Introduction
Cholesterol gallstones are a major public health
problem in all developed countries. In China, ~10-15% of the adult
population suffers from cholesterol gallstones (1,2),
which constitute one of the commonest and most costly digestive
diseases (3). The development of
gallstone is influenced by a number of factors, including smoking
(4,5). However, several studies suggest that
smoking is not a risk factor for gallstones and even has the
opposite effect (6,7). In these studies, older smokers had a
relatively lower prevalence of gallstones, whereas younger smokers
had an increased risk of gallstones (8). Compared with non-smokers, older
smokers who smoke for most of their lives have a lower risk of
gallstone disease (9). In view of
this, it is necessary to understand the possible mechanisms by
which smoking may affect gallstone formation. Smoking may change
the levels of gallstone-related protein in the gallbladder or bile
composition, which could influence gallstone formation (10,11).
Bile acids (BAs), synthesized from cholesterol
molecules, serve a critical role in eliminating excess cholesterol
from the body and process dietary fat by facilitating the formation
of micelles. The formation of gallstones is related to the
metabolic disturbance of BAs. BAs, the final product of cholesterol
metabolism, are the main component of bile, accounting for 50-70%
of the total bile. Almost all patients with gallstone have abnormal
BA metabolism (12). When the
proportions of different BAs are imbalanced, cholesterol cannot
maintain the state of micelles and gradually forms crystals and
precipitates, eventually resulting in cholesterol stones (13,14).
Thus, it is important to tightly regulate BA synthesis.
Megalin and cubilin proteins are expressed in
gallbladder epithelial cells but not in hepatocytes. There are a
number of megalin/cubilin ligands in bile, to mediate endocytosis
of numerous ligands including high-density lipoprotein
(HDL)/apolipoprotein A-I (apoA-I) (15). Dysregulation of megalin and cubilin
at the mRNA and protein levels has been found in either humans or
mice with gallstones (15,16). A study demonstrates that bile acids
can regulate the expression of megalin and cubilin, an effect that
appeared to be mediated by the bile acid nuclear hormone receptor
farnesoid X receptor (FXR) (17).
It is reported that a synthetic FXR agonist could prevent gallstone
formation in susceptible wild-type mice that recapitulate human
cholesterol gallstone disease (5).
Together, they catalyze the synthesis of two major BAs, cholic acid
(CA) and chenodeoxycholic acid (CDCA) (18). Therefore, FXR/megalin/cubilin
pathway serves an important role in maintaining BA homeostasis
(19).
The present study attempted to discover whether
nicotine, as a major component of tobacco smoke, could have a
preventive effect on gallstone-susceptible C57BL6 mice. In
addition, it explored if FXR/megalin/cubilin pathways had
participated in this process.
Materials and methods
Animals and diets
Male C57BL6 mice (8 weeks old, 18-20 g, n=50) were
purchased from the Central Laboratory of Kunming medical
university. Mice were fed on normal rodent feedstuff (cholesterol
<0.02%) or a lithogenic diet (1.25% cholesterol plus 0.5% cholic
acid and 15.8% buffer) for 4 weeks and divided into five groups (10
mice/group): i) ND (mice fed with normal diet), ii) ND + nicotine
(H) (mice fed with normal diet and treated with 6.6 mg/kg/2 days
nicotine), ii) LD (mice fed with lithogenic diet), iii) LD +
nicotine (L) (mice fed with lithogenic diet and treated with 1.1
mg/kg/2 days nicotine), and v) LD + nicotine (H) (mice fed with
lithogenic diet and treated with 6.6 mg/kg/2 days nicotine). Mice
of all groups (except group ND) were treated with nicotine (1.1 mg
and 6.6 mg) for 10 weeks after being weighed every two days
(20-23).
All mice in groups received free access to water and were kept
under the controlled condition at room temperature (22±3˚C), with a
relative humidity of 60-70% and a 12-h light/dark cycle. The mice
were anesthetized with 4% chloral hydrate (300 mg/kg) by
intraperitoneal injection. Mice were sacrificed with 150 mg/kg
pentobarbital sodium by intraperitoneal (i.p.) injection. The
animal experiments were approved by the Institutional Animal Care
and Use Committee of Kunming medical university (approval no.
kmmu2021058).
Collection of gallbladder biles and
gallstone and microscopic studies
At week 10 of the diet feeding, non-fasted animals
were weighed and anesthetized with an i.p. injection of 35 mg/kg
pentobarbital. After cholecystectomy, gallbladder volume was
measured by weighing the whole gallbladder and equating gallbladder
weight (including stones) with gallbladder volume. Gallbladders
were then opened and 5 µl of fresh gallbladder bile was examined
for solid and liquid crystals and gallstones. The pooled
gallbladder biles were centrifuged at 100,000 x g for 30 min at
37˚C and filtered through a preheated (37˚C) Swinnex-GS filter
(0.22 µm) assembly (MilliporeSigma), samples were frozen and stored
at -20˚C for further lipid analyses. Mouse blood was collected from
the vena cava and separated serum samples were stored in a -80˚C
biofreezer. The liver and gallbladder were isolated and frozen in
liquid N2 until required for analysis.
Blood chemical analysis
The serum levels of aspartate aminotransferase
(AST), alanine aminotransferase (ALT), total cholesterol,
HDL-cholesterol, phospholipids and triglycerides were determined
using an automated Hitachi Clinical Analyzer (model 7020; Hitachi
Ltd.).
Lipid analysis
Levels of bile cholesterol, phospholipid, bile salts
as well as cholesterol and triglyceride in bile were determined
respectively following the manufacturer's instructions. Bile from
10 mins' drainage was sampled. Bile cholesterol and phospholipid
concentrations were determined by Cholesterol E-Test Wako kit (cat.
no. 999-02601; FUJIFILM Wako Pure Chemical Corporation) and
Phospholipid C-Test Wako kit (cat. no. 433-36201; FUJIFILM Wako
Pure Chemical Corporation), respectively. The bile salts
concentrations were measured by a Nexera X2 Ultra High-Performance
Liquid Chromatography system (Shimadzu, Kyoto, Japan) according to
the method of Paulusma et al (24). Briefly, 5 µl of diluted bile
(1:100) were spiked with 20 µl of internal standard solution in
methanol:water (1:1 v/v). Protein precipitation was performed with
30 µl of methanol, followed by 10,000 x g centrifugation for 10 min
at 4˚C. The supernatant was used for LC-MS/MS analysis.
Histopathological stain and
Immunohistochemistry analysis
Paraffin-embedded gallbladder and liver sections (5
µm in thickness) were dewaxed twice in xylene solutions for 10 min
each and rehydrated in descending alcohol series. Then paraffin
sections were stained with hematoxylin for 5 min at room
temperature and differentiated with 0.1% hydrochloric acid ethanol
for 1 min, followed with eosin for 5 min at room temperature,
dehydrated in ascending alcohol series, followed by being cleared
twice in xylene solutions for 10 min each. They were observed at
x100 magnification under a light microscope.
To confirm the expression of megalin and FXR, 10%
formalin (48 h at 20-22˚C)-fixed tissues were embedded in paraffin.
The 5 µm paraffin sections were subjected to anti-FXR (1:500, Novus
Biologicals) or anti-megalin antibody (1:200, Abcam) overnight at
4˚C and stained with a DAB (3,3'Diaminobenzidine) kit (MXB
Biotechnologies) for 15 min at 20-22˚C. The positive staining was
taken photograph under a light microscope (magnification, x40).
All samples were blindly inspected by two
independent pathologists. Positive immunostaining was visualized as
brown granules contained in the cytoplasm. The immunostaining was
scored by evaluating the intensity and percentage of positively
stained cells. The intensity of FXR or megalin staining was scored
as follows: 0, none; 1, weak; 2, moderate; and 3, strong. The
percentage scores were assigned, as follows: 1, ≤25; 2, 26-50; 3,
51-75; and 4, >75%. These scores were multiplied to arrive at a
final score ranging between 0 and 12.
Cell culture and treatments
The human gallbladder epithelial cell line GBEC were
purchased from iCell Bioscience Inc. and were cultured in
low-glucose DMEM supplemented with 2 mM glutamine, 1% MEM
non-essential amino acids solution, 1% MEM vitamin solution, 7.5%
FBS and P/S (all from Gibco; Thermo Fisher Scientific, Inc.). The
cells were seeded at ~70-80% confluence in complete media for 24 h
and maintained at 37˚C in 5% CO2. For CDCA and nicotine
treatments, GBECs were plated at 3x104
cells/cm2 for 48 h and subsequently cultured overnight
at 37˚C in the low-serum medium. 10 µM CDCA was added medium for 6
h, then 2 µM nicotine was added. Cells were cultured at 37˚C in 5%
CO2 for another 18 h for reverse
transcription-quantitative (RT-q) PCR and 48 h for western blot
analysis.
RT-qPCR
Total RNA was isolated from 1x105 cells
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) following the manufacturer's instructions and the
concentration of RNA was calculated by spectrophotometry. cDNA was
prepared using the PrimeScript RT reagent kit with gDNA Eraser
(Takara Biotechnology Co., Ltd.). Reverse transcription PCR was
performed u sing SYBR Green Premix Ex Taq (Takara) according to the
manufacturer's protocols Sequences for the primers used are in
Table I. Primer sequences were
synthesized by TsingKe Biological Technology.
 | Table ISequences of primers in the present
study. |
Table I
Sequences of primers in the present
study.
| Gene name | Primer sequence
(5'-3') |
|---|
| FXR | Forward:
GTGGTGGCAAATCCTCCATCAG |
| | Reverse:
AGATTCGCTGGCGTCAGTGTCT |
| CYP7A1 | Forward:
CACCATTCCTGCAACCTTCTGG |
| | Reverse:
ATGGCATTCCCTCCAGAGCTGA |
| CYP7B1 | Forward:
CGGAAATCTTCGATGCTCCAAAG |
| | Reverse:
GCTTGTTCCGAGTCCAAAAGGC |
| CYP8B1 | Forward:
CATGAAGGCTGTGCGTGAGGAA |
| | Reverse:
CATCACGCTGTCCAACACTGGA |
| CYP27A1 | Forward:
TCAGGAGACCATCGGCACCTTT |
| | Reverse:
CCAGTCACTTCCTTGTGCAAGG |
| BSEP | Forward:
CCTTGGTAGAGAAGAGGCGACA |
| | Reverse:
ATGGCTACCCTTTGCTTCTGCC |
| NTCP | Forward:
CCTGATGCCTTTCACTGGCTTC |
| | Reverse:
GGATGGTAGAACAGAGTTGGACG |
| OATP1 | Forward:
GCTGTTCAGTCTTACGAGTGTGC |
| | Reverse:
CAAGGCATACTGGAGGCAAGCT |
| OSTα | Forward:
GCCTGCCATTTTCTCCATCTTGG |
| | Reverse:
CAGCACTGTCATCAGGAAGGTC |
| OSTβ | Forward:
CAAGCATGTTCCTCCTGAGAAGG |
| | Reverse:
CTCTTAGGAAGACCTGGCTGTTG |
| MRP2 | Forward:
TACCAGCGAGTTATCGAAGCGTG |
| | Reverse:
TGCTTCTGACCGCCACTGAGAT |
| MRP3 | Forward:
ACTTCCTCCGAAACTACGCACC |
| | Reverse:
GCTGGCTCATTGTCTGTCAGGT |
| MRP4 | Forward:
CACTCAGGAAACGAACCTTCTCC |
| | Reverse:
TTGCACTGCCTGCGTGTTCTCT |
| NPC1L1 | Forward:
ATCGCACTACCATCCAGGACCTx |
| | Reverse:
CCCAGAGTAGCCTTGGAATCCA |
| ABCG5 | Forward:
TGCCATCCTGACTTACGGAGAG |
| | Reverse:
CTGCTTTGGGTGTCCACTGATG |
| SR-BI | Forward:
ACACCCGAATCCTCGCTGGAAT |
| | Reverse:
CCGTTGGCAAACAGAGTATCGG |
| Megalin | Forward:
CCAATGGACTCACTCTGGACCT |
| | Reverse:
GAATGGAAGGCAGTGCTGATGAC |
| Cubilin | Forward:
TCCGCTTCACATCAGATGGCAG |
| | Reverse:
GGAGCAGTTGAGATTGGGAAGG |
| β-actin | Forward:
CTGTGCCCATCTACGAGGGCTAT |
| | Reverse:
TTTGATGTCACGCACGATTTCC |
For reverse transcription PCR, the PCR mixture was
denatured at 95˚C for 10 sec, annealed at 60˚C for 20 sec and then
extended at 72˚C for 30 sec. This process was repeated for a total
of 40 cycles. The relation of NPC1 like intracellular cholesterol
transporter 1 (NPC1L1) and sterol regulatory element-binding
protein 2 (SREBP-2) mRNA expression with β-actin was calculated
based on the threshold cycle (Ct) values. The relative mRNA
expression of β-actin was calculated by the inverse log of ΔΔCq
(25). All experiments were
performed in triplicate.
Western blot analysis
Protein were lysed using RIPA lysis buffer (Beyotime
Biotechnology, Inc.). Protein concentration was measured using the
bicinchoninic acid (BCA) protein assay (Beyotime Biotechnology,
Inc.). Protein samples (20 µg) were separated on 8% SDS-PAGE gels
and transferred to polyvinylidene difluoride membranes
(MilliporeSigma). Non-specific binding to the membrane was blocked
for 1 h at room temperature with 5% fat-free milk in TBST
(Tris-buffered saline with 0.1% Tween 20 detergent) and then the
membranes were incubated with 1:2,000 FXR primary antibody (cat.
no. NB300-259; Novus Biologicals, LLC), 1:1,000 megalin primary
antibody (cat. no. ab56014; Abcam) and 1:1,000 cubilin primary
antibody (ab251050, Abcam) respectively at 4˚C overnight. Then, the
membrane was washed four times with TBST and incubated with a
1:5,000 dilution of the HRP conjugated secondary antibody (cat. no.
ab205718; Abcam) at room temperature for 45 mins. After the
membrane was washed twice with TBST, membrane-bound antibody was
visualized using an enhanced chemiluminescent kit (MilliporeSigma)
according to manufacturer's instructions. The densities were
quantified via photodensitometric scanning using Quantity One-4.2.3
software (Bio-Rad Laboratories, Inc.).
Statistical analysis
Values are given as the mean ± SD. Statistical
differences between multiple groups were compared using
Kruskal-Wallis analysis. When statistical significance was
identified based on the nonparametric test, Dunn's test was used
for multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of nicotine on the liver
weight-to-body weight ratio and liver function
The liver weight-to-body weight ratio was not
significantly different among the different treatment groups.
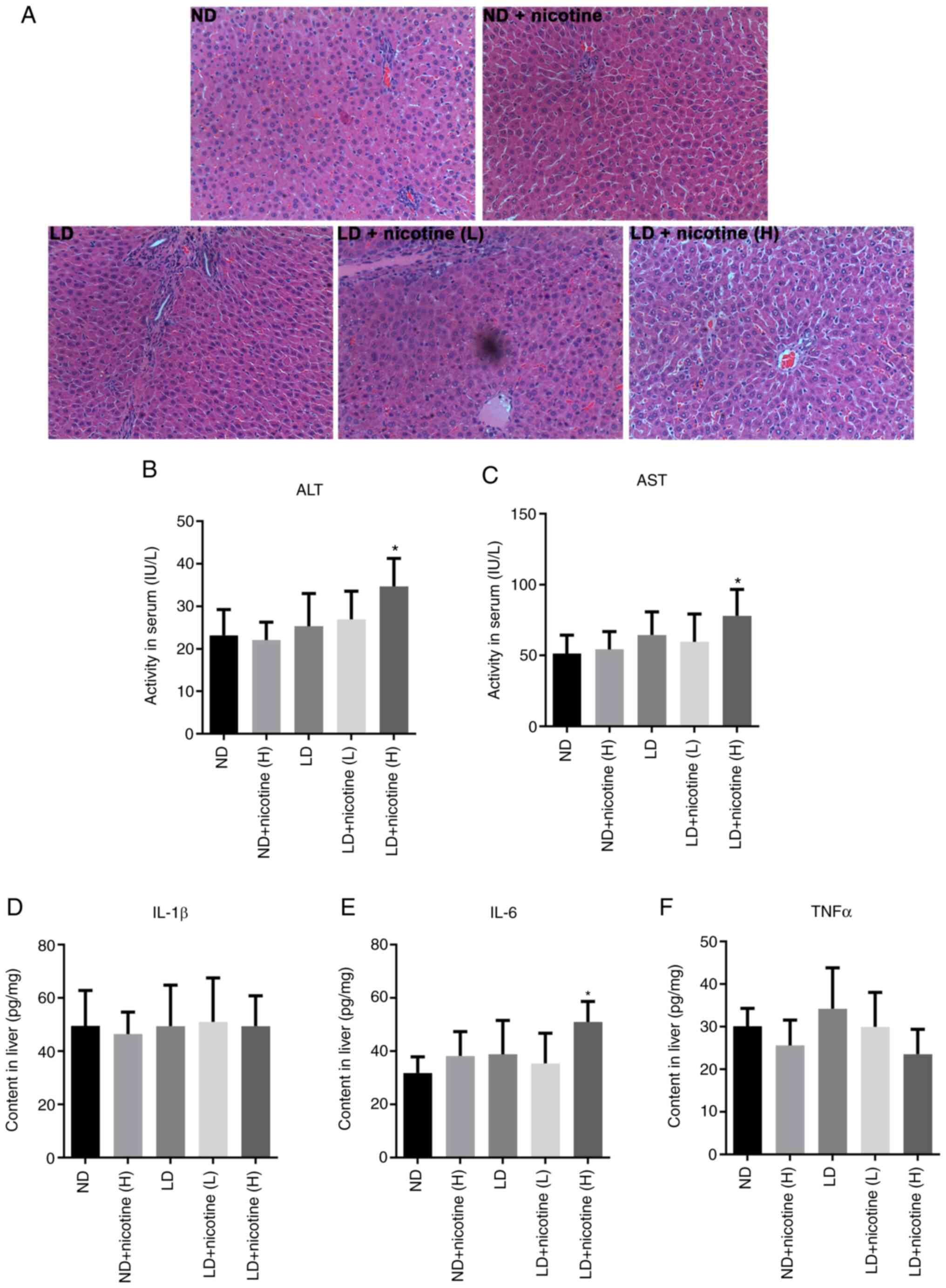
H&E staining of the liver in Fig.
1 did not reveal obvious histomorphological differences among
different groups, except the high-dose nicotine treatment which
caused liver injury (Fig. 1A).
Serum ALT and AST levels did not change after
low-dose nicotine treatment, but increased in the high-dose
nicotine treatment group (Fig. 1B
and C). In addition, no
significant differences were observed in the levels of inflammatory
factors following nicotine treatment (Fig. 1D-F). This suggested that low-dose
nicotine treatment showed no significant toxic side effects on
liver function after 10 weeks.
Effects of nicotine on gallstone
formation
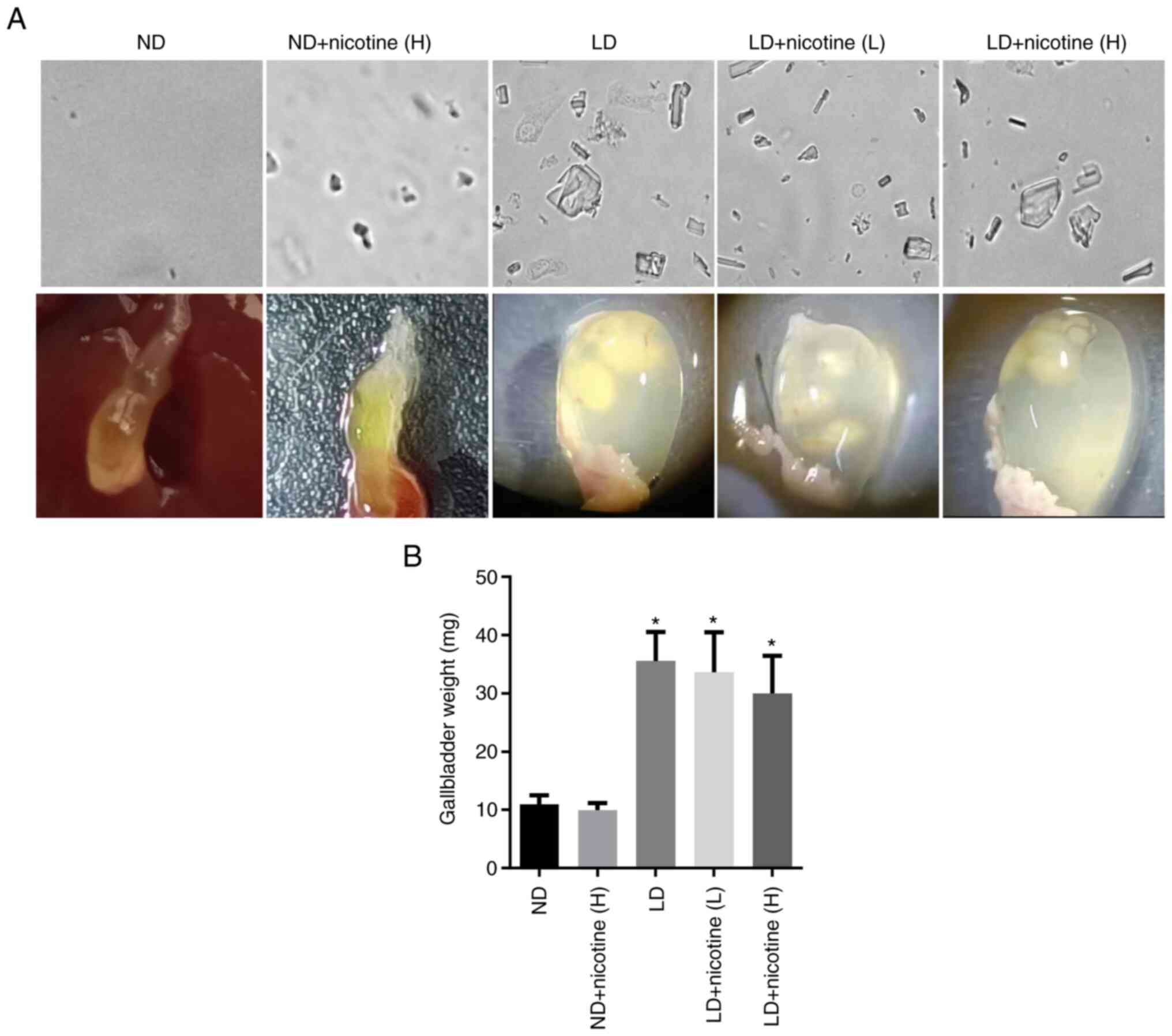
Mice fed with a normal diet treated with high-dose
nicotine showed no abnormal behavior and no gallbladder stone
formation. Crystals were observed in the lithogenic diet groups.
Gross analysis of the gallbladder showed that LD + nicotine groups
did not exhibit significant lower stone formation compared to the
LD group (Fig. 2A). The
gallbladder volume of mice in the LD + nicotine (H) group was lower
than that in the LD group, while low concentrations of nicotine did
not change gallbladder volume (Fig.
2B; Table II).
 | Table IIGallstone formation rate, gallbladder
size and volume in different groups. |
Table II
Gallstone formation rate, gallbladder
size and volume in different groups.
| | Gallbladder
size |
|---|
| Groups | Formation rate
(%) | Length (mm) | Width (mm) | Volume (µl) |
|---|
| ND | 0 | 6.3±0.6 | 6.0±0.50 | 30.02±4.93 |
| ND + nicotine
(H) | 0 | 6.2±0.4 | 5.9±0.7 | 28.95±4.09 |
| LD | 100 | 7.8±1.1 | 3.4±0.4 | 67.00±12.84 |
| LD + nicotine
(L) | 90 | 7.7±0.9 | 5.5±0.9 | 61.50±9.52 |
| LD + nicotine
(H) | 90 | 7.6±1.0 | 6.8±1.8 | 53.82±7.25 |
The effect of nicotine on lipid levels
in bile and serum
Compared with the LD group, the cholesterol level in
the gallbladder bile from the LD + nicotine (H) group was
significantly reduced, (Fig. 3A).
The cholesterol level did not significantly decrease in LD +
nicotine (L) group compared with the LD group. Compared with the LD
group, there were no significant differences in the phospholipids
and bile salts in the bile of the experimental groups (Fig. 3B and C).
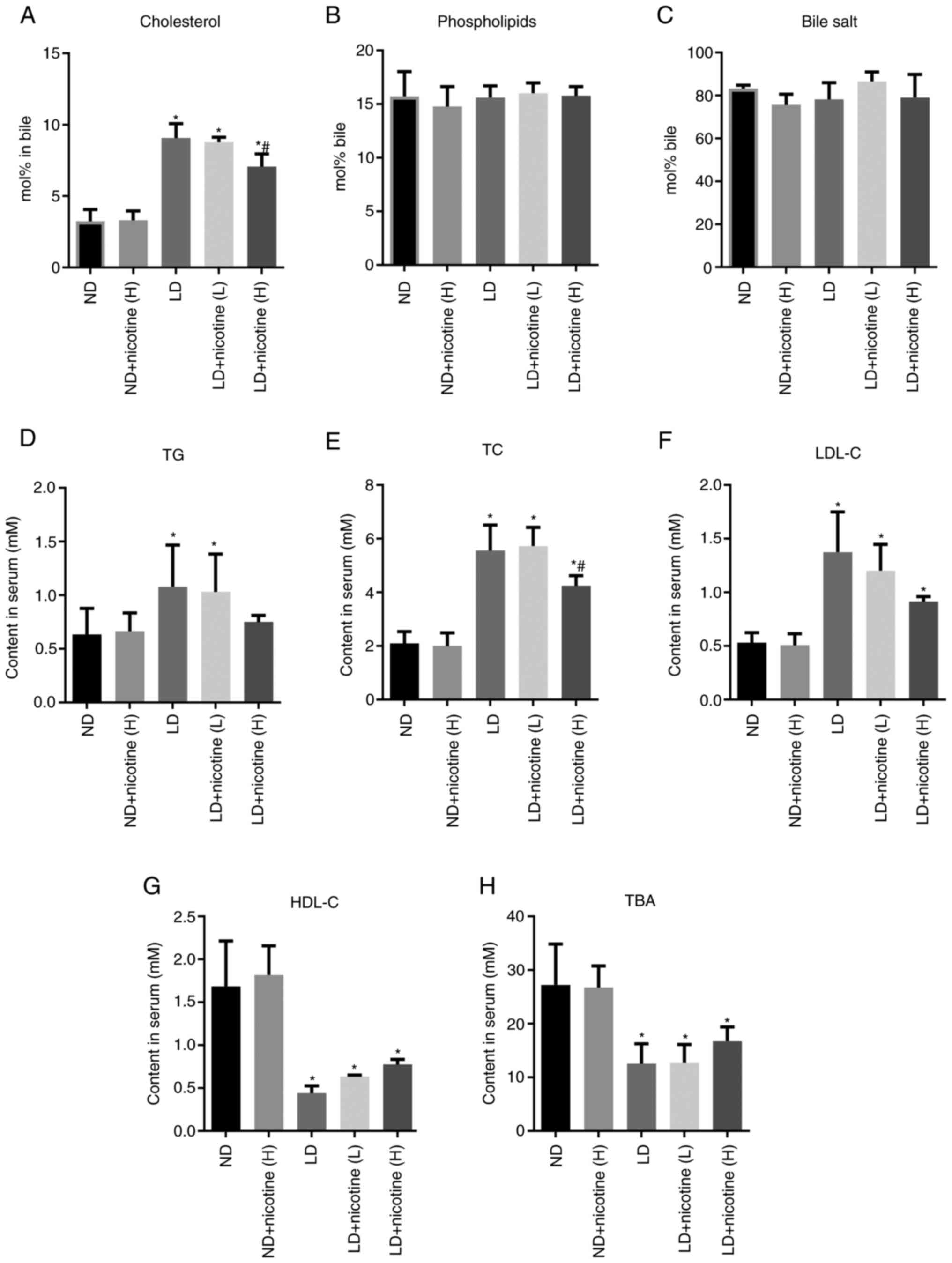 | Figure 3Effects of nicotine on the levels of
cholesterol and lipids in the serum and bile. The bile level of (A)
cholesterol, not (B) phospholipid or (C) bile salts was
significantly elevated in LD groups and reduced in the nicotine
treatment group. Compared with the ND group, the serum levels of
(D) TG, (E) TC and (F) LDL-C increased, while (G) HDL-C and (H) TBA
decreased. Notably, nicotine could attenuate LD-increased TC levels
in LD + nicotine (H) group. Data are presented as the mean ±
standard deviation (n=10 mice per group). *P<0.05 vs.
the ND group; #P<0.05 vs. the LD group. LD,
lithogenic diet; ND, normal diet; H, 6.6 mg/kg/2 days nicotine; L,
1.1 mg/kg/2 days nicotine; TG, triglycerides; TC, total
cholesterol; LDL-C, low-density lipoprotein-cholesterol; HDL-C,
high-density lipoprotein cholesterol; TBA, total bile acids. |
Serum lipid levels between different groups were
also compared, including triglycerides (TG), total cholesterol
(TC), low-density lipoprotein-cholesterol (LDL-C) and high-density
lipoprotein cholesterol (HDL-C) and total bile acid (TBA). The
results showed that compared with the ND group, the serum levels of
TG, TC and LDL-C in the LD group significantly increased, while
HDL-C and TBA significantly decreased (Fig. 3D-H). After low-concentration
nicotine treatment, the levels of these indicators are unchanged.
Compared with the LD group, the serum TG, TC and LDL-C of the mice
treated with the high-dose nicotine group decreased significantly,
while the levels of HDL-C and TBA increased (Fig. 3D-H).
The effect of nicotine on mRNA
expression of BA-related gene in liver tissues
The present study examined major genes involved in
bile acid metabolism in liver tissue from mice with or without
nicotine treatment. As shown in Fig.
4, compared to the ND group, FXR, cholesterol 7α-hydroxylase
(CYP7A1) and oxysterol 7α-hydroxylase (CYP7B1) and
Na+-taurocholate cotransporting polypeptide (NTCP) mRNA
levels increased, while CYP7A1 and sterol regulatory
element-binding protein 2 (SREBP-2) decreased in the LD group.
These effects were reversed by high-dose nicotine treatment
(Fig. 4). Notably, no changes in
some genes expression were observed following nicotine treatment,
including 3-hydroxy-3-methylglutaryl-CoA reductase, sterol
12-α-hydroxylase (CYP8B1), organic solute transporter α (OSTα),
bile salt export pump (BSEP), organic anion transporting
polypeptide 1 (OATP1), multidrug resistance-associated protein
(MRP)2, MRP3, MRP4. This suggests that nicotine may induce changes
in cholesterol in other ways in addition to partially affecting
bile acids.
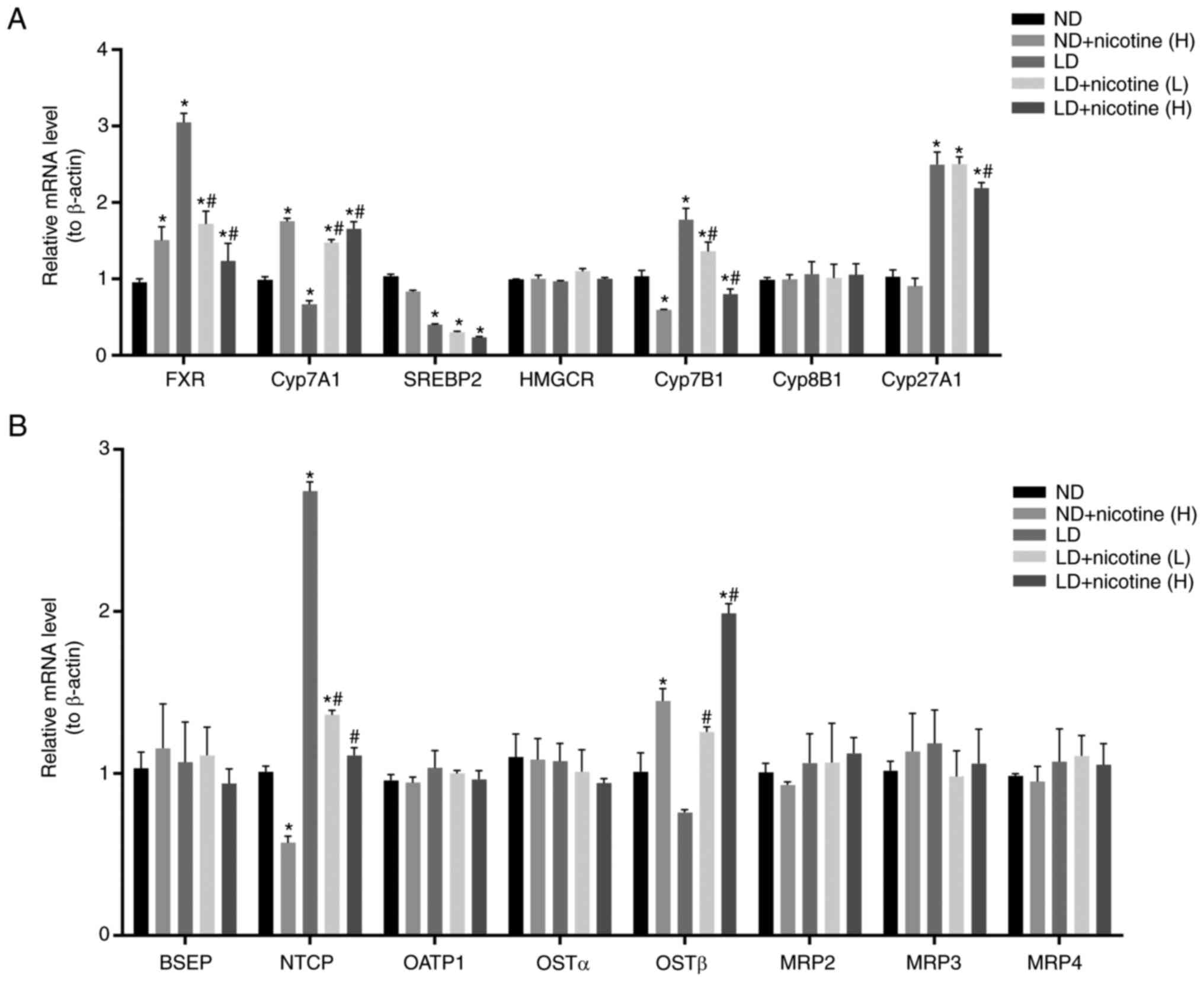 | Figure 4Effect of nicotine on mRNA expression
of bile acid metabolism-related gene in liver tissues. mRNA levels
of (A) bile acid synthesis-related genes and (B) bile acid
transport-related genes in the livers of mice injected with
nicotine were measured by quantitative polymerase chain reaction
(n=3). Data are expressed as the mean ± standard deviation of the
mean. *P<0.05 vs. the ND group; #P<0.05
vs. the LD group. ND, normal diet; LD, lithogenic diet. FXR,
farnesoid X receptor; CYP7A1, cholesterol 7α-hydroxylase; SREBP2.
sterol regulatory element-binding protein 2; HMGCR,
3-hydroxy-3-methylglutaryl-CoA reductase; CYP7B1, oxysterol
7α-hydroxylase; CYP27A1, cytochrome P450 family 27 subfamily A
member 1; BSEP, bile salt export pump; NTCP,
Na+-taurocholate cotransporting polypeptide; OATP1,
organic anion transporting polypeptide 1; OST, organic solute
transporter; MRP, multidrug resistance-associated protein. |
Effect of nicotine on bile acid
metabolism in the gallbladder epithelial tissues
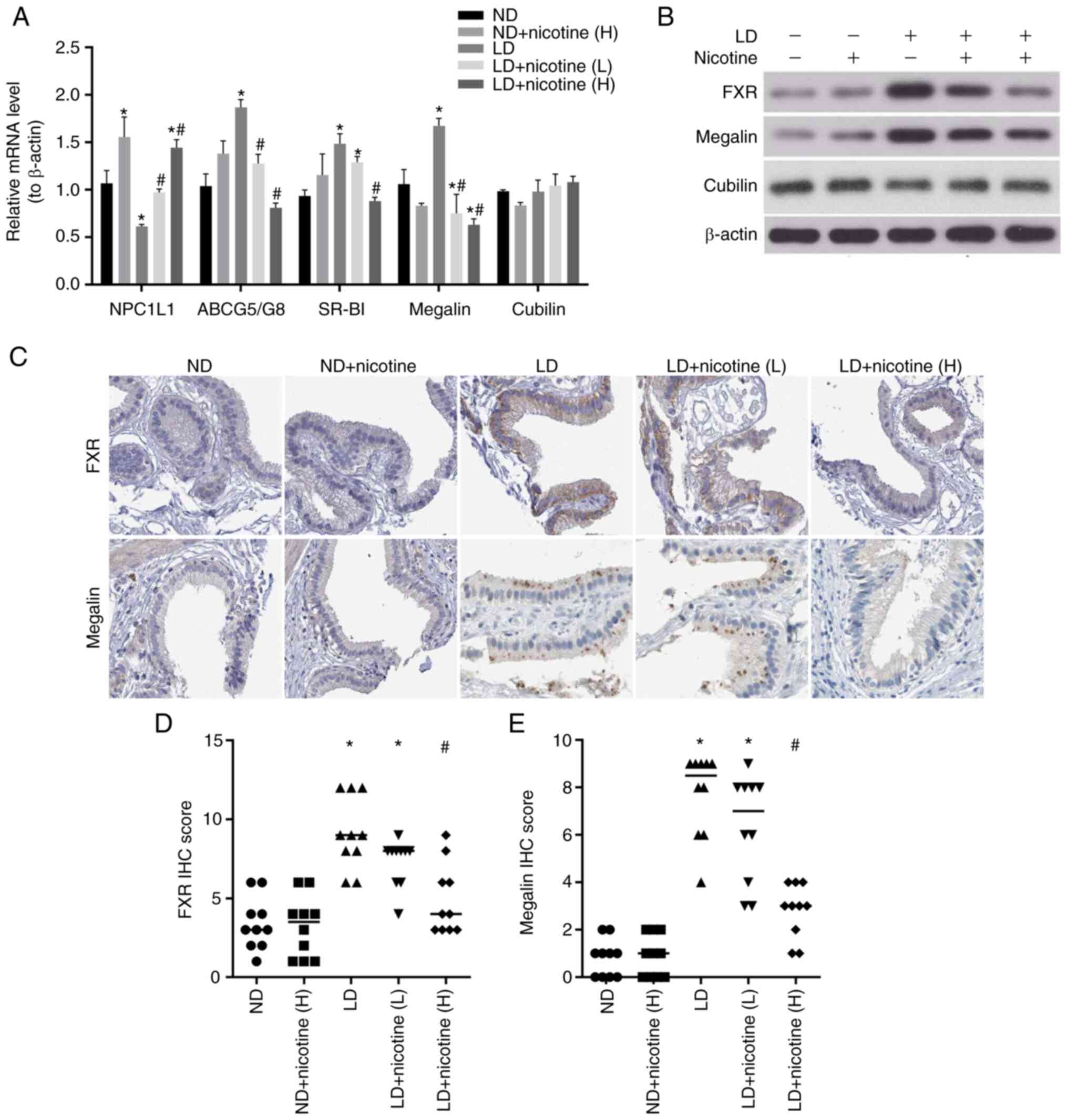
The present study investigated the cholesterol
metabolism in the gallbladder epithelial cells. It detected the
mRNA levels of NPC1L1, ABCG5/G8, SR-BI and megalin/cubilin
expression by RT-qPCR. The results showed that compared with the ND
group, the expression level of NPC1L1 in the LD group significantly
decreased, while megalin, ABCG5/G8 and SR-BI increased. The
administration of low and high concentrations of nicotine could
attenuate their expression levels (Fig. 5A).
Generally, the FXR-megalin/cubilin signaling pathway
regulates cholesterol balance and participates in the formation of
stones (17). Therefore, the
present study also detected the levels of FXR-megalin/cubilin
expression by western blotting and immunohistochemistry. Similar to
mRNA expression, nicotine did not alter cubilin protein levels, but
restored LD-downregulated FXR and megalin levels (Fig. 5B and C). These results suggest that nicotine
can affect the formation process of gallstones by regulating
cholesterol metabolism in the gallbladder.
Effect of nicotine on the regulation
of FXR/megalin/cubilin protein expression in the gallbladder
epithelial cells
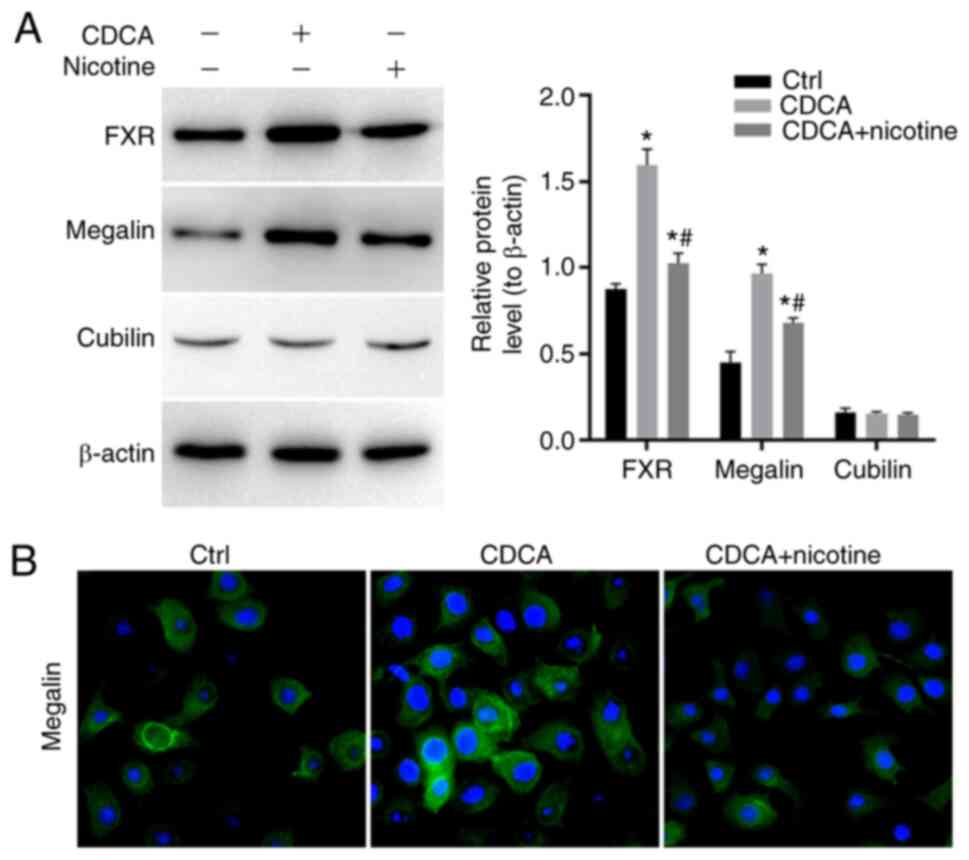
As shown in Fig.
6A, the FXR agonists CDCA increased the expression of megalin
in GBEC. CDCA clearly increased the expression of FXR and megalin
in GBEC, while not changing the expression of cubilin. The nicotine
inhibited CDCA-induced expression of FXR and megalin in GBEC.
Immunofluorescence analyses of megalin expression in GBEC showed
that megalin is expressed in perinuclear and vesicular, decreased
by nicotine (Fig. 6B). It
suggested that nicotine could regulate FXR and megalin, which might
be involved in the regulation of the expression of the genes
related to bile acid metabolism.
Discussion
Nicotine has a little beneficial effect on human
diseases, including colitis and oral ulcers (26-28).
The present study investigated the effects of nicotine on
cholesterol metabolism and gallstone prevention in
gallstone-susceptible C57L mice. It found that nicotine did not
prevent cholesterol gallstone formation, but decreased biliary
cholesterol secretion, retarding phase transition of cholesterol
and that this is likely due to nicotine changing the expression of
FXR/megalin pathway.
The present study first investigated the effects of
nicotine on liver function. H&E staining and liver function
assay showed that nicotine did not induce liver damage. Currently,
research on the effects of nicotine on the liver is inconsistent. A
few studies suggest that nicotine has toxicity in the liver
(29-31).
A longer study period would have allowed for potentially increased
differences in liver damage levels. By contrast, several studies
suggest that chronic nicotine treatment has no significant effect
on the liver (32) and even
alleviates poison-induced liver injury (33-35).
The inconsistency of these findings may be due to differences in
the concentration, duration of nicotine treatment and the types of
substances that induce liver injury.
The present study next examined the effect of
nicotine on gallstone formation and gallbladder bile composition.
Neither inhibitory nor increased effect of nicotine on the rate of
gallstone formation was observed in LD diet mice. Almost all
studies suggest that smoking increases the progression of
gallbladder disease, including gallstones (36,37).
However, earlier research showed that nicotine can protect against
the formation of gallstones (9,38).
There may be two reasons for this: i) As well as nicotine, tobacco
contains a variety of other substances that promote gallstone
formation. ii) Smoking prolongs the maximal gallbladder emptying
time and delays gallbladder contraction, then results in bile
stasis in gallbladder, which causes most gallbladder disorders
(39).
The effect of nicotine on bile cholesterol
metabolism was investigated. There were significant changes in the
composition of bile after 10 weeks of nicotine treatment with a
reduction in total bile cholesterol concentrations. The data showed
high concentrations of nicotine increased the TBA levels. Since
gallbladder volume and bile secretion were similar before and after
nicotine treatment, the increase in TBA may be caused by changes in
the ratio of different types of BAs. The rise in total BA
concentration in bile tends to reduce gallstone formation by
increasing cholesterol solubility (40,41).
FXR can regulate the synthesis of BAs in a
tissue-specific manner, regulating bile acid reabsorption,
maintaining bile acid cycle homeostasis and reducing cholesterol
and fat production (42). In the
present study, except BA synthase FXR, CYP7A1 and CYP7B1 and efflux
transporters NTCP and organic solute transporter β (OSTβ), nicotine
did not change the expression of the efflux transporter BSEP,
OATP1, OSTα, MRP2/3/4 and alternative pathway synthase cytochrome
P450 Family 27 Subfamily A Member 1 (CYP27A1) and CYP8B1. In
addition to bile acid metabolism mediated by the enterohepatic
circulation, a study suggested that attention should be paid to
cholesterol metabolism in gallbladder epithelial cells (43), as the gallbladder has the capacity
to actively absorb cholesterol from bile and thereby modulate bile
cholesterol content. Thus far, the expression of cubilin and
megalin, ATP binding cassette subfamily G (ACBG)5/8, scavenger
receptor class B type I (SR-BI), has been reported in the apical
side of gallbladder epithelial cells (44). However, further elucidation of the
mechanism is required.
The qPCR data showed that nicotine could attenuate
LD-downregulation of NPC1L1 and reduce the expression of megalin,
ABCG5/G8 and SR-BI. In addition, megalin and cubilin, which are
expressed on the gallbladder but not on hepatocytes, are also
regulated by bile acids and their receptor FXR (16). This indicates that
FXR/megalin/cubilin may serve a central role in the pathophysiology
of gallstones. In the study of Tsaroucha et al (15), the megalin and cubilin mRNA levels
are low in gallstone tissues, but the study of Erranz et al
(16) showed megalin protein
levels were upregulated by a lithogenic diet. This is mainly
because CA, not cholesterol, increases the expression of megalin,
but does not affect the expression of cubilin (16). The present study used a similar
lithogenic diet, which contained the natural FXR agonists CA. The
data demonstrated that FXR/megalin protein expression increased in
the LD group during gallstone formation, low or high-dose nicotine
could decrease megalin mRNA and protein expression, but cubilin
expression did not change. These data indicated that nicotine may
act on cholesterol metabolism in gallbladder cells and may be
involved in the process of gallstones formation. However, further
assessment of the direct effect of megalin and cubilin regulated by
nicotine on gallstone formation is required.
There are several limitations to the present study.
First, it did not conduct an in-depth analysis for microscopic
examination of the gallbladder and list all abnormal findings such
as cells, crystals or other components. Second, it did not analyze
the difference in stones composition and load between the three
groups. Third, although a number of useful aspects of the
lithogenic process can be studied using animal models, they are not
100% identical to humans. Moreover, a number of changes apparently
associated with gallstone formation may be a function of
heredity.
The present study demonstrated that nicotine did not
prevent LD-induced gallstone formation. However, nicotine could
regulate FXR, sterol regulatory element-binding protein 2, CYP7A1,
NTCP and OSTβ in the liver and ABCG5/8, NPC1L1, SR-BI and megalin
in the gallbladder. These genes are associated synthesis,
transformation and transportation of bile acid. Thus, despite
unlikely therapeutic applications, nicotine might have potential
beneficial effects for anti-lithogenic activity.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by The Research
Foundation of China Tobacco Company [grant no. 110201901021
(JY-08)]; The Research Foundation of China Tobacco Yunnan
Industrial Co., Ltd. (grant no. 2020539200340213) and Yunnan
Provincial Fund for High Level Reserve Talents in Health Science
(grant no. H-2018068).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
QG, XL and BY conceived and designed the study. QG,
PB and QM contributed to the experiments, acquisition of data and
comments and editorial review of the manuscript. YG and JJ analyzed
data and revised the manuscript critically for important
intellectual content. QG, PB, XL and BY contributed to
interpretation of data and drafted the article. All authors read
and approved the final version of the manuscript. BY and QG confirm
the authenticity of all the raw data.
Ethics approval and consent to
participate
The animal experiments were approved by the
Institutional Animal Care and Use Committee of Kunming Medical
University (approval no. kmmu2021058).
Patient consent for publication
Not applicable.
Competing interests
Note that QG, QM, YG, JJ and XL are employees of the
Yunnan Key Laboratory of Tobacco Chemistry, R&D Center of China
Tobacco Yunnan Industrial Co. Ltd. who also funded the present
study, which explored the effect of nicotine on cholesterol
gallstone formation in C57BL/6J mice fed on a lithogenic diet.
References
|
1
|
Di Ciaula A and Portincasa P: Recent
advances in understanding and managing cholesterol gallstones.
F1000Res. 7(F1000 Faculty Rev-1529)2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hsing AW, Gao YT, Han TQ, Rashid A, Sakoda
LC, Wang BS, Shen MC, Zhang BH, Niwa S, Chen J and Fraumeni JF Jr:
Gallstones and the risk of biliary tract cancer: A population-based
study in China. Br J Cancer. 97:1577–1582. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sutherland JM, Mok J, Liu G, Karimuddin A
and Crump T: A cost-utility study of laparoscopic cholecystectomy
for the treatment of symptomatic gallstones. J Gastrointest Surg.
24:1314–1319. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rebholz C, Krawczyk M and Lammert F:
Genetics of gallstone disease. Eur J Clin Invest.
48(e12935)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Arrese M, Cortés V, Barrera F and Nervi F:
Nonalcoholic fatty liver disease, cholesterol gallstones, and
cholecystectomy: New insights on a complex relationship. Curr Opin
Gastroenterol. 34:90–96. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yuan S, Giovannucci EL and Larsson SC:
Gallstone disease, diabetes, calcium, triglycerides, smoking and
alcohol consumption and pancreatitis risk: Mendelian randomization
study. NPJ Genom Med. 6(27)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kono S, Eguchi H, Honjo S, Todoroki I, Oda
T, Shinchi K, Ogawa S and Nakagawa K: Cigarette smoking, alcohol
use, and gallstone risk in Japanese men. Digestion. 65:177–183.
2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kim HS, Cho SK, Kim CS and Park JS: Big
data and analysis of risk factors for gallbladder disease in the
young generation of Korea. PLoS One. 14(e0211480)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Rhodes M and Venables C: Symptomatic
gallstones-a disease of non-smokers? Digestion. 49:221–226.
1991.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jørgensen T: Gall stones in a Danish
population. Relation to weight, physical activity, smoking, coffee
consumption, and diabetes mellitus. Gut. 30:528–534.
1989.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mohr GC, Kritz-Silverstein D and
Barrett-Connor E: Plasma lipids and gallbladder disease. Am J
Epidemiol. 134:78–85. 1991.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Claudel T, Zollner G, Wagner M and Trauner
M: Role of nuclear receptors for bile acid metabolism, bile
secretion, cholestasis, and gallstone disease. Biochim Biophys
Acta. 1812:867–878. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cedó L, Farràs M, Lee-Rueckert M and
Escolà-Gil JC: Molecular insights into the mechanisms underlying
the cholesterol-lowering effects of phytosterols. Curr Med Chem.
26:6704–6723. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rudling M, Laskar A and Straniero S:
Gallbladder bile supersaturated with cholesterol in gallstone
patients preferentially develops from shortage of bile acids. J
Lipid Res. 60:498–505. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tsaroucha AK, Chatzaki E, Lambropoulou M,
Despoudi K, Laftsidis P, Charsou C, Polychronidis A, Papadopoulos N
and Simopoulos CE: Megalin and cubilin in the human gallbladder
epithelium. Clin Exp Med. 8:165–170. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Erranz B, Miquel JF, Argraves WS, Barth
JL, Pimentel F and Marzolo MP: Megalin and cubilin expression in
gallbladder epithelium and regulation by bile acids. J Lipid Res.
45:2185–2198. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Marzolo MP and Farfán P: New insights into
the roles of megalin/LRP2 and the regulation of its functional
expression. Biol Res. 44:89–105. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Housset C, Chrétien Y, Debray D and
Chignard N: Functions of the gallbladder. Compr Physiol.
6:1549–1577. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Willnow TE and Christ A: Endocytic
receptor LRP2/megalin-of holoprosencephaly and renal Fanconi
syndrome. Pflugers Arch. 469:907–916. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tsounapi P, Honda M, Dimitriadis F, Kimura
Y, Shimizu S, Kawamoto B, Hikita K, Saito M, Sofikitis N and
Takenaka A: MP07-15 alterations in oxidative stress parameters in
the testis and epididymis in a nicotine-exposed rat model. Can
nicotine-abstinence overcome the oxidative damage? J Urol.
197(e87)2017.
|
|
21
|
Moschetta A, Bookout AL and Mangelsdorf
DJ: Prevention of cholesterol gallstone disease by FXR agonists in
a mouse model. Nat Med. 10:1352–1358. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Young JW, Finlayson K, Spratt C, Marston
HM, Crawford N, Kelly JS and Sharkey J: Nicotine improves sustained
attention in mice: Evidence for involvement of the alpha7 nicotinic
acetylcholine receptor. Neuropsychopharmacology. 29:891–900.
2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Berrendero F, Mendizábal V, Robledo P,
Galeote L, Bilkei-Gorzo A, Zimmer A and Maldonado R:
Nicotine-induced antinociception, rewarding effects, and physical
dependence are decreased in mice lacking the preproenkephalin gene.
J Neurosci. 25:1103–1112. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Paulusma CC, Groen A, Kunne C, Ho-Mok KS,
Spijkerboer AL, Rudi de Waart D, Hoek FJ, Vreeling H, Hoeben KA,
van Marle J, et al: Atp8b1 deficiency in mice reduces resistance of
the canalicular membrane to hydrophobic bile salts and impairs bile
salt transport. Hepatology. 44:195–204. 2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Baron JA: Beneficial effects of nicotine
and cigarette smoking: The real, the possible and the spurious. Br
Med Bull. 52:58–73. 1996.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wolf R, Wolf D and Ruocco V: The benefits
of smoking in skin diseases 1. Clin Dermatol. 16:641–647.
1998.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wasilewski A, Zielińska M, Storr M and
Fichna J: Beneficial effects of probiotics, prebiotics, synbiotics,
and psychobiotics in inflammatory bowel disease. Inflamm Bowel Dis.
21:1674–1682. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mosher LR: Nicotinic acid side effects and
toxicity: A review. Am J Psychiatry. 126:1290–1296. 1970.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rutledge SM and Asgharpour A: Smoking and
liver disease. Gastroenterol Hepatol (NY). 16:617–625.
2020.PubMed/NCBI
|
|
31
|
Li G, Chan YL, Wang B, Saad S, George J,
Oliver BG and Chen H: E-cigarettes damage the liver and alter
nutrient metabolism in pregnant mice and their offspring. Ann N Y
Acad Sci. 1475:64–77. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yue J, Miksys S, Hoffmann E and Tyndale
RF: Chronic nicotine treatment induces rat CYP2D in the brain but
not in the liver: An investigation of induction and time course:
2006 Innovations in neuropsychopharmacology award. J Psychiatry
Neurosci. 33:54–63. 2008.PubMed/NCBI
|
|
33
|
Chen XM, Li FQ, Yan S, Wu XC and Tang CL:
Nicotine alleviates the liver inflammation of non-alcoholic
steatohepatitis induced by high-fat and high-fructose in mice.
Beijing Da Xue Xue Bao Yi Xue Ban. 48:777–782. 2016.PubMed/NCBI(In Chinese).
|
|
34
|
Zhang D, Dai J, Cao Y, Wang Z and Qiao Z
and Qiao Z: Nicotine exposure of male mice protects offspring
against carbon tetrachloride-induced acute liver injury. J Biochem
Mol Toxicol. 36(e23069)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhao J, Park S, Kim JW, Qi J, Zhou Z, Lim
CW and Kim B: Nicotine attenuates concanavalin A-induced liver
injury in mice by regulating the α7-nicotinic acetylcholine
receptor in Kupffer cells. Int Immunopharmacol.
78(106071)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Shabanzadeh DM and Novovic S: Alcohol,
smoking and benign hepato-biliary disease. Best Pract Res Clin
Gastroenterol. 31:519–527. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Aune D, Vatten LJ and Boffetta P: Tobacco
smoking and the risk of gallbladder disease. Eur J Epidemiol.
31:643–653. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kono S, Shinichi K, Ikeda N, Yanai F and
Imanishi K: Prevalence of gallstone disease in relation to smoking,
alcohol use, obesity, and glucose tolerance: A study of
self-defense officials in Japan. Am J Epidemiol. 136:787–794.
1992.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Degirmenci B, Albayrak R, Haktanir A, Acar
M and Yucel A: Acute effect of smoking on gallbladder emptying and
refilling in chronic smokers and nonsmokers: A sonographic study.
World J Gastroenterol. 12:5540–5543. 2006.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Trautwein EA, Kunath-Rau A and
Erbersdobler HF: Increased fecal bile acid excretion and changes in
the circulating bile acid pool are involved in the
hypocholesterolemic and gallstone-preventive actions of psyllium in
hamsters. J Nutr. 129:896–902. 1999.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Di Ciaula A, Garruti G, Lunardi Baccetto
R, Molina-Molina E, Bonfrate L, Wang DQ and Portincasa P: Bile acid
physiology. Ann Hepatol. 16 (Suppl 1):s4–s14. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Stofan M and Guo GL: Bile acids and FXR:
Novel targets for liver diseases. Front Med (Lausanne).
7(544)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Dikkers A and Tietge UJF: The neglected
cousin of the hepatocyte: How gallbladder epithelial cells might
contribute to cholesterol gallstone formation. Dig Dis Sci.
58:296–298. 2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Miquel J, Moreno M, Amigo L, Molina H,
Mardones P, Wistuba II and Rigotti A: Expression and regulation of
scavenger receptor class B type I (SR-BI) in gall bladder
epithelium. Gut. 52:1017–1024. 2003.PubMed/NCBI View Article : Google Scholar
|