Introduction
Acute myeloid leukemia (AML), characterized by
blocked differentiation and clonal proliferation of hematopoietic
stem/progenitor cells, is the commonest hematological malignancy
and seriously endangers human health. So far, multiple methods
including combined chemotherapy, targeted therapy and hematopoietic
stem cell transplantation have not been effective in the treatment
of AML (1). Therefore, exploring
new therapies for AML is a focus of research. Bone marrow
microenvironment (BMME) is a vital research field. Abnormal BMME
has long been considered as one of the important factors affecting
the onset of AML. Relevant studies have also confirmed that some
components of BMME can promote the survival of AML cells and induce
chemotherapy resistance by secreting cytokines and interacting with
AML cells (2,3).
Natural drug extracts play an important role in the
prevention and treatment of tumors in various systems, and have
their own distinctiveness in the clinical treatment of leukemia.
The regulation of the tumor microenvironment through multi-target
effect on tumor cells is one of the important characteristics of
anti-tumor therapy of traditional Chinese medicine. Cimigenol (Cim)
is one of the main effective components of natural Cimicifugae
Rhizoma (4). Research results
have shown that Cim has a clear inhibitory effect on the biological
activity of tumor cells (5-7).
However, the role of Cim in AML and relevant mechanisms remains to
be elucidated. In clinical treatment, it was found that Cim did not
directly affect AML cells promoted by BMSCs (in vitro
research data not shown as it is a part of another study), and due
to this result, the present study did not use Cim to treat MV-4-11
or U937 cells. Therefore, in present study, the role of Cim in
inducing AML cell apoptosis and inhibiting AML-related angiogenesis
was explored. Cim might affect the occurrence and progression of
AML by regulating BMME, but the specific regulatory mechanism is
unclear and requires further exploration.
Materials and methods
Reagents and instruments
Cim was purchased from MilliporeSigma, and RPMI 1640
culture medium and fetal bovine serum from Gibco; Thermo Fisher
Scientific, Inc. EdU kit, TUNEL kit and Annexin V-APC/7-AAD
apoptosis kit were purchased from Jiangsu KeyGEN Biotech Co., Ltd.
RNA extraction kit, reverse transcription kit, One Step TB Green
PrimeScript RT-PCR kit II (SYBR Green) were purchased from Takara
Bio, Inc. Primers were synthesized by Sangon Biotech (Shanghai)
Co., Ltd. Rabbit anti-human GAPDH was purchased from Jiangsu KeyGEN
Biotech Co., Ltd., and rabbit anti-human C-X-C chemokine receptor
type 4 (CXCR4), stromal cell-derived factor-1α (SDF-1α), vascular
cell adhesion molecule 1 (VCAM1), leukocyte function-associated
antigen-1 (LFA-1), Fms like tyrosine kinase receptor 3 (FLT3),
nucleophosmin 1 (NPM-1), CCAAT/enhancer-binding protein alpha
(C/EBPA), AKT, phosphorylated (p-)AKT, mTOR and p-mTOR were
purchased from Abcam; very-late-antigen-4 (VLA-4) was purchased
from ProteinTech Group, Inc. Flow cytometer FC500 was from Beckman
Coulter, Inc., ND2000 ultra-microspectrophotometer was from Thermo
Fisher Scientific, Inc., and gel image analysis system was produced
by GeneGenius. BL8040 (CXCR4 antagonist) was from Roche
Diagnostics.
Cell culture
Human AML cell lines MV-4-11 and U937 were purchased
from ATCC and primary BMSCs cell were purchased from Procell Life
Science & Technology Co., Ltd. (cat. no. CP-H166) and cultured
in RPMI 1640 medium containing 10% fetal bovine serum in a 5%
CO2 incubator at 37˚C. According to cell proliferation,
the medium was changed from time to time for subculture. Cells in
the logarithmic growth phase were collected for subsequent
experiment. MV-4-11 and U937 cell lines were not contaminated and
the STR profiles were positive. The present study was approved by
ethics Committee of Affiliated Hospital of Nanjing University of
Chinese Medicine (approval no. 2021010606).
Cell treatment
MV-4-11 or U937 cells were inoculated onto 96-well
plate (2x105/well), and subsequent experiments were
performed when confluence reached >80%. The cells in the NC
group were routinely cultured. In the BMSC group, MV-4-11 or U937
cells were co-cultured with BMSCs. In the BMSC + DMSO group,
MV-4-11 or U937 cells were co-cultured with BMSCs, to which was
added 250 µl DMSO. In the BMSC + Low group, MV-4-11 or U937 cells
were co-cultured with BMSCs and to 250 µl of the mixed solution was
added 5 mg/l Cim (final concentration). In the BMSC + Middle group,
MV-4-11 or U937 cells were co-cultured with BMSCs and to 250 µl of
the mixed solution was added 10 mg/l Cim (final concentration). In
the BMSC + High group, MV-4-11 or U937 cells were co-cultured with
BMSCs and to 250 µl of the mixed solution was added 20 mg/l Cim
(final concentration). In the BMSC + BL8040 group, MV-4-11 or U937
cells were co-cultured with BMSCs, to which was added 10 nM BL8040.
In the BMSC + Cim group, MV-4-11 or U937 cells were co-cultured
with BMSCs and to 250 µl of the mixed solution was added 20 mg/l
Cim (final concentration). In the BMSC + Cim + BL8040 group,
MV-4-11 or U937 cells were co-cultured with BMSCs and to 250 µl of
the mixed solution was added 20 mg/l Cim (final concentration) and
10 nM BL8040. After the cells in each group were treated for 48 h
at room temperature, the subsequent experiment was conducted.
The co-culture method for the BMSCs cells was to
inoculate on a treated glass slide and when the cells adhered,
place the slide into the dish of AML cells and co-culture them.
Detection of cell proliferation by EdU
staining
After corresponding treatment in each group for 48
h, 50 µmol/l EdU staining solution was added to each well for
incubation for 2 h at room temperature, followed by washing with
PBS for 3 times. Then, 4% paraformaldehyde was added for fixation
for 30 min, and 50 µl 2 mg/ml glycine was added for incubation on a
shaking table for 5 min. Additionally, 100 µl 0.5% TritonX-100 was
added for penetration enhancement at room temperature, followed by
PBS washing for 3 times. Afterwards, each well had 100 µl
Hoechst33342 staining solution added for reaction at room
temperature in the dark for 30 min, followed by washing with PBS
for 3 times. Finally, observation and capturing of images were
conducted under a fluorescence microscope, with three duplicated
wells in each group.
TUNEL assay
After corresponding treatment for 48 h, MV-4-11 or
U937 cells were collected from each group, fixed with 4%
paraformaldehyde for 30 min at room temperature, and then washed
twice with PBS. After adding 100 µl TUNEL balanced buffer and
incubation at room temperature for 5 min, 50 µl reaction buffer was
finally added for incubation in the dark for 60 min at room
temperature. Following centrifugation (8,000 x g, 4˚C, 2 min), the
supernatant was discarded, followed by washing with
5x10-3 mg/l BSA. The morphological changes of cells were
observation and capturing of images were conducted under a
fluorescence microscope.
Flow cytometry
After corresponding treatment for 48 h, MV-4-11 or
U937 cells were collected from each group. In 1 h after Annexin
V/PI staining at room temperature, 10,000 cells were collected and
fixed in each group, and the apoptotic rate of hepatoma cells was
detected by flow cytometry. Samples were repeated three times in
each group. The quantification was analyzed by FlowJo 7.6.5
software (FlowJo LLC). The apoptosis rate=early + late apoptotic
cells/all cells x100%
Reverse transcription-quantitative
(RT-q) PCR
After corresponding treatment for 48 h, MV-4-11 or
U937 cells (10,000 cells) were collected from each group. Total RNA
was extracted from cells using TRIzol® (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. Then, 0.5 µg RNA was converted into cDNA at 37˚C for
1 h using PrimeScript RT MasterMix (Takara Bio, Inc.). qPCR was
performed using ChamQ SYBR® qPCR MasterMix (Vazyme
Biotech, Co., Ltd.). Primer sequences are listed in Table I. The reaction system (20 µl)
included: 10 µl SYBR FAST qPCR Mix (2X), 1 µl upstream primer (10
µmol/l), 1 µl downstream primer (10 µmol/l), 2 µl cDNA template and
6 µl ddH2O. The reaction conditions were as follows:
95˚C for 5 min, 95˚C for 15 sec, 60˚C for 1 min for 40 cycles.
Finally, the relative expression of each gene was analyzed using
the 2-ΔΔCq method (8).
The experiment was repeated three times.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene | Forward
(5'-3') | Reverse
(5'-3') | Size |
|---|
| AKT |
CAGGATGTGGACCAACGTGA |
AAGGTGCGTTCGATGACAGT | 137 bp |
| c/EBPα |
GACAAGAACAGCAACGAGTACC |
GTCATTGTCACTGGTCAGCTC | 132 bp |
| CXCR4 |
TTCCAGTTTCAGCACATCATGG |
GTCGATGCTGATCCCAATGTAG | 192 bp |
| FLT3 |
GTGAATCCTTACCCTGGCATTC |
GTCAAATTAGGGAAGGATGGCC | 164 bp |
| LFA-1 |
GGTTGACGTGGTGTATGAGAAG |
GAAACCAACCTTGTACAGCACT | 109 bp |
| mTOR |
AACCTCCTCCCCTCCAATGA |
TCAGCGGTAAAAGTGTCCCC | 92 bp |
| NPM-1 |
CACCAAAAGGACCTAGTTCTGT |
TGCCAGAGATCTTGAATAGCCT | 157 bp |
| SDF-1α |
GATTCTTCGAAAGCCATGTTGC |
TCAATGCACACTTGTCTGTTGT | 121 bp |
| VCAM-1 |
AGTTCTTGTTTGCCGAGCTAAA |
AAATCTCTGGAGCTGGTAGACC | 197 bp |
| VLA-4 |
AACATGAGCAGATTGGTAAGGC |
CAGACAGAAGCTCCAAAGTACG | 112 bp |
| GAPDH |
CAAATTCCATGGCACCGTCA |
AGCATCGCCCCACTTGATTT | 109 bp |
Western blotting
After corresponding treatment for 48 h, MV-4-11 or
U937 cells were collected from each group. Total protein was
extracted from cells using RIPA buffer (Changsha Auragene
Biological Technology Co., Ltd.) and quantified using a BCA Protein
Assay kit (Beijing Dingguo Changsheng Biotechnology, Co., Ltd.).
The lysates were incubated at 95˚C for 5 min, an equal amount of
total protein (30 µg/lane), separated using 10% SDS-PAGE (Bio-Rad
Laboratories) and transferred onto PVDF membranes
(MilliporeSigma).
After being blocked with 5% skimmed milk at room
temperature for 2 h, the membrane was incubated with primary
antibodies CXCR4 (1:1,000; cat. no. ab16502), SDF-1α (1:1,000; cat.
no. ab25117), VLA-4 (1:1,000; ProteinTech Group, Inc., cat. no.
19676-1-AP), VCAM1 (1:1,000; cat. no. ab134047), LFA-1 (1:1,000;
cat. no. ab235456), FLT3 (1:1,000; cat. no. ab52648), NPM-1
(1:1,000; cat. no. ab10530), C/EBPA (1:1,000; cat. no. ab40761),
AKT (1:1,000; cat. no. ab179463), mTOR(1:1,000; cat. no. ab2732),
p-AKT(1:1,000; cat. no. ab81283), p-mTOR(1:1,000; cat. no.
ab109268) or GAPDH (1:500; cat. no. ab8245) at 4˚C overnight. The
membrane was washed with TBST three times for 10 min. Subsequently,
HRP-labeled goat anti-rabbit IgGⅡ antibody (1:5,000) was added for
incubation at room temperature for 1 h, and the membrane was washed
with TBST three times for 10 min. Finally, Proteins were visualized
using an ECL reagent kit (Shanghai Yeasen Biotech Co., Ltd.) and
were semi-quantified using ImageJ software (1.46r; National
Institutes of Health).
Statistical analysis
Experiments were performed in triplicate at minimum.
Data are presented as the mean ± standard deviation and were
analyzed using GraphPad Prism 8.0 (GraphPad Software, Inc.). For
statistical analysis, pairwise comparisons between two groups were
analyzed using the unpaired Student's t-test. One-way ANOVA
followed by Tukey's post hoc test was used for comparisons between
>2 groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Different Cim concentrations
suppresses AML cell proliferation protected by BMSC
Compared with NC group, EdU positive cell rates of
BMSC and BMSC + DMSO groups were significantly upregulated in
MV-4-11 and U937 cell lines (P<0.001, respectively, Fig. 1A and B). With Cim supplement, compared with
BMSC group, EdU positive cell rates of Cim treated groups were
significantly depressed in MV-4-11 and U937 cell lines (P<0.05,
P<0.01 or P<0.001, respectively, Fig. 1A and B) and was dose-dependent (P<0.05,
respectively, Fig. 1A and B).
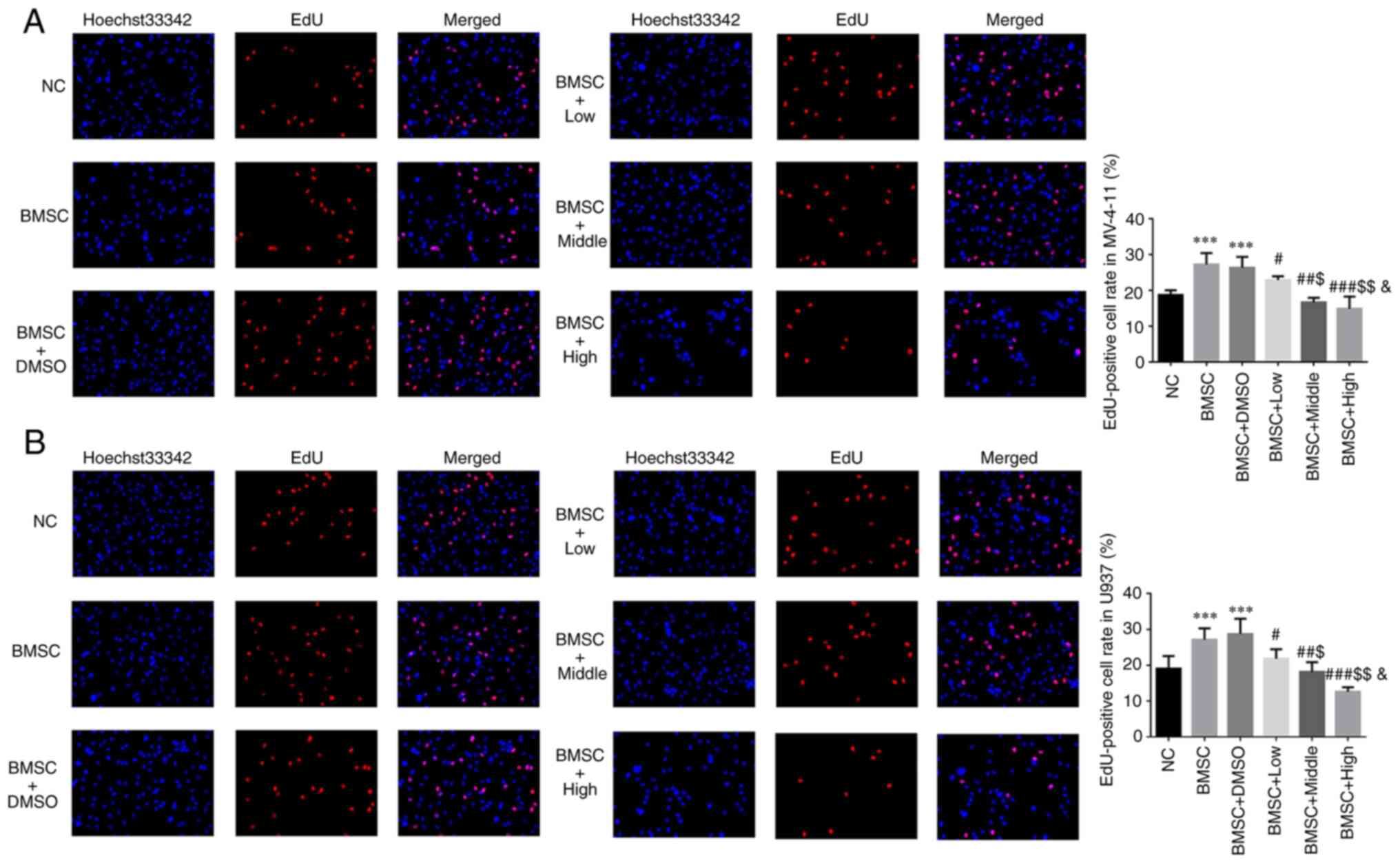 | Figure 1Different concentrations of Cim
suppress AML cell proliferation protected by BMSC. EdU-positive
cell rate in (A) MV-4-11 and (B) U937 cell line (%, magnification,
x200). ***P<0.001 vs. NC; #P<0.05,
##P<0.01, ###P<0.001 vs. BMSC;
$P<0.05, $$P<0.01, vs. BMSC + Low;
&P<0.05, vs. BMSC + Middle. Cim, cimigenol; AML,
acute myeloid leukemia; BMSC, bone marrow stromal cells; NC, cells
in normal culture medium; BMSC, cells co-cultured with BMSC; BMSC +
DMSO, cells co-cultured with BMSCs and treated with 250 µl DMSO;
BMSC + Low, cells co-cultured with BMSCs and treated with 5 mg Cim;
BMSC + Middle, cells co-cultured with BMSCs and treated with 10 mg
Cim; BMSC + High, cells co-cultured with BMSCs and treated with 20
mg Cim. |
Different Cim concentrations increase
AML cell apoptosis protected by BMSC by flow cytometry
Compared with NC group, apoptosis rates of BMSC and
BMSC + DMSO groups were significantly downregulated in MV-4-11 and
U937 cell lines (P<0.001, respectively, Fig. 2A and B). With Cim supplement, compared with
BMSC group, apoptosis rates of Cim treated groups were
significantly increased in MV-4-11 and U937 cell lines (P<0.05,
P<0.01 or P<0.001, respectively, Fig. 2A and B) with dose-dependent (P<0.05,
respectively, Fig. 2A and B).
 | Figure 2Different concentrations of Cim
suppress increased AML cell apoptosis protected by BMSC by flow
cytometry. Apoptosis cell rates in (A) MV-4-11 and (B) U937 (%).
***P<0.001, vs. NC; #P<0.05,
##P<0.01, ###P<0.001, vs. BMSC;
$P<0.05, $$P<0.01, vs. BMSC + Low;
&P<0.05, vs. BMSC + Middle. Cim, cimigenol; AML,
acute myeloid leukemia; BMSC, bone marrow stromal cells; NC, cells
in normal culture medium; BMSC, cells co-cultured with BMSC; BMSC +
DMSO, cells co-cultured with BMSCs and treated with 250 µl DMSO;
BMSC + Low, cells co-cultured with BMSCs and treated with 5 mg Cim;
BMSC + Middle, cells co-cultured with BMSCs and treated with 10 mg
Cim; BMSC + High, cells co-cultured with BMSCs and treated with 20
mg Cim. |
Different Cim concentrations increase
number of apoptotic AML cells protected by BMSC by TUNEL assay
Compared with the NC group, the number of positive
apoptotic cells in BMSC and BMSC + DMSO groups was significantly
downregulated in MV-4-11 and U937 cell lines (P<0.001,
respectively, Fig. 3A and B). With Cim supplement, compared with
BMSC group, the number of positive apoptotic cells in Cim treated
groups was significantly increased in MV-4-11 and U937 cell lines
(P<0.05, P<0.01 or P<0.001, respectively; Fig. 3A and B) dose-dependently (P<0.05,
respectively; Fig. 3A and B).
 | Figure 3Different concentrations of Cim
suppress increased apoptosis AML cell number protected by BMSC by
TUNEL assay. Positive apoptotic cell number in (A) MV-4-11 and (B)
U937 (magnification, x200). ***P<0.001, vs. NC;
#P<0.05, ##P<0.01,
###P<0.001, vs. BMSC; $P<0.05,
$$P<0.01, vs. BMSC + Low; &P<0.05,
vs. BMSC + Middle. Cim, cimigenol; AML, acute myeloid leukemia;
BMSC, bone marrow stromal cells; NC, cells in normal culture
medium; BMSC, cells co-cultured with BMSC; BMSC + DMSO, cells
co-cultured with BMSCs and treated with 250 µl DMSO; BMSC + Low,
cells co-cultured with BMSCs and treated with 5 mg Cim; BMSC +
Middle, cells co-cultured with BMSCs and treated with 10 mg Cim;
BMSC + High, cells co-cultured with BMSCs and treated with 20 mg
Cim; BMSCs, breaking bone marrow stromal cells. |
Different Cim concentrations affect
relative gene expression by RT-qPCR assay
Compared with the NC group, CXCR4, SDF-1α, mTOR,
AKT, VLA-4, VCAM-1, LFA-1 and C/EBPα mRNA expression in BMSC and
BMSC + DMSO groups was significantly upregulated and FLT3 and NPM-1
mRNA expression in BMSC and BMSC + DMSO groups was significantly
downregulated in MV-4-11 and U937 cell lines (P<0.001,
respectively, Fig. 4A and B). With Cim, compared with the BMSC
group, CXCR4, SDF-1α, mTOR, AKT, VLA-4, VCAM-1, LFA-1 and C/EBPα
mRNA expression was significantly downregulated and FLT3 and NPM-1
mRNA expression was significantly upregulated in MV-4-11 and U937
cell lines (P<0.05, P<0.01 or P<0.001, respectively,
Fig. 4A and B) dose-dependently (P<0.05,
respectively, Fig. 4A and B).
 | Figure 4Different concentrations of Cim
suppress affect relative gene expression by reverse
transcription-quantitative PCR. Relative gene expression in (A)
MV-4-11 and (B) U937. ***P<0.001, vs. NC;
#P<0.05, ##P<0.01,
###P<0.001, vs. BMSC; $P<0.05,
$$P<0.01, vs. BMSC + Low; &P<0.05,
vs. BMSC + Middle. Cim, cimigenol; AML, acute myeloid leukemia;
BMSC, bone marrow stromal cells; NC, cells in normal culture
medium; BMSC, cells co-cultured with BMSC; BMSC + DMSO, cells
co-cultured with BMSCs and treated with 250 µl DMSO; BMSC + Low,
cells co-cultured with BMSCs and treated with 5 mg Cim; BMSC +
Middle, cells co-cultured with BMSCs and treated with 10 mg Cim;
BMSC + High, cells co-cultured with BMSCs and treated with 20 mg
Cim; BMSCs, breaking bone marrow stromal cells. |
Different Cim concentrations affect
relative protein expression by western blotting
Compared with the NC group, CXCR4, SDF-1α, p-mTOR,
p-AKT, VLA-4, VCAM-1, LFA-1 and C/EBPα protein expression of BMSC
and BMSC + DMSO groups was significantly upregulated, and FLT3 and
NPM-1 protein expression of BMSC and BMSC + DMSO groups was
significantly downregulated in MV-4-11 and U937 cell lines
(P<0.001, respectively, Fig. 5A
and B). With Cim, compared with
the BMSC group, CXCR4, SDF-1α, p-mTOR, p-AKT, VLA-4, VCAM-1, LFA-1
and C/EBPα protein expression was significantly downregulated and
FLT3 and NPM-1 protein expression was significantly upregulated in
MV-4-11 and U937 cell lines (P<0.05, P<0.01 or P<0.001,
respectively, Fig. 5A and B) dose-dependently (P<0.05,
respectively, Fig. 5A and B).
 | Figure 5Different concentrations of Cim
suppress affected relative protein expression by western blotting.
Relative protein expression in (A) MV-4-11 and (B) U937.
***P<0.001, vs. NC; #P<0.05,
##P<0.01, ###P<0.001, vs. BMSC;
$P<0.05, $$P<0.01, vs. BMSC + Low;
&P<0.05, vs. BMSC + Middle. Cim, cimigenol;
CXCR4, C-X-C chemokine receptor type 4; SDF-1α, stromal
cell-derived factor-1α; p-, phosphorylated; VLA-4,
very-late-antigen-4; VCAM1, vascular cell adhesion molecule 1;
LFA-1, leukocyte function-associated antigen-1; FLT3, Fms like
tyrosine kinase receptor 3; NPM-1, nucleophosmin 1; C/EBPA,
CCAAT/enhancer-binding protein alpha; NC, cells in normal culture
medium; BMSC, cells co-cultured with BMSC; BMSC + DMSO, cells
co-cultured with BMSCs and treated with 250 µl DMSO; BMSC + Low,
cells co-cultured with BMSCs and treated with 5 mg Cim; BMSC +
Middle, cells co-cultured with BMSCs and treated with 10 mg Cim;
BMSC + High, cells co-cultured with BMSCs and treated with 20 mg
Cim; BMSCs, breaking bone marrow stromal cells. |
Effect of CXCR4 on the anti-tumor
effects of Cim in cell proliferation
Compared with the NC group, EdU positive cell rates
in BMSC groups were significantly upregulated in MV-4-11 and U937
cell lines (P<0.001, respectively, Fig. 6A and B). With BL8040 (CXCR4 inhibitor) and/or
Cim treatment, compared with BMSC group, EdU positive cell rates of
BMC + BL8040, BMSC + Cim and BMSC + Cim + BL8040 groups were
significantly suppressed (P<0.001, respectively, Fig. 6A and B).
 | Figure 6Effect of CXCR4 on the anti-tumor
effects of Cim in cell proliferation. EdU-positive cell rate in (A)
MV-4-11 and (B) U937 (%, x200). ***P<0.001, vs. NC;
###P<0.001, vs. BMSC. CXCR4, C-X-C chemokine receptor
type 4; Cim, cimigenol; NC, cells in normal culture medium; BMSC,
cells co-cultured with BMSC; BMSC + DMSO, cells co-cultured with
BMSCs and treated with 250 µl DMSO; BMSC + Low, cells co-cultured
with BMSCs and treated with 5 mg Cim; BMSC + Middle, cells
co-cultured with BMSCs and treated with 10 mg Cim; BMSC + High,
cells co-cultured with BMSCs and treated with 20 mg Cim; BMSCs,
breaking bone marrow stromal cells. |
Effect of CXCR4 on the anti-tumor
effects of Cim in cell apoptosis by flow cytometry
Compared with the NC group, apoptosis rates in BMSC
groups were significantly downregulated in MV-4-11 and U937 cell
lines (P<0.001, respectively, Fig.
7A and B). With BL8040 (CXCR4
inhibitor) and/or Cim treatment, compared with BMSC group,
apoptosis rates of BMC + BL8040, BMSC + Cim and BMSC + Cim + BL8040
groups were significantly increased (P<0.001, respectively,
Fig. 7A and B).
 | Figure 7Effect of CXCR4 on the anti-tumor
effects of Cim in cell apoptosis by flow cytometry. Apoptosis cell
rate in (A) MV-4-11 and (B) U937 (%). ***P<0.001, vs.
NC; ###P<0.001, vs. BMSC. C-X-C chemokine receptor
type 4; Cim, cimigenol; NC, cells in normal culture medium; BMSC,
cells co-cultured with BMSC; BMSC + DMSO, cells co-cultured with
BMSCs and treated with 250 µl DMSO; BMSC + Low, cells co-cultured
with BMSCs and treated with 5 mg Cim; BMSC + Middle, cells
co-cultured with BMSCs and treated with 10 mg Cim; BMSC + High,
cells co-cultured with BMSCs and treated with 20 mg Cim; BMSCs,
breaking bone marrow stromal cells. |
Effect of CXCR4 on the anti-tumor
effects of Cim in cell apoptosis by TUNEL assay
Compared with the NC group, number of positive
apoptotic cells in the BMSC groups was significantly downregulated
in MV-4-11 and U937 cell lines (P<0.001, respectively, Fig. 8A and B). With BL8040 (CXCR4 inhibitor) and/or
Cim treatment, compared with BMSC group, the number of positive
apoptotic cells in the BMC + BL8040, BMSC + Cim and BMSC + Cim +
BL8040 groups was significantly increased (P<0.001,
respectively, Fig. 8A and B).
 | Figure 8Effect of CXCR4 on the anti-tumor
effects of Cim in cell apoptosis by TUNEL assay. Number of positive
apoptotic cells in (A) MV-4-11 and (B) U937 (magnification, x200).
***P<0.001, vs. NC; ###P<0.001, vs.
BMSC. C-X-C chemokine receptor type 4; Cim, cimigenol; NC, cells in
normal culture medium; BMSC, cells co-cultured with BMSC; BMSC +
DMSO, cells co-cultured with BMSCs and treated with 250 µl DMSO;
BMSC + Low, cells co-cultured with BMSCs and treated with 5 mg Cim;
BMSC + Middle, cells co-cultured with BMSCs and treated with 10 mg
Cim; BMSC + High, cells co-cultured with BMSCs and treated with 20
mg Cim; BMSCs, breaking bone marrow stromal cells. |
CXCR4 inhibitor affects relative gene
expression
Compared with the NC group, CXCR4, SDF-1α, mTOR,
AKT, VLA-4, VCAM-1, LFA-1 and C/EBPα mRNA expression in BMSC groups
was significantly upregulated, and FLT3 and NPM-1 mRNA expression
in BMSC groups was significantly downregulated in MV-4-11 and U937
cell lines (P<0.001, respectively, Fig. 9A and B). With BL8040 (CXCR4 inhibitor) and/or
Cim treatment, compared with the BMSC group, CXCR4, SDF-1α, mTOR,
AKT, VLA-4, VCAM-1, LFA-1 and C/EBPα mRNA expression was
significantly downregulated and FLT3 and NPM-1 mRNA expression was
significantly upregulated in MV-4-11 and U937 cell lines
(P<0.001, respectively, Fig. 9A
and B).
 | Figure 9CXCR4 inhibitor affected relative
gene expression. Relative gene expression in (A) MV-4-11 and (B)
U937. ***P<0.001, vs. NC; ###P<0.001,
vs. BMSC. C-X-C chemokine receptor type 4; Cim, cimigenol; NC,
cells in normal culture medium; BMSC, cells co-cultured with BMSC;
BMSC + DMSO, cells co-cultured with BMSCs and treated with 250 µl
DMSO; BMSC + Low, cells co-cultured with BMSCs and treated with 5
mg Cim; BMSC + Middle, cells co-cultured with BMSCs and treated
with 10 mg Cim; BMSC + High, cells co-cultured with BMSCs and
treated with 20 mg Cim; BMSCs, breaking bone marrow stromal
cells. |
CXCR4 inhibitor affects relative
protein expression
Compared with the NC group, CXCR4, SDF-1α, mTOR,
AKT, VLA-4, VCAM-1, LFA-1 and C/EBPα protein expression of BMSC
groups was significantly upregulated, and FLT3 and NPM-1 protein
expression of BMSC groups was significantly downregulated in
MV-4-11 and U937 cell lines (P<0.001, respectively, Fig. 10A and B). With BL8040 (CXCR4 inhibitor) and/or
Cim treatment, compared with BMSC group, CXCR4, SDF-1α, mTOR, AKT,
VLA-4, VCAM-1, LFA-1 and C/EBPα protein expression was
significantly downregulated and FLT3 and NPM-1 protein expression
was significantly upregulated in MV-4-11 and U937 cell lines
(P<0.001, respectively, Fig.
10A and B).
 | Figure 10CXCR4 inhibitor affects relative
protein expression. Relative protein expression in (A) MV-4-11 and
(B) U937. ***P<0.001, vs. NC;
###P<0.001, vs. BMSC. Cim, cimigenol; CXCR4, C-X-C
chemokine receptor type 4; SDF-1α, stromal cell-derived factor-1α;
p-, phosphorylated; VLA-4, very-late-antigen-4; VCAM1, vascular
cell adhesion molecule 1; LFA-1, leukocyte function-associated
antigen-1; FLT3, Fms like tyrosine kinase receptor 3; NPM-1,
nucleophosmin 1; C/EBPα, CCAAT/enhancer-binding protein alpha; NC,
cells in normal culture medium; BMSC, cells co-cultured with BMSC;
BMSC + DMSO, cells co-cultured with BMSCs and treated with 250 µl
DMSO; BMSC + Low, cells co-cultured with BMSCs and treated with 5
mg Cim; BMSC + Middle, cells co-cultured with BMSCs and treated
with 10 mg Cim; BMSC + High, cells co-cultured with BMSCs and
treated with 20 mg Cim; BMSCs, breaking bone marrow stromal
cells. |
Discussion
Under the physiological state, BMSCs in BMME can
produce a variety of adhesion molecules and chemokines, thus
mediating multiple signal cascades to ensure and maintain the
normal localization and homeostasis of HSCs in BM. In the
pathological state of AML, these products can be hijacked and
shared by AML cells, so that AML cells can obtain environmental
conditions conducive to their own survival, expansion and
progression, finally leading to the weakening of apoptosis in AML
cells (9,10). The bindings and interactions of the
chemokine receptor family represented by CXCR4 and its relevant
ligands play a representative role in this process. CXCR4 directly
or indirectly activates a variety of signal cascades by binding
with its ligands to ensure the correct localization, homeostasis
maintenance and normal survival of HSCs in BMME (11). CXCR4 may eventually cause the
progression, difficulties in treatment and recurrence of AML by
affecting the proliferation, migration, chemotaxis and angiogenesis
as well as increasing the chemotherapy resistance of leukemia cells
(12).
SDF-1α is one of the members of the chemokine family
in BMME and also the only ligand of CXCR4. In BMME, many components
such as BMSCs, immature osteoblasts and bone marrow endothelial
cells can secrete SDF-1α (13).
Among these cell components, BMSCs are the main source (14). SDF-1α is often expressed in bone
marrow microvascular hot spots that attract the aggregation of AML
cells in BMME (15). It can
promote the survival and expansion of AML cells by guiding AML
cells to a favorable environment in BMME (16). In addition, the adhesion between
AML cells and bone marrow stroma in BMME is vital for the survival
and proliferation of AML cells and SDF-1α participates in the
regulation of this mechanism. This regulation can be briefly
summarized as: SDF-1α regulates the adhesion of AML cells to matrix
components in BMME by activating adhesion molecules, such as
integrins CD44 and VLA-4, so that AML cells can obtain
‘concealment’ and produce chemotherapy resistance (17). The binding of CXCR4 and its ligand
SDF-1α can initiate multiple Ca2+-dependent or
independent signal events, leading to actin cytoskeleton
reorganization and activating integrins, which results in its
appropriate interaction with the endothelium of BM sinus and
stromal cells, finally affecting the survival, chemotaxis, homing
and proliferation of cells (18).
A relevant study (19)
demonstrated that CXCR4 inhibitor AMD3465 can promote the
peripheral mobilization of AML cells and enhance the anti-leukemia
effect of chemotherapeutic drugs. A further study (3) found that blocked CXCR4/SDF-1α
interaction affects the activity of related downstream signaling
pathways such as PI3K/AKT and MAPK and increases the mobilization
rate of AML cells, finally leading to the increase in chemotherapy
sensitivity. Experiments have confirmed that other CXCR4
antagonists and monoclonal antibodies, including LY2510924, CX-01,
POL6326 and NOX-A12, can also effectively inhibit the growth of AML
cells and produce sustained pharmacodynamic effects on peripheral
mobilization of cells (20-22).
Some related studies showed that the CXCR4/SDF-1α signaling pathway
stimulation could improve cancer cell biological activating in
vitro and in vivo studies (23,24).
The results of the present study showed that after AML cells were
co-cultured with BMSCs, the apoptosis of AML cells MV-4-11 and U937
was protected, the proliferation was increased and the CXCR4/SDF-1α
signaling pathway was activated. Therefore, it was hypothesized
that BMSCs possess a protective effect on AML cells.
Cim has an inhibitory effect on the activity of
tumor cells in vivo and in vitro (5-7).
The present study showed that Cim also had a certain killing effect
on AML cells under the protection of BMSCs and that its mechanism
might be through the inhibition of the CXCR4/SDF-1α signaling
pathway, which further inhibited downstream AKT and mTOR activities
and acted on the terminal VLA-4(25), VCAM-1(26), LFA-1(27), FLT3(28), NPM-1(29) and c/EBPα (30). Therefore, it is hypothesized that
Cim can promote the apoptosis and inhibit the proliferation of AML
cells by inhibiting the CXCR4/SDF-1α signaling pathway.
However, there were some limitations to the present
study. It only studied the effect of cimigenol on AML cell lines
via CXCR4/SDF-1α pathway; the anti-tumor effects of cimigenol might
be regulated by other pathways, meanwhile there were some
differences between in vitro in AML. Future in vivo
studies will address the bioavailability of cimigenol.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Youth Fund of
Natural Science Foundation of Jiangsu Province (grant no.
BK20201096).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BM, HD and XMS contributed to the conceptualization
and the design of the present study. BM, HD and XD performed the
experiments and analyzed the data. XD, SQ, XCS and XMS were
responsible for the acquisition, analysis and interpretation of the
data. HD and XCS contributed to the drafting of the manuscript. XD
and SQ confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of the Affiliated Hospital of Nanjing University of
Chinese Medicine (approval no. 2021010606).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kumar B, Garcia M, Weng L, Jung X,
Murakami JL, Hu X, McDonald T, Lin A, Kumar AR, DiGiusto DL, et al:
Acute myeloid leukemia transforms the bone marrow niche into a
leukemia-permissive microenvironment through exosome secretion.
Leukemia. 32:575–587. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hanahan D and Coussens LM: Accessories to
the crime: Functions of cells recruited to the tumor
microenvironment. Cancer Cell. 21:309–322. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Uy GL, Rettig MP, Motabi IH, McFarland K,
Trinkaus KM, Hladnik LM, Kulkarni S, Abboud CN, Cashen AF,
Stockerl-Goldstein KE, et al: A phase 1/2 study of
chemosensitization with the CXCR4 antagonist plerixafor in relapsed
or refractory acute myeloid leukemia. Blood. 119:3917–3924.
2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Schmid D, Woehs F, Svoboda M, Thalhammer
T, Chiba P and Moeslinger T: Aqueous extracts of Cimicifuga
racemosa and phenolcarboxylic constituents inhibit production of
proinflammatory cytokines in LPS-stimulated human whole blood. Can
J Physiol Pharmacol. 87:963–972. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Sakurai N, Kozuka M, Tokuda H, Mukainaka
T, Enjo F, Nishino H, Nagai M, Sakurai Y and Lee KH: Cancer
preventive agents. Part 1: Chemopreventive potential of cimigenol,
cimigenol-3,15-dione, and related compounds. Bioorg Med Chem.
13:1403–1408. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jöhrer K, Stuppner H, Greil R and Çiçek
SS: Structure-guided identification of black cohosh (actaea
racemosa) triterpenoids with in vitro activity against multiple
myeloma. Molecules. 25(766)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li X, Wang W, Fan Y, Wei Y, Yu LQ, Wei JF
and Wang YF: Anticancer efficiency of cycloartane triterpenoid
derivatives isolated from Cimicifuga yunnanensis Hsiao on
triple-negative breast cancer cells. Cancer Manag Res.
10:6715–6729. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zaitseva L, Murray MY, Shafat MS, Lawes
MJ, MacEwan DJ, Bowles KM and Rushworth SA: Ibrutinib inhibits
SDF1/CXCR4 mediated migration in AML. Oncotarget. 5:9930–9938.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Peled A and Tavor S: Role of CXCR4 in the
pathogenesis of acute myeloid leukemia. Theranostics. 3:34–39.
2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sugiyama T, Kohara H, Noda M and Nagasawa
T: Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4
chemokine signaling in bone marrow stromal cell niches. Immunity.
25:977–988. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Konoplev S, Rassidakis GZ, Estey E,
Kantarjian H, Liakou CI, Huang X, Xiao L, Andreeff M, Konopleva M
and Medeiros LJ: Overexpression of CXCR4 predicts adverse overall
and event-free survival in patients with unmutated FLT3 acute
myeloid leukemia with normal karyotype. Cancer. 109:1152–1156.
2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jiang Z, Zhou W, Guan S, Wang J and Liang
Y: Contribution of SDF-1α/CXCR4 signaling to brain development and
glioma progression. Neurosignals. 21:240–258. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Greenbaum A, Hsu YM, Day RB, Schuettpelz
LG, Christopher MJ, Borgerding JN, Nagasawa T and Link DC: CXCL12
in early mesenchymal progenitors is required for haematopoietic
stem-cell maintenance. Nature. 495:227–230. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sipkins DA, Wei X, Wu JW, Runnels JM, Côté
D, Means TK, Luster AD, Scadden DT and Lin CP: In vivo imaging of
specialized bone marrow endothelial microdomains for tumour
engraftment. Nature. 435:969–973. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tavor S, Eisenbach M, Jacob-Hirsch J,
Golan T, Petit I, Benzion K, Kay S, Baron S, Amariglio N, Deutsch
V, et al: The CXCR4 antagonist AMD3100 impairs survival of human
AML cells and induces their differentiation. Leukemia.
22:2151–5158. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Avigdor A, Goichberg P, Shivtiel S, Dar A,
Peled A, Samira S, Kollet O, Hershkoviz R, Alon R, Hardan I, et al:
CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of
human CD34+ stem/progenitor cells to bone marrow. Blood.
103:2981–2989. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhan T, Cao C, Li L, Gu N, Civin CI and
Zhan X: MIM regulates the trafficking of bone marrow cells via
modulating surface expression of CXCR4. Leukemia. 30:1327–1334.
2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zeng Z, Shi YX, Samudio IJ, Wang RY, Ling
X, Frolova O, Levis M, Rubin JB, Negrin RR, Estey EH, et al:
Targeting the leukemia microenvironment by CXCR4 inhibition
overcomes resistance to kinase inhibitors and chemotherapy in AML.
Blood. 113:6215–6224. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Galsky MD, Vogelzang NJ, Conkling P,
Raddad E, Polzer J, Roberson S, Stille JR, Saleh M and Thornton D:
A phase I trial of LY2510924, a CXCR4 peptide antagonist, in
patients with advanced cancer. Clin Cancer Res. 20:3581–3588.
2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Karpova D, Bräuninger S, Wiercinska E,
Krämer A, Stock B, Graff J, Martin H, Wach A, Escot C, Douglas G,
et al: Mobilization of hematopoietic stem cells with the novel
CXCR4 antagonist POL6326 (balixafortide) in healthy
volunteers-results of a dose escalation trial. J Transl Med.
15(2)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hoellenriegel J, Zboralski D, Maasch C,
Rosin NY, Wierda WG, Keating MJ, Kruschinski A and Burger JA: The
spiegelmer NOX-A12, a novel CXCL12 inhibitor, interferes with
chronic lymphocytic leukemia cell motility and causes
chemosensitization. Blood. 123:1032–1039. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gros SJ, Graeff H, Drenckhan A, Kurschat
N, Blessmann M, Rawnaq T and Izbicki JR: CXCR4/SDF-1α-mediated
chemotaxis in an in vivo model of metastatic esophageal carcinoma.
In Vivo. 26:711–718. 2012.PubMed/NCBI
|
|
24
|
Zhu H, Sun Q, Tan C, Xu M, Dai Z, Wang Z,
Fan J and Zhou J: Tacrolimus promotes hepatocellular carcinoma and
enhances CXCR4/SDF-1α expression in vivo. Mol Med Rep.
10:585–592. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Holt RU, Fagerli UM, Baykov V, Rø TB, Hov
H, Waage A, Sundan A and Børset M: Hepatocyte growth factor
promotes migration of human myeloma cells. Haematologica.
93:619–622. 2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fan X, Chen X, Feng Q, Peng K, Wu Q,
Passerini AG, Simon SI and Sun C: Downregulation of GATA6 in
mTOR-inhibited human aortic endothelial cells: Effects on
TNF-α-induced VCAM-1 expression and monocytic cell adhesion. Am J
Physiol Heart Circ Physiol. 316:H408–H420. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wagner JA, Rosario M, Romee R,
Berrien-Elliott MM, Schneider SE, Leong JW, Sullivan RP, Jewell BA,
Becker-Hapak M, Schappe T, et al: CD56bright NK cells exhibit
potent antitumor responses following IL-15 priming. J Clin Invest.
127:4042–4058. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Chen W, Drakos E, Grammatikakis I,
Schlette EJ, Li J, Leventaki V, Staikou-Drakopoulou E, Patsouris E,
Panayiotidis P, Medeiros LJ and Rassidakis GZ: mTOR signaling is
activated by FLT3 kinase and promotes survival of FLT3-mutated
acute myeloid leukemia cells. Mol Cancer. 9(292)2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Marzec M, Kasprzycka M, Liu X, El-Salem M,
Halasa K, Raghunath PN, Bucki R, Wlodarski P and Wasik MA:
Oncogenic tyrosine kinase NPM/ALK induces activation of the
rapamycin-sensitive mTOR signaling pathway. Oncogene. 26:5606–5614.
2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Watanabe M, Hisatake M and Fujimori K:
Fisetin suppresses lipid accumulation in mouse adipocytic 3T3-L1
cells by repressing GLUT4-mediated glucose uptake through
inhibition of mTOR-C/EBPα signaling. J Agric Food Chem.
63:4979–4987. 2015.PubMed/NCBI View Article : Google Scholar
|
























