Introduction
Recent advances in endoscopic diagnosis have increased the detection rate of superficial gastrointestinal cancer. Additionally, with the standardization of treatment technology and advancement of devices, the results of endoscopic treatment centered on endoscopic submucosal dissection (ESD) are also improving (1). In Japan, esophageal ESD-endoscopic treatment for superficial squamous cell carcinoma of the esophagus (SSCE)-was first reported by Oyama in 2005(2) and was covered by insurance in 2008. Currently, ESD is standardized and the first treatment option for SSCE. With increasing ESD performance, the 5-year survival rate of SSCE in Japan has dramatically improved to ~80% (3,4). The standard treatment for advanced esophageal cancer is surgical resection or chemoradiotherapy (CRT) (5). Surgical resection is curative; however, postoperative quality of life (QOL) (6) often reduces because of its high invasiveness. In patients undergoing CRT, QOL is easily maintained and the response rate to squamous cell carcinoma is high. Even with the infiltration depth of T1, the local residual recurrence rate after CRT is as high as ~30% (7), and deeper infiltration degrees lead to higher local recurrence rates (8), indicating that local control is difficult. There is no standard treatment for locally recurrent lesions after CRT; however, additional surgical resection is often selected for intolerant patients. Nevertheless, the risk of suture failure increases because of irradiation, and the mortality rate due to perioperative complications is high (7-12%) (9,10). Moreover, lymph node metastases and metastases to other organs are often observed during recurrence. Hence, surgery is not indicated in many cases.
To address these problems, salvage endoscopic resection has become a treatment modality for residual recurrent lesions after CRT (11). Minimally invasive endoscopic treatment can achieve high long-term survival without severe complications (12,13). Moreover, photodynamic therapy (PDT) is a valuable treatment option for treating local residual/recurrent esophageal cancer after CRT (14-17). PDT, a local treatment modality, uses a tumor-affinitive photosensitizer (PS) that is selectively incorporated into cancer cells and a laser beam with a wavelength matching the absorption wavelength of PS that is irradiated to cause a photochemical reaction within the tumor and destroy the tumor cells (17). Currently in Japan, PDT is covered by insurance for lung cancer (early stage, advanced cancer), malignant brain tumor, early esophageal cancer, recurrent esophageal cancer, stomach cancer, and early cervical cancer. We are actively practicing salvage endoscopic treatment at our hospital; moreover, we started performing salvage PDT (sal-PDT) in 2007 as advanced medical care. This study evaluated the long-term treatment results after conventional ESD for SSCE and after salvage endoscopic treatment for locally recurrent lesions after CRT. We also introduced treatment strategies for esophageal cancer, including salvage endoscopic treatment.
Materials and methods
Ethics statements
This study was approved by the Nagasaki University Hospital Ethics Committee (protocol code: 21041908, approval date: April 20, 2021), and written informed consent was obtained from all enrolled patients.
Conventional esophageal ESD. Patients
Patients with SSCE (clinical stage 0, cT1aN0M0) treated with ESD between June 2007 and August 2019 at Nagasaki University Hospital were enrolled in this study. Patients with Barrett's adenocarcinoma, intraepithelial neoplasia, and follow-up period <6 months were excluded. For preoperative diagnosis of superficial SSCE, routine endoscopy, Lugol-stained endoscopy [0.5% Lugol solution (Lugol)], narrow-band imaging (NBI), magnifying endoscopy, computed tomography (CT), and pretreatment positron emission tomography (PET) were performed. In addition, we retrospectively analyzed the recurrence rate of metastasis and long-term prognosis.
ESD procedure
ESD was performed with intravenous anesthesia using midazolam and pethidine, and the dose was adjusted appropriately according to the patient's degree of sedation and pain. We sprayed 0.5% of Lugol and marked the cancer-free margin. Then, we injected 5 ml of purified sodium hyaluronate (MucoUp®; Boston Scientific, Marlborough, MA, USA) with 0.0025% epinephrine into the submucosa, made a mucosal incision, and performed submucosal dissection using Flash Knife BT-S (DK2620JI; Fujifilm Medical Co., Ltd., Tokyo, Japan). During the entire procedure, carbon dioxide was used as insufflating gas. En-bloc resection includes all marks, and an en-bloc resection with histologically cancer-free margins is defined as complete resection (R0) (18). Complications included postoperative bleeding and delayed perforation, in addition to bleeding and perforation during treatment. Bleeding was defined as the need for blood transfusion due to a decrease in the hemoglobin level of 2 g/dl. Perforation was diagnosed when space was observed endoscopically through a visual hole, subcutaneous emphysema was present, and/or CT showed mediastinal emphysema after ESD.
Salvage endoscopic treatment. Patients
Patients who underwent salvage endoscopic treatment at Nagasaki University Hospital between June 2007 and August 2019 were enrolled in this study. Patients with a follow-up period of <6 months after salvage endoscopic treatment were excluded. The salvage endoscopic treatment was indicated for patients with advanced esophageal cancer who had local recurrence after CRT and had no evident lymph node or multi-organ metastasis confirmed by CT or PET-CT. Patients underwent endoscopic depth assessment (white light imaging, NBI magnified imaging, and endoscopic ultrasound sonography) and salvage ESD if no apparent deep sub-mucosal (SM) invasion was observed. In addition, salvage PDT was performed for SM infiltration cases, or cases judged difficult for ESD due to severe fibrosis. All patients were allowed to choose other treatment modalities, including salvage endoscopic treatment and additional surgical resection, and consented cases were included in the treatment.
Sal-ESD/Sal-PDT procedure
Salvage endoscopic treatment was defined as endoscopic treatment (ESD/PDT) performed for a lesion that recurred locally after irradiation for advanced esophageal cancer with over 50 Gy radiation. Sal-ESD was performed in the same manner as conventional ESD. In addition to the cases without lymph node/other organ metastasis, sal-PDT is indicated for lesions that meet all of the following conditions: the wall depth of the residual recurrent lesion remains at T2, the central axis is less than 3 cm, circumference is less than half, and the lesion has not infiltrated the cervical esophagus (17). We used talaporfin sodium (40 mg/m2, Rezaphyrin®; Meiji Seika Pharma Co., Ltd., Tokyo, Japan) as a photosensitizer, and 4-6 h after intravenous administration of talaporfin sodium, the residual recurrent lesion was irradiated with a PD laser under endoscopic guidance with the LASEREO endoscope system (Fujifilm Co.) and EG-L590ZW (Fujifilm Co.) for PDT. After marking around the lesion with argon plasma coagulation (APC), we started irradiation (power, 150 mW; 100 J/cm2 per location) from the anal side of the lesion and moved toward the oral side (17). Endoscopic follow-up was performed again on the day after PDT, and additional irradiation was performed in the range of 60-400 J when it was judged to be insufficient. Patients were instructed to avoid the sun for 2 weeks after dosing because talaporfin sodium can cause photosensitivity (19).
Histological evaluation after ESD
Excised specimens were soaked in 4% formalin for pathological examination, embedded in paraffin, cut vertically at 2-mm intervals, and stained with hematoxylin and eosin. The histopathological evaluation included the tumor size, invasion depth, lymphovascular invasion (LVI), degree of differentiation, and horizontal/vertical stump diagnosed according to the Japanese Classification of Esophageal Cancer (20). Infiltration depths were classified as epithelium (EP), lamina propria (LPM), muscular mucosae (4), superficial submucosal tissue (≤200 µm, SM1), and deep submucosal tissue (>200 µm, SM2). The final histological diagnosis was reached by agreement between at least two pathologists.
Patient follow-up
Adjuvant therapy (surgery or chemoradiotherapy) after conventional ESD has been proposed for non-curative resection cases (one or more of positive lymphovascular invasion, SM massive infiltration, and pathological infiltration (INF)-β or γ). Patients with curative resection underwent endoscopic follow-up and clinical visits every 3-6 months for the first year. One year later, they continued follow-up every 4-6 months, depending on the degree of invasion. Patients who underwent salvage endoscopic treatment continued follow-up endoscopy every 3-4 months. Local recurrence was defined as a tumor that developed within the ESD scar. Asynchronous recurrence was defined as a tumor that recurred at a new site after ≥6 months of complete remission. Every patient also underwent chest and abdominal CT, performed annually to detect metastases. Especially for the patients who underwent salvage PDT, in addition to endoscopic observation, the biopsy was performed randomly from the treated area to evaluate the presence or absence of recurrence. Local complete response (L-CR) was defined as no recurrence in endoscopic findings, biopsy results, and CT findings. We assessed patient survival after endoscopic treatment through regular clinic visits, medical records, or telephone contact until December 2019.
Statistical analysis
All statistical analyses were performed using JMP® Pro 17.0.0 for MacOS (SAS Institute Inc., Cary, NC, USA). Cumulative cancer-related survival was estimated using the Kaplan-Meier curve and assessed using the log-rank test. In addition, the Mann-Whitney U test or Pearson's chi-square test was used to examine the differences between the groups. A P-value of <0.05 was considered statistically significant.
Results
Conventional ESD. Patients
Conventional ESD was performed for superficial esophageal cancer in 565 patients (910 lesions) from September 2007 to July 2019 at Nagasaki University Hospital (Fig. 1). After excluding patients with an observation period <6 months and cancers other than squamous cell carcinoma (Barrett's cancer or intraepithelial neoplasia), 480 patients (825 lesions) were examined. Overall, 392 patients (81.7%, 676 lesions) underwent curative resection. The patients with a non-curative resection were allowed to choose additional treatment (CRT or surgical resection) after they were provided sufficient information regarding the procedures. Patients were more inclined towards choosing CRT, which is relatively less invasive, particularly elderly patients and patients with underlying general conditions (such as patients with underlying conditions unsuitable for general anesthesia). In total, CRT was performed in 65 patients, and surgical resection was performed in 11 patients as an additional treatment for lesions with non-curative resection of ESD.
 |
Figure 1
Study flowchart of the conventional ESD. From September 2007 to July 2017, 565 patients (910 lesions) were treated with conventional esophageal ESD at Nagasaki University Hospital. Curative resection was performed in 392 patients (81.7%, 676 lesions), and 88 patients (149 lesions) underwent non-curative resection. For additional therapy, chemoradiotherapy was performed in 65 patients and surgical resection was performed in 11 patients. SSCE, superficial squamous cell carcinoma of the esophagus; CRT, chemoradiotherapy; ESD, endoscopic submucosal dissection.
|
Treatment results
Background characteristics, short-term outcomes, and adverse events of patients who underwent curative resection are shown in Table I. The en-bloc curative resection rate was 89.5% (606/676). Treatment complications were bleeding (0.6%, 4/676), perforation (0.4%, 3/676), and stenosis (10.7%, 72/676). No local recurrence was observed in the curatively resected cases. The background characteristics of patients who received additional treatment are shown in Table II. More than half of the cases were deeper than pT1a-MM/pT1b-SM1 and positive for LVI, and lymph node/other organ metastases were found in six cases at the time of additional treatment. The adverse events included bleeding 0.6% (4/676), perforation 0.4% (3/676), and stenosis 10.7% (72/676). For the prevention of stenosis, in the cases where the excision diameter exceeded 3/4 of the circumference, local injection of steroid was administered at the ulcer base after excision. In patients who underwent circumferential resection, steroids were systemically administered in addition to their local injection (18).
 |
Table I
Characterization of curative resection cases.
|
Table I
Characterization of curative resection cases.
| Variable |
Value (392 cases, 676 lesions) |
| Age, years (range) |
68 (41-90) |
| Sex, n (%) |
|
| Male |
318 (81.1) |
| Female |
74 (18.9) |
| Location, n (%) |
|
| Cervical/Upper |
38 (9.0) |
| Middle thoracic |
228 (58.2) |
| Lower thoracic/Abdominal |
126 (32.1) |
| Circumferential extension, n (%) |
|
| <3/4 |
428 (63.3) |
| ≥3/4 |
248 (36.7) |
| Median tumor size, mm (range) |
25 (2-110) |
| En-block curative resection rate (%) |
89.5 (606/676) |
| Observation months after ESD (range) |
54.4 (12-145) |
| Adverse event, n (%) |
|
| Bleeding |
4 (0.6) |
| Perforation |
3 (0.4) |
| Stenosis |
72 (10.7) |
 |
Table II
Characterization of additional therapy after endoscopic submucosal dissection.
|
Table II
Characterization of additional therapy after endoscopic submucosal dissection.
| Variable |
CRT (n=65) |
Surgery (n=11) |
Total (n=76) |
| Age, years (range) |
67 (50-83) |
60 (52-72) |
60 (50-83) |
| Sex, n (%) |
|
|
|
| Male |
56 (86.2) |
10 (90.1) |
66 (86.8) |
| Female |
9 (13.8) |
1 (9.1) |
10 (13.2) |
| Depth of tumor invasion, n (%) |
|
|
|
| EP-LPM |
4 (6.2) |
1 (9.1) |
5 (6.6) |
| MM/SM1 |
40 (61.5) |
4 (36.4) |
44 (57.9) |
| ≥SM2 |
21 (32.3) |
6 (54.5) |
27 (33.5) |
| Lymphovascular invasion, n (%) |
33 (50.8) |
8 (72.7) |
41 (53.9) |
| Vertical margin positive, n (%) |
11 (16.9) |
4 (36.4) |
15 (19.7) |
| Observation months after ESD (range) |
56.5 (6-133) |
77.5 (30-139) |
58.8 (6-139) |
| Adverse event, n (%) |
|
|
|
| Bleeding |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Perforation |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Local recurrence rate after ESD, n (%) |
1 (1.5) |
0 (0.0) |
1 (1.3) |
| LN and/or another organ metastasis, n (%) |
5 (7.7) |
1 (9.1) |
6 (7.9) |
Long-term prognosis after conventional ESD and additional treatment
The long-term prognosis of all patients was good, with a 5-year overall survival (OS) rate of 83.1% and a 5-year cause-specific survival (CSS) rate of 98.5% (Fig. 2A). However, OS was lower in patients with SM2 infiltration in the pathological findings than in those without. The 5-year CSS was 84.7%, even in SM2 cases (Fig. 2B). The 5-year CSS rates in the CRT and additional surgical resection groups were 94.6 and 87.5% (Fig. 2C).
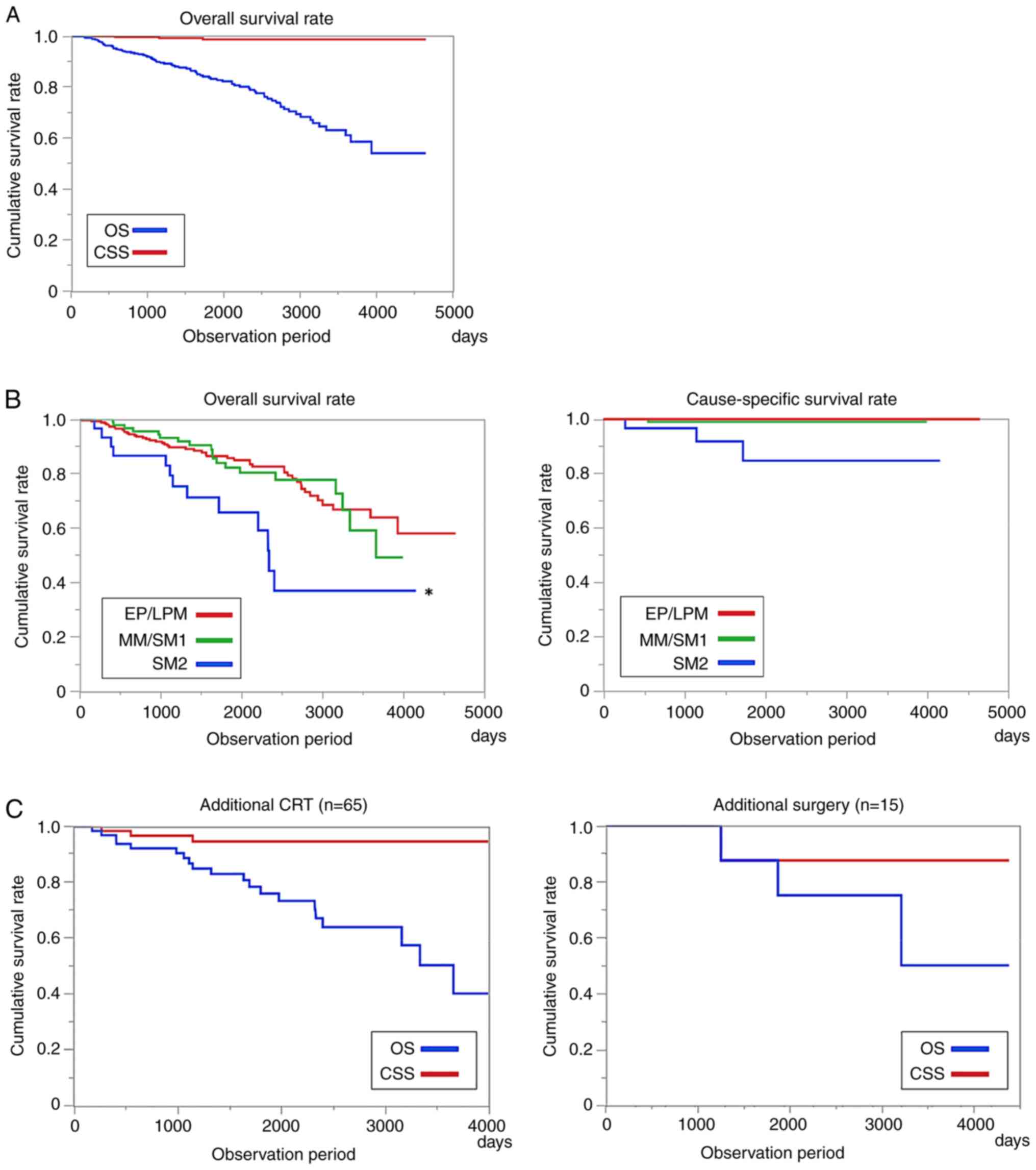 |
Figure 2
(A) Long-term prognosis of conventional endoscopic submucosal dissection. The 5-year OS rate was 83.1%, and the 5-year CSS rate was 98.5%. (B) Long-term prognosis by tumor depth. The 5-year OS was significantly low among patients with SM2 infiltration in the resection pathological findings (*P<0.05, log-rank test). However, compared with the 5-year survival rate, the 5-year CSS was an excellent long-term prognosis even in SM2 patients (84.7%). (C) Long-term prognosis after additional therapy. The 5-year CSS in the additional chemoradiotherapy and additional surgical resection groups were 94.6 and 87.5%. CRT, chemoradiotherapy; OS, overall survival; CSS, cause-specific survival; SM, submucosa; EP, epithelium; LPM, lamina propria; MM, musclaris mucosae.
|
Salvage endoscopic treatment. Patients
From September 2007 to July 2019, 68 patients (77 lesions) underwent salvage endoscopic treatment (ESD/PDT) for locally recurrent lesions after CRT for advanced esophageal cancer. Overall, 42 patients (51 lesions) underwent sal-ESD and 26 patients (26 lesions) underwent sal-PDT (Fig. 3; Table III).
 |
Figure 3
Study flowchart of the salvage endoscopic treatment. Overall, 68 patients underwent salvage endoscopic treatment from 2007 to 2019. The number of salvage endoscopic submucosal dissection was 42 patients (51 lesions), and 26 patients (26 lesions) underwent salvage photodynamic therapy. CRT, chemoradiotherapy; ESD, endoscopic submucosal dissection; PDT, photodynamic therapy.
|
 |
Table III
Characterization of salvage endoscopic therapy (sal-ESD/sal-PDT).
|
Table III
Characterization of salvage endoscopic therapy (sal-ESD/sal-PDT).
| Variable |
Sal-ESD (n=42) |
Sal-PDT (n=26) |
Total (n=68) |
| Age, years (range) |
71.0 (53-85) |
69.5 (55-90) |
70.0 (53-90) |
| Sex, n (%) |
|
|
|
| Male |
34 (81.0) |
23 (88.5) |
57 (83.8) |
| Female |
8 (19.0) |
3 (11.5) |
11 (16.2) |
| Depth of tumor invasion at initial CRT, n (%) |
|
|
|
| T1b |
19 (45.2) |
8 (30.8) |
27 (39.7) |
| T2 |
7 (16.7) |
5 (19.2) |
12 (17.6) |
| ≥T3 |
14 (33.3) |
9 (34.6) |
23 (33.8) |
| Unknown |
2 (4.8) |
4 (15.4) |
6 (8.8) |
| Months from CRT to recurrence (range) |
102.1 (16.3-188.6) |
43.6 (8.5-142.7) |
84.2 (8.5-188.6) |
| En-block resection rate /L-CR rate, n (%) |
40 (95.2) |
24 (92.3) |
64 (95.2) |
| Observation months after salvage therapy (range) |
40.7 (9.4-142.3) |
29.5 (9.8-130.5) |
34.7 (9.4-142.3) |
| Adverse event at salvage therapy, n (%) |
|
|
|
| Bleeding |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Perforation |
0 (0.0) |
2 (7.7) |
2 (2.9) |
| Stenosis |
3 (7.1) |
7 (26.9) |
10 (14.7) |
| Local/ectopic recurrence rate, n (%) |
9 (21.4) |
12 (46.2) |
21 (30.8) |
| LN and/or another organ metastasis, n (%) |
5 (11.9) |
2 (7.7) |
7 (10.3) |
Treatment results
The sal-ESD group had an en-bloc curative resection rate of 95.2%, and the sal-PDT group had an L-CR rate of 92.3% (Table III). Adverse events associated with salvage endoscopic treatment were perforation 2.9% (2/68) and stricture 14.7% (10/68). In particular, postoperative stenosis was frequently observed in the PDT group. However, to prevent stenosis, we ensured that the peripheries did not exceed half the circumference and that the irradiation energy dose at one time was not excessive. In patients with stenosis, endoscopic balloon dilatation was performed. Two patients experienced perforation after PDT; both had esophageal tracheal fistulas, which were closed by placing endotracheal stents followed by conservative treatment. Incidentally, there were no cases of skin phototoxicity. The median observation period after salvage endoscopic treatment was ~34 months (Table III). Therefore, the 3-year OS and CSS were evaluated. The local recurrence rate was higher in the sal-PDT group than in the sal-ESD group (Table III) because the tumor extended to the submucosa during endoscopic treatment in many cases. However, 75% (6/8) of patients with locally recurrent tumors reached L-CR with additional treatment, e.g., APC or additional PDT (data not shown), suggesting the importance of regular endoscopic follow-up. Although the recurrence rates of lymph node and distant metastases were low, it was higher in the sal-PDT group than in the sal-ESD group. The metastasis recurrence rate of salvage therapy was 11.7%. In principle, for local residual or recurrent cases after PDT, additional PDT should be considered. However, based on the patient's general condition (e.g., patients who cannot stand shading) and degree of stenosis, APC ablation or additional chemotherapy (according to standard chemotherapy for esophageal cancer) was provided. However, some patients chose the best supportive care.
Long-term prognosis after salvage endoscopic treatment
A comparative study of the survival rate after salvage endoscopic therapy showed that the 3-year OS rates were 75.4% for sal-ESD and 35.4% for sal-PDT (Fig. 4A). The 3-year CSS rates were 87.3 and 77.1% in the sal-ESD and sal-PDT groups, respectively. During the observation period from the initial treatment of CRT (Fig. 4B), over 80% of patients with sal-ESD/PDT did not die of advanced esophageal cancer for over 5 years.
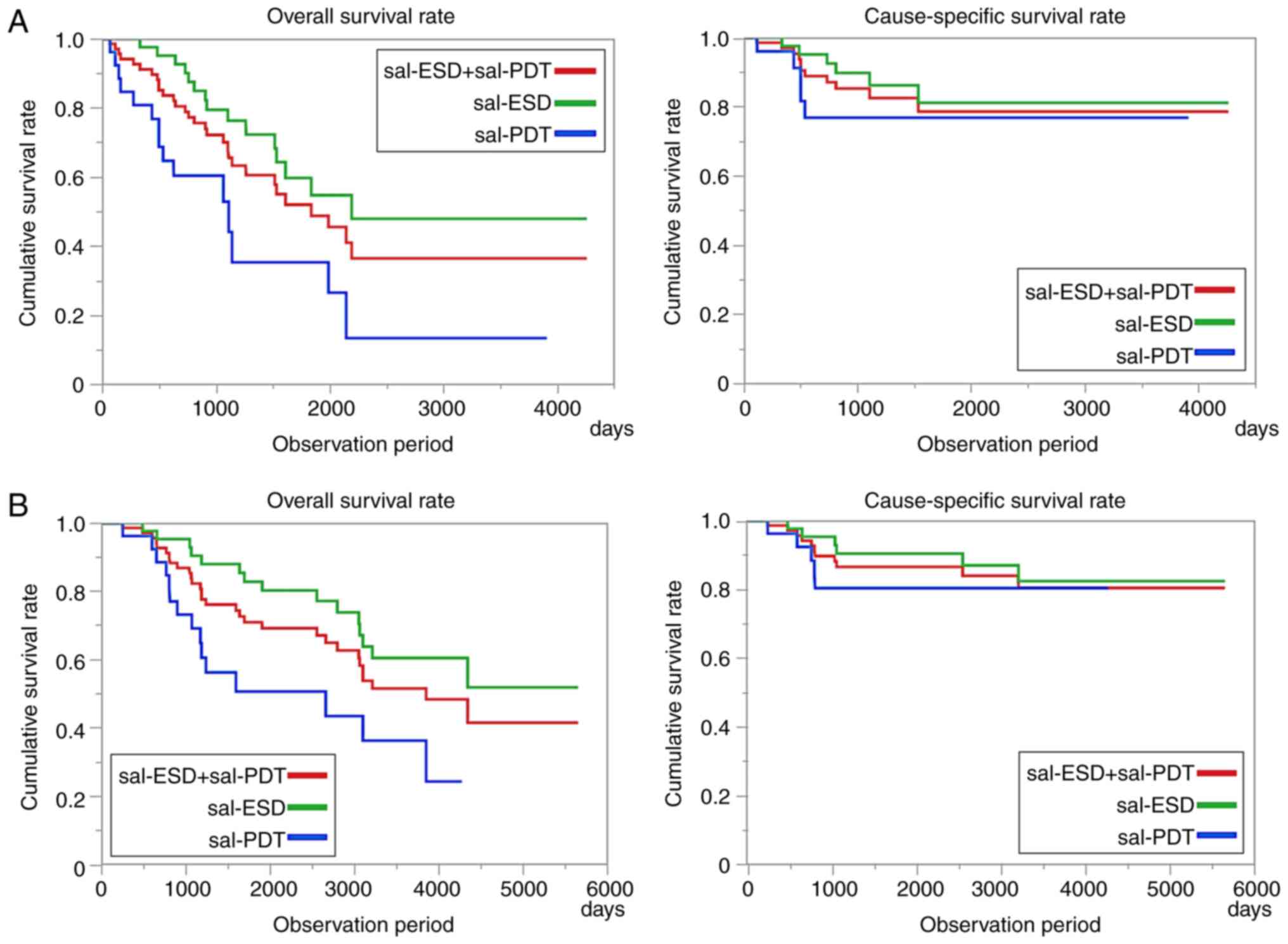 |
Figure 4
(A) Survival rate after salvage endoscopic therapy. The 3-year OS was 62.3% for total salvage therapy (sal-ESD, 75.4%; sal-PDT, 35.4%). The 3-year CSS was 83.5% for total salvage therapy (sal-ESD, 87.3%; sal-PDT, 77.1%). (B) Survival rate after CRT. The 5-year OS was 80.4% for total salvage therapy (sal-ESD, 82.9%; sal-PDT, 50.4%). The 5-year CSS was 86.7% for total salvage therapy (sal-ESD, 90.4%; sal-PDT, 80.4%). OS, overall survival; sal, salvage therapy; ESD, endoscopic submucosal dissection; PDT, photodynamic therapy; CSS, cause-specific survival; CRT, chemoradiotherapy.
|
Examination of factors that affected the survival rate of salvage endoscopic treatment
During the entire observation period, 30 of 68 patients who underwent salvage endoscopic treatment died. Of these, cause of death in 10 patients was esophageal cancer (sal-ESD, 5 cases; sal-PDT, 5 cases). The cases in which patients died from the present illness were evaluated, including their background factors (Table IV). With regards the sal-ESD group, there was no significant difference in age, sex, or the depth of invasion at the time of the first onset; furthermore, the en-bloc resection rate was also low. Only one case with a positive horizontal margin died. With regards the sal-PDT group, there was no significant difference in age, sex, and invasion depth at the time of onset; however, the L-CR rate was significantly lower, and the local recurrence rate and LN and/or another organ metastasis rate after treatment were significantly higher (Table V). In addition, death was significantly higher among patients who developed stenosis after treatment. This is thought to be because residual recurrence could not be evaluated due to stenosis, and additional treatment could not be provided.
 |
Table IV
Examination of factors that affect the survival rate of sal-ESD.
|
Table IV
Examination of factors that affect the survival rate of sal-ESD.
| Variable |
Sal-ESD survival (n=37) |
Sal-ESD death from esophageal cancer (n=5) |
P-value |
| Age, years (range) |
71.0 (53-85) |
69.5 (55-90) |
0.5588 |
| Sex, n (%) |
|
|
0.9542 |
| Male |
30 (81.1) |
23 (88.5) |
|
| Female |
7 (18.9) |
3 (11.5) |
|
| Depth of tumor invasion at initial CRT, n (%) |
|
|
0.5353 |
| T1b |
17 (45.9) |
2 (30.8) |
|
| T2 |
6 (16.2) |
1 (19.2) |
|
| ≥T3 |
12 (32.5) |
2 (34.6) |
|
| Unknown |
2 (5.4) |
0 (15.4) |
|
| Months from CRT to recurrence (range) |
107.3 (32.4-188.6) |
35.0 (16.3-85.3) |
0.0019 |
| En-block resection rate, n (%) |
37(100) |
3 (60.0) |
0.0022 |
| Depth of tumor invasion, n (%) |
|
|
0.0694 |
| EP-LPM |
33 (89.2) |
2 (40.0) |
|
| MM/SM1 |
4 (10.8) |
2 (40.0) |
|
| ≥SM2 |
0 (0.0) |
1 (20.0) |
|
| Lymphovascular invasion, n (%) |
0 (0.0) |
0 (0.0) |
- |
| Vertical margin positive, n (%) |
0 (0.0) |
1 (20.0) |
0.0370 |
 |
Table V
Examination of factors that affected the survival rate of sal-PDT.
|
Table V
Examination of factors that affected the survival rate of sal-PDT.
| |
Sal-PDT survival |
Sal-PDT death from esophageal |
|
| Variable |
(n=21) |
cancer (n=5) |
P-value |
| Age, years (range) |
69.0 (55-90) |
71.0 (67-83) |
0.9818 |
| Sex, n (%) |
|
|
0.5355 |
| Male |
19 (81.1) |
4 (88.5) |
|
| Female |
2 (18.9) |
1 (11.5) |
|
| Depth of tumor invasion at initial CRT, n (%) |
|
|
0.4821 |
| T1b |
7 (45.9) |
1 (30.8) |
|
| T2 |
4 (16.2) |
1 (19.2) |
|
| ≥T3 |
6 (32.5) |
3 (34.6) |
|
| Unknown |
4 (5.4) |
0 (0.0) |
|
| Months from CRT to recurrence (range) |
48.6 (21.8-142.7) |
25.7 (8.5-27.2) |
0.0706 |
| L-CR rate, n (%) |
21(100) |
3 (60.0) |
0.0066 |
| Adverse event at salvage PDT, n (%) |
|
|
|
| Bleeding |
0 (0.0) |
0 (0.0) |
- |
| Perforation |
2 (0.0) |
0 (0.0) |
0.3446 |
| Stenosis |
5 (7.1) |
2 (40.0) |
0.0192 |
| Local/ectopic recurrence rate, n (%) |
8 (38.1) |
4 (80.0) |
0.0845 |
| LN and/or another organ metastasis, n (%) |
0 (0.0) |
2 (40.0) |
0.0066 |
Discussion
In this study, we report the results of ESD treatment for superficial esophageal cancer in our hospital. The batch curative resection rate was 86.9%, which was good. Even for non-curative resection cases with mainly SM infiltration, good long-term results were obtained by early appropriate additional treatment. Esophageal ESD for superficial esophageal cancer has become the standard treatment and can be performed at various facilities. Although this was a single-center study, >600 cases were accumulated, and there have been no previous reports with such a long-term follow-up.
According to the Guidelines for Diagnosis and Treatment of Carcinoma of the Esophagus (20), if pT1a-EP/LPM is negative for LVI and excision margin, the frequency of metastatic recurrence is low and no additional treatment is required. However, pT1a-MM cancer has an increased risk of metastasis, and the frequency of metastasis varies depending on the presence or absence of LVI; thus, additional treatment is strongly recommended (21,22). After endoscopic resection of esophageal cancer, in addition to local and metastatic recurrences, metachronous intraesophageal multiple cancer and metachronous cancer of another organ may occur. Conducting surveillance is also essential for improving prognosis. At our hospital, we usually set follow-up intervals according to the depth of invasion of the resected pathological tissue and perform endoscopy and CT regularly after conventional ESD (see 2.5. Patient follow-up). Since post-treatment ulcers are complicated, especially after salvage PDT, we also perform random biopsies from the treated area. Owing to strict follow-up intervals, most recurrent cases are detected early and are quickly provided additional treatment. CRT or surgical resection is the standard treatment for esophageal cancer deeper than pT2 with LVI (20). CRT for esophageal cancer can preserve organs, and the frequency of lymph node recurrence in the irradiation field is low (23,24); however, the rates of residual recurrence in the esophagus (8) and the number of patients with local residual recurrence are high (25). Since the prognosis is poor, it is important to determine how to perform local control. If this problem can be solved, it is expected that the treatment results for esophageal cancer will further improve (26). Therefore, even if CRT reaches CR, local control can be sufficiently performed by detecting local recurrence early and performing sal-ESD without neglecting regular screening. Based on our experience, there were many cases in which the submucosal layer became fibrotic due to irradiation, and it was not easy to treat. After a thorough preoperative examination, the depth of lesion invasion and degree of fibrosis was predicted, and careful mucosal resection enabled the treatment to be completed with few complications. Sal-PDT was selected for patients with a deeper predicted invasion depth and cases in which detachment was difficult with ESD. Yano et al. reported the effectiveness of sal-PDT in 2002 (27), and since 2015, it has been indicated for recurrent esophageal cancer after CRT as an insurance-covered medical treatment in Japan (17). At our hospital, we introduced PDT as an advanced medical treatment in 2007, and we were able to perform long-term follow-ups. Although there are some reports on the usefulness of sal-PDT, the long-term prognosis has not been examined. Table III shows that many patients had a depth of invasion of T2 or higher and stage II or higher at the first visit. Generally, the 5-year survival rate for advanced esophageal cancer of clinical stage II is 58.6% (18); however, the salvage endoscopic treatment can be used for local control at an early stage to reach a low rate of lymph node/distant metastasis and achieve a high survival rate. Herein, the sal-ESD group had an en-bloc curative resection rate of 96.1%, and the sal-PDT group had an L-CR of 92.3%. The 5-year CSS rates were 81.3 and 77.1% in the sal-ESD and sal-PDT groups, respectively, which were excellent considering that the initial diagnosis was advanced cancer. During the observation period from the initial treatment of CRT, >80% of patients with sal-ESD/PDT did not die of advanced esophageal cancer for >5 years. The reason for the high curative resection rate might be that the endoscopic follow-up had been performed regularly even after CRT and early detection and treatment were performed. Moreover, the average tumor diameter was smaller in the PDT group than in the ESD group. Perforation and stenosis rates were higher in the PDT group than in the ESD group. These complications probably occurred because the lesions that infiltrated deeper were indicated for PDT, and as a result, the effects of laser irradiation extended to the deep submucosa. This suggests the usefulness of salvage endoscopic treatment for locally recurrent lesions after CRT; it is a beneficial treatment option for the treatment of SSCE. Fig. 5 illustrates the treatment strategy for SSCE at our hospital. For lesions up to pT1a-MM/pT1b-SM1 cancer based on a preoperative endoscopic diagnosis, if there is no distant metastasis, endoscopic treatment should be selected, and regular follow-up or additional treatments should be considered according to the postoperative histopathological findings. Salvage endoscopic treatment is performed according to the degree of invasion for local residual/recurrent lesions after CRT. We believe that salvage endoscopic treatment can control esophageal cancer while expanding treatment options and improving QOL. Regular endoscopic follow-up after treatment is essential for that purpose. This study has some limitations. First, this is a single-center study. Nevertheless, many cases have been accumulated. Second, the test method at the time of diagnosis was not identical for all patients (e.g., not all patients underwent endoscopic ultrasound or PET-CT for diagnosis), and there were variations in pretreatment diagnosis. Finally, the long-term prognosis after CRT was not compared with the recurrence-free group and the cases in which salvage endoscopy was not indicated.
 |
Figure 5
Treatment strategy for superficial esophageal cancer in Nagasaki University Hospital. For lesions up to pT1a-MM/pT1b-SM1 cancer by preoperative endoscopic diagnosis, if there is no distant metastasis and sufficient informed outlet is obtained, endoscopic treatment is selected; chemoradiotherapy is selected according to postoperative histopathological findings, and additional treatments such as surgical resection are considered. Furthermore, salvage endoscopic treatment is performed according to the degree of invasion for local residual/recurrent lesions after chemoradiotherapy. †If all the above conditions are negative; †† if any of the above conditions are positive. MM, muscularis mucosae; SM, submucosa; EP, epithelium; LPM, lamina propria; ESD, endoscopic submucosal dissection; EGD, Esophagogastroduodenoscopy; CT, computed tomography; CRT, chemoradiotherapy; PDT, photodynamic therapy; CT, computed tomography; M, mucosa.
|
High en-bloc curative resection and survival rates were obtained for SSCE. Even in non-curative resection cases, long-term survival was obtained with appropriate early additional treatment. Establishing salvage endoscopic treatment makes long-term control of the underlying disease possible while maintaining QOL in patients with recurrent advanced esophageal cancer deeper than SM2 who underwent CRT and those with recurrence after additional CRT after ESD.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Authors' contributions
NY and HI conceptualized the study. JS, YT, TA, MT, MK, and KH collected and organized data from clinical records. JS and YT developed the statistical analysis plan and conducted statistical analyses. JS, NY, MK, HM, KM, YA and KN investigated the data. NY created the methodology and administered the project. HI, YA and KN supervised the conduct of this study. HI, TA, MT, MK, KH, HM and KM validated the study result. NY and YT confirm the authenticity of all the raw data. JS drafted the original manuscript. All authors reviewed the manuscript draft and revised it critically for intellectual content. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and approved by the Nagasaki University Hospital Ethics Committee (approval no. 21041908; approval date: April 20, 2021). Informed consent was obtained in the form of opt-out on the website.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
1
|
Yanai Y, Yokoi C, Watanabe K, Akazawa N and Akiyama J: Endoscopic resection for gastrointestinal tumors (esophageal, gastric, colorectal tumors): Japanese standard and future prospects. Glob Health Med. 3:365–370. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Oyama T, Tomori A, Hotta K, Morita S, Kominato K, Tanaka M and Miyata Y: Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 3 (Suppl 1):S67–S70. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Watanabe M, Toh Y, Ishihara R, Kono K, Matsubara H, Murakami K, Muro K, Numasaki H, Oyama T, Ozawa S, et al: Comprehensive registry of esophageal cancer in Japan, 2014. Esophagus. 19:1–26. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ito Y, Miyashiro I, Ito H, Hosono S, Chihara D, Nakata-Yamada K, Nakayama M, Matsuzaka M, Hattori M, Sugiyama H, et al: Long-term survival and conditional survival of cancer patients in Japan using population-based cancer registry data. Cancer Sci. 105:1480–1486. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Koterazawa Y, Nakamura T, Oshikiri T, Kanaji S, Tanaka S, Ishida T, Yamashita K, Matsuda T, Morita Y, Suzuki S and Kakeji Y: A comparison of the clinical outcomes of esophagectomy and chemoradiotherapy after noncurative endoscopic submucosal dissection for esophageal squamous cell carcinoma. Surg Today. 48:783–789. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
van den Boorn HG, Stroes CI, Zwinderman AH, Eshuis WJ, Hulshof MCCM, van Etten-Jamaludin FS, Sprangers MAG and van Laarhoven HWM: Health-related quality of life in curatively-treated patients with esophageal or gastric cancer: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 154(103069)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kato K, Ito Y, Nozaki I, Daiko H, Kojima T, Yano M, Ueno M, Nakagawa S, Takagi M, Tsunoda S, et al: Parallel-group controlled trial of surgery versus chemoradiotherapy in patients with stage I esophageal squamous cell carcinoma. Gastroenterology. 161:1878–1886.e2. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hironaka S, Ohtsu A, Boku N, Muto M, Nagashima F, Saito H, Yoshida S, Nishimura M, Haruno M, Ishikura S, et al: Nonrandomized comparison between definitive chemoradiotherapy and radical surgery in patients with T2-3 Nany M0 squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys. 57:425–433. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Watanabe M, Mine S, Nishida K, Yamada K, Shigaki H, Matsumoto A and Sano T: Salvage esophagectomy after definitive chemoradiotherapy for patients with esophageal squamous cell carcinoma: Who really benefits from this high-risk surgery? Ann Surg Oncol. 22:4438–4444. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Miyata H, Yamasaki M, Takiguchi S, Nakajima K, Fujiwara Y, Nishida T, Mori M and Doki Y: Salvage esophagectomy after definitive chemoradiotherapy for thoracic esophageal cancer. J Surg Oncol. 100:442–446. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hombu T, Yano T, Hatogai K, Kojima T, Kadota T, Onozawa M, Yoda Y, Hori K, Oono Y, Ikematsu H and Fujii S: Salvage endoscopic resection (ER) after chemoradiotherapy for esophageal squamous cell carcinoma: What are the risk factors for recurrence after salvage ER? Dig Endosc. 30:338–346. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Koizumi S, Jin M, Matsuhashi T, Tawaraya S, Watanabe N, Sawaguchi M, Kanazawa N, Yamada Y, Onochi K, Kimura Y, et al: Salvage endoscopic submucosal dissection for the esophagus-localized recurrence of esophageal squamous cell cancer after definitive chemoradiotherapy. Gastrointest Endosc. 79:348–353. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ego M, Abe S, Nakatani Y, Nonaka S, Suzuki H, Yoshinaga S, Oda I, Kato K, Honma Y, Itami J, et al: Long-term outcomes of patients with recurrent squamous cell carcinoma of the esophagus undergoing salvage endoscopic resection after definitive chemoradiotherapy. Surg Endosc. 35:1766–1776. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Didamson OC and Abrahamse H: Targeted photodynamic diagnosis and therapy for esophageal cancer: Potential role of functionalized nanomedicine. Pharmaceutics. 13(1943)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yano T, Kasai H, Horimatsu T, Yoshimura K, Teramukai S, Morita S, Tada H, Yamamoto Y, Kataoka H, Kakushima N, et al: A multicenter phase II study of salvage photodynamic therapy using talaporfin sodium (ME2906) and a diode laser (PNL6405EPG) for local failure after chemoradiotherapy or radiotherapy for esophageal cancer. Oncotarget. 8:22135–22144. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yagi K, Toriumi T, Aikou S, Yamashita H and Seto Y: Salvage treatment after definitive chemoradiotherapy for esophageal squamous cell carcinoma. Ann Gastroenterol Surg. 5:436–445. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yano T and Muto M: Photodynamic therapy for esophageal cancer. Gastroenterol Endosc. 59:2740–2749. 2017.
|
|
18
|
Yamaguchi N, Isomoto H, Nakayama T, Hayashi T, Nishiyama H, Ohnita K, Takeshima F, Shikuwa S, Kohno S and Nakao K: Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc. 73:1115–1121. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kuwano H, Nishimura Y, Oyama T, Kato H, Kitagawa Y, Kusano M, Shimada H, Takiuchi H, Toh Y, Doki Y, et al: Guidelines for diagnosis and treatment of carcinoma of the esophagus April 2012 edited by the Japan Esophageal Society. Esophagus. 12:1–30. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yamashita H, Kadota T, Minamide T, Sunakawa H, Sato D, Takashima K, Nakajo K, Murano T, Shinmura K, Yoda Y, et al: Efficacy and safety of second photodynamic therapy for local failure after salvage photodynamic therapy for esophageal cancer. Dig Endosc. 34:488–496. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ishihara R, Arima M, et al: ESD/EMR guidelines for esophageal cancer. Gastroenterol Endosc. 62:223–271. 2020.PubMed/NCBI View Article : Google Scholar : (In Korean).
|
|
22
|
Yoshii T, Ohkawa S, Tamai S and Kameda Y: Clinical outcome of endoscopic mucosal resection for esophageal squamous cell cancer invading muscularis mucosa and submucosal layer. Dis Esophagus. 26:496–502. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Takahashi K, Hashimoto S, Mizuno KI, Kobayashi T, Tominaga K, Sato H, Kohisa J, Ikarashi S, Hayashi K, Takeuchi M, et al: Management decision based on lymphovascular involvement leads to favorable outcomes after endoscopic treatment of esophageal squamous cell carcinoma. Endoscopy. 50:662–670. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Onozawa M, Nihei K, Ishikura S, Minashi K, Yano T, Muto M, Ohtsu A and Ogino T: Elective nodal irradiation (ENI) in definitive chemoradiotherapy (CRT) for squamous cell carcinoma of the thoracic esophagus. Radiother Oncol. 92:266–269. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Minashi K, Nihei K, Ogawa G, Takizawa K, Yano T, Amanuma Y, Tsuchida T, Ono H, Iizuka T, Shichijyo S, et al: Final analysis of single-arm confirmatory study of diagnostic endoscopic resection (ER) plus selective chemoradiotherapy (CRT) for stage I esophageal squamous cell carcinoma (ESCC): JCOG0508. J Clin Oncol. 36(4023)2018.
|
|
26
|
Ishikura S, Nihei K, Ohtsu A, Boku N, Hironaka S, Mera K, Muto M, Ogino T and Yoshida S: Long-term toxicity after definitive chemoradiotherapy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol. 21:2697–2702. 2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yano T, Muto M, Minashi K, Ohtsu A and Yoshida S: Photodynamic therapy as salvage treatment for local failures after definitive chemoradiotherapy for esophageal cancer. Gastrointest Endosc. 62:31–36. 2005.PubMed/NCBI View Article : Google Scholar
|



















