Introduction
Epithelioid hemangioendothelioma (EHE), also known
as low-grade anaplastic angiosarcoma, cellular hemangioma,
histiocytoid hemangioma and angioendothelioma, is a rare,
well-differentiated but locally aggressive endothelial tumor
(1). EHE occurs commonly in the
calvarium, spine, femur, tibia and feet, but is rare in the distal
radius (1,2). The therapeutic options vary owing to
the wide spectrum of the tumor behavior (1-3).
For benign-appearing lesions, curettage or marginal resection can
be sufficient, while for more aggressive tumors, wide resection or
even amputation is necessary.
With the progress in imaging and multimodality
therapy, en bloc resection has been widely accepted in treating
intermediate and malignant tumors involving the distal radius. In
the literature, the distal radius was totally sacrificed and the
patient's upper limb function was greatly impaired even if properly
reconstructed (4). Resection with
joint preservation can salvage the majority of wrist function but,
as a challenging procedure, it can also lead to an ultra-critical
sized bone defect which is technically demanding to reconstruct
(5,6). Previous reports regarding wrist joint
sparing surgery are rare (7,8). The
present study reported a case of wrist preservation surgery using a
3D-printed custom-made porous endoprosthesis after en bloc
resection of the EHE in the distal radius. 3D-printed custom-made
porous endoprosthesis is a novel uncemented implant that has
demonstrated great clinical success in the reconstruction of
extensive bone defects (9-11).
Due to its optimized-fit shape and porous surface, it was
considered a feasible alternative for the massive defect of the
distal radius to preserve the intact wrist.
Case presentation
A 14-year-old boy was referred to the Department of
Orthopedics, West China Hospital, Sichuan University in January
2019, complaining of dull pain accompanied with a mass in the right
forearm for a month. The radiographs showed expansive, osteolytic
and poorly demarcated lesions in the middle and distal part of the
right radius (Fig. 1A). Magnetic
resonance imaging (MRI) showed that the tumor did not involve the
growth plate (Fig. 1B and C). Tc-99 bone scans revealed increased
uptake in the forearm (Fig. 1D).
An open biopsy was performed. Immunohistochemistry was carried out
using the EnVision two-step method. Pathological tissues were fixed
using 4% paraformaldehyde solution for 8 h at room temperature. The
tissue blocks were embedded in paraffin wax and the wax sections
were 4 µm thick and blocked with PBS containing 5% goat serum for
30 min at room temperature. Primary antibodies: CD31 (cat. no.
ZM-0044), CD34 (cat. no. ZM-0046), ERG (cat. no. ZM-0103), D2-40
(cat. no. ZM-0469), CAMTA1 (cat. no. ZA-0535), EMA (cat. no.
ZM-0095) and WT-1 (cat. no. ZM-0269) were purchased from OriGene
Technologies, Inc. CR (cat. no. MAB-0716) and S-100 (cat. no.
RAB-0150) were purchased from Fuzhou Maixin Biotech Co., Ltd. The
primary antibodies were diluted according to the instructions
provided by the supplier and incubated overnight at 4˚C. The
polymorphic enzyme complexes (secondary antibody) were incubated
for 30 min at room temperature. Under the microscope the bone
tissue was infiltrated by small cells in the form of patches, some
of which had nuclear sulci with scattered heterogeneous mononuclear
and multinucleated giant cells with hemorrhagic necrosis. A number
of hemangioma-like proliferations were observed. The tumor cells
were epithelioid or long spindle-shaped, with obvious
heterogeneity. The nuclei varied in size and morphology; some were
densely stained and distorted and some were vesicular. Pathological
results [immunohistochemistry: CD31(+); CD34(+); ERG(+); D2-40(+);
CAMTA1(+); EMA(-); S100(-); WT-1(-); CR(-)] verified the diagnosis
of EHE (Fig. 1E). Following
multiple disciplinary team discussions and the wish of the patient,
segmental resection with 3D-printed custom-made porous
endoprosthesis reconstruction surgery was performed in February
2019.
Endoprosthesis design and
fabrication
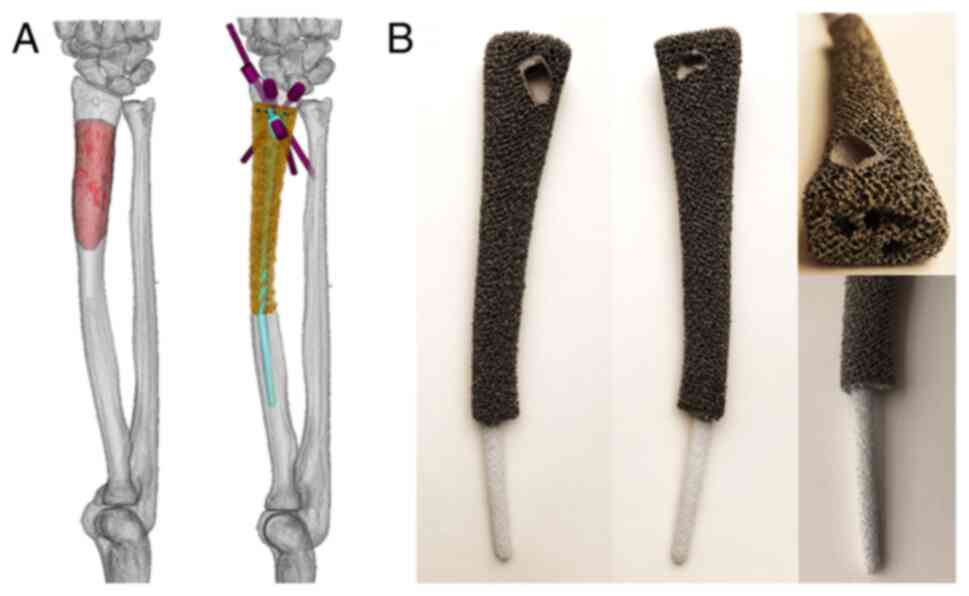
The clinical team designed the endoprosthesis which
was fabricated by Beijing Chunlizhengda Medical Instruments Co.,
Ltd. The main components of the custom-made device were shaft and
stem. For the first step, computerized tomography (CT) data was
used to build virtual 3D radius models in Mimics V20.0 software
(Materialise). The image fusion technique integrated MRI data to
build a virtual tumor model (Fig.
2A). Thereafter, the osteotomy plane was modified in accordance
with the surgical approach and tumor-free bone resection margin.
Subsequently, a preliminary endoprosthesis was generated and
modified to a more streamlined shape. To ensure satisfactory
fitting with the radius, the shape of the endoprosthesis was
optimized via computer simulation. Meanwhile, a longish length of
the implant over defect was produced to create a strain. Next, the
orientation of the screws was designed after considering the
surgical approach and the 3D space anatomical distribution to
obtain a convenient and durable fixation. Then four straight pores
were designed for the fixation using nonabsorbable suture (Ethibond
size 2; Johnson & Johnson, Ltd.) to the stump of the distal
radius in case the primary stability provided by screws was
unsatisfactory. The endoprosthesis was composed of an inside solid
shaft to ensure mechanical strength and outside porous structures
with a pore size of 600 µm and porosity of 70%. The medullary
cavity for the press-fit of the stem was measured with a diameter
of 6 mm in the distal and 4 mm in the proximal. The stem part was
designed as a tapered shape. The diameter was 5.5 mm in the base
and 3.5 mm in the tip.
The endoprosthesis was fabricated using the electron
beam melting technique (Arcam Q10plus; GE Additive). Thereafter,
the stem was coated with titanium and hydroxyapatite powder
(Fig. 2B).
Surgical techniques
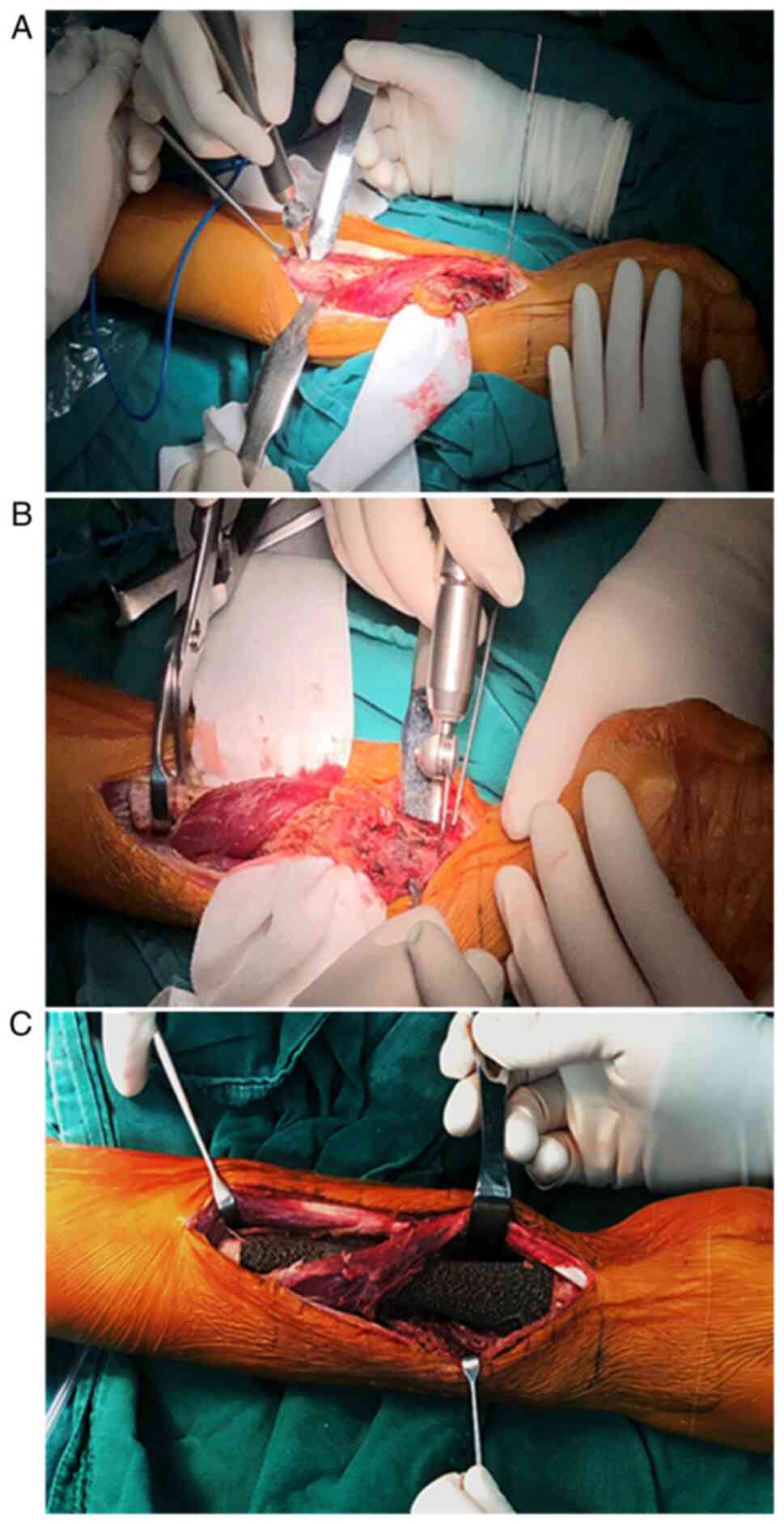
The senior surgeon (Chongqi Tu) performed the
surgery. The patient was placed in the supine position and a
tourniquet was placed in the right upper arm before the surgery. A
longitudinal dorsal approach was used to access the lesion. During
the procedure, the insertion of the pronator quadratus, the partial
origin of flexor pollicis longus and the intraosseous membrane were
released from the radius. According to the preoperative plan,
proximal osteotomy was performed in priority and then distal
osteotomy. The epiphysis was preserved successfully (Fig. 3A and B). Thereafter, the operation area was
soaked in 10% povidone-iodine and pulsatile lavage was performed
with isotonic sodium chloride solution to possibly reduce the
likelihood of wound infection. Using bone-holding clamps to
stabilize the proximal stump, the radial medulla was reamed to
press-fit the endoprosthesis stem. While inserting the stem, the
radial crest referred to avoid implant rotation. After the stem was
fixed appropriately within the canal, the reduction of the distal
end of the endoprosthesis proceeded. After confirming the right
locations of the proximal and distal parts of the endoprosthesis,
we then inserted a 3.5-mm-diameter screw to enhance the primary
stability (Fig. 3C). The released
soft tissues were then reattached to the endoprosthesis. Pulsatile
lavage with 10% povidone-iodine and another pulsatile lavage with
isotonic sodium chloride solution was performed again, with a drain
left in the right forearm thereafter. Resection margin was assessed
and reported by the pathology department of our institution.
Postoperative management
The patient underwent plain radiography and digital
tomosynthesis (Shimadzu metal artifact reduction technology) of the
right forearm postoperatively (Fig.
4A and B). During the first
two weeks postoperatively, the affected limb was immobilized in a
volar resting brace. The grip exercise was carried out from the
first day postoperatively and, at 2 weeks after the surgery,
dorsiflexion and palmar flexion were encouraged without
weight-bearing of the affected limb while immobilizing the wrist.
At 4 weeks postoperatively, the brace was removed and the patient
was allowed to rotate the forearm. The patient then gradually
increased the intensity of training and initiate weight-bearing on
the affected upper limb according to the patient's tolerance and
recovery progress. The patient was followed up monthly in the first
3 months, then trimonthly thereafter. Functional outcome was
assessed by range of motion (ROM), the 1993 version of the
Musculoskeletal Tumor Society (MSTS-93) score (12) (range 0 to 30; a higher score is
desirable), the disabilities of the arm, shoulder and hand (DASH)
questionnaire (13) (range 0 to
100; a lower score is desirable) and Mayo wrist score (14) (range 0 to 100; a higher score is
desirable). Osseointegration was evaluated by digital
tomosynthesis.
Results
Three months after the surgery, functional recovery
of the wrist was favorable. The patient showed a significantly
improved ROM. At the last follow-up at 27 months, wrist joint
function of the affected side was almost normal. The patient
achieved ROM of the affected wrist with active dorsiflexion to 90˚,
palmar flexion to 80˚. At the forearm, the patient had supination
of 90˚ and pronation of 85˚ (Fig.
5A-E). The patient can engage in daily activities such as
push-ups and arm wrestling with a healthy person (Fig. 5F and G). Functional scores were as follows: 95%
in MSTS-93 score, 8 in DASH and 90 in Mayo wrist score. Digital
tomosynthesis showed that the 3D-printed endoprosthesis was well
integrated with both proximal and distal radius. During the
follow-up, no complications were observed.
Discussion
Although the distal radius is not a rare site for
benign bone tumors such as giant cell tumors of bone, it is an
extremely uncommon skeletal site for EHE (1,15).
En bloc resection with reconstruction is needed when a tumor does
not appear benign (1). As a
growing demanding for limb salvage in favor of increasing
postoperative function, a number of reconstructive techniques,
including arthrodesis (16,17),
autograft (18,19), allograft (20,21)
and endoprosthesis (22,23), for massive defects following the en
bloc resection of the tumor in the distal radius have been
described.
Arthrodesis is still an option to salvage proper
grip strength and reduce long-term complications. Total wrist
arthrodesis has been demonstrated to restore 65% grip strength of
the affected wrist comparing to the healthy side (24). However, ROM sacrifice of the wrist
limits patients from performing daily activities and narrows its
application. Therefore, partial wrist arthrodesis was introduced to
result in less restriction of the ROM of the wrist, if the carpal
bones can be retained (16).
Nevertheless, the limited fusion contact area in partial wrist
arthrodesis requires prolonged immobilization to achieve bone
union. Generally, no matter total or partial joint arthrodesis, it
is associated with a high incidence of complications, such as
infection, fracture, delayed union and nonunion (17).
Wrist hemiarthroplasty can preserve improved
postoperative wrist function by providing a more flexible wrist
joint (25). Several materials can
be used for wrist hemiarthroplasty, including allografts,
autografts and endoprosthesis. The osteoarticular allograft offers
reasonable wrist-specific matching and favorable functional
outcomes without donor-site morbidity (26). However, complications including
fractures, nonunion and bony resorption, are not rare using this
technique (26-28).
The fibular head autografts have been demonstrated to have
considerable anatomical similarity to the distal radius (18). However, such a demanding procedure
is associated with wrist instability and inevitable drawbacks of
autograft itself (19,22). In addition, the preservation of
function is not as well as expected. Hemi-arthroplasty using
endoprosthesis is a viable alternative to salvage proper wrist
function (23,25). However, apart from conventional
endoprosthesis-related complications including infection and
aseptic loosening, subluxation is not uncommon with an incidence of
20% (22). The high subluxation
rate of hemiarthroplasty can both impair upper limb function and
cause a cosmetic problem; therefore, joint sparing surgeries
without access to the articular cavity are considered to have the
potential to avoid such disadvantages.
Resection with joint preservation is available when
the growth plate is not involved (29). The joint-sparing resection in
distal radius has only been reported in two case reports (7,8).
Free fibular shaft and devitalized autograft were utilized to
reconstruct the consequent intercalary bone defect. The
postoperative function was deemed good with active dorsiflexion
ranged from 85-90˚ and a palmar flexion ranged from 45-80˚ after a
follow-up duration ranged 14-41 months (20). Higuchi et al (7), using devitalized autograft, present
improved postoperative function with pronation and supination of
90˚, an MSTS score of 100%, a DASH score of 12.5 and a Toronto
Extremity Salvage Score (30) of
93.5. However, as aforementioned, free autograft has its
limitations and devitalized autograft can fail or fracture if the
tumors are osteolytic. Additionally, complete bone union time is
reported to 6 and 9 months, prolonging the duration to achieve
optimal function (14). In the
present case report, a 3D-printed custom-made endoprosthesis was
used to reconstruct the ultra-critical intercalary bone defect and,
consequently, close to normal wrist function after a relatively
short follow-up duration was observed.
Endoprosthetic reconstruction of ultra-critical bone
defect is demanding and prone to mechanical complications (11,31,32).
Currently, no endoprosthesis has been applied to reconstruct an
ultra-critical bone defect in radius. For the patient in the
present study, durable structural reconstruction, optimized
functional restoration and minimized complication incidence was
considered during the endoprosthesis design. First, the distal
interface was highly porous to promote the friction and prevent
migration of the endoprosthesis; second, the increase in
endoprosthesis length confirmed the soft tissue tension to stable
the implant and reserved partial space for ulna growth; third, the
screws went through the bone-implant interface of distal radius to
augment the primary stability and interface compression for further
osseointegration; fourth, the tapered stem with titanium and
hydroxyapatite coating could be press-fitted into the proximal
cavity to ensure fixation durability; finally, the inside solid
structure contributed to the avoidance of endoprosthesis fracture.
Apart from purposeful endoprosthesis design, an early and scheduled
rehabilitation program is also important for the favorable
functional recovery in this patient.
There were some limitations of the present study.
First, the follow-up duration was only 27 months.
Endoprosthetic-related complications might arise in a follow up for
longer periods. Second, the one case involved was insufficient to
verify the efficacy of this surgical techniques. More patients and
a larger multi-institutional study are needed to compare this
approach with other types of reconstruction. Third, finite element
analysis should be performed to optimize endoprosthesis design.
The present study presented a case using 3D-printed
endoprosthesis reconstruction for EHE of the distal radius. The
patient achieved a favorable functional outcome and no
complications were observed. The result suggested that the
3D-printed custom-made porous endoprosthesis may be a feasible
option and benefit selected patients.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported, in part, by the Technology
Research Program of Sichuan Province (grant no. 2020YFS0036), 1·3·5
project for disciplines of excellence, Clinical Research Incubation
Project, West China Hospital, Sichuan University (grant no.
2020HXFH004), Sichuan Science and Technology Innovation Team of
China (grant no. 2019JDTD0008), 1.3.5 project for disciplines of
excellence, West China Hospital, Sichuan University (grant no.
ZYJC18036).
Availability of data and materials
All data used or analyzed during this study are
included in the published article.
Authors' contributions
SW, YL, YZ and CT were involved with the conception
and design of the present study. SW, TG, YW and CZ were involved
with the acquisition of subjects and data. LM, YL, YZ and CT were
involved in the design of the endoprosthesis. TG and CT were
involved in the post-surgical evaluation of the patient. LM, YZ and
CT were involved with the writing and revision of the manuscript.
All authors have read and approved the manuscript. CT and YZ
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the West China
Hospital's Ethics Committee, Sichuan University (Chengdu) and was
permitted to be published. Written informed consent to have the
case details and accompanying images published was obtained from
the patient's parents.
Consent for publication
Written informed consent was obtained from the
patient and his parents for publication of this case report and
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gherman CD and Fodor D: Epithelioid
hemangioendothelioma of the forearm with radius involvement. Case
report. Diagn Pathol. 6(120)2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Castelli P, Caronno R, Piffaretti G and
Tozzi M: Epithelioid hemangioendothelioma of the radial artery. J
Vasc Surg. 41:151–154. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lai FM, Allen PW, Yuen PM and Leung PC:
Locally metastasizing vascular tumor. Spindle cell, epithelioid, or
unclassified hemangioendothelioma? Am J Clin Pathol. 96:660–663.
1991.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhang S, Xu MT, Wang XQ and Wang JJ:
Functional outcome of en bloc excision and custom prosthetic
replacement for giant cell tumor of the distal radius. J Orthop
Sci. 20:1090–1097. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tsuchiya H, Abdel-Wanis M, Kitano S,
Sakurakichi K, Yamashiro T and Tomita K: The natural limb is best:
Joint preservation and reconstruction by distraction osteogenesis
for high-grade juxta-articular osteosarcomas. Anticancer Res.
22:2373–2376. 2002.PubMed/NCBI
|
|
6
|
Nauth A, McKee MD, Einhorn TA, Watson JT,
Li R and Schemitsch EH: Managing bone defects. J Orthop Trauma.
25:462–466. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Higuchi T, Yamamoto N, Hayashi K, Takeuchi
A, Abe K, Taniguchi Y, Araki Y, Tada K and Tsuchiya H: Successful
joint preservation of distal radius osteosarcoma by en bloc tumor
excision and reconstruction using a tumor bearing frozen autograft:
A case report. BMC Surg. 18(12)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yu X, Xu S, Xu M and Yuan Y: Osteosarcoma
of the distal radius treated by en bloc resection and
reconstruction with a fibular shaft preserving the radiocarpal
joint: A case report. Oncol Lett. 7:1503–1506. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lu M, Min L, Xiao C, Li Y, Luo Y, Zhou Y,
Zhang W and Tu C: Uncemented three-dimensional-printed prosthetic
replacement for giant cell tumor of distal radius: A new design of
prosthesis and surgical techniques. Cancer Manag Res. 10:265–277.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang Y, Min L, Lu M, Zhou Y, Wang J, Zhang
Y, Yu X, Tang F, Luo Y, Duan H and Tu C: The functional outcomes
and complications of different reconstruction methods for giant
cell tumor of the distal radius: Comparison of osteoarticular
allograft and three-dimensional-printed prosthesis. BMC
Musculoskelet Disord. 21(69)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhao D, Tang F, Min L, Lu M, Wang J, Zhang
Y, Zhao K, Zhou Y, Luo Y and Tu C: Intercalary reconstruction of
the ‘ultra-critical sized bone defect’ by 3D-printed porous
prosthesis after resection of tibial malignant tumor. Cancer Manag
Res. 12:2503–2512. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Enneking WF, Dunham W, Gebhardt MC,
Malawar M and Pritchard DJ: A system for the functional evaluation
of reconstructive procedures after surgical treatment of tumors of
the musculoskeletal system. Clin Orthop Relat Res. 241–246.
1993.PubMed/NCBI
|
|
13
|
Hudak PL, Amadio PC and Bombardier C:
Development of an upper extremity outcome measure: The DASH
(disabilities of the arm, shoulder and hand) [corrected]. The upper
extremity collaborative group (UECG). Am J Ind Med. 29:602–608.
1996.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cooney WP, Bussey R, Dobyns JH and
Linscheid RL: Difficult wrist fractures. Perilunate
fracture-dislocations of the wrist. Clin Orthop Relat Res. 136–147.
1987.PubMed/NCBI
|
|
15
|
Harness NG and Mankin HJ: Giant-cell tumor
of the distal forearm. J Hand Surg Am. 29:188–193. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhu Z, Zhang C, Zhao S, Dong Y and Zeng B:
Partial wrist arthrodesis versus arthroplasty for distal radius
giant cell tumours. Int Orthop. 37:2217–2223. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zachary SV and Stern PJ: Complications
following AO/ASIF wrist arthrodesis. J Hand Surg Am. 20:339–344.
1995.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Qi DW, Wang P, Ye ZM, Yu XC, Hu YC, Zhang
GC, Yan XB, Zheng K, Zhao LM and Zhang HL: Clinical and
radiographic results of reconstruction with fibular autograft for
distal radius giant cell tumor. Orthop Surg. 8:196–204.
2016.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Humail SM, Ghulam MK and Zaidi IH:
Reconstruction of the distal radius with non-vascularised fibular
graft after resection of giant cell tumour of bone. J Orthop Surg
(Hong Kong). 22:356–359. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lans J, Ballatori SE, Castelein RM, Chen
NC and Lozano Calderon SA: Osteoarticular allograft reconstruction
after distal radius tumor resection: Reoperation and patient
reported outcomes. J Surg Oncol. 123:1304–1315. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
van Isacker T, Barbier O, Traore A, Cornu
O, Mazzeo F and Delloye C: Forearm reconstruction with bone
allograft following tumor excision: A series of 10 patients with a
mean follow-up of 10 years. Orthop Traumatol Surg Res. 97:793–799.
2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang B, Wu Q, Liu J, Chen S, Zhang Z and
Shao Z: What are the functional results, complications, and
outcomes of using a custom unipolar wrist hemiarthroplasty for
treatment of grade III giant cell tumors of the distal radius? Clin
Orthop Relat Res. 474:2583–2590. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Khattak MJ, Umer M and Haroon-ur-Rasheed
and Umar M: Autoclaved tumor bone for reconstruction: An
alternative in developing countries. Clin Orthop Relat Res.
447:138–144. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bhagat S, Bansal M, Jandhyala R, Sharma H,
Amin P and Pandit JP: Wide excision and ulno-carpal arthrodesis for
primary aggressive and recurrent giant cell tumours. Int Orthop.
32:741–745. 2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu W, Wang B, Zhang S, Li Y, Hu B and
Shao Z: Wrist reconstruction after en bloc resection of bone tumors
of the distal radius. Orthop Surg. 13:376–383. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Duan H, Zhang B, Yang HS, Liu YH, Zhang
WL, Min L, Tu CQ and Pei FX: Functional outcome of en bloc
resection and osteoarticular allograft reconstruction with locking
compression plate for giant cell tumor of the distal radius. J
Orthop Sci. 18:599–604. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kocher MS, Gebhardt MC and Mankin HJ:
Reconstruction of the distal aspect of the radius with use of an
osteoarticular allograft after excision of a skeletal tumor. J Bone
Joint Surg Am. 80:407–419. 1998.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Asavamongkolkul A, Waikakul S,
Phimolsarnti R and Kiatisevi P: Functional outcome following
excision of a tumour and reconstruction of the distal radius. Int
Orthop. 33:203–209. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
San-Julian M, Aquerreta JD, Benito A and
Cañadell J: Indications for epiphyseal preservation in metaphyseal
malignant bone tumors of children: Relationship between image
methods and histological findings. J Pediatr Orthop. 19:543–548.
1999.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Davis AM, Wright JG, Williams JI,
Bombardier C, Griffin A and Bell RS: Development of a measure of
physical function for patients with bone and soft tissue sarcoma.
Qual Life Res. 5:508–516. 1996.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ahlmann ER and Menendez LR: Intercalary
endoprosthetic reconstruction for diaphyseal bone tumours. J Bone
Joint Surg Br. 88:1487–1491. 2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sewell MD, Hanna SA, McGrath A, Aston WJ,
Blunn GW, Pollock RC, Skinner JA, Cannon SR and Briggs TW:
Intercalary diaphyseal endoprosthetic reconstruction for malignant
tibial bone tumours. J Bone Joint Surg Br. 93:1111–1117.
2011.PubMed/NCBI View Article : Google Scholar
|