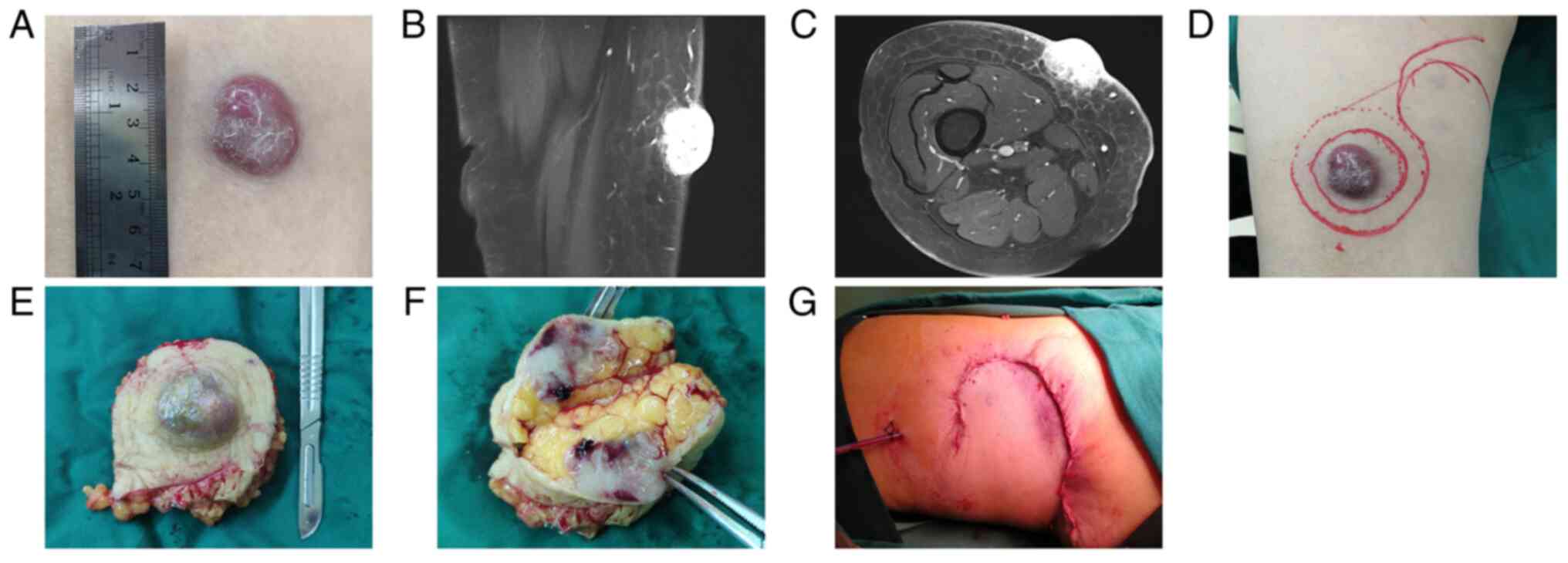
seepage and no smell was observed (Fig. 1A). MRI demonstrated a
space-occupying lesion rich in blood supply in the subcutaneous
adipose layer of the right pretibial thigh (Fig. 1B and C). Under local infiltration anesthesia,
complete resection of the tumor was performed using a 2-cm margin
from the outer edge of the nodule. This reached the subcutaneous
adipose layer and was sutured using a tension-relieving suture
(Fig. 1D-G). The tissues were
fixed with 4% paraformaldehyde, embedded in paraffin, sectioned at
4 µm, and then stained with hematoxylin for 5 min and eosin for 15
min at room temperature. Histopathology demonstrated that the
epidermis was atrophied and thinned and the tumor cells in the
dermis were distributed in clumps. Furthermore, the tumor cells
were arranged in cords or adenoids. The cells were notably deformed
and there were myxoid deposits surrounding the cells (Fig. 2A-D). For immunohistochemistry
(IHC), the paraffin slices were sectioned at a thickness of 4 µm,
deparaffinized and rehydrated, followed by citrate-EDTA antigen
retrieval. After peroxide block, samples were incubated with
primary antibodies overnight at 4˚C and then with horseradish
peroxidase-conjugated goat antirabbit immunoglobulin G (cat. no.
DS-9800; Leica Biosystems, Inc.) for 30 min at 37˚C. The
corresponding primary antibodies used in the experiments were as
follows: Cytokeratin 20 antibody (cat. no. ZA-0574), CD56 antibody
(cat. no. ZM-0057), chromogranin A antibody (cat. no. ZA-0507),
synaptophysin antibody (cat. no. ZA-0506; all from OriGene
Technologies, Inc.). The sections were then stained with a
diaminobenzidine color kit (cat. no. ZLI-9017; OriGene
Technologies, Inc.) and with hematoxylin for 5 min. The process of
secondary antibody staining was based on the BOND-MAX Fully
Automated IHC Stainer (Leica Biosystems). IHC staining demonstrated
that the tumor tissue was positive for cytokeratin 20, CD56,
chromogranin A and synaptophysin (Fig.
2E-H). The diagnosis was determined to be MCC. The stitches
were removed 15 days after surgery and the wound healed adequately.
There was no recurrence after 30 months of follow-up.
Discussion
MCC is a rare and aggressive cutaneous
neuroendocrine tumor, originally described by Toker (3) as a trabecular carcinoma of the skin.
However, the incidence of MCC continues to increase, which is
mainly due to the aging population. A 95% increase in the incidence
of MCC was reported in the US from 2000 to 2013(4). MCC typically occurs in areas of the
skin that are exposed to sunlight, such as the head, neck and
limbs, in middle-aged and elderly patients. MCC may also be
associated with immunosuppression and genetic mutations caused by
Merkel cell polyomavirus infection (5,6).
There is currently a lack of high-quality trials and literature,
and further research into treatment options is required in order to
improve the clinical outcomes of MCC.
At present, the initial management of primary MCC
involves definitive resection of the primary tumor. However, the
most optimal surgery for the treatment of MCC remains
controversial. When selecting a specific surgical margin width for
primary MCC, surgeons frequently rely on their own judgment to
maximize prognosis while minimizing morbidity. Due to the high risk
of local recurrence of MCC, surgical guidelines emphasize complete
resection of the tumor during a one-stage operation to ensure
surgical margin purity is clinically feasible (7). More specifically, it was previously
recommended that the surgical margin width should be 3 cm (8). However, resection margins of >2 mm
frequently require skin transplantation or flap reconstruction to
close the wound (9), which
markedly affects physical appearance. Transplantation or flap
closure also increase postoperative morbidity and the financial
cost to the patient. Furthermore, MCC is highly radiosensitive and
radiotherapy following resection improves local area control and
the overall survival rate (10).
Perez and Zager (2) determined
that there were no significant differences in local recurrence,
disease-specific survival or overall survival among cases with a
margin width of 1, 1.1-1.9 or ≥2 cm. Therefore, this may indicate
that a resection margin of ≥3 cm may not be necessary.
According to the current National Cancer Center
Network guidelines, the standard treatment for primary MCC is
extensive local resection combined with adjuvant radiotherapy, with
a specific resection margin of 1-2 cm (11). Results of a previous study
demonstrated that the clinical local recurrence rate is 4.2-31.7%
(12). These rates may be due to
patients being treated without adjuvant radiation therapy, rapid
growth and metastases of MCC, and the retrospective nature of
previous studies. Furthermore, poor local control may be due to a
positive margin caused by incomplete resection. The positive margin
rate may reach 10.4% following extensive local resection derived
from larger resection in more aggressive-appearing lesions
(2). In addition, a false-negative
margin may occur due to the standard pathological margin
examination evaluating <1% of the surgical margin (13). Furthermore, a competing risk model
was used to directly compare different resection margins based on
stratified patients with MCC and this analysis removed the
influence of non-cancer-related deaths on the outcome. These
results demonstrated that patients <60 years of age with a tumor
diameter of ≤5 cm (T1/2) and patients with a resection margin >2
cm who underwent early adjuvant radiotherapy exhibited improved
survival rates. Furthermore, in patients >75 years of age,
extensive resection was associated with a lower survival rate and
complications were also more likely to occur. A resection margin ≤1
cm may be efficient for stage III MCC. In addition, the results of
surgical resection in T3, T4 or stage IV MCC were not clear.
Therefore, selecting the optimal surgical approach largely depends
on the individual situation of the patient (14). Previous studies have demonstrated
that there should be a safe margin of at least 1 cm in stage I
disease and a larger margin of at least 2 cm in higher stages
(5,15). In summary, extensive local
resection using margins of 1-2 cm remains the standard surgical
technique (16), particularly in
stage I/II MCC. In higher stages of MCC, clear conclusions were
difficult to obtain. However, a larger margin should not be used in
order to not impede postoperative radiotherapy and operative
complications are likely to occur.
For tissue preservation, Mohs or modified Mohs
surgery may be performed. However, this is more likely to be
performed for stage I/II MCC on the head or neck and sentinel lymph
node biopsies should be performed as a priority (17). Furthermore, Mohs micrography
supports a 100% margin assessment and reduces the chance of
residue. In a retrospective study, Terushkin et al (13) reported that Mohs micrography for
the treatment of early stage I/II MCC may achieve survival rates
comparable to extensive local resection and/or adjuvant
radiotherapy, without the requirement for additional radiotherapy
or reoperation to further treat local recurrence. Similarly, a
large-scale retrospective study from the National Cancer Database
demonstrated that there was no difference in survival between
patients who underwent Mohs micrography or extensive local
resection for early stage I/II MCC (18). According to the European
interdisciplinary consensus (19),
the immunohistochemistry needed for the diagnosis of MCC requires
specific staffing and technical support during Mohs surgery
(20), in case of residual tumor
cells in atypical patients. Furthermore, researchers have
questioned the potential correlation between Mohs surgery with
increased tumor metastasis (21).
In high-risk basal cell carcinoma and squamous cell carcinoma, Mohs
surgery demonstrates notable advantages; however, no guidelines or
consensus for Mohs surgery for MCC have been established.
Therefore, Mohs surgery remains a viable alternative option.
Previous research has also emphasized that biopsy staging should be
performed in the tumor center area.
Recent advances in immunotherapy for the treatment
of metastatic melanoma have prompted its application in MCC.
Results of a previous meta-analysis demonstrated that immunotherapy
was safe and effective in reducing the tumor diameter with a
durable response rate (22). At
present, immune checkpoint inhibitors (ICIs) exhibit no clear
advantage as an adjuvant therapy and programmed cell death
protein-1/programmed cell death-ligand 1 inhibitors are still
considered first-line treatment options for advanced MCC (16). Furthermore, pembrolizumab and
avelumab have been approved by the Food and Drug Administration for
the treatment of advanced MCC (16,23).
In a previous study, results were obtained in a multicenter, phase
II, non-controlled study using 26 patients who received a dose of 2
mg/kg pembrolizumab every 3 weeks (23). After expanding the cohort to 50
patients, the overall response rate (ORR) was stable at 56% and the
median pathological complete response was 16.8 months (24). In the phase II JAVELIN Merkel 200
clinical trial, 88 participants with advanced stage IV MCC received
chemotherapy more than once. The results of the trial demonstrated
a pathological complete response rate of 26% at 24 months and 21%
at 36 months. The ORR at 36 months was 32, and 31% at 42 months
when combined with a 1-h intravenous infusion of 10 mg/kg avelumab
every 2 weeks (25). Furthermore,
the efficacy of ipilimumab, INCMGA00012 and ICIs in combination is
currently being investigated. There is insufficient evidence
concerning the efficiency of neoadjuvant and adjuvant therapies in
combination. In a recent phase I/II study comprising 39 patients
with operable MCC, nivolumab was administered 4 weeks prior to
surgery (26). Although
pathological complete response is the main desired outcome, further
clinical trials are required and numerous clinical studies using
nivolumab, pembrolizumab and avelumab for the treatment of stage
I-III MCC are in progress. Research into numerous immunotherapies
has previously provided novel treatment strategies (16).
In the present study, the advanced age and MCC stage
of the patient was considered and a wide local excision using a
2-cm resection margin was performed. The patient refused to receive
further adjuvant radiotherapy. Furthermore, the follow-up period
was 30 months following surgery and no recurrence or complications
occurred.
At present, the surgical treatment of MCC is not
based on high levels of clinical evidence. Extensive local
resection using a 1-2 cm margin remains the standard surgical
technique, particularly in stage I/II MCC. A larger resection
margin is not recommended, as it may delay radiotherapy or lead to
further complications. In addition, immunotherapy-based research
has provided novel treatment strategies. In the future, multicenter
prospective randomized clinical trials are required to determine
the optimal operative and perioperative plan, following a
comprehensive assessment of the local recurrence rate, survival
rate and disease-specific survival rate (2).
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YS and YZ performed the data analyses and wrote the
manuscript. JM performed the surgery and contributed to the
original conception of the study. YZ and JM confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Guang'anmen Hospital, China Academy of Chinese Medical
Sciences (Beijing, China; approval no. 2022-SDTS-06).
Patient consent for publication
Written informed consent for the publication of the
patient's clinical information and images was obtained from the
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Harvey JA, Mirza SA, Erwin PJ, Chan AW,
Murad MH and Brewer JD: Recurrence and mortality rates with
different treatment approaches of Merkel cell carcinoma: A
systematic review and meta-analysis. Int J Dermatol. 61:687–697.
2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Perez MC and Zager JS: Aso author
reflections: Resection margins in merkel cell carcinoma: Is a 1 cm
margin wide enough? Ann Surg Oncol. 25 (Suppl
3)(S901)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Toker C: Trabecular carcinoma of the skin.
Arch Dermatol. 105:107–110. 1972.PubMed/NCBI
|
|
4
|
Paulson KG, Park SY, Vandeven NA, Lachance
K, Thomas H, Chapuis AG, Harms KL, Thompson JA, Bhatia S, Stang A
and Nghiem P: Merkel cell carcinoma: Current US incidence and
projected increases based on changing demographics. J Am Acad
Dermatol. 78:457–463.e2. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Schadendorf D, Lebbe C, Zur Hausen A,
Avril MF, Hariharan S, Bharmal M and Becker JC: Merkel cell
carcinoma: Epidemiology, prognosis, therapy and unmet medical
needs. Eur J Cancer. 71:53–69. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rollison DE, Giuliano AR and Becker JC:
New virus associated with merkel cell carcinoma development. J Natl
Compr Canc Netw. 8:874–880. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bichakjian CK, Olencki T, Alam M, Andersen
JS, Berg D, Bowen GM, Cheney RT, Daniels GA, Glass LF, Grekin RC,
et al: Merkel cell carcinoma, version 1.2014. J Natl Compr Canc
Netw. 12:410–424. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Becker J, Mauch C, Kortmann RD, Keilholz
U, Bootz F, Garbe C, Hauschild A and Moll I: Short german
guidelines: Merkel cell carcinoma. J Dtsch Dermatol Ges. 6 (Suppl
1):S15–S16. 2008.PubMed/NCBI View Article : Google Scholar : (In English,
German).
|
|
9
|
Doepker MP, Thompson ZJ, Fisher KJ,
Yamamoto M, Nethers KW, Harb JN, Applebaum MA, Gonzalez RJ, Sarnaik
AA, Messina JL, et al: Is a wider margin (2 cm vs. 1 cm) for a
1.01-2.0 mm melanoma necessary? Ann Surg Oncol. 23:2336–2342.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Strom T, Carr M, Zager JS, Naghavi A,
Smith FO, Cruse CW, Messina JL, Russell J, Rao NG, Fulp W, et al:
Radiation therapy is associated with improved outcomes in merkel
cell carcinoma. Ann Surg Oncol. 23:3572–3578. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bichakjian CK, Olencki T, Aasi SZ, Alam M,
Andersen JS, Blitzblau R, Bowen GM, Contreras CM, Daniels GA,
Decker R, et al: Merkel cell carcinoma, version 1.2018, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
16:742–774. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shaikh WR, Sobanko JF, Etzkorn JR, Shin TM
and Miller CJ: Utilization patterns and survival outcomes after
wide local excision or mohs micrographic surgery for merkel cell
carcinoma in the United States, 2004-2009. J Am Acad Dermatol.
78:175–177.e3. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Terushkin V, Brodland DG, Sharon DJ and
Zitelli JA: Mohs surgery for early-stage Merkel cell carcinoma
(MCC) achieves local control better than wide local excision +/-
radiation therapy with no increase in MCC-specific death. Int J
Dermatol. 60:1010–1012. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yan L, Sun L, Guan Z, Wei S, Wang Y and Li
P: Analysis of cutaneous merkel cell carcinoma outcomes after
different surgical interventions. J Am Acad Dermatol. 82:1422–1434.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Schwartz JL, Wong SL, Mclean SA, Hayman
JA, Lao CD, Kozlow JH, Malloy KM, Bradford CR, Frohm ML, Fullen DR,
et al: NCCN guidelines implementation in the multidisciplinary
Merkel cell carcinoma program at the university of Michigan. J Natl
Compr Canc Netw. 12:434–441. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tai P: A practical update of surgical
management of merkel cell carcinoma of the skin. ISRN Surg.
2013(850797)2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dellambra E, Carbone ML, Ricci F, Ricci F,
Di Pietro FR, Moretta G, Verkoskaia S, Feudi E, Failla CM, Abeni D
and Fania L: Merkel cell carcinoma. Biomedicines.
9(718)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Singh B, Qureshi MM, Minh TT and Sahni D:
Demographics and outcomes of stage I and II Merkel cell carcinoma
treated with Mohs micrographic surgery compared with wide local
excision in the National cancer database. J Am Acad Dermatol.
79:126–134.e3. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lebbe C, Becker JC, Grob JJ, Malvehy J,
Del Marmol V, Pehamberger H, Peris K, Saiag P, Middleton MR,
Bastholt L, et al: Diagnosis and treatment of Merkel cell
carcinoma. European consensus-based interdisciplinary guideline.
Eur J Cancer. 51:2396–2403. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kinas CG and Carroll BT: General
guidelines for quality assurance of immunohistochemistry in a Mohs
lab. Dermatol Surg. 43:507–511. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hughes MP, Hardee ME, Cornelius LA,
Hutchins LF, Becker JC and Gao L: Merkel cell carcinoma:
Epidemiology, target, and therapy. Curr Dermatol Rep. 3:46–53.
2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Garza-Davila VF, Valdespino-Valdes J,
Barrera FJ, Ocampo-Candiani J and Garza-Rodríguez V: Clinical
impact of immunotherapy in Merkel cell carcinoma patients: A
systematic review and meta-analysis. J Am Acad Dermatol.
87:121–130. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lee AY and Berman RS: The landmark series:
Non-melanoma skin cancers. Ann Surg Oncol. 27:22–27.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nghiem P, Bhatia S, Lipson EJ, Sharfman
WH, Kudchadkar RR, Brohl AS, Friedlander PA, Daud A, Kluger HM,
Reddy SA, et al: Durable tumor regression and overall survival in
patients with advanced Merkel cell carcinoma receiving
pembrolizumab as first-line therapy. J Clin Oncol. 37:693–702.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
D'Angelo SP, Bhatia S, Brohl AS, Hamid O,
Mehnert JM, Terheyden P, Shih KC, Brownell I, Lebbé C, Lewis KD, et
al: Avelumab in patients with previously treated metastatic Merkel
cell carcinoma: Long-term data and biomarker analyses from the
single-arm phase 2 JAVELIN Merkel 200 trial. J Immunother Cancer.
8(e000674)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Topalian SL, Bhatia S, Amin A, Kudchadkar
RR, Sharfman WH, Lebbé C, Delord JP, Dunn LA, Shinohara MM,
Kulikauskas R, et al: Neoadjuvant nivolumab for patients with
resectable merkel cell carcinoma in the checkmate 358 trial. J Clin
Oncol. 38:2476–2487. 2020.PubMed/NCBI View Article : Google Scholar
|