1. Introduction
Lung cancer is one of the commonest malignancies
with the fastest growth in morbidity and mortality, and a great
threat to human health and life (1). According to the GLOBOCAN 2020 global
cancer morbidity and mortality statistical analysis compiled by the
International Agency for Research on Cancer, lung cancer is still
the main cause of cancer mortalities (18% of the total cancer
mortalities) (2). Nearly half of
newly diagnosed lung cancer cases are at an advanced stage at which
the therapeutic effect is limited and the treatment process is
painful (1). Although great
progress has been made in the molecular mechanism of lung cancer,
therapeutic interventions for lung cancer have only achieved modest
benefits (3). Conventional
chemotherapy also has the disadvantage of cytotoxicity to normal
tissues (4). Therefore, finding
more effective and safe drugs to prevent, inhibit or reverse the
occurrence of lung cancer is still the focus of research.
The root of Salvia miltiorrhiza Bunge was
first recorded in the Shennong's Herbs (5,6). It
has the functions of promoting blood circulation, removing blood
stasis and calming the mind. Promoting blood circulation and
removing blood stasis is one of the common therapeutic principles
in traditional Chinese medicine (TCM) for malignant tumors
(7-9).
Studies of the anti-tumor effects of Salvia miltiorrhiza
date back to the 1960s (10).
Since the 1980s, a number of studies have shown that
S. miltiorrhiza has a prominent anti-tumor effect,
especially the against lung cancer (5,11-13).
Therefore, a number of experiments have been conducted to explore
its mechanism against lung cancer. However, research on the
anti-tumor effects of S. miltiorrhiza (Chinese name:
Danshen) can be traced back to the 1970s. At that time, it was
proposed that S. miltiorrhiza injection could promote lung
metastasis in animal transplanted tumors (14), which attracted clinical interest.
Subsequently, a study suggested that Tanshinone IIA sulfonate had
no effect on promoting growth and metastasis of Lewis cancer and
noted that different experimental methods of previous researchers
resulted in different experimental results (15). Since the beginning of the 21st
century, the research on anti-tumor effects of S.
miltiorrhiza has been continuous and the results of these
studies have been fruitful (16-19).
The purpose of the present review was to summarize the mechanism of
the active ingredients of S. miltiorrhiza against lung
cancer in the past 20 years or so in order evaluate the clinical
value of S. miltiorrhiza against lung cancer.
S. miltiorrhiza is the dry root and rhizome
of S. miltiorrhiza. It has been used in traditional Chinese
medicine to treat obstruction of qi in the chest, heartache,
abdominal pain, insomnia, irregular menstruation, skin ulcers and
other diseases with the functions of analgesic and eliminating
carbuncles (20). In modern times,
the use of S. miltiorrhiza has been studied in the treatment
of various cardiovascular and endocrine diseases, including
coronary artery disease (21,22),
angina (23), hepatitis (24,25),
cancer and menstrual disorders (26,27).
Related experimental research has also made good progress. In the
past 20 years, a number of studies have focused on the mechanism of
active components of S. miltiorrhiza against lung cancer
(19,28).
2. Active ingredients of Salvia
miltiorrhiza
S. miltiorrhiza contains a number of chemical
constituents. According to the Chinese Pharmacopoeia (2020)
(29), S. miltiorrhiza can
be divided into lipophilic terpenes and water-soluble phenolic
acids, fatty acids, lactones, polysaccharides, flavonoids and other
components (Table I; Fig. 1). In addition, S.
miltiorrhiza contains nitrogen-containing compounds, lactone
compounds, polysaccharides, flavonoids, steroids, triterpenes and
other active ingredients. S. miltiorrhiza has
anti-atherosclerosis, cardioprotective and neuroprotective effects
(5,11). In addition, S. miltiorrhiza
can also lower blood sugar, regulate intracellular calcium ion
concentration, inhibit inflammatory response, resist oxidation and
scavenge oxygen free radicals together with other pharmacological
effects (30-33).
Modern pharmacological studies have shown that S.
miltiorrhiza can be used for the treatment of various diseases,
including cerebrovascular disease (34,35),
coronary heart disease (36,37),
Parkinson's disease (38),
Alzheimer's disease (39-41),
peptic ulcers (42), scars
(43), chronic kidney disease
(44,45), liver cirrhosis (46), osteoporosis, lung cancer and other
diseases (40). S.
miltiorrhiza is one of the traditional Chinese medicines that
can be used as a health food, as announced by the National Health
Commission of China (47). It is
compatible with other traditional Chinese medicines for the
treatment of various diseases.
 | Table IClassification of Salvia
miltiorrhiza. |
Table I
Classification of Salvia
miltiorrhiza.
| Classification of
components of Salvia miltiorrhiza | Specific
ingredients |
|---|
| Diterpenoids | Tanshinone IIA,
Tanshinone IIB, Cryptotanshinone, Tanshinone I and
dihydrotanshinone |
| Phenolic acids | Rosmarinic acid,
salvianolic acid A, salvianolic acid B, danshensu and
protocatechualdehyde |
| Other | Linoleic acid,
linolenic acid, salvia lactone, neosalvianen, Baicalin
β-Sitosterol, ursolic acid and carotene |
Phenolic acids. The main water-soluble
components of S. miltiorrhiza are phenolic acids; among
which salvianolic acids, salvianolic acid A (Sal A) and salvianolic
acid B (Sal B) are the most abundant components (47,48).
Salvianolic acid in S. miltiorrhiza has recognized
biological activity (11). Whether
in vivo or in vitro, most salvianolic acids have
anti-inflammatory, antioxidant and free radical scavenging
activities, which can protect cells from a variety of harmful
factors (40,45,46).
Although salvianolic acid can directly scavenge free radicals, they
may not be present in the body at a high concentration (49). The antioxidant activity of
salvianolic acid may increase the expression of antioxidant enzymes
by activating nuclear factor E2-related factor 2 (Nrf2)/heme
oxygenase-1 (HO-1) signaling pathway (9), thus reducing the expression of
peroxidase (50).
Diterpenoids. At present, >40 diterpenoids
have been found in S. miltiorrhiza and they can be divided
into two types according to their structure, namely o-quinone-type
tanshinone and p-quinone-type rolitazone (17). Tanshinones are lipophilic light
unstable active ingredients in S. miltiorrhiza including
tanshinone IIA (T IIA), cryptotanshinone (CT), dihydrotanshinone
(ICTS) and tanshinone I (T I), among others. The content of T IIA
in tanshinone was the highest, followed by CT (51).
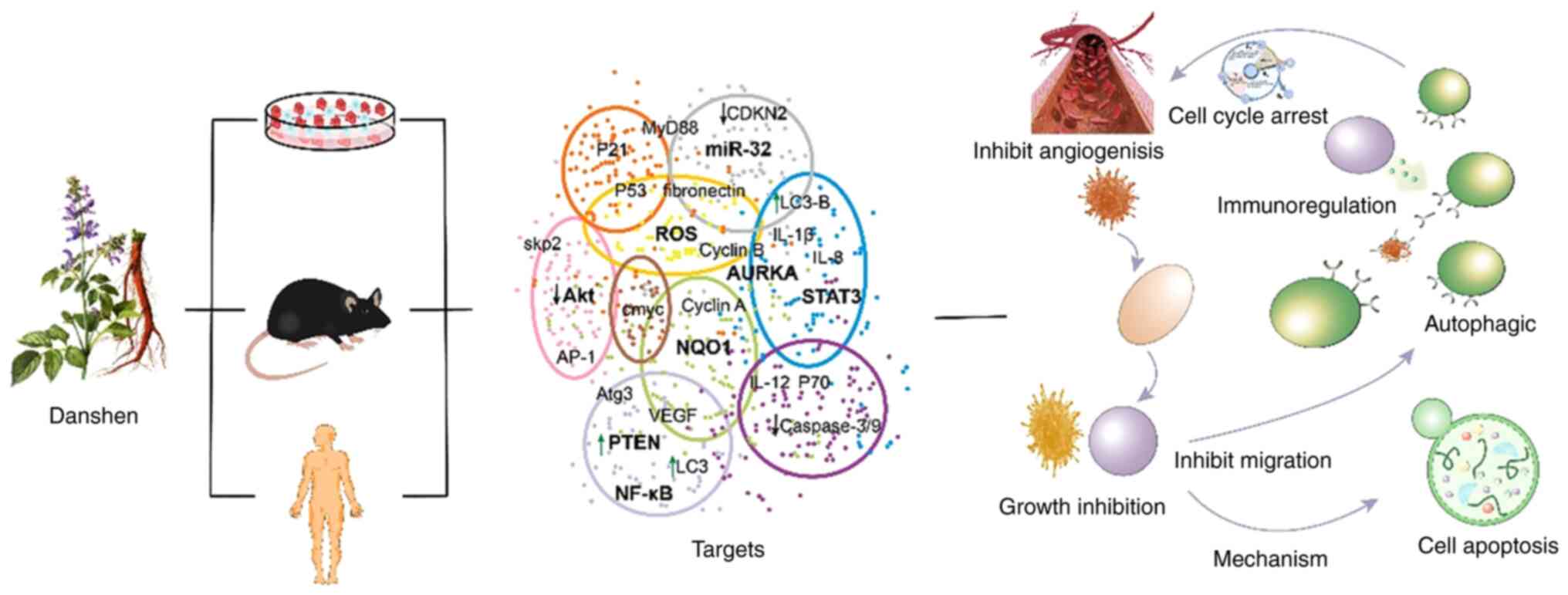
3. Mechanisms of active ingredients of
Salvia miltiorrhiza against lung cancer
A number of studies have shown that different parts
and different components of S. miltiorrhiza have effects
against different types of non-small cell lung cancer (NSCLC) and
the same component against the same type of lung cancer cells may
involve multiple mechanisms of action, such as inhibiting cell
proliferation, promoting cell apoptosis, inducing cell autophagy,
regulating immune-related, fighting tumor angiogenesis and
inhibiting cell migration and invasion (47,49,52,53)
(Fig. 2).
Inhibiting lung cancer cell proliferation and
growth. The continuous proliferation and growth of tumor cells
is the basis of tumor invasion and malignant transformation
(54). The mechanism of S.
miltiorrhiza against the growth of lung cancer has been widely
studied (47,55,56).
Sal A, Sal B, CT and T IIa have been found to inhibit lung cancer
A549 cells, SPC-A-1 cells, H1299 cells in vitro and Lewis
cells in vivo (57,58). The main target proteins involved
include p53, p21, the cyclin-dependent kinase inhibitor (CDKN)
family, cyclin family, c-myc and Aurora-a (59). Target genes include c-myc, AURKA,
EGFR, and microRNA (miR)-34a (60,61).
Signal pathways include P46 [JNK/stress-activated protein kinases
(SAPK)], phosphatase and tensed homolog (PTEN)/Akt,
miR-146a-5p/EGFR axis and phosphoinositide 3-kinase/Akt (62).
Sal A, one of the active components from S.
miltiorrhiza, suppresses the growth of mouse tumors (63). S-3-1 (a
2-allyl-3,4-dihydroxybenzaldehyde) is a synthetic intermediate of a
Sal A derivative with strong inhibitory effects on the growth of
cancer cells in vitro (64,65).
In 2002, Li et al (64)
found that S-3-1 at 20 mg/ml could significantly enhance gap
junction-to-cell communication, reverse the transformed phenotype
and inhibit tumors in human lung epithelial cancer W1-38 cells and
human lung adenocarcinoma A549 cells. S-3-1 inhibits the expression
of c-myc gene in A549 cells (49).
S-3-1 also inhibits the expression of P46 (JNK/SAPK) in A549 cells
(66). Bi et al (67) noted that Sal A negatively mediates
A549 lung cancer cell line growth or apoptosis, probably by
positively regulating PTEN protein level. Sal A treatment
significantly decreases A549 cell growth, promoting partial
apoptosis and increasing mitochondrial membrane permeability.
Western blot analysis showed that Sal A upregulates the PTEN
protein level, while consistently downregulating Akt
phosphorylation.
Shi et al (68) found that the growth rate and colony
formation rate of SPC-A-1 cells are significantly reduced following
T IIA treatment. T IIA can inhibit the growth and clonal formation
of SPC-A-1 cells and may inhibit DNA synthesis by significantly
upregulating the expression of p53 and P21 and downregulating the
expression of CDKN2(68). Chen
et al (55) observed that
following treatment of A549 cells with CT, the expression of cyclin
B1, CDK1 and Cdc25C is downregulated at the gene and protein
levels, while p21 is upregulated. The Cdc25 phosphatase family
regulates the dephosphorylation of cyclin B/cyclin-dependent kinase
(CDK1) and triggers entry into mitosis and inhibits p53-induced
growth inhibition (69). P21
(CIP1/WAF1) acts as a regulator of cell cycle progression and is
controlled by the tumor suppressor protein p53. Growth inhibition
of p21 can promote cell differentiation and death, prevent cell
proliferation (59). Qi et
al (66) showed that CT can
inhibit the expression of EGFR in NSCLC cells and downregulation of
EGFR can inhibit cell proliferation and cell cycle. EGFR is a
direct target of mirNA-146A-5p and CT can inhibit the proliferation
and growth of lung cancer by regulating miR-146A-5p/EGFR axis.
Zhang et al (70) showed that CT not only inhibits the
basic phosphorylation levels of insulin-like growth factor 1
receptor (IGF-1R) and RAC-α serine/threonine protein kinase (Akt),
but also blocks igF-1-induced igF-1R and Akt phosphorylation,
thereby inhibiting the proliferation and migration of lung cancer
cells. Another study demonstrated a novel mechanism of miR-34a
regulation in human malignancies in which NF-κB could regulate
miR-34a expression (71). miR-34a
targets TGFβR2 which inhibits the apoptosis of NSCLC cells. Studies
have confirmed that miR-34a can inhibit the tumor progression of
lung cancer (72,73). miR-25, miR-32 and miR-92a/b are in
the same miR family (74). Based
on this, they may serve a role in specific cancers, mainly
inhibiting cell proliferation and promoting apoptosis and
preventing the cell cycle process (9,63).
Shi et al (68) showed that
ICTS I, T I, T IIA and CT all have a proliferation inhibitory
effect on the SPC-A-1 cell line. The results showed that the
structure of aromatic ring A can enhance cytotoxicity and the
structure of Furan ring C can affect cytotoxicity. Aurora-A is
recognized as an important molecular target for cancer treatment
(75-77)
and is a member of the carcinogenic family of novel mitotic
serine/threonine kinases. A large body of evidence indicates the
role of Aurora-A in centrosome maturation (78), spindle formation (79) and G2-M transition
(80). Aurora-A is frequently
overexpressed in different types of cancers (81-85).
The suppression of Aurora-A using short hairpin RNA inhibits tumor
growth (86-89).
Li et al (56) showed that
tanshinone significantly downregulates the expression level of
Aurora-A in vitro, whereas TI significantly downregulates
the protein level of Aurora-A in vivo. CT and T I inhibit
cell proliferation by preventing the cell cycle in the S phase,
while T IIA prevents the cell cycle in the G2-M phase.
Cdc2, also known as CDK1 (cyclin-dependent kinase), serves an
important role in the progression of the cell cycle and is
considered an indispensable molecular target for the design of
therapeutic anticancer drugs (90). Li et al (56) found that the expression of CDK2 is
particularly inhibited by CT or T I treatment. Tung et al
(91) compared the effects of T I
and T IIA against lung cancer cells and concluded that T I inhibits
the growth of lung cancer cells in a dose-dependent manner by
inhibiting the expression of VEGF, Cyclin A and Cyclin B proteins
and its effect is superior compared with T IIA. Their research
confirmed that T I can eliminate lung function damage and the
formation of lung adenocarcinoma.
Tian et al (92) found that T I could change lung
adenocarcinoma gene expression and signal pathway. Simultaneously,
hydrochloride ester can inhibit the growth of lung adenocarcinoma
in nude mice and downregulate the expression levels of ATP7A and
ATP7B.
Gao et al (93) confirmed that Tan IIA, as an EGFR
signaling inhibitor, inhibits NSCLC cells by targeting
egFR-Akt-mcl1 axis to inhibit EGFR signaling pathway. Tan IIA
destroys the stability of McL-1, shortens the half-life and
promotes the ubiquitination and degradation of McL-1. Tan IIA
reduces the cell viability and colony formation of EGFR wild-type
and activates mutant cell lines and inhibits tumor growth in
vivo. Wang et al (94)
observed that sodium S. miltiorrhiza inhibits the activity,
migration and invasion of A549 and NCI-H1299 cells, promotes
apoptosis and reduces the expression of proliferating cell nuclear
antigen, MMP9 and Bcl-2, while upregulating Bax expression and
inhibiting the PI3K/AKT pathway in A549 and NCI-H1299 cells.
However, there was no cytotoxic effect on beAS-2B cell
proliferation activity.
Promoting cell apoptosis. Inducing cell cycle
arrest and apoptosis is one of the important mechanisms of
anticancer compounds (95). CT, T
IIA and methanol extract of S. miltiorrhiza (CTN) have been
found to be effective in inhibiting NSCLC including in vitro
tumor cells A549 cells, SPC-A-1 cells, H596-NQO1, Glc-82 cells,
in vivo nude mouse lung tumors and nude mouse Glc-82
xenografts (19,68,96).
Salvia miltiorrhiza promotes apoptosis of lung cancer cells,
mainly through ferroptosis receptors, mitochondria and endoplasmic
reticulum, stimulating p53, Bax, Fas, CCAAT/enhancer binding
protein homologous protein (CHOP), caspase family, NQO1, ATF-4 and
other target proteases as well as ERS-induced apoptosis pathway,
such as IRE1α, Caspase 12 and PI3K/Akt (97,98).
Zinnah and Park (99) noted that CT can transform
drug-resistant lung A549 cancer cells into tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL)-sensitive cells.
Mechanistically, CT-induced TRAIL receptor2 (DR5) does not depend
on p53, but depends on the induction of CCAAT/CHOP. The
cancellation of CHOP abolishes CT-induced DR5 expression and
TRAIL-mediated cell death-related enhancement. The in vitro
and in vivo experiments of Chen et al (55) showed that CT enhances the
expression of p53 and Bax and downregulates the expression of
Bcl-2. This apoptosis may be mediated by caspases. CT can cause
growth inhibition of human lung cancer, cell cycle arrest,
apoptosis and tumor formation in vitro and in
vivo.
He et al (100) treated SPC-A-1 cells with T IIA
and observed a large number of apoptotic cells using electron
microscopy. Flow cytometry showed that the apoptosis index of
tanshinone group was significantly higher than that of cisplatin
(DDP) group and control group. They hypothesized that T IIA can
induce the apoptosis of lung cancer SPC-A-1 cells via upregulating
p53, Bax, Fas and downregulating the expression of Bcl-2.
Results of in vivo experiments showed that
anticancer ketones have an antitumor effect on Lewis lung cancer in
mice and its mechanism may be related to the induction of tumor
cell apoptosis (101). Chiu and
Su (57) noted that T IIA
significantly inhibits the proliferation of A549 cells in a dose-
and time-dependent manner. FACS results showed that when A549 cells
were incubated with different concentrations of T IIA (control,
2.5, 5 and 10 mg/ml) for 48 h, the sub-G1 phase
increases. T IIA induces the production of ROS, Ca2+ and
reduces mitochondrial membrane potential (MMP). Western blotting
results showed that after 6, 12 and 24 h of culture with T IIA (5
mg/ml), p53 and Bax protein expression increased, but the
proto-oncogene Bcl-2 significantly decreased. T IIA may induce
apoptosis by reducing MMP and inducing a higher Bax/Bcl-2 ratio,
thereby inhibiting the proliferation of NSCLC A549 cells (97). Liu et al (102) observed through in vivo
experiments that T IIA induced NQO1 (+) A549 cells and H596-NQO1
cells to produce excessive ROS, DNA damage and apoptosis, but NQO1
(-) H596 cells without TIIA did not produce excessive ROS, DNA
damage and apoptosis. T IIA treatment significantly delayed the
growth of A549 tumor xenotransplantation, activated ROS triggered
p53 independent and caspase-dependent mitochondrial apoptosis cell
death pathway, cytochrome c release, and subsequent caspase
activation and PARP-1 cleavage. Zhang et al (103) noted out that T IIA inhibits the
growth of A549 cells, induces JNK signal activation and triggers
caspase cascade apoptosis mediated by cytochrome c release. During
the induction process, changes in mitochondrial morphology and loss
of MMP are observed. In addition, T IIA induces apoptosis in A549
cells and this has been confirmed by typical morphological changes,
in which cytochrome c is released from mitochondria and Bax is
translocated into mitochondria. Caspase activity data shows that T
IIA activates mitochondrial-mediated apoptosis of caspase-9 and
caspase-3, but does not activate receptor-mediated apoptosis of
caspase-8, which can be largely rescued by SP600125 (a JNK
inhibitor). Kim et al (104) showed that T IIA increases
TRAIL-induced cell death by selectively activating PERK/ATF4 and
inhibiting STAT3-mediated DR5 upregulation and Survivin
downregulation, indicating that the combined intervention of T IIA
and TRAIL is a new treatment strategy for human NSCLC. NSCLC cells
shows resistance to TRAIL-mediated cell death, but the combined
treatment of T IIA and TRAIL synergistically reduces cell viability
and increases the apoptosis of TRAIL-resistant NSCLC cells. T IIA
can greatly induce death receptor (DR) 5, but not DR4. Liu et
al (105) found that NAMPT
inhibition can synergistically induce NQO1 activation to induce
apoptotic cell death. NAMPT catalyzes the first rate-limiting step
in the conversion of nicotinamide to NAD(+), which is essential for
a number of enzymes and regulatory proteins involved in various
cellular processes, including deacetylation of the enzyme SIRT I,
which can regulate a variety of tumor suppressors, including p53
and forkhead box (FOX)O. In Liu's study, NQO1 substrates T IIA and
β-lapachone (β-lap) induce rapid depletion of the NAD(+) pool, but
adaptively significantly upregulate NAMPT. The non-toxic inhibitory
effect of FK866 on NAMPT significantly enhances the apoptosis
induced by the NQO1 targeting agent. Compared with T IIA or β-lap
treatment alone, co-treatment with FK866 induces more significant
NAD(+) depletion and SIRT I activity inhibition, thereby increasing
the accumulation of acetylated FOXO1 and activating apoptosis
pathways.
Tanshinone significantly induces apoptosis of lung
cancer cells in vitro, which is related to the
downregulation of Bcl-2 and survivin gene expression and protein
levels and increases the Bax/Bcl-2 ratio, which is a more reliable
indicator of apoptosis (106-108).
CT, T I and T IIA treatment induces dose-dependent apoptosis. Among
the three tanshinones, T IIA is the most effective at inducing
apoptosis. At a concentration of 2 µM, its apoptosis increased by 5
times (from 2 to 10%) (90).
TRAIL is a promising anticancer drug. It has a
unique cancer cell-specific pro-apoptotic effect, but its potential
is greatly inhibited by acquired drug resistance (109). Stimulation with CT, T I or T IIA
can effectively enhance the activity of TRAIL, thereby reducing the
activity and inhibiting the proliferation of TRAIL-resistant
TOV-21G and SKOV3. Among them, T IIA is the most effective and its
IC50 is 2.00±0.36 µM (110). Zhang et al (103) found that tan IIA may induce
cytochrome caspase cascade apoptosis through the JNK pathway. It
induces apoptosis through mitochondrial release of cytochrome c and
Bax migration towards mitochondria.
Shen et al (111) showed that diterpene tanshinone
(DT) can induce apoptosis in nine human cancer cell lines with an
IC50 of 4.37-29 µg/ml, of which PC9 and MCF-7 have the
lowest values of IC. Fluorescence staining showed that the DT had a
lethal effect on PC9 cells. Western blotting showed that caspase
3/9 and ATF-4 protein expression gradually decreased. However, the
expression of PARP, cleaved caspase 3/9 and phosphorylated
(p-)eIF2α, p-JNK and caspase-12 gradually increased in a
dose-dependent manner. Lou et al (112) noted that the endoplasmic
reticulum stress-mediated apoptosis pathway is an effective way to
promote tumor cell apoptosis and may be an important target for DT
against lung cancer. The team used human lung adenocarcinoma PC9
cell line and nude mouse xenograft model as examples to verify the
anti-lung cancer effect of DT in vivo and in vitro
and clarified the IRE1α/caspase 12 apoptosis pathway induced by ERS
Its underlying mechanism. The results showed that, in vivo,
DT can promote PC9 cell apoptosis in a concentration-dependent
manner, upregulate the expression of Bip, IRE1 and TRAF2 proteins
in tumor tissues and reduce tumor weight and weight loss. In
vitro, DT inhibits the proliferation of PC9 cell lines in a
concentration-dependent manner, destroys the mitochondrial
structure of PC9 cells, promotes the expression of Bax, IRE1α, Bip,
TRAF2 and caspase 12 proteins and reduces the expression of Bcl-2
protein. DT shows good anti-lung cancer effects both in vivo
and in vitro. The mechanism is related to the activation of
IRE1α/caspase 12 apoptosis pathway induced by ERS and the promotion
of apoptosis.
CTN is a methanol extract of S. miltiorrhiza
and is an active compound that induces apoptosis through the
mitochondrial apoptosis pathway and PTEN-mediated PI3K/Akt pathway
inhibition. CTN induces significant (P<0.05) and dose-dependent
apoptosis in Glc-82 cells. Cell cycle analysis shows that CTN
induces G2/M phase arrest and significantly (P<0.05)
increases p53 and p21 levels and activates caspase-3/9 and PARP1
expression, suggesting that mitochondria are involved in apoptosis
signals. In addition, CTN reduces the expression of anti-apoptotic
proteins Bcl-2 and Bcl-xl and increases the expression of
pro-apoptotic protein Bax. The results also showed that CTN can
increase the expression level of PTEN and reduce the
phosphorylation level of Akt in Thr 308 and Ser 473 domains
(19).
Xie et al (113) showed that tan IIA can restrain
cell proliferation, induce apoptosis and arrest cell cycle at the S
phase. It may block VEGF/VEGFR signal pathway, cause cell cycle
arrest and indirectly inhibit downstream signal pathway and then
upregulate the expression of apoptosis genes, downregulate
anti-apoptosis genes and then inhibit the development, and promote
the apoptosis, of tumor cells.
Kim et al (104) showed that tan IIA induced TRAIL
sensitization of lung cancer cells by selective induction of
endoplasmic reticulum stress. So, tan IIA may induce apoptosis of
TRAIL via upregulating DR5 and downregulating Survivin via
selective activation of PERK/ATF and inhibition of STAT3,
respectively.
Chiu and Su (57)
showed that tan IIA induces apoptosis by the abduction of ROS and
by diminishing the mitochondrial membrane potential in A549 cells.
Tan IIA might decrease the expression ofBcl-2 and increase Bax, p53
and Cyto-c and may work via the abduction of ROS and a higher scale
of Bax/Bcl-2.
Liu et al (102) suggested that the apoptosis
pathway by NQO1-activated and p53-independent mechanism determines
the antitumor function of tan IIA against NSCLC. Tan IIA may
activate ROS triggered, p53-independent and caspase-dependent
mitochondria apoptotic mechanism by increased Bax/Bcl-xL,
disruption of mitochondrial membrane potential, release of
cytochrome c and caspase excitation and PARP-1 cleavage.
Some studies have also found that sodium T IIA
sulfonate (STS) directly binds to fragile histidine triad
diadenosine triphosphatase (FHIT) protein and inhibits the activity
of FHIT AP3A hydrolase through competitive binding with the FHIT
substrate binding site and induces tumor cell apoptosis (114,115).
Wu et al (116) found that dihydroisotanshinone I
can inhibit the growth of A549 cells and H460 cells through
apoptosis and ferroptosis and inhibit the transfer of A549 cells in
a nude mouse model.
Inducing cell autophagy. Autophagy is
essential for maintaining intracellular homeostasis and is also a
mechanism of cell survival, involving the degradation and recycling
of cytoplasmic longevity proteins and organelles (117). Constitutive autophagy shows a
clear pro-survival effect in cell damage and the imbalance of
autophagy is considered a detrimental role in cell function and
survival (118). There is
convincing clinical and experimental evidence that macrophages
promote cancer development and malignant progression. In response
to activation signals, macrophages are ‘re-educated’ and polarized
to the M1 phenotype (pro-inflammatory) or M2 phenotype
(anti-inflammatory). Tumor-associated macrophages mainly exhibit an
M2-like phenotype (119) and a
special subpopulation of macrophages may be an important new
therapeutic target.
Li et al (120) proposed a new M2 macrophage
tumor-promoting model, called ‘autophagy angiogenesis effect’;
polarized M2 macrophages induce the occurrence of abnormal
autophagy, resulting in the degradation of autophagosome the level
of NO and ROS in vascular endothelial cells increases, which in
turn leads to abnormal vasodilation to stimulate hyperplasia and
tumor progression. Consistent with this hypothesis, the study found
that M2 macrophages lead to abnormal angiogenesis accompanied by
abnormal autophagy, which eventually leads to tumorigenesis, while
clodronate liposomes cause macrophage depletion,
chloroquine-induced autophagy prevention or salvianolic acid
B-induced vascular protection significantly reduced the occurrence
of abnormal angiogenesis and lung cancer.
Hao et al (121) found that CT induces pre-death
autophagy by activating JNK signaling mediated by increased
intracellular ROS production. CT induces the formation of
intracellular (ROS) in a concentration- and time-dependent manner,
N-acetyl-L-cysteine (NAC), catalase, biphenyldiiodonium (DPI),
pyrrole Alkyl dimethyl thiocarbamate (PDTC) and dichloromalo
reverse this. In addition, NAC, JNK siRNA and SP600125 suppress
CT-induced autophagy. NAC reverses CT-induced phosphorylation of
JNK. NAC, 3-methyladenine (3-MA) and SP600125 partially reverse
CT-induced cell death. CT (10 mg/kg) significantly inhibits tumor
growth by 48.3% in A549 xenograft nude mice and this was completely
reversed by NAC (50 mg/kg) combined therapy.
STAT3 is a potential drug target for chemotherapy.
Guo et al (122) found
that ICTS significantly inhibits STAT3 activity. ICTS inhibits the
constitutive and inducible phosphorylation of STAT3 at Y705 without
affecting the phosphorylation of STAT3 at S727 in A549 lung cancer
cells. In addition, ICTS inhibits the nuclear translocation of
STAT3. ICTS induces autophagy, as manifested by the accumulation of
autophagic vesicles and increased expression of LC3 protein and
autophagosomes. The autophagy inhibitor chloroquine can partially
reverse ICTS-induced cell death. Docking assay predicts that both
ICTS and CTS bind to the SH2 domain of STAT3. ICTS forms hydrogen
bonds and pi-pi interactions with nearby amino acid residues of
Lys591, Arg609 and Ser636. These findings indicate that ICTS (a
natural compound) is a potent STAT3 inhibitor. ICTS induces
apoptosis and promotes autophagy in A549 cells. Compared with CTS,
ICTS has a stronger inhibitory effect on STAT3 phosphorylation and
A549 cytotoxicity. ICTS induces autophagy, as manifested by the
accumulation of autophagic vesicles and increased expression of LC3
protein and autophagosomes. The autophagy inhibitor chloroquine can
partially reverse ICTS-induced cell death.
Zhang et al (123) found that tanshinone can
upregulate the expression of miR-137 and miR-137 can significantly
inhibit the proliferation of NSCLC cell lines through autophagy.
The study proposes ULK2 and IBTK as potential targets for miR-137.
ULK2 can act on the 3-kinase upstream of phosphatidylinositol that
regulates the formation of autophagosomes and autophagosome
precursors. As an inhibitor of BTK tyrosine kinase activity, IBTK
plays a role in the development of B cells and interferes with
BTK-mediated calcium mobilization and NF-κB-mediated
transcription.
The findings of Gao et al (124) indicate that total tanshinone
(TDT)-induced apoptosis of 95D cells and protective autophagy are
mediated by increased intracellular ROS production. TDT induces the
production of ROS, while N-acetylcysteine (NAC) reverses it. NAC
also reversed TDT-induced Δψ depolarization, monosaccharide-based
cardavelin (MDC) staining, Bax upregulation, PARP lysis, Beclin-1,
LC3-II and cell viability. Compared with T IIA, TDT showed more
cytotoxic effects on 95D cells. Annexin V/7-AAD double staining,
MMP depolarization (Δψ), upregulation of pro-apoptotic proteins
(e.g., cleaved PARP, cleaved caspase-3, Bax and Bad) and The
downregulation of anti-apoptotic protein Bcl-2 is evidence of
TDT-induced apoptosis. TDT-induced autophagy was demonstrated by
up-regulation of MDC staining and autophagy-related proteins (such
as LC3-II, Beclin-1, Atg3, Atg5, Atg7 and Atg12). Autophagy
inhibitors 3-MA and bafilomycin A1 enhance TDT-induced cell death.
3-MA pretreatment enhances TDT-induced upregulation of Bax and PARP
cleavage.
Wu et al (116) found that compared with the
control group, the T IIA different concentration groups had a
significant proliferation inhibitory effect on A549 cells and
showed a time-dose-dependent relationship; T IIA can reduce the
expression level of cyclin D1 of A549 cells and cause cell cycle
arrest in G0 phase; it can also increase the ratio of LC3-Ⅱ/LC3-Ⅰ
of A549 cells, decrease the expression of p62 and induce autophagy
in cells. T IIA may induce cell cycle arrest by upregulating
autophagy in human NSCLC A549 cells, thereby inhibiting cell
proliferation and exerting anti-tumor effects (123).
Immune regulation. Tumor-associated
macrophages (TAM) are derived from peripheral blood mononuclear
cells recruited from tumors (119). The tumor promotion function of
macrophages at the primary site includes supporting
tumor-associated angiogenesis and promoting tumor cell invasion,
migration and intravascular migration. TAMs may provide a
microenvironment for the invasion and development of NSCLC
(125). There is evidence that
macrophages are affected by the tumor microenvironment, so they can
stimulate tumor metastasis by releasing multiple compounds
(including cytokines) (126).
Chemokine (CC motif) ligand 2 (CCL2), previously known as monocyte
chemoattractant protein-1, was first identified by its ability to
attract monocytes in vitro (127,128). In lung cancer, the
CCL2 signaling pathway is an important mechanism by which TAMs
activate lung cancer cell growth and metastasis through two-way
crosstalk between macrophages and lung cancer cells (129).
Man et al (130) noted that cryptotanshinone
effectively inhibits tumor growth of H446 cells and proliferation
of CD4+ T cells. Cryptotanshinone treatment increases
the cytotoxicity of CD4+ T cells, but does not affect
the cytotoxicity of CD8+ T cells. Meanwhile,
cryptotanshinone induces p-JAK2 and p-STAT4 protein expression in
CD4+ T cells. These results suggest that
cryptotanshinone inhibits lung tumor cell growth by activating the
JAK2/STAT4 pathway to increase CD4+ T cell toxicity.
CT not only upregulates p53, downregulates cyclinB1
and Cdc2 and inhibits the proliferation of mouse Lewis lung cancer
(LLC) cells, but it also induces LLC cell cycle arrest and is
involved in the activation of NF-κB, P38 and JNK. CO stimulation
and MHC molecules upregulated by CT stimulates dendritic cells to
produce TNFα, IL-1β and IL-12P70. CT, when used in combination with
low-dose anti-PD-L1, is effective against LLC tumors and induces
subsequent long-term anti-LLC specific immunity. CT therapy
promotes T cell infiltration and increases expression of genes
typical for Th1 polarization in LLC tumor tissue (131). Wu et al (132) found that DT represses the
phosphorylation of STAT3, the protein expression of S-phase kinase
associated protein-2 (Skp2), including CCL2 and suppresses the
macrophage recruitment ability of lung cancer cells. S.
miltiorrhiza mediates the interruption of crosstalk between
lung cancer cells and macrophages and the blocking of lung cancer
cell proliferation. T I significantly inhibits the migration,
invasion and gelatinase activity of CL1-5 cells stimulated by
macrophage conditioned culture medium in vitro and reduces
the tumorigenesis and metastasis of cl1-5 mice with severe combined
immunodeficiency. T I decreases the transcriptional activity of
interleukin-8 and stimulates DNA binding activity in CL1-5 cells by
decreasing activator protein-1 and nuclear factor κB. T I has
anticancer effects in vitro and in vivo mainly
mediated by interleukin-8, RAS-mitogen-activated protein kinase and
Rac1 signaling pathway (133).
Inhibition of tumor angiogenesis.
Angiogenesis is a key step in tumor growth. Compared to normal
tissues, tumor vasculature is abnormal because of its high
permeability, tortuosity and high inter-tissue pressure. The
majority of patients with lung cancer who have failed treatment
have distant metastases (134).
Tumor cell metastasis is a complex, multi-step process involving
the interaction between cancer cells and their surrounding
microenvironment (135).
Angiogenesis is a key step for tumor growth and metastasis
(136). The expression level of
VEGF-A 165 is positively correlated with the growth and spread of
cancer cells (137,138). The development of drugs targeting
VEGF-A 165 is an important focus of research.
Li et al (56) showed that T I inhibits the growth
of H1299 tumors in in vivo and in vitro experiments,
which is related to the inhibition of tumor angiogenesis. Jin et
al (139) found that T I
inhibits the expression of angiogenic factor IL-8 through the NF-κB
and AP-1 pathways, thereby inhibiting tumorigenesis, angiogenesis
and metastasis. Chen et al (65) showed that T I inhibited the growth
of lung cancer cells in a dose-dependent manner by inhibiting the
expression of VEGF, Cyclin a and Cyclin B proteins, and its effect
was better than T IIA. In addition, the transgenic mouse model of
lung cancer induced by human vascular endothelial growth factor
A165 (hVEGF-A165) gene was further tested for the treatment of lung
cancer in vivo. TI significantly reduced the overexpression
of hVEGF-A1-65 in transgenic mice to the normal level. Therefore,
the key mechanism of anti-tumor effect of TI treatment on
angiogenesis and angiogenesis (56).
Sal A promotes the apoptosis of NSCLC cells,
inhibits the migration and invasion ability of NSCLC cells and
ultimately prevents the formation of vasculogenic mimicry by
reducing the levels of EphA2, vascular endothelial cadherin and
MMP2. Salvianolic acid A significantly blocked the expression of
P-PI3K, p-Akt and p-mTOR in NSCLC cells, thereby affecting the
PI3K/Akt/mTOR pathway. Therefore, Sal A can block the formation of
vascular mimicry in human NSCLC through PI3K/AKT/mTOR pathway
(91). Salvicine is a
pharmacologically active derivative from S. miltiorrhiza and
can effectively reduce the capillary-like tube formation of HMEC.
In addition, it (30 µM) significantly reduces the mRNA expression
of bFGF in A549 cells, while the mRNA expression of VEGF remains
unchanged (140).
Lung cancer cell invasion. Cell invasion is
the first and most important step of metastasis. This is a complex
process that involves the invasion of nearby cells by invasive
cancer cells, which spread through the extracellular matrix (to the
auxiliary sites) (30,32). Zhang et al (141) propose that T II A can reduce the
adhesion of lung cancer cells; methylpyrazine, T II A and rudulin
can inhibit the invasion of PGCL3 cells in Boyden Chamber assay and
conclude that blood activating drugs can inhibit or promote the
invasion and metastasis of PGCL3 and PAa cells. Wang et al
(142) found through in
vitro studies that in the process of inhibiting human NSCLC,
STAT3 is not the only target of CT. It has been confirmed that CT
upregulates the expression levels of miR-30d-5p, miR-126-3p,
miR-133a, miR-338-3p and miR-451a and downregulates the expression
levels of miR-21-5p and miR-96-5p, miR-182-5p and miR-205-5p. Among
them, miR-133a was most significantly upregulates. miR-133a targets
and downregulates the expression of MMP14; however, MMP15, MMP16
and MMP24 are unaffected. It has been determined that this process
is independent of tissue inhibitors of metalloproteinase. CT can
inhibit the invasion of human NSCLC, which may be due to the
inhibition of MMP14 expression. T IIA can inhibit the growth of
human NSCLC A549 and H1299 cells in a concentration-dependent
manner and significantly reduce the number and size of cell clone
clusters and inhibit the proliferation ability of A549 cells in
vitro. Following T IIA treatment, A549 cell invasion and
migration ability are reduced, the expression level of integrin α2,
integrin β1, MMP2, MMP9, β-catenin and N-cadherin mRNA in the cells
decreases and the expression level of E-cadherin mRNA increases
(143).
4. Salvia miltiorrhiza combined
against lung cancer
In recent years, researchers have turned their
attention to S. miltiorrhiza combined with traditional
anti-cancer therapy and S. miltiorrhiza compounds to fight
lung cancer and have achieved certain results (65). S. miltiorrhiza can
effectively reverse drug resistance (such as DDP and gefitinib)
resistance, can improve the efficacy of radio-chemotherapy,
effectively avoid drug resistance, enhance the body's anti-cancer
sensitivity and has no toxic effect on the body (71,144). The main mechanism of action
includes the reduction of the lung cancer multidrug resistance gene
multidrug resistance-associated protein 1 (MDR1), inhibition of the
c-met/AKT/mTOR signaling pathway and inhibition of the Nrf2
pathway, thereby inhibiting the growth and proliferation of lung
cancer cells and promoting cancer cell apoptosis (145). The mechanism of Dihydrotanshinone
I against lung cancer includes inhibition of PTEN/PI3K/AKT
pathway-induced apoptosis in lung cancer cells and inhibition of
STAT3/VEGF/CDK2 to exert anti-angiogenesis and apoptosis effects
(146).
S. miltiorrhiza combined with anti-cancer
therapy. Drug resistance is one of the main reasons for
chemotherapy failure in the treatment of NSCLC. Chen et al
(147) divided human lung cancer
A549 cells into normal control group and drug group and determined
their MDR1 expression level by reverse transcription-quantitative
PCR, which confirmed that Sal A reduced lung cancer multidrug
resistance gene MDR1 may be regulated by miRNA expression and
target gene The effect of the method provides an experimental basis
for further elucidating the mechanism of Chinese medicines in
reversing multidrug resistance. Tang et al (148) found that Sal A can improve the
chemotherapeutic efficacy of DDP, indicating that Sal A and DDP
have a synergistic effect, which mainly enhances the A549/DDP
cells. The sensitivity of DDP effectively prevents the upregulation
of multidrug resistance-associated protein 1 (MDR1) in A549/DDP
cells.
Xia et al (149) indicate that CT may be developed
as a potential sensitizer in cooperation with anti-cancer drugs to
combat chemoresistance cancer by inhibiting the Nrf2 pathway. CT
can enhance the sensitivity of A549/DDP cells to DDP. In A549/DDP
cells, the endogenous expression levels of Nrf2 and its target
genes, including GCLC, GCLM, HO-1, NQO1 and MRP1, are much higher
than that of A549 cells and CT partly restores the sensitivity of
A549/DDP cells to DDP by muting Nrf2. Compared with DDP
monotherapy, the combination of CT and DDP leads to cell death and
apoptosis by sensitizing A549/DDP cells to DDP. Nrf2 knockout can
eliminate this reversal effect. At the same time, it was also found
that CT triggers other signals related to chemical resistance, such
as MAPKs, Akt and STAT3 pathways.
CT can prevent radiation-induced lung injury (RILI)
(150). CT can effectively
maintain lung function in RILI rats, reduce early lung inflammation
and infiltration and significantly reduce the levels of IL-6 and
IL-10. In addition, CT is superior to prednisone in reducing
collagen deposition and pulmonary fibrosis, while HYP (collagen
indicator) and α-SMA (myofibroblast marker) are significantly
reduced. In terms of mechanism, CT inhibits the expression of
fibrosis signals TGF-β1 and NOX-4 and at the same time increases
the level of anti-fibrotic enzyme MMP-1 in lung tissue. CT
treatment is superior compared with PND in enhancing MMP-1 levels.
However, CT has little effect on the activation of CTGF and the
inhibition of COX-2. Finally, CT treatment significantly reduces
radiation-induced activation of CCL3 and its receptor CCR1.
T IIA can be developed as a new drug in
postoperative adjuvant therapy together with other anti-tumor drugs
and improve the sensitivity of chemotherapy drugs to NSCLC with
fewer side effects. T IIA combined with doxorubicin can
synergistically inhibit migration, induce apoptosis and prevent the
cell cycle in S and G2 phases of A549 cells. The two
groups of monotherapy and combination therapy upregulate the
expression of cleaved caspase-3 and Bax and downregulate the
expression of VEGF, VEGFR2, p-PI3K, p-Akt, Bcl-2 and caspase-3
protein. The molecular docking algorithm shows that, compared with
doxorubicin, T IIA can be docked to the active sites of all tested
proteins through H-bond and aromatic interactions (143). The experiment of Li (96) proved the anti-tumor activity of T
IIA combined with cyclophosphamide on Lewis lung cancer mice and
its effect on cellular immune function and concluded that the
combination of T IIA and cyclophosphamide can downregulate Bcl-2 in
lung cancer tissues and upregulates the expression of Bax, inhibits
neovascularization of tumor tissue, enhances immune function and
has significant anti-tumor activity. The combined treatment of T
IIA and DDP has been shown to synergistically disrupt cell
migration and invasion, arrest the cell cycle at S phase and induce
apoptosis in A549 and PC9 cells. Kyoto Encyclopedia of Genes and
Genomes pathway analysis and molecular docking indicate that T IIA
may mainly affect the phosphatidylinositol 3-kinase-Akt signaling
pathway. In all treatment groups, the expression levels of Bax and
cleaved caspase-3 were upregulated, while the expression levels of
Bcl-2, caspase-3, p-Akt and p-PI3K proteins were downregulated
(151).
Gefitinib resistance is a major obstacle to the
treatment of NSCLC. T IIA combined with gefitinib enhances the
cytotoxic effect in gefitinib-resistant NSCLC cells and enhances
the inhibitory effect on the proliferation, migration and invasion
of gefitinib-resistant NSCLC cells. In addition, T IIA enhances the
pro-apoptotic effect of gefitinib in gefitinib-resistant NSCLC
cells by increasing the level of cleaved caspase 3. Simultaneously,
T IIA increases the sensitivity of HCC827/gefitinib cells to
gefitinib by downregulating the VEGFR2/Akt pathway. In vivo
experiments further confirmed that the combination of gefitinib and
T IIA inhibits tumor growth in the mouse xenograft model of
HCC827/gefitinib (152).
S. miltiorrhiza can also be used with
radiotherapy to enhance the effect of radiotherapy. Cao et
al (153) noted that S.
miltiorrhiza plus radiotherapy significantly prolonged tumor
growth delay and inhibited tumor growth. The study proposes that
S. miltiorrhiza is expected to become a sensitizer for
chemotherapy and radiotherapy to enhance anticancer agents [such as
TNF-α (154), 5-fluorouracil
(155) and γ-ray cytotoxicity
(156)]. S. miltiorrhiza
enhances the radiation response of LLC in a mouse model and its
mechanism may be related to the relief of tumor cell hypoxia after
S. miltiorrhiza plus radiotherapy treatment, which is due to
the improvement of tumor microcirculation and the remodeling of
tumor vasculature.
S. miltiorrhiza injection and DDP chest
injection combined to treat lung cancer malignant pleural effusion
has special advantages; a definite curative effect and few side
effects (157). The clinical
observations of Wang et al (158) suggest that S. miltiorrhiza
can effectively relieve venous thrombosis and can improve the
completion rate of chemotherapy in lung cancer patients. T IIA can
promote the anti-cancer effect of DDP, reduce the nephrotoxicity
and bone marrow suppression caused by DDP and increase the levels
of CD3+, CD4+, CD8+,
CD4+/CD8+ and NK cytokines in nude mice with
lung cancer. The mechanism is related to downregulating the
expression of IL-2 and IL-10 and regulating the Toll-like receptor
4 signaling pathway.
Bi et al (97) research on the compatibility of
S. miltiorrhiza and Panax ginseng (FMG) shows that on
the one hand, FMG may induce the apoptosis of lung cancer A549
cells, thereby affecting the proliferation of lung cancer, by
reducing the formation of microfilaments, which can ultimately
inhibit the proliferation, adhesion and metastasis of lung cancer.
They found that FMG selectively inhibits lung cancer cell
proliferation and induces apoptosis, but it does not have any
cytotoxic effect on normal lung epithelial BEAS-2B cells. Moreover,
FMG inhibits the migration and invasion of lung cancer cells. FMG
significantly promotes p-PTEN expression and subsequently inhibits
the PI3K/AKT signaling pathway. After FMG binds to PTEN protein,
the phosphatase activity of PTEN protein increases, indicating that
PTEN is one of FMG-targeting proteins. In addition, FMG regulates
the expression of some marker proteins related to apoptosis,
migration and invasion. The same team later discovered that the
S. miltiorrhiza-FMG formula can inhibit tumor metastasis and
growth by inhibiting epithelial-mesenchymal transition (EMT)
involved in the PTEN/PI3K/AKT pathway in lung cancer cells
(19,159) and can inhibit the invasion and
migration of lung cancer cells by targeting PTEN. In vivo,
the anti-tumor and anti-metastatic effects of S.
miltiorrhiza formula treatment are related to the inhibition of
EMT (97).
Geng et al (160) used compounded S.
miltiorrhiza injection combined with Shenmai regimen to treat
patients. Later, patients with advanced NSCLC were treated with
compounded S. miltiorrhiza injection and Shenmai injection
intravenously in combination with chemotherapy (pulmonary squamous
cell carcinoma using CAP treatment plan, lung adenocarcinoma using
a MAF plan) to treat NSCLC patients. The total improvement rate of
intravenous injection of compound Salvia miltiorrhiza
injection and Shenmai injection combined with chemotherapy was
69.7%, the total improvement rate of quality of life was 66.7%, and
the median survival time was 10.7 months [5.6 months in the control
group (P<0.01)]. The treatment group significantly reduced the
toxic and side effects of chemotherapy and completed the
chemotherapy course as scheduled. Zhang et al (6) used compound salvia miltiorrhiza and
654-2 injection combined with chemotherapy to treat 27 cases of
NSCLC. The effective rate of the combined treatment was 37%, while
the effective rate of the non-combined group was only 19.7%. It is
hypothesized that the combination of compounded S.
miltiorrhiza and 654-2 injection combined with chemotherapy can
improve the efficacy of NSCLC.
Liang et al (11) used compounded S.
miltiorrhiza dripping pills plus a gefitinib + DDP regimen to
treat advanced NSCLC, The survival time of patients with advanced
NSCLC is prolonged is significantly improved. One study
investigated the role of c-met in DDP-resistant human lung cancer
cell line A549/DDP and the reversal mechanism of salvianolic acid
A, the active component of salvianolic acid. It found that
salvianolic acid A can improve the chemotherapy effect of
cisplatin, indicating that salvianolic acid A and cisplatin have
synergistic effect. In addition, the present study found that
salvianolic acid A enhanced the sensitivity of A549/DDP cells to
cisplatin mainly by inhibiting the c-met/AKT/mTOR signal pathway.
In conclusion, salvianolic acid A inhibits the expression of c-met
and enhanced the sensitivity of lung adenocarcinoma A549 cells to
cisplatin through AKT/mTOR signal pathway (148).
5. Conclusions
Research over the past 20 years has shown that
S. miltiorrhiza has reached the status of ‘single drug,
multiple targets and multiple diseases. S. miltiorrhiza and
its compounds have achieved certain results in cell experiments,
animal experiments and clinical studies. These results showed that
S. miltiorrhiza can inhibit the proliferation and growth of
lung cancer cells, induce cancer cell apoptosis, promote cancer
cell autophagy and regulate immunity, fight tumor angiogenesis and
inhibit cancer cell invasion. In addition, S. miltiorrhiza
can effectively reverse the multi-drug resistance caused by
traditional radiotherapy and chemotherapy, reduce its side effects,
improve the efficacy of radiotherapy and chemotherapy and improve
the quality of life of patients (Fig.
3). These studies provide a good proof for the anti-tumor
effect of S. miltiorrhiza. However, there are still a number
of imperfections in the anti-tumor research into S.
miltiorrhiza: S. miltiorrhiza and its compounds had few
studies on small cell lung cancer and more data is required; it is
not known whether the clinical treatment results of S.
miltiorrhiza for different types of lung cancer are different
and the limitations and side effects of S. miltiorrhiza in
the treatment of lung cancer are rarely reported. Generally, S.
miltiorrhiza contains a variety of active ingredients with the
potential to treat lung cancer and other tumors (19,136,161) and is expected to become a
sensitizer for anti-cancer drugs and therapies; S.
miltiorrhiza in combination with other Chinese medicines or
chemotherapy drugs or radiation therapy can also treat lung cancer
and other tumors in a number of ways. For example, Dan's
participation in combination with oxaliplatin can reduce the
neuropathic pain caused by chemotherapy and reduce the malignant
tumor of glioblastoma cells (162). S. miltiorrhiza has a large
research scope in the treatment of tumors and research into S.
miltiorrhiza in the treatment of lung cancer has broad
prospects.
A previous study evaluated the water extracts of 12
kinds of Chinese herbal medicines (Anemarrhena, Ginkgo, Myrrh,
Pinellia, Rhododendron, Acacia, Ligustrum, Rhubarb, Rubia, Salvia,
Yellow S and Uncaria) and found that all crude water extracts
showed growth-inhibiting active cell lines for some or all kinds of
cancers, indicating the potential use of traditional Chinese
medicine as anti-tumor drugs and suggesting further separation of
their mechanism of action and active anti-tumor compounds (163). The advantage of traditional
Chinese medicine in anti-tumor is that it changes the biological
behavior of tumor cells so as to inhibit their growth and
development and to protect the normal function of the body, so that
patients can survive with tumor, enjoy an extended life, a
reduction in pain and a good quality of life. The main theoretical
principle of traditional Chinese medicine treatment of cancer is
‘Fu Zheng Qu Xie’, which here means ‘enhance the body's protective
anti-cancer immune response and at the same time eliminate cancer
cells or induce cancer cells to become normal cells. Chinese
medicine or prescriptions have this dual ability to treat cancer
and S. miltiorrhiza is the representative Chinese medicine
with this dual ability. In recent years, the combination of
prescriptions and anti-tumor research has provided new
opportunities for the treatment of malignant tumors. The treatment
of tumors with traditional Chinese medicine may become a
breakthrough for humans to fight tumors. The study of traditional
Chinese medicine prevention and treatment of cancer is of great
significance to the health of all mankind.
Acknowledgements
The authors would like to thank Professor Guangji
Zhang (School of Basic Medical Sciences, Zhejiang Chinese Medical
University) for providing financial support for the publication of
the present study. In addition the authors would like to thank the
Zhejiang Chinese Medical University Library for its assistance in
providing document access and account numbers.
Funding
Funding: The present study was supported by the research and
development plan of Zhejiang Province-research on innovative drugs
of traditional Chinese medicine (grant no. 2019C03072) and Key
research project of Zhejiang Provincial Science and Technology Plan
of Traditional Chinese Medicine Study (grant no. 2019ZZ006).
Availability of data and materials
Data sharing is not applicable to this article, as
no data sets were generated or analyzed during the current
study.
Authors' contributions
QA and MW reviewed relevant literature and wrote
the manuscript. YF and CY conceived the present study. GZ suggested
revisions to the manuscript. HS and XX revised the manuscript. All
authors read and approved the final manuscript. Data sharing is not
applicable to this article, as no data sets were generated or
analyzed during the current study.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bade BC and Dela Cruz CS: Lung cancer
2020: Epidemiology, etiology, and prevention. Clin Chest Med.
41:1–24. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Spiro SG and Silvestri GA: One hundred
years of lung cancer. Am J Respir Crit Care Med. 172:523–529.
2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rébé C and Ghiringhelli F: Cytotoxic
effects of chemotherapy on cancer and immune cells: How can it be
modulated to generate novel therapeutic strategies? Future Oncol.
11:2645–2654. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Su CY, Ming QL, Rahman K, Han T and Qin
LP: Salvia miltiorrhiza: Traditional medicinal uses,
chemistry, and pharmacology. Chin J Nat Med. 13:163–182.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang JP, Zhang YY, Zhang Y, Gao YG, Ma
JJ, Wang N, Wang JY, Xie Y, Zhang FH and Chu L: Salvia
miltiorrhiza (Danshen) injection ameliorates iron
overload-induced cardiac damage in mice. Planta Med. 79:744–752.
2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Xiong XJ, Wang Z and Wang J: Innovative
strategy in treating angina pectoris with Chinese patent medicines
by promoting blood circulation and removing blood stasis:
Experience from combination therapy in Chinese medicine. Curr Vasc
Pharmacol. 13:540–553. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tawil N and Rak J: Blood coagulation and
cancer genes. Best Pract Res Clin Haematol.
35(101349)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Qi Y: Progress of modern research on tumor
blood stasis syndrome and its treatment with the method of
promoting blood circulation by removing blood stasis. J Tradit Chin
Med. 15:68–76. 1995.PubMed/NCBI
|
|
10
|
Li ZM, Xu SW and Liu PQ: Salvia
miltiorrhiza Burge (Danshen): A golden herbal medicine in
cardiovascular therapeutics. Acta Pharmacol Sin. 39:802–824.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liang WY, Chen WJ, Yang GH, Zhu D, Mao X,
Shao YY, Wu LF, Zhang XX and Zhang LZ: Research progress on
salvianolic acids of Salvia miltiorrhiza. Zhongguo Zhong Yao
Za Zhi. 41:806–812. 2016.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
12
|
Wang J, Xu J, Gong X, Yang M, Zhang C and
Li M: Biosynthesis, chemistry, and pharmacology of polyphenols from
Chinese Salvia species: A review. Molecules. 24(155)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pang H, Wu L, Tang Y, Zhou G, Qu C and
Duan JA: Chemical analysis of the herbal medicine Salviae
miltiorrhizae Radix et Rhizoma (Danshen). Molecules.
21(51)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Guo R, Li L, Su J, Li S, Duncan SE, Liu Z
and Fan G: Pharmacological activity and mechanism of tanshinone IIA
in related diseases. Drug Des Devel Ther. 14:4735–4748.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu MZ, Huang YS and Xiao WQ: No promoting
effects of sodium tanshinone II-A sulfonate on growth and
metastasis of Lewis carcinoma. Zhongguo Yao Li Xue Bao. 12:534–537.
1991.PubMed/NCBI(In Chinese).
|
|
16
|
Zhou J, Jiang YY, Chen H, Wu YC and Zhang
L: Tanshinone I attenuates the malignant biological properties of
ovarian cancer by inducing apoptosis and autophagy via the
inactivation of PI3K/AKT/mTOR pathway. Cell Prolif.
53(e12739)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yin ZK, Liu ZZ, Yuan X, Feng ZM, Jiang JS,
Zhang X, Zhang PC and Yang YN: Thirteen undescribed diterpenoid
quinones derived from the rhizomes of Salvia miltiorrhiza
and their anti-tumor activities. Phytochemistry.
191(112902)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang Y and Zhang D: Tanshinol inhibits
growth of malignant melanoma cells via regulating miR-1207-5p/CHPF
pathway. Arch Dermatol Res. 312:373–383. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ye YT, Zhong W, Sun P, Wang D, Wang C, Hu
LM and Qian JQ: Apoptosis induced by the methanol extract of
Salvia miltiorrhiza Bunge in non-small cell lung cancer
through PTEN-mediated inhibition of PI3K/Akt pathway. J
Ethnopharmacol. 200:107–116. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen X, Guo J, Bao J, Lu J and Wang Y: The
anticancer properties of Salvia miltiorrhiza Bunge
(Danshen): A systematic review. Med Res Rev. 34:768–794.
2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Qian W, Wang Z, Xu T and Li D:
Anti-apoptotic effects and mechanisms of salvianolic acid A on
cardiomyocytes in ischemia-reperfusion injury. Histol Histopathol.
34:223–231. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tang SM, Xing XY, Deng XH, Zhao NN, Li G,
Yang RC, Sun GB and Sun XB: Research progress of Guanxin Danshen
formula and its effective components in treating coronary artery
heart disease. Zhongguo Zhong Yao Za Zhi. 41:3721–3726.
2016.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
23
|
Ren J, Fu L, Nile SH, Zhang J and Kai G:
Salvia miltiorrhiza in treating cardiovascular diseases: A
review on its pharmacological and clinical applications. Front
Pharmacol. 10(753)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shi MJ, Yan XL, Dong BS, Yang WN, Su SB
and Zhang H: A network pharmacology approach to investigating the
mechanism of Tanshinone IIA for the treatment of liver fibrosis. J
Ethnopharmacol. 253(112689)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tang YX, Liu M, Liu L, Zhen BR, Wang TT,
Li N, Lv N, Zhu Z, Sun G, Wang X and Chen S: Lipophilic
constituents in Salvia miltiorrhiza Inhibit activation of
the hepatic stellate cells by suppressing the JAK1/STAT3 signaling
pathway: A network pharmacology study and experimental validation.
Front Pharmacol. 13(770344)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tseng YJ, Hung YC, Kuo CE, Chung CJ, Hsu
CY, Muo CH, Hsu SF and Hu WL: Prescription of #adix Salvia
miltiorrhiza in Taiwan: A population-based study using the
national health insurance research database. Front Pharmacol.
12(719519)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lee SR, Jeon H, Kwon JE, Suh H, Kim BH,
Yun MK, Lim YJ and Kang SC: Anti-osteoporotic effects of Salvia
miltiorrhiza Bunge EtOH extract both in ovariectomized and
naturally menopausal mouse models. J Ethnopharmacol.
258(112874)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen J and Wang Y, Wang S, Zhao X, Zhao L
and Wang Y: Salvianolic acid B and ferulic acid synergistically
promote angiogenesis in HUVECs and zebrafish via regulating VEGF
signaling. J Ethnopharmacol. 283(114667)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Xu X, Xu H, Shang Y, Zhu R, Hong X, Song Z
and Yang Z: Development of the general chapters of the Chinese
Pharmacopoeia 2020 edition: A review. J Pharm Anal. 11:398–404.
2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Nie JM and Li HF: Therapeutic effects of
Salvia miltiorrhiza injection combined with telmisartan in
patients with diabetic nephropathy by influencing collagen IV and
fibronectin: A case-control study. Exp Ther Med. 16:3405–3412.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang H, Kang Y, Li H, Huang S, Li W, Zheng
M, Huang R, Lei B and Yang X: Salvia miltiorrhiza Derived
carbon dots and their heat stress tolerance of Italian lettuce by
promoting growth and enhancing antioxidant enzyme activity. ACS
Omega. 6:32262–32269. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Duan ZZ, Li YH, Li YY, Fan GW, Chang YX,
Yu B and Gao XM: Danhong injection protects cardiomyocytes against
hypoxia/reoxygenation- and H2O2-induced
injury by inhibiting mitochondrial permeability transition pore
opening. J Ethnopharmacol. 175:617–625. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li L, Sha Z, Wang Y, Yang D, Li J, Duan Z,
Wang H and Li Y: Pre-treatment with a combination of Shenmai and
Danshen injection protects cardiomyocytes against
hypoxia/reoxygenation- and H2O2-induced
injury by inhibiting mitochondrial permeability transition pore
opening. Exp Ther Med. 17:4643–4652. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang Y, Shi Y, Zou J, Zhang X, Liang Y,
Tai J, Cui C, Wang M and Guo D: Network pharmacology exploration
reveals a common mechanism in the treatment of
cardio-cerebrovascular disease with Salvia miltiorrhiza
Burge. and Carthamus tinctorius L. BMC Complement Med Ther.
20(351)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jin Y, Yu L, Xu F, Zhou J, Xiong B, Tang
Y, Li X, Liu L and Jin W: Pharmacokinetics of active ingredients of
Salvia miltiorrhiza and Carthamus tinctorius in
compatibility in normal and cerebral ischemia rats: A comparative
study. Eur J Drug Metab Pharmacokinet. 45:273–284. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sun G, Li X, Wei J, Zhang T, Li B, Chen M,
Wang Y, Chen K and Li Y: Pharmacodynamic substances in Salvia
miltiorrhiza for prevention and treatment of hyperlipidemia and
coronary heart disease based on lipidomics technology and network
pharmacology analysis. Biomed Pharmacother.
141(111846)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Zhang Y, Wang J, Liu YM, Chen YY, Yang XC
and Duan L: The synergistic effects of astragalus mongholicus and
Salvia miltiorrhiza on coronary heart disease identified by
network pharmacology and experiment. Drug Des Devel Ther.
15:4053–4069. 2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Subedi L and Gaire BP: Tanshinone IIA: A
phytochemical as a promising drug candidate for neurodegenerative
diseases. Pharmacol Res. 169(105661)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chong CM, Su H, Lu JJ and Wang Y: The
effects of bioactive components from the rhizome of Salvia
miltiorrhiza (Danshen) on the characteristics of Alzheimer's
disease. Chin Med. 14(19)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Guo Y, Li Y, Xue L, Severino RP, Gao S,
Niu J, Qin LP, Zhang D and Brömme D: Salvia miltiorrhiza: An
ancient Chinese herbal medicine as a source for anti-osteoporotic
drugs. J Ethnopharmacol. 155:1401–1416. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Guo Y, Dong X, Zhang R, Zhong Y, Yang P
and Zhang SY: Salvia miltiorrhiza improves Alzheimer's
disease: A protocol for systematic review and meta-analysis.
Medicine (Baltimore). 99(e21924)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Liu L and Zhang HQ: Effects of Danshen
Decoction on experimental gastric ulcer in rats and mice. Zhong Xi
Yi Jie He Xue Bao. 3:35–38. 2005.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
43
|
He S, Yang Y, Liu X, Huang W and Zhang X,
Yang S and Zhang X: Compound astragalus and Salvia
miltiorrhiza extract inhibits cell proliferation, invasion and
collagen synthesis in keloid fibroblasts by mediating transforming
growth factor-β / Smad pathway. Br J Dermatol. 166:564–574.
2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zhang W, Li J, Yang P, Wang G, Yue Y,
Zhong Y, Liu H, Gui D, Xu Y and Wang N: Efficacy and safety of
Salvia miltiorrhiza for treating chronic kidney diseases: A
systematic review and meta-analysis. Evid Based Complement Alternat
Med. 2022(2117433)2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ahn YM, Kim SK, Lee SH, Ahn SY, Kang SW,
Chung JH, Kim SD and Lee BC: Renoprotective effect of tanshinone
IIA, an active component of Salvia miltiorrhiza, on rats
with chronic kidney disease. Phytother Res. 24:1886–1892.
2010.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Cao T, Lu Y, Zhu M, Cheng J, Ye B, Fang N,
Cui Y, Xue B, Lari Najafi M and Kazemi E: Effects of Salvia
miltiorrhiza and Radix astragali on the TGF-β/Smad/Wnt pathway
and the pathological process of liver fibrosis in rats. Cell Mol
Biol (Noisy-le-grand). 66:46–51. 2020.PubMed/NCBI
|
|
47
|
Shi M, Huang F, Deng C, Wang Y and Kai G:
Bioactivities, biosynthesis and biotechnological production of
phenolic acids in Salvia miltiorrhiza. Crit Rev Food Sci
Nutr. 59:953–964. 2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Du G, Song J, Du L, Zhang L, Qiang G, Wang
S, Yang X and Fang L: Chemical and pharmacological research on the
polyphenol acids isolated from Danshen: A review of salvianolic
acids. Adv Pharmacol. 87:1–41. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Xu H, Li Y, Che X, Tian H, Fan H and Liu
K: Metabolism of salvianolic acid A and antioxidant activities of
its methylated metabolites. Drug Metab Dispos. 42:274–281.
2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Jin YM, Tao XM, Shi YN, Lu Y and Mei JY:
Salvianolic acid B exerts a protective effect in acute liver injury
by regulating the Nrf2/HO-1 signaling pathway. Can J Physiol
Pharmacol. 98:162–168. 2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Jiang Z, Gao W and Huang L: Tanshinones,
critical pharmacological components in Salvia miltiorrhiza.
Front Pharmacol. 10(202)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Shahzadi I, Ali Z, Bukhari S, Narula AS,
Mirza B and Mohammadinejad R: Possible applications of salvianolic
acid B against different cancers. Explor Target Antitumor Ther.
1:218–238. 2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Zeng W, Shan W, Gao L, Gao D, Hu Y, Wang
G, Zhang N, Li Z, Tian X, Xu W, et al: Inhibition of HMGB1 release
via salvianolic acid B-mediated SIRT1 up-regulation protects rats
against non-alcoholic fatty liver disease. Sci Rep.
5(16013)2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Raudenská M, Navrátil J, Gumulec J and
Masařík M: Mechanobiology of cancerogenesis. Klin Onkol.
34:202–210. 2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Chen L, Wang HJ, Xie W, Yao Y, Zhang YS
and Wang H: Cryptotanshinone inhibits lung tumorigenesis and
induces apoptosis in cancer cells in vitro and in
vivo. Mol Med Rep. 9:2447–2452. 2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Li Y, Gong Y, Li L, Abdolmaleky HM and
Zhou JR: Bioactive tanshinone I inhibits the growth of lung cancer
in part via downregulation of Aurora A function. Mol Carcinog.
52:535–543. 2013.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Chiu TL and Su CC: Tanshinone IIA induces
apoptosis in human lung cancer A549 cells through the induction of
reactive oxygen species and decreasing the mitochondrial membrane
potential. Int J Mol Med. 25:231–236. 2010.PubMed/NCBI
|
|
58
|
Han G, Wang Y, Liu T, Gao J, Duan F, Chen
M, Yang Y and Wu C: Salvianolic acid B acts against non-small cell
lung cancer A549 cells via inactivation of the MAPK and Smad2/3
signaling pathways. Mol Med Rep. 25(184)2022.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Warfel NA and El-Deiry WS: p21WAF1 and
tumourigenesis: 20 Years after. Curr Opin Oncol. 25:52–58.
2013.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Guan Z, Chen J, Li X and Dong N:
Tanshinone IIA induces ferroptosis in gastric cancer cells through
p53-mediated SLC7A11 down-regulation. Biosci Rep.
40(BSR20201807)2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Li Z, Zhang Y, Zhou Y, Wang F, Yin C, Ding
L and Zhang S: Tanshinone IIA suppresses the progression of lung
adenocarcinoma through regulating CCNA2-CDK2 complex and AURKA/PLK1
pathway. Sci Rep. 11(23681)2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Li J, Wang K, Chen X, Meng H, Song M, Wang
Y, Xu X and Bai Y: Transcriptional activation of microRNA-34a by
NF-kappa B in human esophageal cancer cells. BMC Mol Biol.
13(4)2012.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Sun C, Su S, Zhu Y, Guo J, Guo S, Qian D,
Yu L, Gu W and Duan JA: Salvia miltiorrhiza stem-leaf active
components of salvianolic acids and flavonoids improved the
hemorheological disorder and vascular endothelial function on
microcirculation dysfunction rats. Phytother Res. 34:1704–1720.
2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Li HY, Li Y, Yan CH, Li LN and Chen XG:
Inhibition of tumor growth by S-3-1, a synthetic intermediate of
salvianolic acid A. J Asian Nat Prod Res. 4:271–280.
2002.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Chen XG, Li Y, Yan CH, Li LN and Han R:
Cancer chemopreventive activities of S-3-1, a synthetic derivative
of danshinone. J Asian Nat Prod Res. 3:63–75. 2001.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Qi P, Li Y, Liu X, Jafari FA, Zhang X, Sun
Q and Ma Z: Cryptotanshinone suppresses non-small cell lung cancer
via microRNA-146a-5p/EGFR axis. Int J Biol Sci. 15:1072–1079.
2019.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Bi L, Chen J, Yuan X, Jiang Z and Chen W:
Salvianolic acid A positively regulates PTEN protein level and
inhibits growth of A549 lung cancer cells. Biomed Rep. 1:213–217.
2013.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Shi H, Zhang Q, Li H, Chu T, Jin H and Mao
S: Growth inhibition of tanshinones on SPC-A-1 cell line and their
structure-activity relationship. Zhongguo Fei Ai Za Zhi. 14:7–12.
2011.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
69
|
Miyazaki T and Arai S: Two distinct
controls of mitotic cdk1/cyclin B1 activity requisite for cell
growth prior to cell division. Cell Cycle. 6:1419–1425.
2007.PubMed/NCBI
|
|
70
|
Zhang J, Wen G, Sun L, Yuan W, Wang R,
Zeng Q, Zhang G and Yu B: Cryptotanshinone inhibits cellular
proliferation of human lung cancer cells through downregulation
ofIGF-1R/PI3K/Akt signaling pathway. Oncol Rep. 40:2926–2934.
2018.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Chen Y, Li H, Li M, Niu S, Wang J, Shao H,
Li T and Wang H: Salvia miltiorrhiza polysaccharide
activates T Lymphocytes of cancer patients through activation of
TLRs mediated-MAPK and-NF-κB signaling pathways. J Ethnopharmacol.
200:165–173. 2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Bandi N and Vassella E: miR-34a and
miR-15a/16 are co-regulated in non-small cell lung cancer and
control cell cycle progression in a synergistic and Rb-dependent
manner. Mol Cancer. 10(55)2011.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Shi Y, Liu C, Liu X, Tang DG and Wang J:
The microRNA miR-34a inhibits non-small cell lung cancer (NSCLC)
growth and the CD44hi stem-like NSCLC cells. PLoS One.
9(e90022)2014.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Wahlquist C, Jeong D, Rojas-Muñoz A, Kho
C, Lee A, Mitsuyama S, van Mil A, Park WJ, Sluijter JP, Doevendans
PA, et al: Inhibition of miR-25 improves cardiac contractility in
the failing heart. Nature. 508:531–535. 2014.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Harrington EA, Bebbington D, Moore J,
Rasmussen RK, Ajose-Adeogun AO, Nakayama T, Graham JA, Demur C,
Hercend T, Diu-Hercend A, et al: VX-680, a potent and selective
small-molecule inhibitor of the Aurora kinases, suppresses tumor
growth in vivo. Nat Med. 10:262–267. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
76
|
Dar AA, Goff LW, Majid S, Berlin J and
El-Rifai W: Aurora kinase inhibitors-rising stars in cancer
therapeutics? Mol Cancer Ther. 9:268–278. 2010.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Görgün G, Calabrese E, Hideshima T, Ecsedy
J, Perrone G, Mani M, Ikeda H, Bianchi G, Hu Y, Cirstea D, et al: A
novel Aurora-A kinase inhibitor MLN8237 induces cytotoxicity and
cell-cycle arrest in multiple myeloma. Blood. 115:5202–5213.
2010.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Hannak E, Kirkham M, Hyman AA and Oegema
K: Aurora-A kinase is required for centrosome maturation in
Caenorhabditis elegans. J Cell Biol. 155:1109–1116. 2001.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Marumoto T, Zhang D and Saya H: Aurora-A-a
guardian of poles. Nat Rev Cancer. 5:42–50. 2005.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Hirota T, Kunitoku N, Sasayama T, Marumoto
T, Zhang D, Nitta M, Hatakeyama K and Saya H: Aurora-A and an
interacting activator, the LIM protein Ajuba, are required for
mitotic commitment in human cells. Cell. 114:585–598.
2003.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Lentini L, Amato A, Schillaci T, Insalaco
L and Di Leonardo A: Aurora-A transcriptional silencing and
vincristine treatment show a synergistic effect in human tumor
cells. Oncol Res. 17:115–125. 2008.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Kaestner P, Stolz A and Bastians H:
Determinants for the efficiency of anticancer drugs targeting
either Aurora-A or Aurora-B kinases in human colon carcinoma cells.
Mol Cancer Ther. 8:2046–2056. 2009.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Compérat E, Bièche I, Dargère D,
Laurendeau I, Vieillefond A, Benoit G, Vidaud M, Camparo P, Capron
F, Verret C, et al: Gene expression study of Aurora-A reveals
implication during bladder carcinogenesis and increasing values in
invasive urothelial cancer. Urology. 72:873–877. 2008.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Lee ECY, Frolov A, Li R, Ayala G and
Greenberg NM: Targeting Aurora kinases for the treatment of
prostate cancer. Cancer Res. 66:4996–5002. 2006.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Matarasso N, Bar-Shira A, Rozovski U,
Rosner S and Orr-Urtreger A: Functional analysis of the Aurora
kinase A Ile31 allelic variant in human prostate. Neoplasia.
9:707–715. 2007.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Kumano M, Miyake H, Terakawa T, Furukawa J
and Fujisawa M: Suppressed tumour growth and enhanced
chemosensitivity by RNA interference targeting Aurora-A in the PC3
human prostate cancer model. BJU Int. 106:121–127. 2010.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Addepalli MK, Ray KB, Kumar B, Ramnath RL,
Chile S and Rao H: RNAi-mediated knockdown of AURKB and EGFR shows
enhanced therapeutic efficacy in prostate tumor regression. Gene
Ther. 17:352–359. 2010.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Li Y, Zhang ZF, Chen J, Huang D, Ding Y,
Tan MH, Qian CN, Resau JH, Kim H and The BT: VX680/MK-0457, a
potent and selective Aurora kinase inhibitor, targets both tumor
and endothelial cells in clear cell renal cell carcinoma. Am J
Transl Res. 2:296–308. 2010.PubMed/NCBI
|
|
89
|
Wang X, Dong L, Xie J, Tong T and Zhan Q:
Stable knockdown of Aurora-A by vector-based RNA interference in
human esophageal squamous cell carcinoma cell line inhibits tumor
cell proliferation, invasion and enhances apoptosis. Cancer Biol
Ther. 8:1852–1859. 2009.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Pérez de Castro I, de Cárcer G, Montoya G
and Malumbres M: Emerging cancer therapeutic opportunities by
inhibiting mitotic kinases. Curr Opin Pharmacol. 8:375–383.
2008.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Tung YT, Chen HL, Lee CY, Chou YC, Lee PY,
Tsai HC, Lin YL and Chen CM: Active component of Danshen (Salvia
miltiorrhiza Bunge), tanshinone I, attenuates lung
tumorigenesis via inhibitions of VEGF, cyclin A, and cyclin B
expressions. Evid Based Complement Alternat Med.
2013(319247)2013.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Tian H, Li Y, Mei J, Cao L, Yin J, Liu Z,
Chen J and Li X: Effects of Salvia miltiorrhiza extract on
lung adenocarcinoma. Exp Ther Med. 22(794)2021.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Gao F, Li M, Liu W and Li W: Inhibition of
EGFR signaling and activation of mitochondrial apoptosis contribute
to tanshinone IIA-mediated tumor suppression in non-small cell lung
cancer cells. OncoTargets Ther. 13:2757–2769. 2020.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Wang M, Tang W, Gong N and Liu P: Sodium
Danshensu inhibits the progression of lung cancer by regulating
PI3K/Akt signaling pathway. Drug Dev Res. 83:88–96. 2022.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Lu Z, Zhou R, Kong Y, Wang J, Xia W, Guo
J, Liu J, Sun H, Liu K, Yang J, et al: S-equol, a secondary
metabolite of natural anticancer isoflavone daidzein, inhibits
prostate cancer growth in vitro and in vivo, though activating the
Akt/FOXO3a pathway. Curr Cancer Drug Targets. 16:455–465.
2016.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Li Q, Hu K, Tang S, Xu LF and Luo YC:
Anti-tumor activity of tanshinone IIA in combined with
cyclophosphamide against Lewis mice with lung cancer. Asian Pac J
Trop Med. 9:1084–1088. 2016.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Bi L, Yan X, Yang Y, Qian L, Tian Y, Mao
JH and Chen W: The component formula of Salvia miltiorrhiza
and Panax ginseng induces apoptosis and inhibits cell
invasion and migration through targeting PTEN in lung cancer cells.
Oncotarget. 8:101599–101613. 2017.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Yan XJ, Yang Y, Bi L, Chen SS, Zhu JJ and
Chen WP: Effects of component formula of Salviae
miltiorrhizae Radix et Rhizoma and Ginseng Radix et Rhizoma on
cell proliferation, apoptosis and skeleton in lung cancer A549
cells. Zhongguo Zhong Yao Za Zhi. 39:4436–4441. 2014.PubMed/NCBI(In Chinese).
|
|
99
|
Zinnah KMA and Park SY: Sensitizing
TRAIL-resistant A549 lung cancer cells and enhancing TRAIL-induced
apoptosis with the antidepressant amitriptyline. Oncol Rep.
46(144)2021.PubMed/NCBI View Article : Google Scholar
|
|
100
|
He J, Zhou Q, Yuan S, Wang Y, Chen X and
Qin J: The growth-inhibiting effect and its molecular mechanism of
Tanshinone on human lung cancer cell line in vitro. Zhongguo Fei Ai
Za Zhi. 5:123–125. 2002.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
101
|
Hu H, Zhang Y, Huang F and Deng H:
Inhibition of proliferation and induction of apoptosis by
tanshinone II A in NCI-H460 cell. Zhong Yao Cai. 28:301–304.
2005.PubMed/NCBI(In Chinese).
|
|
102
|
Liu F, Yu G, Wang G, Liu H, Wu X, Wang Q,
Liu M, Liao K, Wu M, Cheng X and Hao H: An NQO1-initiated and
p53-independent apoptotic pathway determines the anti-tumor effect
of tanshinone IIA against non-small cell lung cancer. PLoS One.
7(e42138)2012.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Zhang J, Wang J, Jiang JY, Liu SD, Fu K
and Liu HY: Tanshinone IIA induces cytochrome c-mediated caspase
cascade apoptosis in A549 human lung cancer cells via the JNK
pathway. Int J Oncol. 45:683–690. 2014.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Kim EO, Kang SE, Im CR, Lee JH, Ahn KS,
Yang WM, Um JY, Lee SG and Yun M: Tanshinone IIA induces TRAIL
sensitization of human lung cancer cells through selective ER
stress induction. Int J Oncol. 48:2205–2212. 2016.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Liu HY, Li QR, Cheng XF, Wang GJ and Hao
HP: NAMPT inhibition synergizes with NQO1-targeting agents in
inducing apoptotic cell death in non-small cell lung cancer cells.
Chin J Nat Med. 14:582–589. 2016.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Israels LG and Israels ED: Apoptosis.
Oncologist. 4:332–339. 1999.PubMed/NCBI
|
|
107
|
Xu X, Lai Y and Hua ZC: Apoptosis and
apoptotic body: Disease message and therapeutic target potentials.
Biosci Rep. 39(BSR20180992)2019.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Pistritto G, Trisciuoglio D, Ceci C,
Garufi A and D'Orazi G: Apoptosis as anticancer mechanism: Function
and dysfunction of its modulators and targeted therapeutic
strategies. Aging (Albany NY). 8:603–619. 2016.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Wu GS: TRAIL as a target in anti-cancer
therapy. Cancer Lett. 285:1–5. 2009.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Chang CC, Lai JS, Tsai CS, Ma SW, Lin JY,
Huang LR, Lu CH, Liao EC and Ho TF: Proapoptotic and
TRAIL-sensitizing constituents isolated from Salvia militiorrhiza
(Danshen). J Biosci Bioeng. 116:516–523. 2013.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Shen L, Lou Z and Zhang G, Xu G and Zhang
G: Diterpenoid tanshinones, the extract from Danshen (Radix
Salviae miltiorrhizae) induced apoptosis in nine human
cancer cell lines. J Tradit Chin Med. 36:514–521. 2016.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Lou ZH, Xia RM, Li XJ, Cheng RB, Shao KD
and Zhang GJ: Anti-lung cancer mechanisms of diterpenoid tanshinone
via endoplasmic reticulum stress-mediated apoptosis signal pathway.
Zhongguo Zhong Yao Za Zhi. 43:4900–4907. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
113
|
Xie J, Liu J, Liu H, Liang S, Lin M, Gu Y,
Liu T, Wang D, Ge H and Mo SL: The antitumor effect of tanshinone
IIA on anti-proliferation and decreasing VEGF/VEGFR2 expression on
the human non-small cell lung cancer A549 cell line. Acta Pharm Sin
B. 5:554–563. 2015.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Chen D, Lin XX, Huang WH, Zhang W, Tan ZR,
Peng JB, Wang YC, Guo Y, Hu DL and Chen Y: Sodium tanshinone IIA
sulfonate and its interactions with human CYP450s. Xenobiotica.
46:1085–1092. 2016.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Zhou X, Pan Y, Wang Y, Wang B, Yan Y, Qu Y
and Ke X: Tanshinones induce tumor cell apoptosis via directly
targeting FHIT. Sci Rep. 11(12217)2021.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Wu CY, Yang YH, Lin YS, Chang GH, Tsai MS,
Hsu CM, Yeh RA, Shu LH, Cheng YC and Liu HT: Dihydroisotanshinone I
induced ferroptosis and apoptosis of lung cancer cells. Biomed
Pharmacother. 139(111585)2021.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Singh R and Cuervo AM: Autophagy in the
cellular energetic balance. Cell Metab. 13:495–504. 2011.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Sridhar S, Botbol Y, Macian F and Cuervo
AM: Autophagy and disease: Always two sides to a problem. J Pathol.
226:255–273. 2012.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Fukuda K, Kobayashi A and Watabe K: The
role of tumor-associated macrophage in tumor progression. Front
Biosci (Schol Ed). 4:787–798. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
120
|
Li GG, Guo ZZ, Ma XF, Cao N, Geng SN,
Zheng YQ, Meng MJ, Lin HH, Han G and Du GJ: The M2 macrophages
induce autophagic vascular disorder and promote mouse sensitivity
to urethane-related lung carcinogenesis. Dev Comp Immunol.
59:89–98. 2016.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Hao W, Zhang X, Zhao W, Zhu H, Liu ZY, Lu
J and Chen X: Cryptotanshinone induces pro-death autophagy through
JNK signaling mediated by reactive oxygen species generation in
lung cancer cells. Anticancer Agents Med Chem. 16:593–600.
2016.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Guo S, Luo W, Liu L, Pang X, Zhu H, Liu A,
Lu J, Ma DL, Leung CH, Wang Y and Chen X: Isocryptotanshinone, a
STAT3 inhibitor, induces apoptosis and pro-death autophagy in A549
lung cancer cells. J Drug Target. 24:934–942. 2016.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Zhang B, Ma Z, Li X, Zhang C, Shao Y, Liu
Z, Li Y and Jin Y: Tanshinones suppress non-small cell lung cancer
through up-regulating miR-137. Acta Biochim Biophys Sin (Shanghai).
48:768–770. 2016.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Gao H, Sun W, Zhao W, Hao W, Leung CH, Lu
J and Chen X: Total tanshinones-induced apoptosis and autophagy via
reactive oxygen species in lung cancer 95D cells. Am J Chin Med.
43:1265–1279. 2015.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Yuan A, Hsiao YJ, Chen HY, Chen HW, Ho CC,
Chen YY, Liu YC, Hong TH, Yu SL, Chen JJ and Yang PC: Opposite
effects of M1 and M2 macrophage subtypes on lung cancer
progression. Sci Rep. 5(14273)2015.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Pollard JW: Tumour-educated macrophages
promote tumour progression and metastasis. Nat Rev Cancer. 4:71–78.
2004.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Valente AJ, Graves DT, Vialle-Valentin CE,
Delgado R and Schwartz CJ: Purification of a monocyte chemotactic
factor secreted by nonhuman primate vascular cells in culture.
Biochemistry. 27:4162–4168. 1988.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Yoshimura T, Robinson EA, Tanaka S,
Appella E, Kuratsu J and Leonard EJ: Purification and amino acid
analysis of two human glioma-derived monocyte chemoattractants. J
Exp Med. 169:1449–1459. 1989.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Schmall A, Al-Tamari HM, Herold S,
Kampschulte M, Weigert A, Wietelmann A, Vipotnik N, Grimminger F,
Seeger W, Pullamsetti SS and Savai R: Macrophage and cancer cell
cross-talk via CCR2 and CX3CR1 is a fundamental mechanism driving
lung cancer. Am J Respir Crit Care Med. 191:437–447.
2015.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Man Y, Yang L, Zhang D and Bi Y:
Cryptotanshinone inhibits lung tumor growth by increasing
CD4+ T cell cytotoxicity through activation of the
JAK2/STAT4 pathway. Oncol Lett. 12:4094–4098. 2016.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Liu S, Han Z, Trivett AL, Lin H, Hannifin
S, Yang D and Oppenheim JJ: Cryptotanshinone has curative dual
anti-proliferative and immunotherapeutic effects on mouse Lewis
lung carcinoma. Cancer Immunol Immunother. 68:1059–1071.
2019.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Wu CY, Cherng JY, Yang YH, Lin CL, Kuan
FC, Lin YY, Lin YS, Shu LH, Cheng YC, Liu HT, et al: Danshen
improves survival of patients with advanced lung cancer and
targeting the relationship between macrophages and lung cancer
cells. Oncotarget. 8:90925–90947. 2017.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Lee CY, Sher HF, Chen HW, Liu CC, Chen CH,
Lin CS, Yang PC, Tsay HS and Chen JJ: Anticancer effects of
tanshinone I in human non-small cell lung cancer. Mol Cancer Ther.
7:3527–3538. 2008.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Chu YW, Yang PC, Yang SC, Shyu YC, Hendrix
MJ, Wu R and Wu CW: Selection of invasive and metastatic
subpopulations from a human lung adenocarcinoma cell line. Am J
Respir Cell Mol Biol. 17:353–360. 1997.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Chen JJ, Peck K, Hong TM, Yang SC, Sher
YP, Shih JY, Wu R, Cheng JL, Roffler SR, Wu CW and Yang PC: Global
analysis of gene expression in invasion by a lung cancer model.
Cancer Res. 61:5223–5230. 2001.PubMed/NCBI
|
|
136
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Tammela T, Enholm B, Alitalo K and
Paavonen K: The biology of vascular endothelial growth factors.
Cardiovasc Res. 65:550–563. 2005.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676.
2003.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Jin L, Chen C, Huang L, Bu L, Zhang L and
Yang Q: Salvianolic acid A blocks vasculogenic mimicry formation in
human non-small cell lung cancer via PI3K/Akt/mTOR signalling. Clin
Exp Pharmacol Physiol. 48:508–514. 2021.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Zhang Y, Wang L, Chen Y and Qing C:
Anti-angiogenic activity of salvicine. Pharm Biol. 51:1061–1065.
2013.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Zhang P, Pei Y and Qi Y: Influence of
blood-activating drugs on adhesion and invasion of cells in lung
cancer patients. Zhongguo Zhong Xi Yi Jie He Za Zhi. 19:103–105.
1999.PubMed/NCBI(In Chinese).
|
|
142
|
Wang H, Zhang Y, Zhang Y, Liu W and Wang
J: Cryptotanshinone inhibits lung cancer invasion via
microRNA-133a/matrix metalloproteinase 14 regulation. Oncol Lett.
18:2554–2559. 2019.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Xie J, Liu JH, Liu H, Liao XZ, Chen Y, Lin
MG, Gu YY, Liu TL, Wang DM, Ge H and Mo SL: Tanshinone IIA combined
with adriamycin inhibited malignant biological behaviors of NSCLC
A549 cell line in a synergistic way. BMC Cancer.
16(899)2016.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Zhang XZ, Qian SS, Zhang YJ and Wang RQ:
Salvia miltiorrhiza: A source for anti-Alzheimer's disease
drugs. Pharm Biol. 54:18–24. 2016.PubMed/NCBI View Article : Google Scholar
|
|
145
|
He SB, Zhang BX, Wang HH, Wang Y and Qiao
YJ: Study on mechanism of Salvia miltiorrhiza treating
cardiovascular disease through auxiliary mechanism elucidation
system for Chinese medicine. Zhongguo Zhong Yao Za Zhi.
40:3713–3717. 2015.PubMed/NCBI(In Chinese).
|
|
146
|
Chuang MT, Ho FM, Wu CC, Zhuang SY, Lin
SY, Suk FM and Liang YC: 15,16-Dihydrotanshinone I, a compound of
Salvia miltiorrhiza bunge, induces apoptosis through
inducing endoplasmic reticular stress in human prostate carcinoma
cells. Evid Based Complement Alternat Med.
2011(865435)2011.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Chen FY, Bi L, Qian L, Gao J, Jiang YC and
Chen WP: Identification of multidrug resistance gene MDR1
associated microRNA of salvianolic acid A reversal in lung cancer.
Zhongguo Zhong Yao Za Zhi. 41:3279–3284. 2016.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
148
|
Tang XL, Yan L, Zhu L, Jiao DM, Chen J and
Chen QY: Salvianolic acid A reverses cisplatin resistance in lung
cancer A549 cells by targeting c-met and attenuating Akt/mTOR
pathway. J Pharmacol Sci. 135:1–7. 2017.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Xia C, Bai X, Hou X, Gou X, Wang Y, Zeng
H, Huang M and Jin J: Cryptotanshinone reverses cisplatin
resistance of human lung carcinoma A549 cells through
down-regulating Nrf2 pathway. Cell Physiol Biochem. 37:816–824.
2015.PubMed/NCBI View Article : Google Scholar
|
|
150
|
Jiang Y, You F, Zhu J, Zheng C, Yan R and
Zeng J: Cryptotanshinone ameliorates radiation-induced lung injury
in rats. Evid Based Complement Alternat Med.
2019(1908416)2019.PubMed/NCBI View Article : Google Scholar
|
|
151
|
Liao XZ, Gao Y, Huang S, Chen ZZ, Sun LL,
Liu JH, Chen HR, Yu L, Zhang JX and Lin LZ: Tanshinone IIA combined
with cisplatin synergistically inhibits non-small-cell lung cancer
in vitro and in vivo via down-regulating the phosphatidylinositol
3-kinase/Akt signalling pathway. Phytother Res. 33:2298–2309.
2019.PubMed/NCBI View Article : Google Scholar
|
|
152
|
Wang R, Luo Z, Zhang H and Wang T:
Tanshinone IIA reverses gefitinib-resistance in human
non-small-cell lung cancer via regulation of VEGFR/Akt pathway.
Onco Targets Ther. 12:9355–9365. 2019.PubMed/NCBI View Article : Google Scholar
|
|
153
|
Cao HY, Ding RL, Li M, Yang MN, Yang LL,
Wu JB, Yang B, Wang J, Luo CL and Wen QL: Danshensu, a major
water-soluble component of Salvia miltiorrhiza, enhances the
radioresponse for Lewis lung carcinoma xenografts in mice. Oncol
Lett. 13:605–612. 2017.PubMed/NCBI View Article : Google Scholar
|
|
154
|
Kim JH, Jeong SJ, Kwon TR, Yun SM, Jung
JH, Kim M, Lee HJ, Lee MH, Ko SG, Chen CY and Kim SH:
Cryptotanshinone enhances TNF-α-induced apoptosis in chronic
myeloid leukemia KBM-5 cells. Apoptosis. 16:696–707.
2011.PubMed/NCBI View Article : Google Scholar
|
|
155
|
Su CC: Tanshinone IIA potentiates the
efficacy of 5-FU in Colo205 colon cancer cells in vivo
through downregulation of P-gp and LC3-II. Exp Ther Med. 3:555–559.
2012.PubMed/NCBI View Article : Google Scholar
|
|
156
|
Ye Y, Xu W, Zhong W, Li Y and Wang C:
Combination treatment with dihydrotanshinone I and irradiation
enhances apoptotic effects in human cervical cancer by HPV E6
down-regulation and caspases activation. Mol Cell Biochem.
363:191–202. 2012.PubMed/NCBI View Article : Google Scholar
|
|
157
|
Xia R and Chen X: Effects of Danshen
injection on the malignant obstructive jaundice in the SD rat
model. J Huazhong Univ Sci Technolog Med Sci. 26:686–689.
2006.PubMed/NCBI View Article : Google Scholar
|
|
158
|
Wang T, Fu X and Wang Z: Danshen Formulae
for Cancer: A Systematic Review and Meta-Analysis of High-Quality
Randomized Controlled Trials. Evid Based Complement Altern Med.
2019(2310639)2019.PubMed/NCBI View Article : Google Scholar
|
|
159
|
Yang Y, Qiu S, Qian L, Tian Y, Chen Y, Bi
L and Chen W: OCF can repress tumor metastasis by inhibiting
epithelial-mesenchymal transition involved in PTEN/PI3K/AKT pathway
in lung cancer cells. PLoS One. 12(e0174021)2017.PubMed/NCBI View Article : Google Scholar
|
|
160
|
Geng QX, Zhu XL and Zhang XH: Effect of
combined therapy of shenmai and compound danshen injection on
myocardial reperfusion injury after percutaneous coronary
intervention in patients with acute myocardial infarction. Zhongguo
Zhong Xi Yi Jie He Za Zhi. 24:496–499. 2004.PubMed/NCBI(In Chinese).
|
|
161
|
Zhang X, Luo W, Zhao W, Lu J and Chen X:
Isocryptotanshinone induced apoptosis and activated MAPK signaling
in human breast cancer MCF-7 cells. J Breast Cancer. 18:112–118.
2015.PubMed/NCBI View Article : Google Scholar
|
|
162
|
Di Cesare Mannelli L, Piccolo M, Maione F,
Ferraro MG, Irace C, De Feo V, Ghelardini C and Mascolo N:
Tanshinones from Salvia miltiorrhiza Bunge revert
chemotherapy-induced neuropathic pain and reduce glioblastoma cells
malignancy. Biomed Pharmacother. 105:1042–1049. 2018.PubMed/NCBI View Article : Google Scholar
|
|
163
|
Shen YD, Tian YX, Bu XZ and Gu LQ: Natural
tanshinone-like heterocyclic-fused ortho-quinones from
regioselective Diels-Alder reaction: Synthesis and cytotoxicity
evaluation. Eur J Med Chem. 44:3915–3921. 2009.PubMed/NCBI View Article : Google Scholar
|