Introduction
Being a rare malignancy, adrenocortical carcinoma
(ACC) exhibits aggressiveness as well as a poor prognosis. A number
of patients present local invasion or metastasis at the time of the
diagnosis (1). The annual ACC
incidence is 0.7-2.0 cases in every 1 million individuals,
accounting for 0.2% of cancer deaths in the Netherlands (2). According to the staging criteria of
the Union for International Cancer Control and the American Joint
Committee on Cancer (3), R0
resection can be achieved in stage I or II with an ideal prognosis.
However, for stage III or IV resection the 5-year survival rate is
low, reported to be 6-15% (4,5).
Therefore, it is particularly important to identify molecular
markers for the study of ACC pathogenesis and auxiliary clinical
treatment.
Aldo-keto reductase family 1 member B10 (AKR1B10),
which belongs to the AKR superfamily, consists of 316 amino acids
and its gene is located on chromosome 7q33(6). AKR1B10 stimulation suppresses ACC
cell proliferation and promotes apoptosis (7). BioGRID (8) predicted a potential interaction
between fibronectin type III domain-containing protein 5 (FNDC5)
and AKR1B10. A transmembrane protein, FNDC5 is also a prohormone
that is released from irisin (9).
FNDC5 expression is elevated in ovarian cancer tissue and
suppresses epithelial ovarian cancer cell proliferation, migration
and invasion (10). Irisin induces
G2/M cell cycle arrest and suppresses proliferation and
invasion of glioblastoma cells (11). FNDC5 expression is reduced in
non-small cell lung cancer cells (NSCLCs) cells and increases the
sensitivity of NSCLC cells to paclitaxel (12). Irisin/FNDC5 suppress the viability,
invasion and migration as well as epithelial-mesenchymal transition
(EMT) of osteosarcoma cells (13).
Irisin also induces G1 arrest and inhibits proliferation
and migration of pancreatic cancer cells via the 5'-AMP-activated
protein kinase (AMPK)/mTOR signalling pathway (14). Nevertheless, to the best of our
knowledge, the role of FNDC5 in ACC remains unclear.
The present study aimed to explore the role of FNDC5
in the proliferation, migration, invasion and EMT of ACC cells and
the underlying mechanisms.
Materials and methods
Bioinformatics
Gene Expression Profiling Interactive Analysis
(GEPIA) database analyzed the expression of FNDC5 in the tumour
tissue of patients with ACC and the correlation between FNDC5 and
AKR1B10. Encyclopedia of RNA Interactomes database predicted the
correlation between FNDC5 expression and the overall survival in
patients with ACC.
Cell culture and transfection
ACC cell line (NCI-H295R) provided by BeNa Culture
Collection was cultivated in DMEM (Gibco; Thermo Fisher Scientific,
Inc.) which was supplemented with 10% FBS (Beyotime Institute of
Biotechnology) and 1% penicillin/streptomycin (Beyotime Institute
of Biotechnology) at 37˚C with 5% CO2.
Full-length cDNA of human FNDC5 was cloned into the
pcDNA3.1 vector (Thermo Fisher Scientific, Inc.) to generate an
FNDC5 overexpression vector (Oe-FNDC5). A pcDNA3.1 empty vector was
used as the negative control (Oe-NC). Small interfering (si)RNAs
specific for AKR1B10 (si-AKR1B10#1, 5'-CAGGATATCGGCACATTGACTGG-3'
and si-AKR1B10#2, GGCCTATGTCTATCAGAATGAAC) as well as its si-NC
(5'-AAGACAUUGUGUGUCCGCCTT-3') were constructed by Guangzhou RiboBio
Co., Ltd. NCI-H295R cells in logarithmic growth phase were seeded
in 6-well plates (1x106 cells/well) and cultured until
the cell confluence reached 80%. A total of 20 µg Oe-FNDC5, Oe-NC,
si-AKR1B10 and si-NC was transfected into NCI-H295R cells
separately using Lipofectamine 3000 reagent (Thermo Fisher
Scientific, Inc.) and incubated for 6 h at 37˚C. At 48 h
post-transfection, the collection of cells was implemented for
ensuing experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT of RNA, which was isolated from NCI-H295R cells
utilizing TRIzol® reagent (Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions, into cDNA was
performed using the PrimeScript Reverse Transcriptase kit (Takara
Bio, Inc.), according to the manufacturer's protocol. qPCR was
performed using the SYBR® PremixEX Taq™ kit (Takara Bio,
Inc.) The qPCR thermocycling conditions were as follows: Initial
denaturation at 95˚C for 10 min; followed by 40 cycles of 95˚C for
15 sec and 64˚C for 30 sec. FNDC5 and AKR1B10 mRNA levels were
quantified using the 2-ΔΔCq method and normalized to the
internal reference gene (15). The
following primer pairs (Sangon Biotech) were used for qPCR: FNDC5
forward, 5'-CCGCCAGTATGACATCATTGAA-3' and reverse,
5'-GTCACCTCACACCACTCAGG-3'; AKR1B10 forward,
5'-CATGAAGTGGGGGAAGCCAT-3' and reverse, 5'-CGTTACAGGCCCTCCAGTTT-3';
and GAPDH forward, 5'-GGAGCGAGATCCCTCCAAAAT-3' and reverse,
5'-GGCTGTTGTCATACTTCTCATGG-3'.
Western blot analysis
Total protein was isolated from NCI-H295R cells
utilizing RIPA buffer (Beyotime Institute of Biotechnology) and
quantified using a BCA Protein assay kit (Beyotime Institute of
Biotechnology). Total protein (30 µg/lane) was separated by
SDS-PAGE on 5-10% gels and transferred onto a PVDF membrane. The
membranes were blocked with 5% BSA (Thermo Fisher Scientific, Inc.)
for 1.5 h at room temperature and incubated overnight with primary
antibodies against FNDC5 (cat. no. ab174833; 1:1,000), E-cadherin
(cat. no. ab40772; 1/1,000), N-cadherin (cat. no. ab76011;
1/5,000), Snail (cat. no. ab216347; 1/1,000), AKR1B10 (cat. no.
ab192865; 1/1,000), phosphorylated (p)-AMPK (cat. no. ab133448;
1/1,000), AMPK (cat. no. ab207442; 1/1,000), p-mTOR (cat. no.
ab109268; 1/1,000), mTOR (cat. no. ab134903; 1/10,000) and GAPDH
(cat. no. ab9485; 1/2,500) from Abcam at 4˚C. Subsequently, the
membranes were incubated with a secondary anti-rabbit horseradish
peroxidase-conjugated antibody (cat. no. ab6721; 1/2,000; Abcam)
for 2 h at room temperature. The protein bands were visualized
using BeyoECL Plus (Beyotime Institute of Biotechnology) and ImageJ
software 1.8.0 (National Institutes of Health) was used for
analysis of band intensity with GAPDH as the loading control.
Cell Counting Kit-8 (CCK-8) assay
Following transfection, NCI-H295R cells
(2x104 cells/well) were plated into 96-well plates and
incubated for 24, 48 and 72 h at 37˚C. Afterwards, 10 µl CCK-8
solution (Beyotime Institute of Biotechnology) was added to each
well for 2 h. The optical density at 450 nm was measured with a
microplate reader.
5-ethynyl-2'-deoxyuridine (EdU)
incorporation assay
Following transfection, NCI-H295R cells
(2x104 cells/well) were seeded into 96-well plates and
incubated with 20 µM EdU for 2 h at room temperature and DNA was
stained using 10 µmol/l DAPI for 10 min at room temperature. The
observation of cell proliferative capability was performed
utilizing an inverted fluorescence microscope (magnification,
x200). Green cells were the EdU/DAPI-positive cells.
Wound healing assay
Transfected NCI-H295R cells were seeded into 6-well
plates at 1x105 cells/well and when cell confluency
reached 90%, a wound was made using a 10-µl pipette tip and the
remaining cells were cultured in serum-free DMEM (Gibco; Thermo
Fisher Scientific, Inc.) at 37˚C. Under an inverted light
microscope (magnification, x100), the wounds were observed at 0 and
24 h. ImageJ software version 1.8.0 (National Institutes of Health)
was used for the determination of cell migration rate. The
migration rate was calculated as follows: (Wound width at 0 h-wound
width at 24 h)/wound width at 0 h x100%.
Transwell assay
A total of 1x105 transfected NCI-H295R
cells were plated in the upper chambers of Transwell plates that
were pre-coated with Matrigel at 37˚C for 1 h. Serum-free DMEM
(Gibco; Thermo Fisher Scientific, Inc.) was added into the top
chambers while the bottom chambers were filled with 800 µl DMEM
containing 10% FBS. Following 48 h incubation at 37˚C, the
migratory cells were exposed for 10 min to 4% paraformaldehyde
fixation at room temperature as well as 10 min of 0.4% crystal
violet staining at room temperature. Migratory cells were observed
utilizing an inverted light microscope (magnification, x100).
Cell apoptosis analysis
Following plasmid transfection for 48 h, the
transfected HL-60 cells (1x106) were washed with PBS and
resuspended in 500 µl binding buffer. Afterwards, the solution was
transferred to a flow cytometry tube and mixed with 5 µl Annexin
V-fluorescein isothiocyanate staining solution (BD Biosciences).
Cells were treated with 10 µl propidium iodide solution (50 µg/ml;
Dojindo Laboratories, Inc.) for 30 min at room temperature in the
dark. The percentages of apoptotic cells were quantitated using a
FACSCalibur flow cytometer (BD Biosciences) and FlowJo software
(version 7.0; Tree Star, Inc.). The apoptosis rate was determined
by calculating the percentage of early and late apoptotic
cells.
Measurement of caspase-3 activity
Following centrifugation at 20,000 x g for 15 min at
4˚C, activity of caspase-3 in cell supernatants was assessed using
the Caspase-3 Colorimetric Assay kit (Abcam), according to the
manufacturer's instructions. The optical density at 400 nm was
measured with a microplate reader (Molecular Devices, LLC).
Co-immunoprecipitation
Following transfection, NCI-H295R cells were lysed
with RIPA lysis buffer (Beyotime Institute of Biotechnology) and
the supernatant was collected by centrifugation at 13,000 x g for
10 min at 4˚C. 500 µg cell lysate was incubated with antibodies
against 2 µg AKR1B10 (cat. no. NBP1-44998; Novus Biologicals),
PDIA6 (cat. no. ab227545; Abcam) or IgG (cat. no. ab172730; Abcam)
at 4˚C overnight. Then, 50 µg protein A magnetic beads were added
for capturing the complexes of AKR1B10 and PDIA6. After the IP
reaction, 50 µg protein G/A agarose beads were centrifuged at 1,000
x g for 3 min at 4˚C to the bottom of the tube. The supernatant was
then carefully absorbed, and the agarose beads were washed three
times with 1 ml lysis buffer. A total of 15 µl 2X SDS sample buffer
was finally added for boiling at 100˚C for 5 min. Afterwards, the
collected complexes were subjected to western blot analysis. The
input was regarded as the positive control; IgG was the negative
control.
Statistical analysis
Data from three independent replicates are presented
as the mean ± standard deviation. GraphPad Prism software (version
8.0.1; GraphPad Software, Inc.) was used for statistical analysis.
Comparisons between multiple groups were performed using one-way
ANOVA followed by Tukey's test. P<0.05 was considered to
indicate a statistically significant difference.
Results
FNDC5 is lowly expressed in ACC and is
associated with poor prognosis
GEPIA database showed that the expression of FNDC5
was lower in the tumour tissue of patients with ACC than that in
normal tissues (Fig. 1A).
Encyclopedia of RNA Interactomes database indicated that low
expression of FNDC5 was significantly associated with poor overall
survival in patients with ACC (Fig.
1B).
Overexpression of FNDC5 inhibits
proliferation of ACC cells
After transfecting Oe-FNDC5 into NCI-H295R cells,
FNDC5 expression was significantly increased (Fig. 2A and B). Moreover, the viability and
proliferation of NCI-H295R cells decreased following FNDC5
overexpression (Fig. 2C and
D).
Overexpression of FNDC5 inhibits
invasion, migration and EMT of ACC cells
The NCI-H295R cell invasion and migration were
decreased after overexpressing FNDC5 (Fig. 3A and B). The expression levels of
EMT-associated proteins showed that FNDC5 overexpression
significantly promoted the expression of E-cadherin while
inhibiting the expression of N-cadherin and Snail in NCI-H295R
cells (Fig. 3C).
Overexpression of FNDC5 promotes
apoptosis of ACC cells
Compared with the control and Oe-NC group, the
proportion of apoptotic HL-60 cells was significantly increased
following FNDC5 overexpression (Fig.
4A). Consistently, FNDC5 overexpression also increased
caspase-3 activity (Fig. 4B).
Bcl-2 expression was decreased in the Oe-FNDC5 group while the Bax
expression was increased (Fig.
4C).
FNDC5 interacts with AKR1B10 in ACC
cells
GEPIA database indicated that FNDC5 had a positive
correlation with AKR1B10 expression in patients with ACC (Fig. 5A). After overexpressing FNDC5,
AKR1B10 expression in NCI-H295R cells increased (Fig. 5B and C). The expression of AKR1B10 was measured
by incubation with anti-FNDC5 and expression of FNDC5 was analyzed
by incubation with anti-AKR1B10, which indicated that FNDC5
interacted with AKR1B10 (Fig.
5D).
Downregulation of AKR1B10 reverses the
effect of FNDC5 overexpression on ACC cells by modulating the
AMPK/mTOR pathway
Following transfection with si-AKR1B10#1 or
si-AKR1B10#2, AKR1B10 expression in NCI-H295R cells was decreased
and was lower in the si-AKR1B10#2 group (Fig. 6A and B). Therefore, si-AKR1B10#2 transfection
was used for subsequent experiments. FNDC5 overexpression increased
the p-AMPK expression levels but decreased expression of p-mTOR
(Fig. 6C); these effects were then
counteracted by AKR1B10 silencing. AKR1B10 silencing improved the
decreased viability and proliferation of NCI-H295R cells caused by
FNDC5 overexpression (Fig. 6D and
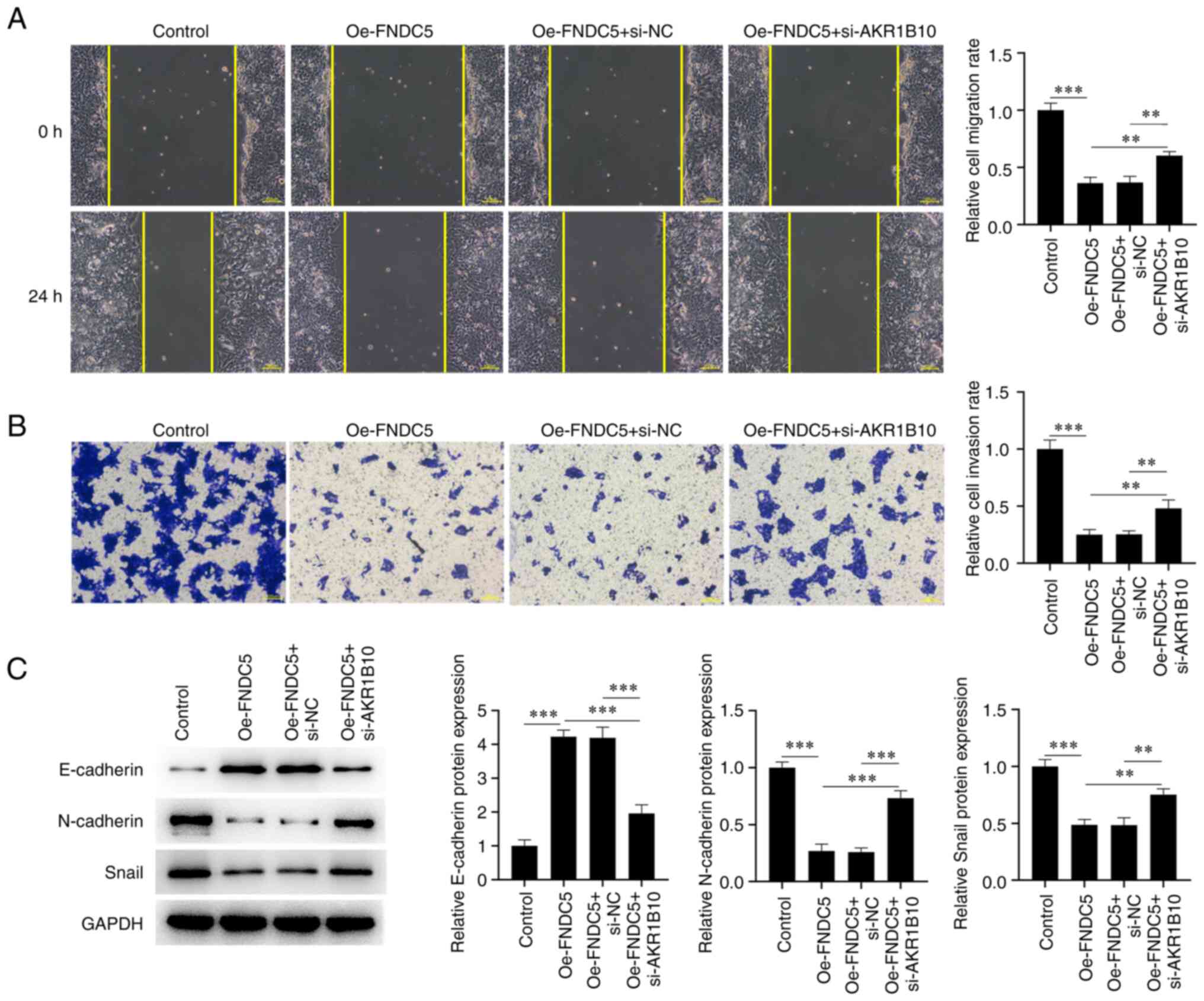
E). Cell migration and invasion
were also increased in NCI-H295R cells co-transfected with
siAKR1B10#2 and Oe-FNDC5 (Oe-FNDC5 + si-AKR1B10 group) compared
with Oe-FNDC5 + si-NC group (Fig.
7A and B). Moreover,
expression of E-cadherin was downregulated while expression of
N-cadherin and Snail was upregulated in the Oe-FNDC5 + si-AKR1B10
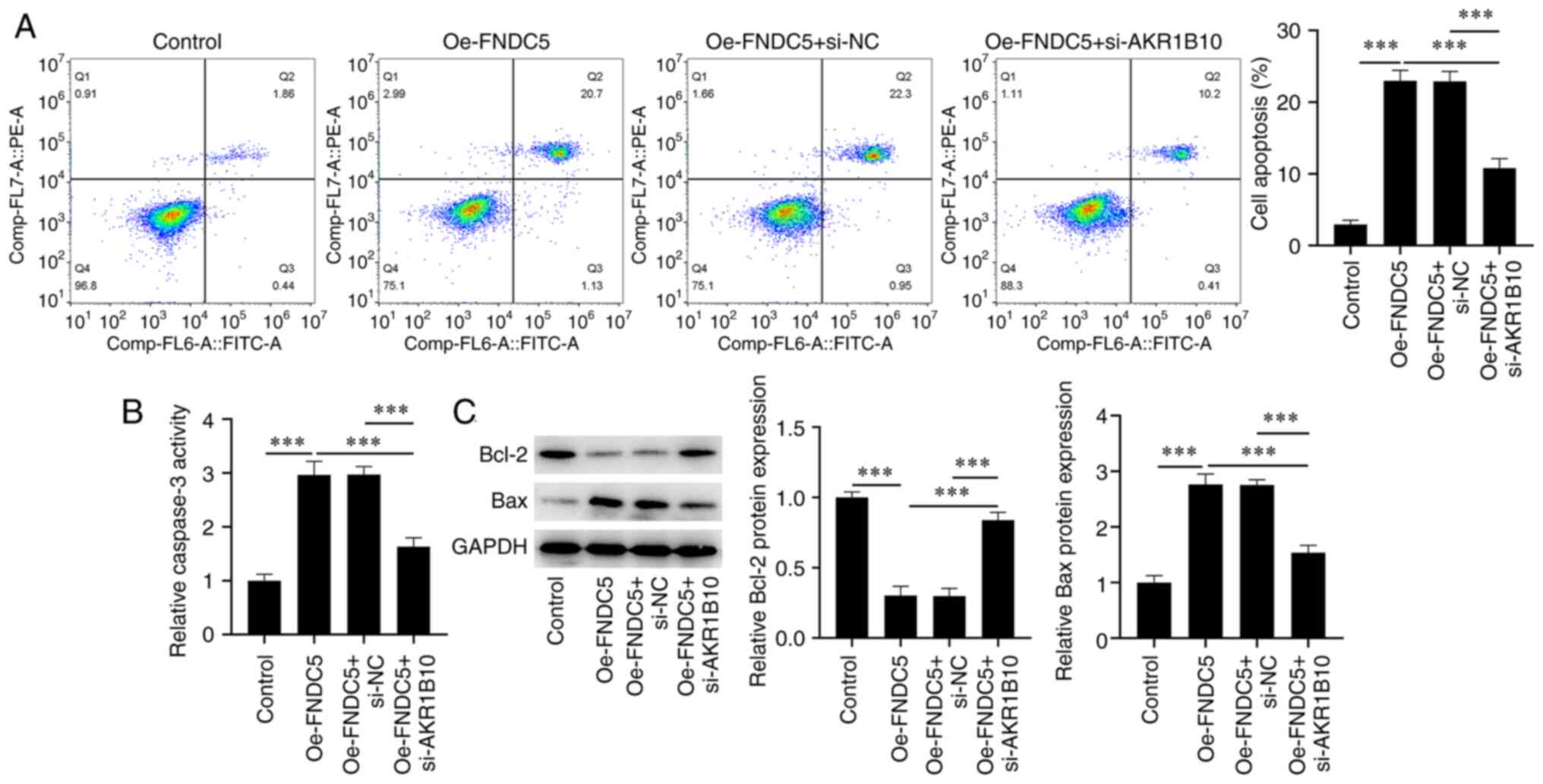
compared with Oe-FNDC5 + si-NC group (Fig. 7C). By contrast, the proportion of
apoptotic NCI-H295R cells was decreased by AKR1B10 silencing
compared with the Oe-FNDC5 + si-NC group (Fig. 8A). Western blot analysis indicated
that AKR1B10 silencing in NCI-H295R cells transfected with Oe-FNDC5
increased Bcl-2 expression but decreased Bax expression and
caspase-3 activity (Fig. 8B and
C).
Discussion
Irisin, which is proteolyzed by FNDC5, can convert
white adipose tissue to brown, thus exerting its regulatory impacts
on metabolic disease (16). It was
discovered that FNDC5 is associated with the occurrence as well as
the advancement of tumors. Compared with normal tissue, irisin
expression in esophageal, gastric, colon and breast cancer is
notably increased (17,18). At the same time, irisin may have a
suppressive impact on proliferation, migration and invasion of
breast and lung cancer, as well as osteosarcoma and other cells
(19-21).
The aforementioned studies demonstrated that irisin may be involved
in the development of tumors. FNDC5 is highly expressed in renal
(22), colorectal (23) and breast cancer (24). FNDC5 expression is increased in
sorafenib-resistant hepatocellular carcinoma (HCC) cells and
knockdown of FNDC5 enhances levels of ferroptosis in
sorafenib-resistant HCC cells (25). In the present study, GEPIA database
were used to analyze FNDC5 expression in ACC; FNDC5 expression was
decreased in tumour tissues from patients with ACC. The present
study demonstrated that FNDC5 may have suppressive effects on the
proliferation, invasion and migration of NCI-H295R cells as well as
EMT. Additionally, FNDC5 promoted apoptosis of NCI-H295R cells.
AKR1B10 is also reported to be involved in the
development of multiple cancers (26,27).
AKR1B10 expression is reduced in gastric cancer tissues and AKR1B10
suppresses the proliferation, migration and EMT of gastric cancer
cells (26). AKR1B10 is decreased
in colorectal cancer tissue and AKR1B10 knockdown facilitates
proliferation and migration of colorectal cancer cells (27). AKR1B10 suppresses cell viability
and colony formation while facilitating apoptosis of NCI-H295R
cells (7). In the present study,
the knockdown of AKR1B10 weakened the effect of FNDC5
overexpression on proliferation, invasion, migration, EMT and
apoptosis of NCI-H295R cells.
AMPK/mTOR signalling pathway is a key regulator in a
variety of tumors (28,29). A previous study discovered that
frankincense, pine needle and geranium essential oil regulate the
AMPK/mTOR pathway to inhibit proliferation of breast cancer cells
(28). AMPK activator OSU-53
activates AMPK and regulates mTOR and its downstream signalling
pathways to inhibit proliferation and viability of thyroid cancer
cells (29). The combination of
metformin and aspirin significantly inhibits AMPK/STAT3-dependent
phosphorylation of mTOR, reduce the expression of myeloid cell
leukaemia-1 and Bcl-2 and suppresses proliferation, migration and
invasion of pancreatic adenocarcinoma (30). In the present study, FNDC5
overexpression activated the AMPK/mTOR signalling pathway to
suppress proliferation, invasion, migration and EMT but promote the
apoptosis of NCI-H295R cells; these effects were counteracted by
AKR1B10 knockdown.
The present study only investigated and discussed
the effects and regulatory mechanisms of FNDC5 and AKR1B1 in ACC
cells. Further in vivo tumour model experiments and
validation of clinical tissue samples should be performed in future
investigations to support the findings of the present study.
In conclusion, FNDC5 positively regulated AKR1B10
expression to inhibit the proliferation, invasion and migration of
NCI-H295R cells by activating the AMPK/mTOR pathway.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DC designed and conceived the study and wrote the
manuscript. DC, RH, FR and HW performed the experiments. CW and YZ
analyzed data. All authors have read and approved the final
manuscript. DC and RH confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vaidya A, Nehs M and Kilbridge K:
Treatment of adrenocortical carcinoma. Surg Pathol Clin.
12:997–1006. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kerkhofs TM, Verhoeven RH, Van der Zwan
JM, Dieleman J, Kerstens MN, Links TP, Van de Poll-Franse LV and
Haak HR: Adrenocortical carcinoma: A population-based study on
incidence and survival in the Netherlands since 1993. Eur J Cancer.
49:2579–2586. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474.
2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fassnacht M, Dekkers OM, Else T, Baudin E,
Berruti A, de Krijger R, Haak HR, Mihai R, Assie G and Terzolo M:
european society of endocrinology clinical practice guidelines on
the management of adrenocortical carcinoma in adults, in
collaboration with the european network for the study of adrenal
tumors. Eur J Endocrinol. 179:G1–G46. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kiesewetter B, Riss P, Scheuba C, Mazal P,
Kretschmer-Chott E, Haug A and Raderer M: Management of
adrenocortical carcinoma: are we making progress? Ther Adv Med
Oncol. 13(17588359211038409)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Giménez-Dejoz J, Weber S, Fernández-Pardo
Á, Möller G, Adamski J, Porté S, Parés X and Farrés J: Engineering
aldo-keto reductase 1B10 to mimic the distinct 1B15 topology and
specificity towards inhibitors and substrates, including retinoids
and steroids. Chem Biol Interact. 307:186–194. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen D, Shen Z, Cheng X, Wang Q, Zhou J,
Ren F, Sun Y, Wang H and Huang R: Homeobox A5 activates p53 pathway
to inhibit proliferation and promote apoptosis of adrenocortical
carcinoma cells by inducing Aldo-Keto reductase family 1 member B10
expression. Bioengineered. 12:1964–1975. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Oughtred R, Stark C, Breitkreutz BJ, Rust
J, Boucher L, Chang C, Kolas N, O'Donnell L, Leung G, McAdam R, et
al: The BioGRID interaction database: 2019 update. Nucleic Acids
Res. 47:D529–D541. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Komolka K, Albrecht E, Schering L,
Brenmoehl J, Hoeflich A and Maak S: Locus characterization and gene
expression of bovine FNDC5: Is the myokine irisin relevant in
cattle? PLoS One. 9(e88060)2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhu T, Zhang W, Zhang Y, Lu E, Liu H, Liu
X, Yin S and Zhang P: Irisin/FNDC5 inhibits the
epithelial-mesenchymal transition of epithelial ovarian cancer
cells via the PI3K/Akt pathway. Arch Gynecol Obstet. 306:841–850.
2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Huang CW, Chang YH, Lee HH, Wu JY, Huang
JX, Chung YH, Hsu ST, Chow LP, Wei KC and Huang FT: Irisin, an
exercise myokine, potently suppresses tumor proliferation,
invasion, and growth in glioma. FASEB J. 34:9678–9693.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fan GH, Zhu TY and Huang J: FNDC5 promotes
paclitaxel sensitivity of non-small cell lung cancers via
inhibiting MDR1. Cell Signal. 72(109665)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cheng G, Xu D, Chu K, Cao Z, Sun X and
Yang Y: The effects of MiR-214-3p and Irisin/FNDC5 on the
biological behavior of osteosarcoma cells. Cancer Biother
Radiopharm. 35:92–100. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu J, Song N, Huang Y and Chen Y: Irisin
inhibits pancreatic cancer cell growth via the AMPK-mTOR pathway.
Sci Rep. 8(15247)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Boström P, Wu J, Jedrychowski MP, Korde A,
Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, et al: A
PGC1-α-dependent myokine that drives brown-fat-like development of
white fat and thermogenesis. Nature. 481:463–468. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Aydin S, Kuloglu T, Ozercan MR, Albayrak
S, Aydin S, Bakal U, Yilmaz M, Kalayci M, Yardim M, Sarac M, et al:
Irisin immunohistochemistry in gastrointestinal system cancers.
Biotech Histochem. 91:242–250. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kuloglu T, Celik O, Aydin S, Hanifi
Ozercan I, Acet M, Aydin Y, Artas G, Turk A, Yardim M, Ozan G, et
al: Irisin immunostaining characteristics of breast and ovarian
cancer cells. Cell Mol Biol (Noisy-le-grand). 62:40–44.
2016.PubMed/NCBI
|
|
19
|
Kong G, Jiang Y, Sun X, Cao Z, Zhang G,
Zhao Z, Zhao Y, Yu Q and Cheng G: Irisin reverses the IL-6 induced
epithelial-mesenchymal transition in osteosarcoma cell migration
and invasion through the STAT3/Snail signaling pathway. Oncol Rep.
38:2647–2656. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shao L, Li H, Chen J, Song H, Zhang Y, Wu
F, Wang W, Zhang W, Wang F, Li H and Tang D: Irisin suppresses the
migration, proliferation, and invasion of lung cancer cells via
inhibition of epithelial-to-mesenchymal transition. Biochem Biophys
Res Commun. 485:598–605. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gannon NP, Vaughan RA, Garcia-Smith R,
Bisoffi M and Trujillo KA: Effects of the exercise-inducible
myokine irisin on malignant and non-malignant breast epithelial
cell behavior in vitro. Int J Cancer. 136:E197–E202.
2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Altay DU, Keha EE, Karagüzel E, Menteşe A,
Yaman SO and Alver A: The diagnostic value of FNDC5/Irisin in renal
cell cancer. Int Braz J Urol. 44:734–739. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wozniak S, Nowinska K, Chabowski M and
Dziegiel P: Significance of Irisin (FNDC5) expression in colorectal
cancer. In Vivo. 36:180–188. 2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cebulski K, Nowińska K, Jablońska K,
Romanowicz H, Smolarz B, Dzięgiel P and Podhorska-Okołów M:
Expression of Irisin/FNDC5 in breast cancer. Int J Mol Sci.
23(3530)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu H, Zhao L, Wang M, Yang K, Jin Z, Zhao
C and Shi G: FNDC5 causes resistance to sorafenib by activating the
PI3K/Akt/Nrf2 pathway in hepatocellular carcinoma cells. Front
Oncol. 12(852095)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shao X, Wu J, Yu S, Zhou Y and Zhou C:
AKR1B10 inhibits the proliferation and migration of gastric cancer
via regulating epithelial-mesenchymal transition. Aging (Albany
NY). 13:22298–22314. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yao Y, Wang X, Zhou D, Li H, Qian H, Zhang
J, Jiang L, Wang B, Lin Q and Zhu X: Loss of AKR1B10 promotes
colorectal cancer cells proliferation and migration via regulating
FGF1-dependent pathway. Aging (Albany NY). 12:13059–13075.
2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ren P, Ren X, Cheng L and Xu L:
Frankincense, pine needle and geranium essential oils suppress
tumor progression through the regulation of the AMPK/mTOR pathway
in breast cancer. Oncol Rep. 39:129–137. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Plews RL, Mohd Yusof A, Wang C, Saji M,
Zhang X, Chen CS, Ringel MD and Phay JE: A novel dual AMPK
activator/mTOR inhibitor inhibits thyroid cancer cell growth. J
Clin Endocrinol Metab. 100:E748–E756. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yue W, Zheng X, Lin Y, Yang CS, Xu Q,
Carpizo D, Huang H, DiPaola RS and Tan XL: Metformin combined with
aspirin significantly inhibit pancreatic cancer cell growth in
vitro and in vivo by suppressing anti-apoptotic proteins Mcl-1 and
Bcl-2. Oncotarget. 6:21208–21224. 2015.PubMed/NCBI View Article : Google Scholar
|