Introduction
The incidence of stress cardiomyopathy (SC), an
acute cardiomyopathy caused by mental stimulation or physical
stress also known as Takotsubo syndrome, increased considerably
during the COVID-19 pandemic (1-3).
It is reported that suffering the pandemic, the proportion of SC in
acute coronary syndromes patients increased from 1,8% before to
7,8% now (4). This disease has
increased complications in patient, leading to increased adverse
outcomes, medical costs and waste of medical resources (5). The clinical symptoms of SC are
similar to those of myocardial ischemia (6). Its manifestations include chest pain,
dyspnoea, abnormal electrocardiogram (ST-segment elevation),
changes in myocardial enzymology and left ventricular (LV) motor
dysfunction (7,8). In addition, a body of evidence has
shown that the clinical prognosis of SC is worse compared with that
of myocardial ischemia (9,10). Apart from the
Renin-Angiotensin-Aldosterone System inhibitors, no therapeutic
interventions have been effective in decreasing mortality now,
improving prognosis or preventing recurrence in the acute or
chronic stages of SC (11). SC has
become a serious public health problem (12).
It has been hypothesized that SC development can be
attributed to the cardiotoxicity caused by stress-induced excessive
generation of catecholamines (CAs) (13). When CA levels spike
superphysiologically in circulation and combined with β1-AR in the
bottom of the heart, the contractility of bottom of the heart
increase (14), which accelerates
heart rate, inducing insufficient coronary artery perfusion and LV
dilation and mild-moderate cardiac biomarker elevation (15,16).
Therefore, CAs (including isoprenaline, epinephrine,
norepinephrine, dopamine and phenylephrine) are commonly used in
current studies to induce SC models (17,18).
However, isoprenaline is the most commonly used drug to induce SC
models with apical heart dysfunction (19).
In general, there have been some achievements in the
research on SC but the criteria and evaluation indicators for
establishing the mouse animal model of this disease have been
unclear and vary widely (20-22).
So it is crucial to establish successful animal SC models to
elucidate the pathological mechanism underlying the disease and for
the development of effective drug therapy.
In the present study, SC mouse models were generated
via daily intraperitoneal injection of isoprenaline (ISO) at
varying doses for 14 consecutive days. The present study aimed to
determine the optimal modelling dose and establish a stable SC
mouse model and evaluation criteria consistent with human
pathological characteristics.
Materials and methods
Experimental animals
A total of 72 female C57BL/6 mice (age, 6 weeks;
body weight, 20±2 g) were purchased from Zhejiang Ziyuan Laboratory
Animal Technology Co., Ltd. [animal license no. SCXK (Zhe)
2019-0004]. Animals were bred in the Experimental Animal Center of
Anhui University of Traditional Chinese Medicine. The protocol was
approved by the Experimental Animal Ethics Committee of Anhui
University of Traditional Chinese Medicine [approval no.
AHUCM-mouse-2022045].
Animal grouping and modelling
Mice were housed in individual cages (six mice/cage)
under standard laboratory conditions and were given food and water
ad libitum. Mice were kept at 20-22˚C, with 50% humidity and
a fixed 12/12-h light/dark cycle. Mice were randomly divided into
two parts (six groups each part, six mice each group) according to
a random number table, including a control group of untreated mice
and five groups treated with varying doses of ISO (5, 10, 25, 50
and 100 mg/kg; APExBIO Technology LLC). ISO was administered
intraperitoneally once daily for 14 consecutive days (Fig. 1A). The mice in the first part were
observed to make a survival curve, and other thirty-six mice in the
second part were used for open field test, echocardiography, ECG,
haematoxylin and eosin staining, and ELISA. All mice were weighed
daily until the end of the experiment. The whole experiment lasted
14 days.
 | Figure 1Effect of ISO on body weight and
growth of mice. (A) Experimental flow chart. Following daily i.p.
injection of ISO for 14 consecutive days, an open field test was
performed 30 min after the last injection and echocardiography and
electrocardiogram were performed at 60 min. Serum and heart samples
were collected within 90 min. (B) Survival rate. (C) Survival
curve. (D) Baseline body weight. (E) Body weight change within 14
days. (F) Percent change in body weight. For the control and 5 and
25 mg/kg ISO group, n=6. For the 10 mg/kg ISO group, n=4. For the
50 mg/kg ISO group, n=5. For the 100 mg/kg ISO group, n=2.
*P<0.05 vs. control. ISO, isoprenaline; OFT, open
field test; ECG, electrocardiogram; Echo, echocardiography; i.p.,
intraperitoneal. |
Open field test
At 30 min after ISO injection on day 14, the mice
were placed in the central compartment of an open field box
(Shanghai Xinsoft Information Technology Co., Ltd.), and their
activity was recorded for 5 min using a camera system. At the end
of the experiment, computer software (Super Maze V2.0, Shanghai
Xinsoft Information Technology Co., Ltd.) was used to analyse the
video and indexes of the total distance (track length of free
movement of mice in open field), degree across the grid (total
number of mesh traversed freely by mice in open field), time in the
centre (the amount of time of the mice in the intermediate zone in
the open field) and the grid number in the centre (the total number
of intermediate mesh traversed by mice in open field) were assessed
for each group.
Echocardiography
Alteration in LV function of the mice was measured
using echocardiography [Vinno Technology (Suzhou) Co., Ltd.] after
the open field test. LV function was assessed by measuring the
following parameters: LV internal end-diastolic diameter (LVIDd,
mm); LV internal end-systolic diameter (LVIDs, mm); LV
end-diastolic volume (LVEDV, ml); LV end-systolic volume (LVESV,
ml); ejection fraction (EF, %) and fractional shortening (FS, %).
Mice were anaesthetized with 1% isoflurane gas during the
electrocardiogram (ECG) and echocardiography detection to minimise
effect on the heart rate, autonomic nervous system and blood oxygen
saturation (23).
ECG
After the echocardiography, a PowerLab system
(ADInstruments, Ltd.) was used to record the lead ECG on all limbs
of mice to monitor their cardiac function. The electrode was
inserted into the right upper and left lower limbs subcutaneously
and right lower limbs intramuscularly. Once the waveform was
stabilized, ECG was recorded continuously for 15 min to observe the
following parameters: ST segment; QT interval and Q wave
amplitude.
Animal euthanasia
The experiments followed the principle of minimizing
pain and fear in animals. Euthanasia was performed using 5%
isoflurane inhalation for >1 min. After euthanasia the absence
of heartbeat and breathing were used to confirm death. The bodies
were transported to the designated recycling room at the Laboratory
Animal Center of Anhui University of Traditional Chinese
Medicine.
Of seven mice that died during ISO administration,
two, one and four belonged to the 10, 50 and 100 mg/kg ISO groups,
respectively. In the 10 mg/kg ISO group, one mouse died due to
adverse reactions following ISO injection, causing a sharp increase
in myocardial contractility and oxygen consumption in a short
period, resulting in arrhythmia and myocardial ischemic necrosis.
The remaining three mice in the 10 mg/kg ISO group were euthanised
due to weight loss caused by insufficient food and water intake. A
total of one mouse in the 50 mg/kg group and three mice from the
100 mg/kg group were euthanised due to extreme physical discomfort
caused by myocardial ischemia as well as tension and stress induced
by long-term injection of high ISO doses. The fourth mouse in the
100 mg/kg group suffered extremely slow heartbeat when ECG and
echocardiography were performed under isoflurane anaesthesia.
Haematoxylin and eosin (H&E)
staining
Histopathological changes in myocardial tissue were
observed using H&E staining (cat. nos. BA4097, BA4099, Zhuhai
Beso Biotechnology Co. LTD). The myocardial tissue samples were
fixed in 4% paraformaldehyde for 24 h (4˚C). Samples were placed in
70, 85 and 95% ethanol for gradient dehydration. The samples were
were embedded in melted paraffin wax for 3 h (50-60˚C). Then, the
embedded sample is cut into pieces 20 µm thick and placed onto a
slide. Thereafter, sections were dewaxed three times in xylene
(room temperature) and then washed with anhydrous ethanol for 3 min
(room temperature). The sections were immersed in 95, 80, and 70%
ethanol for 1-3 min and washed with pure water for 1 min (room
temperature). Sections were stained with haematoxylin for 3 min at
room temperature. Following washing with water, samples were
treated with PBS for 1 min (room temperature), followed by staining
with eosin for 1 min at room temperature. Finally, the samples were
immersed in 70, 85, and 95 ethanol for gradient dehydration and
sealed using neutral gum. The morphology of myocardial tissue was
observed under fluorescent microscope using bright field (NIKON
ECLIPSE C1, Nikon Corporation; magnification, x400).
ELISA
Within 90 min of the last ISO injection, mice were
anaesthetized with 2% isoflurane and sacrificed via decapitation
after blood (1 ml) was extracted through the orbital vein. The
blood samples were centrifuged (1,006.2 x g for 10 min, room
temperature) and the supernatant was collected and stored at -80˚C
until further analysis. Brain natriuretic peptide (BNP), cardiac
calcitonin T (cTnT), cortisol, CA or C-reactive protein (CRP) ELISA
reagent (JYM0380Mo, JYM1157Mo, JYM0759Mo, JYM0392Mo, JYM0563Mo,
respectively; all Wuhan Jiyinmei Technology Co., Ltd.) was placed
at room temperature and equilibrated for 30 min, as per the
manufacturer's instructions. Serum samples (50 µl) were placed in a
96-well plate with 10 µl antibody (all 1:5) against BNP, cTnT,
cortisol, CA or CRP. Following incubation at 37˚C, 30 min), plate
washing, adding color developing agent (37˚C, 10 min and dark
treatment), and adding termination fluid, the absorbance of the
mixture was measured at 450 nm using an automatic microplate reader
(RT-600, Shenzhen Redu Life Science Co., Ltd.), and the
concentration of the sample was calculated according to a standard
calibration curve.
Statistical analysis
SPSS 25.0 (IBM Corp.) statistical software was used
for analysis and GraphPad Prism 8.0 (GraphPad Software, Inc.;
Dotmatics) was used for data plotting. Data are expressed as the
mean ± standard deviation (n=6). The differences between groups
were analysed using one-way ANOVA and Tukey's post hoc test.
Pearson's correlation coefficient was used to analyse correlation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Inhibitory effect of different doses
of ISO on body weight and growth
The survival rate of each group was assessed after
daily administration of ISO for 14 consecutive days to draw a
survival curve. The survival rates of 5, 10, 25, 50 and 100 mg/kg
ISO groups were 100.0, 66.7, 100.0, 83.3 and 33.3%, respectively
(Fig. 1B). There was no
significant difference in the mean survival time between the 5, 10,
25, 50 and 100 mg/kg groups (14, 10.3±5.8, 14, 13.5±1.2 and
10.2±4.6, respectively; Fig. 1C)
with 0 mg/kg group.
At baseline, no significant differences in body
weight were observed between groups (Fig. 1D). During the experiment,
standardized feeding was adopted to ensure that the diet were at
the same level as much as possible. However, an increase in body
weight was observed in all ISO-treated mice (Fig. 1E); the increase in body weight was
significantly lower in the 25 and 50 mg/kg ISO groups compared with
that in the control (Fig. 1F).
Following intraperitoneal injection of ISO, the mice showed signs
of excessive resting on the ground, trembling, urination, cowering
in the corner and increased sensitivity to stress. After 5 days of
ISO administration, a more prominent stress response in the form of
increased aggressiveness was observed in the 10 mg/kg ISO
group.
Effect of different doses of ISO on
stress response in mice
The open field test revealed changes in behavioural
parameters in all ISO groups compared with those in the control
group (Fig. 2A). Compared with the
control group, mice in all the ISO groups showed a significant
decrease in the total distance travelled, degree across the grid,
time spent at the centre of the field and grid number in the centre
(Fig. 2B-E). These results showed
that the level of spontaneous activity was significantly decreased
after ISO administration and activity and exploration of the mice
were confined to the peripheral grid at the bottom of the box. ISO
injection for 7 days also resulted in decreased voluntary movement
(Fig. S1A-D), with the most
prominent decrease observed in the 5, 50 and 100 mg/kg ISO
groups.
Effect of different doses of ISO on
left ventricular systolic function in mice
Echocardiography of ISO-treated mice showed a
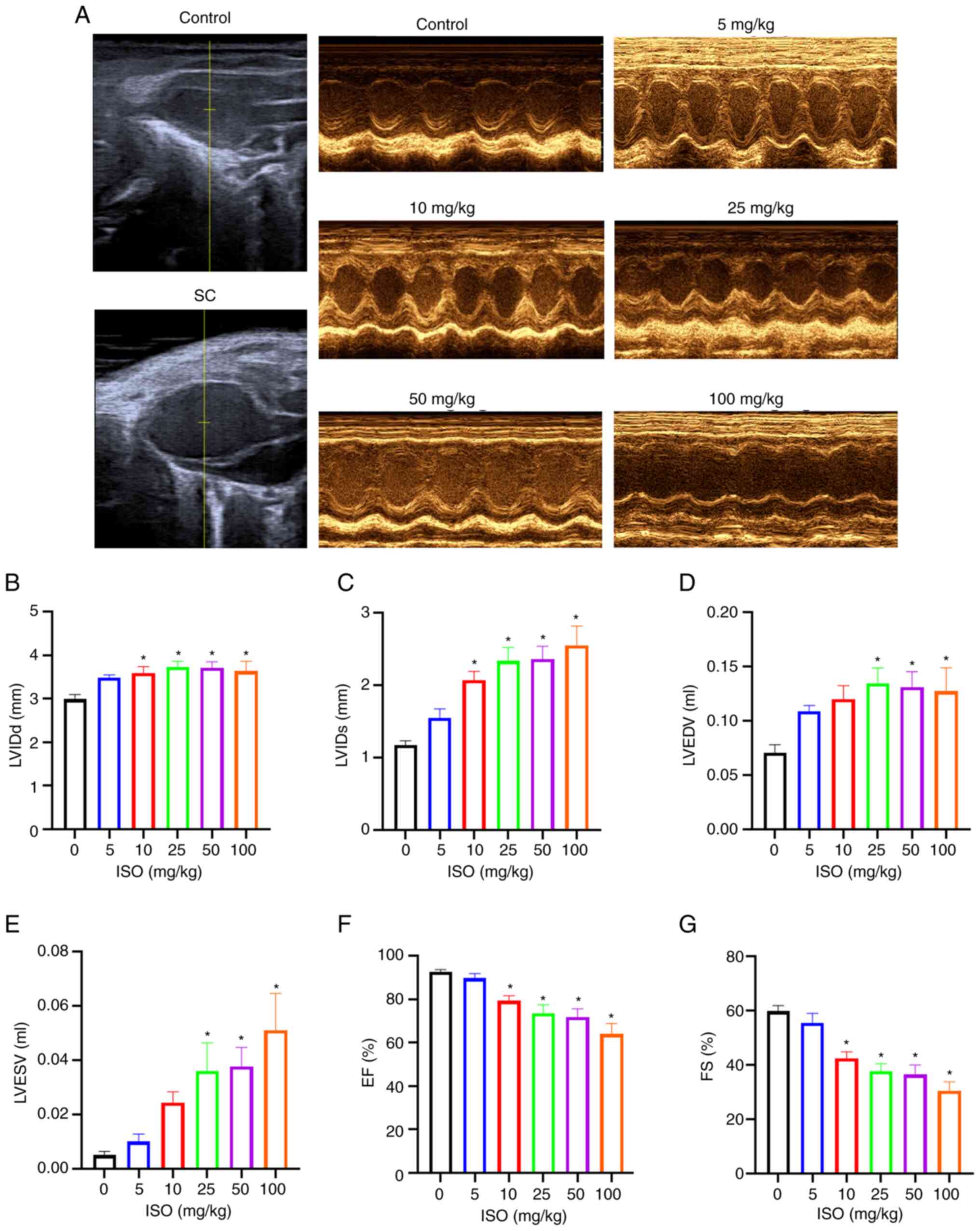
balloon-like enlargement of the apex of the heart (Fig. 3A), abnormal contractile movement of
the apex or middle part of the heart and normal contractile
function of the base of the heart. Compared with the control group,
LVIDd and LVIDs were significantly increased in the 10, 25, 50 and
100 mg/kg ISO groups while LVEDV and LVESV were significantly
increased in the 25, 50 and 100 mg/kg ISO groups (Fig. 3B-E). On the other hand, compared
with the control group, EF and FS were significantly decreased in
the 10, 25, 50 and 100 mg/kg ISO groups (Fig. 3F and G).
These results indicated that the ISO group exhibited
enlarged apex and LV dyskinesia and a significant increase in the
diameter and volume of the left ventricle, which was consistent
with the pathological characteristics of SC (24). After 7 days of ISO administration,
diastolic function of mice in all ISO groups remained normal
compared with that in the control (Fig. S2A and C). Furthermore, mice in the 50 mg/kg ISO
group showed LV systolic dysfunction and significantly increased LV
diameter and volume with the control group (Fig. S2B and D-F).
Effect of ISO on the ECG in mice
ECG of the mice in all groups was recorded after 14
days of ISO treatment. ECG of all ISO-treated mice differed from
that of the control group (Fig.
4A). Compared with the control, the ST height was significantly
increased in the 25, 50 and 100 mg/kg ISO groups, the QT interval
was significantly increased in all ISO-treated groups and the Q
wave was significantly increased in the 5, 25, 50 and 100 mg/kg ISO
groups (Fig. 4B-D).
These results indicated that ISO administration for
14 days induced myocardial ischemia (ST segment elevation),
atrioventricular block (prolonged QT interval) and damage to the
heart (abnormal Q wave), which is consistent with the ECG
characteristics of SC (25).
Following ISO administration for only 7 days 25 and 100 mg/kg group
exhibited significant increases in ST height, QT interval and Q
wave compared with control (Fig.
S3A-D).
Following a single ISO injection, ECG revealed an
inclined depression in the upper ST segment within 30-60 min,
followed by normalization of the ECG. In addition, SC pathology was
not maintained post-single injection (Fig. S4A-D).
Effect of ISO on cardiac pathological
changes in mice
H&E staining showed that the heart of mice in
the control group was normal-sized, with a clear short cylindrical
myocardial cell structure, with neat horizontal stripes and oval
nuclei in the centre. Compared with the control, myocardial tissue
of the mice in all ISO-treated groups exhibited abnormal myocardial
cells, dissolution of myocardial fibres and cytoplasm, widening of
myocardial cell space and oedema and hyperaemia of the interstitium
(Fig. 5).
Effect of different doses of ISO on
serum indexes in mice
Compared with those in the control, cortisol, cTnT,
CA and CRP levels were significantly increased in the 5, 10, 25, 50
and 100 mg/kg ISO groups while BNP levels were increased in the 10,
25, 50 and 100 mg/kg ISO groups (Fig.
6A-E).
Stress is associated with heart injury
in mice with SC
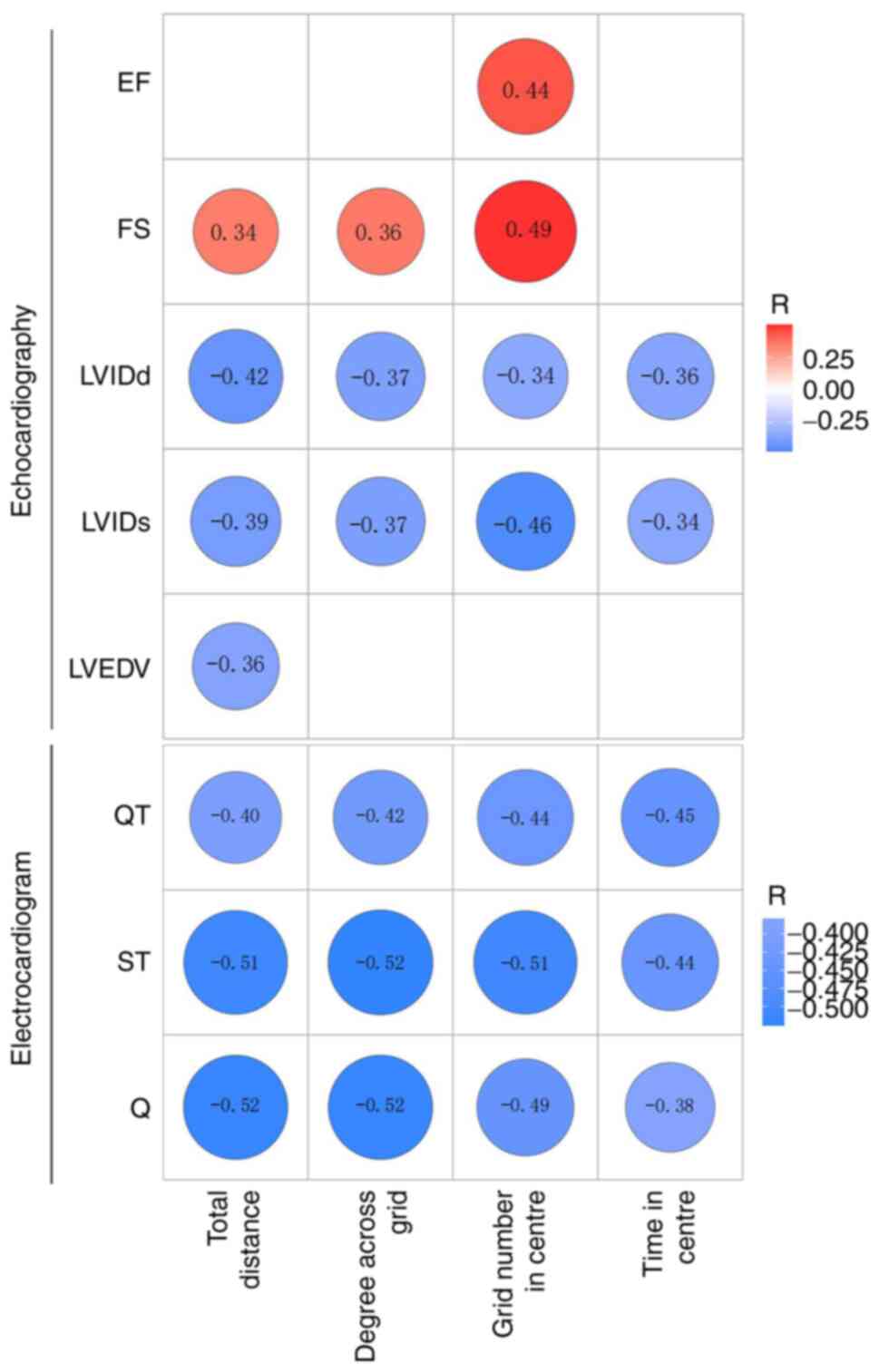
Correlation analysis was performed to assess the
correlation between total distance, the number of degrees across
the grid, the time in the centre and number of grids in the centre
of the open field with LVIDd, LVIDs, LVEDV, EF and FS. The total
distance correlated positively with FS (R=0.34) and negatively with
LVIDd, LVIDs and LVEDV (R=-0.42, R=-0.39 and R=-0.36, respectively;
Fig. 7). The number of degrees
across the grid correlated positively with FS (R=0.36) and
negatively with LVIDd and LVIDs (R=-0.37 and R=-0.37,
respectively). Furthermore, grid number in the centre correlated
positively with EF and FS (R=0.44 and R=0.49, respectively) and
negatively with LVIDd and LVIDs (R=-0.34 and R=-0.46,
respectively); however, the time in the centre was negatively
correlated with LVIDd and LVIDs (R=-0.36 and R=-0.34,
respectively).
Further correlation analysis was performed to
determine if total distance, the number of degrees across the grid,
time in the centre and the number of grids in the centre in the
open field test correlated with QT interval, ST segment and Q wave
amplitude. The total distance was negatively correlated with QT
interval, ST segment and Q wave amplitude (R=-0.40, R=-0.51 and
R=-0.52, respectively). Moreover, the number of degrees across the
grid was negatively correlated with QT interval, ST segment and Q
wave amplitude (R=-0.42, R=-0.52 and R=-0.52, respectively).
Furthermore, the number of grids in the centre was negatively
correlated with QT interval, ST segment and Q wave amplitude
(R=-0.44, R=-0.51 and R=-0.49, respectively). Finally, the time in
the centre negatively correlated with QT interval, ST segment and Q
wave amplitude (R=-0.45, R=-0.44 and R=-0.38, respectively;
Fig. 7).
Discussion
The success in establishing an SC model is
demonstrated by assessing the consistency of myocardial
manifestations with clinicopathological features under stress
(26). Clinical diagnosis is based
on psychological stress levels, upregulation of CA, cardiac
hypertrophy, apical balloon-like change, motor dysfunction, ECG
manifestations of myocardial blood deficiency, increase in BNP
levels and other manifestations (27,28).
Intense emotional or physical stress overstimulates the sympathetic
nervous system, leading to the excessive release of CAs, which is
hypothesized to trigger SC (29-31).
ISO simulates the stress-state levels of CAs. Furthermore, it
causes pathological myocardial damage in mice, similar to the
stress-state myocardial injury in individuals with hypertrophic
cardiomyopathy (32). Thus,
isopropyl CA hormone epinephrine is often used as the primary
component of SC-induction drugs. On the other hand, a joint
scientific statement from the Heart Failure Association Takotsubo
Syndrome Study Group and Myocardial Function Working Group of the
European Society of Cardiology has reported that 90% of patients
with SC are female (33). Compared
with male patients, female patients have a higher incidence of
angina, depression and other concomitant symptoms (34-37).
For this reason, the present study used female mice as research
subjects.
Previous studies (38-42)
have reported that SC models can be constructed using either
continuous intraperitoneal injection of a small dose of ISO (5-100
mg/kg) or a single intraperitoneal injection of a large dose of ISO
(200-400 mg/kg; Table I). The
present study showed that a single intraperitoneal ISO injection
was not enough to induce SC, which was in agreement with a previous
study (43). In addition, studies
have shown that animal models prepared with high-dose ISO injection
exhibit a high mortality rateA recent study found that injection of
400 mg/kg ISO proved lethal and the mice died on account of acute
myocardial ischemia within 5 min of 400 mg/kg ISO injection
(44). Hence, the present study
aimed to determine the optimum dosage regimen of ISO to establish a
stable mouse SC model for investigating the pathogenesis of the
disease, exploring the dose-effect association and devising more
effective treatment approaches.
 | Table IPreviously used methods for
administering ISO to induce stress cardiomyopathy. |
Table I
Previously used methods for
administering ISO to induce stress cardiomyopathy.
| First author/s,
year | Mouse model | Dose, mg/kg | Dosage regimen | (Refs.) |
|---|
| Liao et al,
2022 | C57BL/6J | 200 | Single
intraperitoneal injection of ISO | (38) |
| Shao et al,
2013 | C57BL/6J | 400 | Single
intraperitoneal injection of ISO | (39) |
| Khurana et
al, 2021 | 129/Sv | 25 | Continuous
subcutaneous injection of ISO for 5 days | (40) |
| Walsh-Wilkinson
et al, 2021 | C57BL/6J | 30 | Micro-osmotic pump
inserted subcutaneously to inject ISO for 21 consecutive days | (41) |
| Deng et al,
2004 | Konmin | 30 | Continuous
intraperitoneal injection of ISO for 3 days | (42) |
Open-field testing is a widely used classical method
to study rodent exploration behaviour and assess their emotional
state (45,46). Total distance travelled is
calculated to assess rodent activity based on the assumption that
the central area is more threatening to rodents than the peripheral
areas (44). The level of movement
in the central areas is used to assess anxiety (47). All ISO groups showed a decrease in
values of all the open field test parameters, including total
distance travelled, the number of degrees across the grid, the time
in the centre and number of grids in the centre, which indicated
that ISO injection for 14 consecutive days induced a stress
response in mice.
ECG and echocardiography are common methods to
evaluate cardiac function (48).
After onset of SC, ST segment on ECG immediately elevates and the
QT interval prolongs for an extended time (49,50),
which is accompanied by pathological Q wave (51,52)
and atrioventricular block (53,54).
SC is characterized by decreased LV systolic function and abnormal
ventricular motor function, as well as decreased EF (55) and increased LV diameter and volume
at end-diastolic and -systolic stages, which manifests in the form
of an abnormal balloon-like shape of the left ventricle (56).
The present study showed a high mortality rate in
mice injected with 100 mg/kg ISO and a slow growth rate in mice
injected with 25, 50 and 100 mg/kg ISO. The open-field test showed
that all ISO-treated mice exhibited a notable stress response.
Echocardiography revealed alterations in cardiac function of mice
in the 25 and 50 mg/kg ISO groups. The same groups showed a
significant increase in inner diameter and volume of the left
ventricle and a significant decrease in EF and FS, which are
typical manifestations of SC (57). Moreover, ECG showed a significant
increase in ST segment in the 25, 50, and 100 mg/kg ISO groups,
which indicated myocardial ischemia (58,59).
Furthermore, all ISO groups exhibited marked prolongation of QT
interval; however, pathological Q wave was observed in the 5, 25,
50, and 100 mg/kg ISO groups. Correlation analysis showed that
stress was associated with cardiac function change in the present
animal model.
An increase in levels of serum BNP, which is
produced by ventricular myocytes and indicates impaired cardiac
function (60,61), is a common indicator of SC
(62-64).
At the onset of SC, BNP and peak cTnT levels increase significantly
and they act as biomarkers that distinguish SC from acute
myocardial ischemia (65). The
levels of cortisol, another classic stress biomarker, also increase
during the development of SC (66,67).
CRP is a powerful predictor and risk factor for myocardial injury
(68). A key factor in SC
progression is the overactivation of the sympathetic nervous system
so high levels of CA can effectively reflect the degree of
sympathetic nerve activation (69,70).
The present study indicated that ISO administration
led to a significant increase in cortisol, cTnT, CRP and CA levels
in all ISO groups, indicating that the sympathetic nervous system
was activated following ISO treatment in mice, which produced a
stress state and caused myocardial damage and inflammation.
Moreover, increased BNP levels found in 10, 25, 50 and 100 mg/kg
ISO groups indicated damage to heart function caused by ISO
injection.
In conclusion, intraperitoneal injection of 25 or 50
mg/kg ISO for 14 consecutive days in mice induced a stable SC
model. Compared with the 50 mg/kg ISO group, the 25 mg/kg ISO group
exhibited a lower mortality rate with more prominent changes in ECG
and levels of serum markers. Nonetheless, a stable mouse SC model
can be established via 25 or 50 mg/kg ISO administration. The model
in the current study showed several advantages, such as a simple
and affordable modelling method, good stability and low mortality.
It not only demonstrated the effect of different doses of ISO on
stress response and cardiac function but also screened the most
appropriate dose to establish a viable model and provide a basis
for future SC research.
Supplementary Material
Open field test following
intraperitoneal ISO injection for seven days. (A) Total distance.
(B) Degree across the grid. (C) Time in centre. (D) Grid number in
the centre. For control and 5, 25 and 50 mg/kg group, n=6. For the
10 mg/kg group, n=4. For 100 mg/kg ISO group, n=5.
*P<0.05 vs. control. ISO, isoprenaline.
Echocardiography after 7 days of
intraperitoneal ISO injection. (A) LVIDd. (B) LVIDs. (C) LVEDV. (D)
LVESV. (E) EF. (F) FS. For the control and 5, 25 and 50 mg/kg
group, n=6. For the 10 mg/kg ISO group, n=4. For the 100 mg/kg ISO
group, n=5. *P<0.05 vs. control. LVIDd, left
ventricular internal end-diastolic diameter; LVIDs, left
ventricular internal end-systolic diameter; LVEDV, left ventricular
end-diastolic volume; LVESV, left ventricular end-systolic volume;
EF, ejection fraction; FS, fractional shortening; ns,
non-significant; ISO, isoprenaline.
Electrocardiogram after 7 days of
intraperitoneal ISO injection. (A) ST segment height. (B) QT
interval. (C) Q wave amplitude. For the control and 5, 25 and 50
mg/kg ISO group, n=6. For 10 mg/kg ISO group, n=4. For the 100
mg/kg group, n=5. *P<0.05 vs. control. ISO,
isoprenaline.
Electrocardiogram following single
intraperitoneal injection. (A) ST segment height. (B) QT interval.
(C) Q wave amplitude. For the 100 mg/kg ISO group, n=3. For other
groups, n=6. *P<0.05 vs. control. ISO, isoprenaline;
ns, not significant.
Acknowledgements
The authors would like to thank Mr Wang Wenhui, Mr
Xie Yuhua and Mr Zhong Wen from Anhui University of Chinese
Medicine (Hefei, China) for collecting data.
Funding
Funding: The present study was supported by the National Key
RESEARCH and Development Program of China (grant no.
2018YFC1704600) and Traditional Chinese Medicine Leading Talents
Construction Project of Anhui Province (grant no.
ZYYLJRC201911).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HW and SC confirm the authenticity of all the raw
data. SC, HW and MZ conceived and designed the study. HW and HS
collected data. HW, HS, CZ and SW analysed and interpreted the
data. HS, CZ and SW revised the manuscript. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Study protocols were reviewed and approved by the
Experimental Animal Ethics Committee of Anhui University of
Traditional Chinese Medicine (approval no.
AHUCM-mouse-2022045).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Barbieri L, Galli F, Conconi B, Gregorini
T, Lucreziotti S, Mafrici A, Pravettoni G, Sommaruga M and Carugo
S: Takotsubo syndrome in COVID-19 era: Is psychological distress
the key? J Psychosom Res. 140(110297)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pasqualetto MC, Secco E, Nizzetto M,
Scevola M, Altafini L, Cester A and Rigo F: Stress cardiomyopathy
in COVID-19 disease. Eur J Case Rep Intern Med.
7(001718)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Desai HD, Sharma K, Jadeja DM, Desai HM
and Moliya P: COVID-19 pandemic induced stress cardiomyopathy: A
literature review. Int J Cardiol Heart Vasc.
31(100628)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jabri A, Kalra A, Kumar A, Alameh A,
Adroja S, Bashir H, Nowacki AS, Shah R, Khubber S, Kanaa'N A, et
al: Incidence of stress cardiomyopathy during the coronavirus
disease 2019 pandemic. JAMA Netw Open. 3(e2014780)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shah RM, Shah M, Shah S, Li A and Jauhar
S: Takotsubo syndrome and COVID-19: Associations and implications.
Curr Probl Cardiol. 46(100763)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
de Chazal HM, Del Buono MG, Keyser-Marcus
L, Ma L, Moeller FG, Berrocal D and Abbate A: Stress cardiomyopathy
diagnosis and treatment: JACC state-of-the-art review. J Am Coll
Cardiol. 72:1955–1971. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rivera AMC, Ruiz-Bailén M and Aguilar LR:
Takotsubo cardiomyopathy-a clinical review. Med Sci Monit.
17:RA135–RA157. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rahbar-Karbasdehi E and Rahbar-Karbasdehi
F: Clinical challenges of stress cardiomyopathy during coronavirus
2019 epidemic. Cell Mol Biomed Rep. 1:88–90. 2021.
|
|
9
|
Okura H: Update of takotsubo syndrome in
the era of COVID-19. J Cardiol. 77:361–369. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chhabra L: Prognostication in takotsubo
syndrome. Rev Cardiovasc Med. 23(110)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
de Gregorio C, Pistelli L, Borgi M, Trio
O, Akashi YJ and Andò G: TakoTsubo syndrome: A well-known disease
but not everything is clear yet. Rev Cardiovasc Med.
23(184)2022.
|
|
12
|
Templin C, Ghadri JR, Diekmann J, Napp LC,
Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann
CA, et al: Clinical features and outcomes of takotsubo (stress)
cardiomyopathy. N Eng J Med. 373:929–938. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lyon AR, Citro R, Schneider B, Morel O,
Ghadri JR, Templin C and Omerovic E: Pathophysiology of Takotsubo
syndrome: JACC state-of-the-art review. J Am Coll Cardiol.
77:902–921. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shams Y and Henareh L: Plasma
catecholamine levels in patients with takotsubo syndrome:
Implications for the pathogenesis of the disease. Int J Cardiol.
181:35–38. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Motiejunaite J, Amar L and Vidal-Petiot E:
Adrenergic receptors and cardiovascular effects of catecholamines.
Ann Endocrinol (Paris). 82:193–197. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kumar A, Pappachan JM and Fernandez CJ:
Catecholamine-induced cardiomyopathy: An endocrinologist's
perspective. Rev Cardiovasc Med. 22:1215–1228. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lyon AR, Rees PSC, Prasad S, Poole-Wilson
PA and Harding SE: Stress (Takotsubo) cardiomyopathy-a novel
pathophysiological hypothesis to explain catecholamine-induced
acute myocardial stunning. Nat Clin Pract Cardiovasc Med. 5:22–29.
2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Redfors B, Ali A, Shao Y, Lundgren J, Gan
LM and Omerovic E: Different catecholamines induce different
patterns of takotsubo-like cardiac dysfunction in an apparently
afterload dependent manner. Int J Cardiol. 174:330–336.
2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ali A, Redfors B, Lundgren J, Alkhoury J,
Oras J, Gan LM and Omerovic E: Effects of pretreatment with
cardiostimulants and beta-blockers on isoprenaline-induced
takotsubo-like cardiac dysfunction in rats. Int J Cardiol.
281:99–104. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Xiang S, Bian Z, Zhou H, Deng W, Zhu J and
Tang Q: . Combined utilization of bisoprolol and glutathione
attenuates ventricular remodeling of takotsubo cardiomyopathy in
mice. Chin J Cardiovasc Med. 20:358–365. 2015.
|
|
21
|
Ueyama T: Emotional stress-induced
Tako-tsubo Cardiomyopathy: animal model and molecular mechanism.
Annals of the New York Academy of Sciences. 1018:437–444.
2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ueyama T, Kasamatsu K, Hano T, Yamamoto K,
Tsuruo Y and Nishio I: . Emotional Stress Induces Transient Left
Ventricular Hypocontraction in the Rat Via Activation of Cardiac
Adrenoceptors A Possible Animal Model of 'Tako-Tsubo'
Cardiomyopathy. Circulation Journal. 66:712–713. 2002.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Murakami M, Niwa H, Kushikata T, Watanabe
H, Hirota K, Ono K and Ohba T: Inhalation anesthesia is preferable
for recording rat cardiac function using an electrocardiogram. Biol
Pharm Bull. 37:834–839. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Citro R, Lyon AR, Meimoun P, Omerovic E,
Redfors B, Buck T, Lerakis S, Parodi G, Silverio A, Eitel I, et al:
Standard and advanced echocardiography in takotsubo (stress)
cardiomyopathy: Clinical and prognostic implications. J Am Soc
Echocardiogr. 28:57–74. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sharkey SW: Electrocardiogram mimics of
acute ST-segment elevation myocardial infarction: Insights from
cardiac magnetic resonance imaging in patients with tako-tsubo
(stress) cardiomyopathy. J Electrocardiol. 41:621–625.
2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hau J, Schapiro SJ and Van Hoosier Jr GL:
Handbook of laboratory animal science: Animal Models, Volume III.
CRC press, 2004.
|
|
27
|
Rahbar-Karbasdehi E and Rahbar-Karbasdehi
F: Clinical challenges of stress cardiomyopathy during coronavirus
2019 epidemic. Cell Mol Biomed Rep. 1:88–90. 2021.
|
|
28
|
Lyon AR, Bossone E, Schneider B, Sechtem
U, Citro R, Underwood SR, Sheppard MN, Figtree GA, Parodi G, Akashi
YJ, et al: Current state of knowledge on Takotsubo syndrome: A
position statement from the taskforce on takotsubo syndrome of the
heart failure association of the european society of cardiology. J
Heart Fail. 18:8–27. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Akashi YJ, Nef HM and Lyon AR:
Epidemiology and pathophysiology of Takotsubo syndrome. Nat Rev
Cardiol. 12:387–397. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hurst RT, Prasad A, Askew JW, Sengupta PP
and Tajik AJ: Takotsubo cardiomyopathy: A unique cardiomyopathy
with variable ventricular morphology. JACC Cardiovasc Imaging.
3:641–649. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang X, Pei J and Hu X: The brain-heart
connection in takotsubo syndrome: The central nervous system,
sympathetic nervous system, and catecholamine overload. Cardiol Res
Pract. 2020(4150291)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Xu W, Li XP, Li EZ, Liu YF, Zhao J, Wei LN
and Ma L: Protective effects of allicin on ISO-induced rat model of
myocardial infarction via JNK signaling pathway. Pharmacology.
105:505–513. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Omerovic E, Citro R, Bossone E, Redfors B,
Backs J, Bruns B, Ciccarelli M, Couch LS, Dawson D, Grassi G, et
al: Pathophysiology of Takotsubo syndrome -a joint scientific
statement from the heart failure association takotsubo syndrome
study group and myocardial function working group of the european
society of cardiology-part 2: Vascular pathophysiology, gender and
sex hormones, genetics, chronic cardiovascular problems and
clinical implications. Eur J Heart Fail. 24:272–286.
2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Pimple P, Hammadah M, Wilmot K, Ramadan R,
Al Mheid I, Levantsevych O, Sullivan S, Garcia EV, Nye J, Shah AJ,
et al: Chest pain and mental stress-induced myocardial ischemia:
Sex differences. Am J Med. 131:540–547. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Rainville JR, Lipuma T and Hodes GE:
Translating the transcriptome: Sex differences in the mechanisms of
depression and stress. Biol Psychiatry. 91:25–35. 2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Vaccarino V, Sullivan S, Hammadah M,
Wilmot K, Al Mheid I, Ramadan R, Elon L, Pimple PM, Garcia EV, Nye
J, et al: Mental stress induced-myocardial ischemia in young
patients with recent myocardial infarction: Sex differences and
mechanisms. Circulation. 137:794–805. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Gebhard C: Women and acute coronary
syndromes: Still up to no good. Eur Heart J. 38:1066–1068.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Liao X, Chang E, Tang X, Watanabe I, Zhang
R, Jeong HW, Adams RH and Jain MK: Cardiac macrophages regulate
isoproterenol-induced Takotsubo-like cardiomyopathy. JCI Insight.
7(e156236)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Shao Y, Redfors B, Ståhlman M, Täng MS,
Miljanovic A, Möllmann H, Troidl C, Szardien S, Hamm C, Nef H, et
al: A mouse model reveals an important role for
catecholamine-induced lipotoxicity in the pathogenesis of
stress-induced cardiomyopathy. Eur J Heart Fail. 15:9–22.
2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Khurana I, Maxwell S, Royce S,
Mathiyalagan P, Karagiannis T, Mazarakis N, Vongsvivut J,
Harikrishnan KN, Okabe J, Al-Hasani K, et al: SAHA attenuates
Takotsubo-like myocardial injury by targeting an epigenetic Ac/Dc
axis. Signal Transduct Target Ther. 6:1–4. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Walsh-Wilkinson E, Arsenault M and Couet
J: Segmental analysis by speckle-tracking echocardiography of the
left ventricle response to isoproterenol in male and female mice.
PeerJ. 9(e11085)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Deng SX and Tian T: An experimental study
on the expression of TNF-α in myocardial injury during stress. J
Chongqing Medical University. 3:315–317. 2004.(In Chinese).
|
|
43
|
Wallner M, Duran JM, Koller S, Mohsin S,
Lis S, Sharp TE, Berretta RM and Houser SR: Single-dose
isoproterenol does not depress cardiac function in mice. Circul
Res. 117:A311. 2015.
|
|
44
|
Ye KJ, Yang J, Liu Z, et al: Evaluation of
cardiac electrical activity in mice with isoproterenol-induced
stress cardiomyopathy by optical mapping technique. Chin J Geriatr
Heart Brain Vessel Dis. 24:412–417. 2022.(In Chinese).
|
|
45
|
Gould TD, Dao DT and Kovacsics CE: The
open field test. Mood And Anxiety Related Phenotypes In Mice.
42:1–20. 2009.
|
|
46
|
Thippeswamy BS, Mishra B, Veerapur VP and
Gupta G: Anxiolytic activity of Nymphaea alba Linn. in mice as
experimental models of anxiety. Indian J Pharmacol. 43:50–55.
2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Lipkind D, Sakov A, Kafkafi N, Elmer GI,
Benjamini Y and Golani I: New replicable anxiety-related measures
of wall vs. center behavior of mice in the open field. J Appl
Physiol (1985). 97:347–359. 2004.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Leren IS, Saberniak J, Haland TF,
Edvardsen T and Haugaa KH: Combination of ECG and echocardiography
for identification of arrhythmic events in early ARVC. JACC
Cardiovasc Imaging. 10:503–513. 2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Mitsuma W, Kodama M, Ito M, Tanaka K,
Yanagawa T, Ikarashi N, Sugiura K, Kimura S, Yagihara N, Kashimura
T, et al: Serial electrocardiographic findings in women with
Takotsubo cardiomyopathy. Am J Cardiol. 100:106–109.
2007.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Abraham J, Mudd JO, Kapur N, Klein K,
Champion HC and Wittstein IS: Stress cardiomyopathy after
intravenous administration of catecholamines and beta-receptor
agonists. J Am Coll Cardiol. 53:1320–1325. 2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Gianni M, Dentali F, Grandi AM, Sumner G,
Hiralal R and Lonn E: Apical ballooning syndrome or takotsubo
cardiomyopathy: A systematic review. Eur Heart J. 27:1523–1529.
2006.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Thakar S, Chandra P, Hollander G and
Lichstein E: Electrocardiographic changes in Takotsubo
cardiomyopathy. Pacing Clin Electrophysiol. 34:1278–1282.
2011.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Kodama S, Miyoshi K, Shiga Y, Maruyama S,
Sumi S, Tojou H, Yamanouchi Y and Urata H: Takotsubo cardiomyopathy
complicated by high-grade atrioventricular block: A report of two
cases. Exp Clin Cardiol. 14:e35–e38. 2009.PubMed/NCBI
|
|
54
|
Bexton RS and Camm AJ: First degree
atrioventricular block. Eur Heart J. 5:107–109. 1984.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Lee M: Time course of functional recovery
in takotsubo (stress) cardiomyopathy: A serial speckle tracking
echocardiography and electrocardiography study. J Cardiovasc
Imaging. 28:50–60. 2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Semelka RC, Tomei E, Wagner S, Mayo J,
Caputo G, O'Sullivan M, Parmley WW, Chatterjee K, Wolfe C and
Higgins CB: Interstudy reproducibility of dimensional and
functional measurements between cine magnetic resonance studies in
the morphologically abnormal left ventricle. Am Heart J.
119:1367–1373. 1990.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Chockalingam A, Xie GY and Dellsperger KC:
Echocardiography in stress cardiomyopathy and acute LVOT
obstruction. Int J Cardiovasc Imaging. 26:527–535. 2010.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Santoro F, Stiermaier T, Tarantino N,
Guastafierro F, Graf T, Möller C, Di Martino LFM, Thiele H, Di
Biase M, Eitel I and Brunetti ND: Impact of persistent ST elevation
on outcome in patients with Takotsubo syndrome. Results from the
GErman Italian STress Cardiomyopathy (GEIST) registry. Int J
Cardiol. 255:140–144. 2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Ross J Jr: Electorcardiographic ST-segment
analysis in the characterization of myocardial ischemia and
infarction. Circulation. 53:I73–I81. 1976.PubMed/NCBI
|
|
60
|
Hall C: Essential biochemistry and
physiology of (NT-pro) BNP. Eur J Heart Fail. 6:257–260.
2004.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Vuolteenaho O, Ala-Kopsala M and Ruskoaho
H: BNP as a biomarker in heart disease. Adv Clin Chem. 40:1–36.
2005.PubMed/NCBI
|
|
62
|
Rahbar-Karbasdehi E and Rahbar-Karbasdehi
F: Clinical challenges of stress cardiomyopathy during coronavirus
2019 epidemic. Cell Mol Biomed Rep. 1:88–90. 2021.
|
|
63
|
Glaveckaitė S, Šerpytis P, Pečiūraitė D,
Puronaitė R and Valevičienė N: Clinical features and three-year
outcomes of Takotsubo (stress) cardiomyopathy: Observational data
from one center. Hellenic J Cardiol. 57:428–434. 2016.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Ahmed KA, Madhavan M and Prasad A: Brain
natriuretic peptide in apical ballooning syndrome (Takotsubo/stress
cardiomyopathy): Comparison with acute myocardial infarction. Coron
Artery Dis. 23:259–264. 2012.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Randhawa MS, Dhillon AS, Taylor HC, Sun Z
and Desai MY: Diagnostic utility of cardiac biomarkers in
discriminating Takotsubo cardiomyopathy from acute myocardial
infarction. J Card Fail. 20:2–8. 2014.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Đurić I, Obradović S and Gligić B:
Dynamics of electrocardiographic changes, brain-natriuretic peptide
and cortisol levels in a patient with stress (takotsubo)
cardiomyopathy: A case report. Vojnosanit Pregl. 70:511–515.
2013.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Madhavan M, Borlaug BA, Lerman A, Rihal CS
and Prasad A: Stress hormone and circulating biomarker profile of
apical ballooning syndrome (Takotsubo cardiomyopathy): Insights
into the clinical significance of B-type natriuretic peptide and
troponin levels. Heart. 95:1436–1441. 2009.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Świątkiewicz I, Magielski P and Kubica J:
C-reactive protein as a risk marker for post-infarct heart failure
over a multi-year period. Int J Mol Sci. 22(3169)2021.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Wittstein IS: Stress cardiomyopathy: A
syndrome of catecholamine-mediated myocardial stunning? Cell Mol
Neurobiol. 32:847–857. 2021.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Al Houri HN, Jomaa S, Jabra M, Alhouri AN
and Latifeh Y: Pathophysiology of stress cardiomyopathy: A
comprehensive literature review. Ann Med Surg (Lond).
2022(104671)2022.PubMed/NCBI View Article : Google Scholar
|