Introduction
Ischaemic stroke is one of the main causes of
disability and morbidity worldwide (1). Multiple mechanisms, including
inflammation, oxidative stress and excitotoxicity, are considered
to be associated with cerebral ischaemic injury (2). Among them, inflammation plays an
important role in the pathogenesis of ischaemic stroke.
Previous studies have revealed that inflammation
mediated by inflammasomes is an important mechanism for secondary
neuronal injury in ischaemic strokes (3,4). An
important family of inflammasomes are the NOD-like receptors (NLR),
represented by NOD-like receptor protein (NLRP)1 and NLRP3(5). Inflammasomes were first discovered to
be multi-protein complexes in 2002 (5-7).
The NLRP1 inflammasome, which is abundant in the brain, is the
first member of the NLR family (6).
It has been shown that overexpression of microRNA
(miR)-9a-5p ameliorates NLRP1 inflammasome-mediated ischaemic
injury (7). Inhibition of the
NLRP1 inflammasome has been reported to ameliorate the inflammatory
response to cells and animal models (8-12).
In studies of stroke it has been reported that activation of NLRP1
inflammasome can lead to neuronal cell death and behavioural
deficits (13). All of these
studies imply that the NLRP1 inflammasome plays an important role
in the process of ischaemic injury and may be a potential target
for the treatment of ischaemic strokes.
Ligustroflavone (LIG) is an active compound derived
from Ligustrum lucidum (14,15)
that has a variety of pharmacological activities (16-19).
Previous research demonstrated that LIG reduces necroptosis in rat
brain after ischaemic strokes by targeting the receptor-interacting
serine/threonine-protein kinase (RIPK)1/RIPK3/mixed lineage kinase
domain-like pseudokinase pathway (20). Whether LIG could act through NLRP1
in MCAO model will be investigated in the present study.
Materials and methods
Mouse models
A total of 48 specific-pathogen-free male C57BL/6
mice (6 weeks old, 20-22 g) were supplied by Beijing Vital River
Laboratory Animal Technology Co. Ltd. All of the animals were
housed in specific pathogen-free conditions with standard
temperature (22±1˚C), humidity (50-60%) and light conditions (12 h
light/dark cycle), with access to food and water ad libitum.
The animal studies were conducted in accordance with the Ethics
Committee of Medical College of Xi'an Peihua University (approval
no. PH202107; Xi'an, China).
Ischemic strokes animal model
establishments and drug administration
Middle cerebral artery occlusion (MCAO) surgery was
performed as previously described (21). In brief, the randomly selected mice
were anesthetized with 2% isoflurane mixed with oxygen and
nitrogen. The right common carotid artery was clipped with an
artery clamp, and the external carotid artery (ECA) was ligatured.
A nylon suture with a blunted tip (0.40-mm diameter) was gently
advanced from a tiny incision in the ECA to the internal carotid
artery. The filament was left in place to cause an obstruction for
60 min and then removed for the reperfusion. A constant-temperature
blanket was used to keep the mice body temperature at 37±1˚C until
the mice recovered from surgery.
Mice were randomly assigned to four groups (n=6 per
group) according to the different operation processes and drug
administration. The sham group was subjected to the same operation
with the exception that no nylon suture was inserted. The trial
group received 30 mg/kg LIG [MedChemExpress; intragastrically
(i.g)]. The dose of LIG applied was based on previous literature
(20). The MCAO group was
subjected to 1 h ischaemia plus 24 h reperfusion. The MCAO + LIG
group received the LIG (30 mg/kg; i.g.) 15 min before the
operation.
After experiment completion, mice were sacrificed by
exsanguination under deep anaesthesia (sodium pentobarbital
intraperitoneal injection, 50 mg/kg). Mice death was confirmed by
lack of heart beat.
Neurobehavioral evaluation
The modified neurological severity scoring (mNSS)
trial consists of ten different tasks that can evaluate the motor
(muscle status, abnormal movement), sensory (visual, tactile and
proprioceptive), balance, and reflex functions of mice (22). Neurological deficits were graded
from 0 to 18 (0=normal function; 18=maximal deficit). One point was
scored for each abnormal behaviour or for the lack of a tested
reflex. Therefore, higher scores imply greater neurological injury.
An open-field trial based on the pattern of exploration (centre vs.
periphery) was used to assess anxiety-like behaviour. Mice were
tracked under moderate lighting for 15 min in a 40-cm2
open field using software (ANY-Maze; Stoelting Co.). General
activity was assessed by fixing the total distance travelled. A
Rotarod trial was used to assess motor coordination and learning.
On the day of testing, mice were given four 300-sec accelerating
Rotarod tests with an inter-trial interval of 30 min. The average
latency to the first fall from the rod was recorded. All
experimenters were blinded to the four different test groups of
mice.
Hoechst 33258 staining
The brain sections were prepared, and 4-µm sections
were stained with Hoechst 33258 (Beyotime Institute of
Biotechnology). Brain tissues were fixed in 4% paraformaldehyde
overnight at 4˚C and embedded in paraffin. The paraffin sections
were incubated with 0.5 ml Hoechst 33258 solution for 5 min at room
temperature and washed twice with PBS. The cells were imaged and
counted manually using fluorescence microscopy (CX21; Olympus
Corporation).
Western blotting
Brain tissues were homogenized in RIPA lysis buffer
(cat. no. R0278; Thermo Fisher Scientific, Inc.) comprising 150 mM
NaCl, 1.0% IGEPAL® CA-630, 0.5% Sodium Deoxycholate,
0.1% SDS and 50 mM Tris. The samples were lysed on ice for 30 min,
and then centrifuged at 12,000 x g at 4˚C for 10 min. Protein
concentration was quantified using a BCA assay kit (cat. no. 23227;
Pierce; Thermo Fisher Scientific, Inc.). Proteins (30 µg per lane)
were separated by 12% electrophoresis and then transferred to PVDF
membrane (cat. no. 05317; MilliporeSigma). After blocking with 5%
defatted milk diluted with TBST buffer (0.1% tween) for 1 h at room
temperature, membranes were incubated overnight at 4˚C with the
following primary antibodies against: Bax (1:1,000; cat. no.
ab32503; Abcam), Bcl-2 (1:800; cat. no. ab182858; Abcam), caspase-3
(1:200; cat. no. ab184787; Abcam), NLRP1 (1:500; cat. no.
NBP1-97593IR; Novus Biologicals), apoptosis-associated speck-like
protein containing a CARD (ASC; 1:500; cat. no. ab175449; Abcam),
caspase-1 (1:800; cat. no. ab138483; Abcam), IL-1β (1:500; cat. no.
ab283818; Abcam), IL-18 (1:800; cat. no. ab191860; Abcam), IL-6
(1:500; cat. no. ab259341; Abcam), TNF-α (1:800; cat. no. ab215188;
Abcam) and β-actin (1:2,000; cat. no. MA5-15739; Invitrogen; Thermo
Fisher Scientific, Inc.) were used. On the following day, the PVDF
membranes were incubated with HRP-conjugated secondary antibodies
(1:10,000; cat. no. 31461; Invitrogen; Thermo Fisher Scientific,
Inc.) for 1 h at room temperature. Subsequently membranes were
incubated with ECL reagents (cat. no. WBULS0100; MilliporeSigma)
and bands visualized using a western blot detection system (Bio-Rad
Laboratories, Inc.). The protein quantities were analysed using
ImageJ software (version 1.8.0.112; National Institutes of
Health).
Reverse transcription-quantitative
PCR
Total RNA was extracted from brain tissues using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. cDNA was
synthesized using a PrimeScript™ II 1st Strand cDNA
Synthesis kit (Takara Biotechnology Co., Ltd.) according to the
manufacturer's protocol. RT-qPCR were carried out with a SuperReal
Premix Plus kit (Vazyme Biotech Co., Ltd.). The thermocycling
conditions for qPCR were as follows: 15 min at 95˚C to activate the
chemically modified hot-start Taq DNA polymerase, followed by 40
cycles of duration for 15 sec at 95˚C and 30 sec of annealing and
extension at 60˚C. The specific primer sequences used were: NLRP1
forward, 5'-TGGCACATCCTAGGGAAATC-3' and reverse,
5'-TCCTCACGTGACAGCAGAAC-3'); ASC forward,
5'-GTCACAGAAGTGGACGGAGTG-3' and reverse,
5'-CTCATCTTGTCTTGGCTGGTG-3'; Caspase-1 forward,
5'-GACTGGGACCCTCAAGTTTT-3' and reverse, 5'-CCAGCAGCAACTTCATTTCT-3';
β-actin forward, 5'-TCAGCAAGCAGGAGTACGATG-3' and reverse,
5'-AACAGTCCGCCTAGAAGCACTT-3'. β-actin was amplified as the internal
control. The original Cq values of the sample were adjusted to
internal control and relative transcript levels were analysed by
2-ΔΔCq method (23).
Enzyme-linked immunosorbent assay
(ELISA)
Protein samples were extracted from brain tissues
and the concentration determined using a BCA assay kit (Pierce;
Thermo Fisher Scientific, Inc.). The levels of inflammatory
cytokines IL-1β (cat. no. ab197742), IL-18 (cat. no. ab216165),
IL-6 (cat. no. ab222503) and TNF-α (cat. no. ab208348) were
assessed using commercial ELISA kits (Abcam) according to the
manufacturer's protocol.
Statistical analysis
All data were analysed with the SPSS statistical
software (version 18.0; SPSS, Inc.). The comparisons of two groups
were analysed using unpaired Student's t-test. The two factor
experiments and comparisons of multiple groups were analysed by
one-way ANOVA followed by Tukey's test. Data are expressed as mean
± SD (unless otherwise shown). P<0.05 was considered to indicate
a statistically significant difference.
Results
LIG plays a neuroprotective role in
MCAO mice
Neurological tests were performed to assess the
effects of LIG on the neurological functional impairment of MCAO
model mice. In the mNSS trial, the mNSS scores of MCAO group mice
were significantly higher compared with those of the sham group.
Treatment with LIG decreased the increase of mNSS scores induced by
MCAO (Fig. 1A). The residence time
of mice in MCAO group on the Rotarod was significantly reduced
compared with the sham group. Whereas treatment with LIG/MCAO
significantly increased the residence time on the Rotarod compared
with the MCAO group (Fig. 1B). In
the open-field behavioural task trail, mice in MCAO group
demonstrated a decrease in the perimeter zone and total travel
distance compared with the sham group. However, LIG treatment
significantly increased the perimeter zone and total travel
distance (Fig. 1C). All together,
these data implied that LIG may mitigate MCAO-induced neurological
deficits.
LIG mitigates neuronal injury in MCAO
mice
The effects of LIG on MCAO-induced neuronal damage
were also investigated. Compared with the sham group, the number of
apoptotic neurons was significantly increased in the MCAO group.
However, treatment with LIG decreased the percentage of neuronal
apoptosis induced by MCAO (Fig. 2A
and B). Relative to the sham
group, the Bcl-2/Bax ratio was decreased in the MCAO group,
although levels of caspase-3 and Bax were raised. These changes in
the MCAO group could be inhibited by treatment with LIG (Fig. 2C and D). All these data suggested that LIG may
prevent neuronal injury in MCAO mice.
LIG restrains the activation of NLRP1
inflammasome in MCAO mice
To determine the effects of LIG on the activation of
the NLRP1 inflammasome, the expression levels of the inflammasome
complex were assessed. NLRP1, ASC and caspase-1 protein and mRNA in
the MCAO model mice were significantly increased relative to the
sham group (Fig. 3). Treatment
with LIG significantly prevented the upregulation of the expression
levels of NLRP1, ASC and caspase-1 induced by MCAO (Fig. 3A-C). Moreover, the increase in mRNA
levels of NLRP1, ASC and caspase-1 in MCAO model mice were
significantly inhibited by treatment with LIG, and these were
consistent with protein expression levels (Fig. 3D-F). These results indicated that
the NLRP1 inflammasome was activated in the MCAO model, and LIG may
prevent the activation of NLRP1 inflammasome.
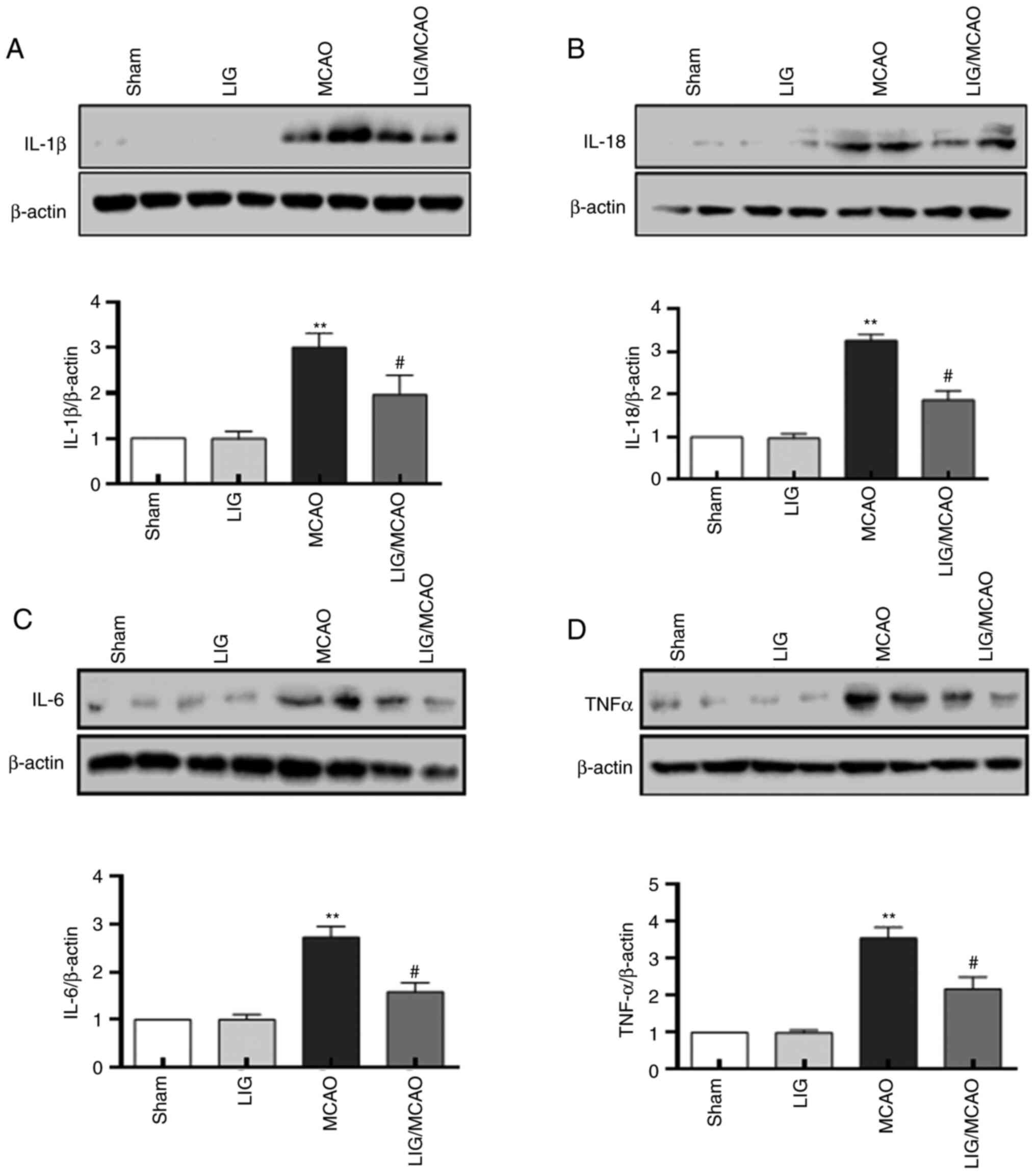
LIG reduces the levels of inflammatory
cytokines in MCAO mice
The effects of LIG on the pro-inflammatory cytokines
in MCAO model mice were further investigated. The protein levels of
IL-1β, IL-18, IL-6 and TNFα were first detected using western
blotting. The expression levels of IL-1β, IL-18, IL-6 and TNF-α
were significantly higher in the MCAO mouse group compared with the
sham group, but LIG significantly attenuated this effect (Fig. 4). The expression levels of IL-1β,
IL-18, IL-6 and TNF-α were detected using an ELISA kit, and were
consistent with the western blotting results (Fig. 5). These data suggested that NLRP1
inflammasome-mediated pro-inflammatory cytokines in MCAO mice may
be inhibited by treatment with LIG.
Discussion
To the best of our knowledge, the present study was
the first to provide evidence that LIG may protect the brain from
ischaemic stroke via a mechanism involving NLRP1. The results
demonstrated that neurological dysfunction and neuronal damage were
associated with MCAO treatment, along with elevated levels of NLRP1
inflammasome complexes (NLRP1, ASC and caspase-1) and inflammatory
cytokines (IL-1β, IL-18, IL-6 and TNF-α). Furthermore,
administration of LIG after cerebral ischaemia protected against
neuronal damage, improved functional recovery and inhibited
elevation in levels of NLRP1 inflammasome complexes and
inflammatory cytokines.
MCAO is widely recognized as an experimental animal
model for ischaemic injury. Previous studies have shown that MCAO
mice show neuronal damage followed by neurological impairment
(24,25). LIG has previously been shown to
give neuroprotection in vivo (20). The present study data revealed that
LIG protected against MCAO-induced neuronal damage and neurological
dysfunction. LIG minimized the neurological deficits and reduced
the neuronal damage of MCAO mice. These results indicated that LIG
exerts a neuroprotective effect in MCAO mice.
It is well recognized that neuroinflammation is
involved in the pathogenesis of ischaemic stroke (26). Studies have shown that inhibiting
the activation of NLRP1 inflammasomes following ischaemic stroke
can regulate the proinflammatory cytokines and play a
neuroprotective effect (13,27,28).
Herein, the present study demonstrated that the neuroprotective
effects of LIG were associated with a significant reduction in the
levels of NLRP1 inflammasome proteins in an MCAO model of ischaemic
stroke.
In view of the inflammasome as an important mediator
of inflammation, the targeted therapies for the inflammasome
provide potential treatment methods for cerebral ischaemia
(29). These targeted therapies
include: Signal transduction pathways (such as NF-κB and MAPK)
(30); inflammasome components
(such as NLRPs, ASC and caspase-1) (31,32);
secondary messengers (such as reactive oxygen species) and
cytokines (IL-1β and IL-18) (33).
A previous study demonstrated that intracerebroventricular
injection of antibodies to the NLRP1 receptor can penetrate the
blood-cerebrospinal fluid barrier to interfere with the assembly of
NLRP1 in neurons, thereby reducing the activation of caspase-1,
inhibiting the maturation of IL-1β and IL-18 and reducing the
infarction area of the mouse cerebral ischaemia model (27,34).
NLRP1 inflammasomes increase the production and secretion of
inflammatory factors IL-1β and IL-18 precursors through a series of
mechanisms to mediate the death of ischemic neurons (35). IL-1β is involved in the death of
neurons through oxidative stress in ischemic stroke (36). However, recent studies have
revealed that IL-1β binds to the IL-1 receptor 1 (IL-1R1)
expressing neurons during cerebral ischaemia which was harmful to
damaged brain tissue (37,38). Another study revealed that the
neuroprotective effect of IL-1β is associated with its
concentration and the reaction time after ischaemic stroke
(39). The increased production of
IL-18 in neurons promotes macrophages to produce pro-inflammatory
factors (such as TNF-α and IL-6) and neurotoxic mediators by
causing the up-regulation of IFN-γ, which leads to serious nerve
tissue damage (40).
In conclusion, the results of the present study
revealed that the expression levels of NLRP1, ASC and caspase-1
were upregulated in the MCAO model mice and were inhibited by
treatment with LIG. Furthermore, the expression levels of
pro-inflammatory cytokines, IL-1β, IL-18, IL-6 and TNF-α in MCAO
model mice were also inhibited by LIG. These data indicated that
the neuroprotective effects of LIG may be associated with the
inhibitory effects of LIG on the NLRP1 inflammasome-mediated
inflammatory processes, although the precise mechanism requires
further investigation.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by a grant from the Natural
Science Foundation of Shaanxi Provincial Department of Education
(grant no. 21JK0820), the Scientific Research Project of Xi'an
Peihua University (grant no. PHKT2101) and Department of Science
and Technology of Shaanxi Province (grant no. 2022JQ-876).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FB and YB carried out the experimental work and
performed the data collection and interpretation. FB and YZ
participated in the design and coordination of experimental work,
and acquisition of data. YB and WL carried out the study design,
the analysis, and interpretation of data and drafted the
manuscript. FB and WL confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Medical College of Xi'an Peihua University
(approval no. PH202107).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bu ZQ, Yu HY, Wang J, He X, Cui YR, Feng
JC and Feng J: Emerging role of ferroptosis in the pathogenesis of
ischemic stroke: A new therapeutic target? ASN Neuro.
13(17590914211037505)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Khoshnam SE, Winlow W, Farzaneh M, Farbood
Y and Moghaddam HF: Pathogenic mechanisms following ischemic
stroke. Neurol Sci. 38:1167–1186. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chen YJ, Nguyen HM, Maezawa I, Grössinger
EM, Garing AL, Köhler R, Jin LW and Wulff H: The potassium channel
KCa3.1 constitutes a pharmacological target for neuroinflammation
associated with ischemia/reperfusion stroke. J Cereb Blood Flow
Metab. 36:2146–2161. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yang L, Tang J, Chen Q, Jiang B, Zhang B,
Tao Y, Li L, Chen Z and Zhu G: Hyperbaric oxygen preconditioning
attenuates neuroinflammation after intracerebral hemorrhage in rats
by regulating microglia characteristics. Brain Res. 1627:21–30.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Latz E, Xiao TS and Stutz A: Activation
and regulation of the inflammasomes. Nat Rev Immunol. 13:397–411.
2013.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Kummer JA, Broekhuizen R, Everett H,
Agostini L, Kuijk L, Martinon F, van Bruggen R and Tschopp J:
Inflammasome components NALP 1 and 3 show distinct but separate
expression profiles in human tissues suggesting a site-specific
role in the inflammatory response. J Histochem Cytochem.
55:443–452. 2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cao Y, Zhang H, Lu X, Wang J, Zhang X, Sun
S, Bao Z, Tian W, Ning S, Wang L and Cui L: Overexpression of
MicroRNA-9a-5p ameliorates NLRP1 inflammasome-mediated ischemic
injury in rats following ischemic stroke. Neuroscience.
444:106–117. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Brickler T, Gresham K, Meza A,
Coutermarsh-Ott S, Williams TM, Rothschild DE, Allen IC and Theus
MH: Nonessential role for the NLRP1 inflammasome complex in a
murine model of traumatic brain injury. Mediators Inflamm.
2016(6373506)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hu W, Zhang Y, Wu W, Yin Y, Huang D, Wang
Y and Li W and Li W: Chronic glucocorticoids exposure enhances
neurodegeneration in the frontal cortex and hippocampus via NLRP-1
inflammasome activation in male mice. Brain Behav Immun. 52:58–70.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cribbs DH, Berchtold NC, Perreau V,
Coleman PD, Rogers J, Tenner AJ and Cotman CW: Extensive innate
immune gene activation accompanies brain aging, increasing
vulnerability to cognitive decline and neurodegeneration: A
microarray study. J Neuroinflammation. 9(179)2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tan MS, Tan L, Jiang T, Zhu XC, Wang HF,
Jia CD and Yu JT: Amyloid-β induces NLRP1-dependent neuronal
pyroptosis in models of Alzheimer's disease. Cell Death Dis.
5(e1382)2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Qiao C, Zhang Q, Jiang Q, Zhang T, Chen M,
Fan Y, Ding J, Lu M and Hu G: Inhibition of the hepatic Nlrp3
protects dopaminergic neurons via attenuating systemic inflammation
in a MPTP/p mouse model of Parkinson's disease. J
Neuroinflammation. 15(193)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fann DY, Lim YA, Cheng YL, Lok KZ,
Chunduri P, Baik SH, Drummond GR, Dheen ST, Sobey CG, Jo DG, et al:
Evidence that NF-κB and MAPK signaling promotes NLRP inflammasome
activation in neurons following ischemic stroke. Mol Neurobiol.
55:1082–1096. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pieroni A and Pachaly P: Isolation and
structure elucidation of ligustroflavone, a new apigenin
triglycoside from the leaves of Ligustrum vulgare L. Pharmazie.
55:78–80. 2000.PubMed/NCBI
|
|
15
|
Pieroni A, Pachaly P, Huang Y, Van Poel B
and Vlietinck AJ: Studies on anti-complementary activity of
extracts and isolated flavones from Ligustrum vulgare and Phillyrea
latifolia leaves (Oleaceae). J Ethnopharmacol. 70:213–217.
2000.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang H, Xing WW, Li YS, Zhu Z, Wu JZ,
Zhang QY, Zhang W and Qin LP: Effects of a traditional Chinese
herbal preparation on osteoblasts and osteoclasts. Maturitas.
61:334–339. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Siu WS, Wong HL, Lau CP, Shum WT, Wong CW,
Gao S, Fung KP, Lau CB, Hung LK, Ko CH and Leung PC: The effects of
an antiosteoporosis herbal formula containing epimedii herba,
ligustri lucidi fructus and psoraleae fructus on density and
structure of rat long bones under tail-suspension, and its
mechanisms of action. Phytother Res. 27:484–492. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Zhang Y, Diao TY, Wang L, Che CT and Wong
MS: Protective effects of water fraction of fructus ligustri lucidi
extract against hypercalciuria and trabecular bone deterioration in
experimentally type 1 diabetic mice. J Ethnopharmacol. 158:239–245.
2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sha NN, Zhao YJ, Zhao DF, Mok DK, Shi Q,
Wang YJ and Zhang Y: Effect of the water fraction isolated from
fructus ligustri lucidi extract on bone metabolism via antagonizing
a calcium-sensing receptor in experimental type 1 diabetic rats.
Food Funct. 8:4703–4712. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang YY, Liu WN, Li YQ, Zhang XJ, Yang J,
Luo XJ and Peng J: Ligustroflavone reduces necroptosis in rat brain
after ischemic stroke through targeting RIPK1/RIPK3/MLKL pathway.
Naunyn Schmiedebergs Arch Pharmacol. 392:1085–1095. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liu Y, Lü L, Hettinger CL, Dong G, Zhang
D, Rezvani K, Wang X and Wang H: Ubiquilin-1 protects cells from
oxidative stress and ischemic stroke caused tissue injury in mice.
J Neurosci. 34:2813–2821. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu W, Chen Y, Meng J, Wu M, Bi F, Chang
C, Li H and Zhang L: Ablation of caspase-1 protects against
TBI-induced pyroptosis in vitro and in vivo. J Neuroinflammation.
15(48)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cai G, Cai G, Zhou H, Zhuang Z, Liu K, Pei
S, Wang Y, Wang H, Wang X, Xu S, et al: Mesenchymal stem
cell-derived exosome miR-542-3p suppresses inflammation and
prevents cerebral infarction. Stem Cell Res Ther.
12(2)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu F, Schafer DP and McCullough LD: TTC,
fluoro-Jade B and NeuN staining confirm evolving phases of
infarction induced by middle cerebral artery occlusion. J Neurosci
Methods. 179:1–8. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rajkovic O, Potjewyd G and Pinteaux E:
Regenerative medicine therapies for targeting neuroinflammation
after stroke. Front Neurol. 9(734)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Abulafia DP, de Rivero Vaccari JP, Lozano
JD, Lotocki G, Keane RW and Dietrich WD: Inhibition of the
inflammasome complex reduces the inflammatory response after
thromboembolic stroke in mice. J Cereb Blood Flow Metab.
29:534–544. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Singhal G, Jaehne EJ, Corrigan F, Toben C
and Baune BT: Inflammasomes in neuroinflammation and changes in
brain function: A focused review. Front Neurosci.
8(315)2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liu L and Chan C: The role of inflammasome
in Alzheimer's disease. Ageing Res Rev. 15:6–15. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dinarello CA: The IL-1 family and
inflammatory diseases. Clin Exp Rheumatol. 20 (5 Suppl 27):S1–S13.
2002.PubMed/NCBI
|
|
31
|
Fink SL and Cookson BT:
Caspase-1-dependent pore formation during pyroptosis leads to
osmotic lysis of infected host macrophages. Cell Microbiol.
8:1812–1825. 2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mariathasan S, Newton K, Monack DM, Vucic
D, French DM, Lee WP, Roose-Girma M, Erickson S and Dixit VM:
Differential activation of the inflammasome by caspase-1 adaptors
ASC and Ipaf. Nature. 430:213–218. 2004.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang R, Xu M, Wang Y, Xie F, Zhang G and
Qin X: Nrf2-a promising therapeutic target for defensing against
oxidative stress in stroke. Mol Neurobiol. 54:6006–6017.
2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Alomar SY, Gentili A, Zaibi MS, Kępczyńska
MA and Trayhurn P: IL-1β (interleukin-1β) stimulates the production
and release of multiple cytokines and chemokines by human
preadipocytes. Arch Physiol Biochem. 122:117–122. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ma Z, Li K, Chen P, Pan J, Li X and Zhao
G: Propofol attenuates inflammatory damage via inhibiting
NLRP1-Casp1-Casp6 signaling in ischemic brain injury. Biol Pharm
Bull. 43:1481–1489. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ma H, Su D, Wang Q, Chong Z, Zhu Q, He W
and Wang W: Phoenixin 14 inhibits ischemia/reperfusion-induced
cytotoxicity in microglia. Arch Biochem Biophys.
689(108411)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Franke M, Bieber M, Kraft P, Weber ANR,
Stoll G and Schuhmann MK: The NLRP3 inflammasome drives
inflammation in ischemia/reperfusion injury after transient middle
cerebral artery occlusion in mice. Brain Behav Immun. 92:223–233.
2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wang Q, Yu D, Liang J, Cheng Q, Zhou F and
Lin H: Significance of expression of AIM2, IL-1β, and IL-18 in
plasma of patients with acute cerebral infarction. Zhong Nan Da Xue
Xue Bao Yi Xue Ban. 46:149–155. 2021.PubMed/NCBI View Article : Google Scholar : (In English,
Chinese).
|
|
39
|
Shaftel SS, Kyrkanides S, Olschowka JA,
Miller JN, Johnson RE and O'Banion MK: Sustained hippocampal IL-1
beta overexpression mediates chronic neuroinflammation and
ameliorates Alzheimer plaque pathology. J Clin Invest.
117:1595–1604. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
40
|
Nakanishi K, Yoshimoto T, Tsutsui H and
Okamura H: Interleukin-18 regulates both Th1 and Th2 responses.
Annu Rev Immunol. 19:423–474. 2001.PubMed/NCBI View Article : Google Scholar
|