Introduction
Cancer has become an important factor threatening
human health. There are about 19.3 million new cancer cases
worldwide each year, and about 10 million patients die of cancer
(1). Although with the development
of medicine, the quality of life of patients with common malignant
tumors such as lung cancer, breast cancer, and esophageal cancer
has been significantly improved, the treatment effect for patients
with malignant tumors with a low incidence such as malignant
peritoneal mesothelioma still not as good as it should be. Some
patients had to resort to palliative care and end-of-life care
(2). Pain is one of the most
common symptoms of malignant peritoneal mesothelioma. It is
reported that 92.7% of patients with this condition have
experienced varying degrees of cancer pain. Therefore, analgesia
plays an important role in the treatment of malignant peritoneal
mesothelioma. Opioids are the first choice for the treatment of
moderate and severe pain, but they also readily cause adverse
reactions, such as constipation, nausea, urinary retention,
respiratory depression, and addiction. Excessive or unreasonable
administration can cause poisoning. This is mainly characterized by
respiratory depression, pupillary constriction, a drop in blood
pressure, and in severe cases, circulatory failure, shock, and even
death. Few cases of morphine poisoning have been reported, and even
fewer are cases of morphine poisoning in patients with malignant
peritoneal mesothelioma. Here, we present a case of morphine
poisoning in patients with malignant peritoneal mesothelioma in
order to provide guidelines for clinical practice.
Case report
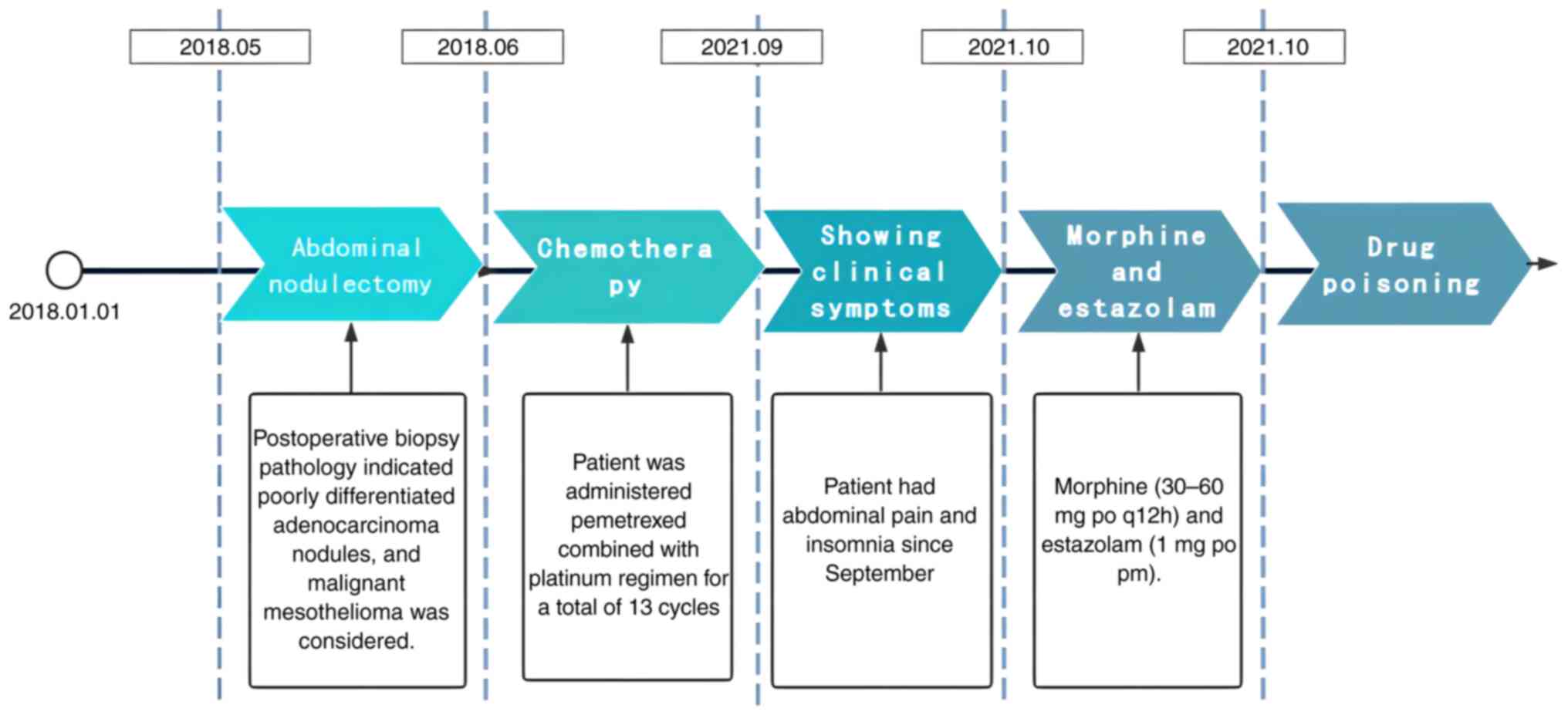
The patient, a 64-year-old man, had been diagnosed
with malignant peritoneal mesothelioma 3 years and 5 months
previously. He had undergone abdominal nodulectomy in May 2018.
Postoperative biopsy pathology indicated poorly differentiated
adenocarcinoma nodules, and malignant mesothelioma was considered
(Fig. 1A and B). Immunohistochemistry indicated that
the tumor was positive for AEI/AE3, calretinin, CK5/6, WT1, D2-40,
and EMA and negative for Vim, CEA, and calponin. The Ki67 index was
10%. These findings were consistent with malignant mesothelioma.
Because the patient was considered to have an advanced tumor and
had a malignant abdominal effusion, he could not undergo surgery
(Fig. 1C and D). There were no contraindications for
chemotherapy. Therefore, the patient received a pemetrexed combined
with platinum regimen for a total of 13 cycles, and the last cycle
of chemotherapy was administered in September 2021. During
chemotherapy, he was also treated with traditional Chinese medicine
and gamma knife surgery in another hospital (the specific treatment
is unknown). The patient had abdominal pain and poor sleep.
Therefore, to alleviate cancer pain and insomnia, he began to take
morphine hydrochloride sustained-release tablets (30-60 mg po q12h)
in addition to estazolam tablets intermittently (1 mg po prn)
(Fig. 2). He was admitted to the
Fourth Hospital of Hebei Medical University in October 2021, for
continued treatment.
The next day, the patient appeared to be in a deep
coma, with constricted pupils, poor light reflex, and negative
orbit compression reflex. The physical examination was difficult
due to patients loss of consciousness and no response to voice
command. ECG monitoring indicated the following results: Pulse: 122
beats/min, Respiratory rate: 13 times/min, and Blood pressure:
100/69 mmHg. Blood gas analysis indicated a pH of 7.290, PaCO2 of
59.4 mmHg, PaO2 of 33.5 mmHg, lactic acid of 1.61 mmol/l, and BE of
0.8 mmol/l. Liver and kidney function tests indicated an ALT level
of 13.1 U/l, an AST level of 23 U/l, an SCR level of 136.1 µmol/l,
and a urea level of 10.7 mmol/l. The blood chemical composition
test detected a morphine component of 3.5 mg/l (therapeutic dose is
less than 1.0 mg/l) and a diazepam component of 6.6 mg/l
(therapeutic dose is less than 2.0 mg/l). Therefore, we diagnosed
respiratory failure secondary to drug poisoning. The patient
immediately underwent oral tracheal intubation and
ventilator-assisted breathing, hypotension treatment, gastric
lavage, acid inhibition to protect the gastric mucosa, and fluid
replacement. At the same time, the patient is gave hemoperfusion
twice by the doctor on duty, additionally, naloxone (0.4 mg) and
flumazenil (0.3 mg) were injected into the patient by intravenous
injection in order to promote awakening. After about 6 h, the
patient's consciousness gradually recovered and his vital signs
stabilized to a normal state. His status improved, and he was
discharged from the hospital.
Discussion
Malignant tumors have become one of the main threats
to human health. There were 19.3 million new cancer cases and
nearly 10 million cancer-related deaths worldwide in 2020(1). As the most common cancer symptom,
pain seriously affects the quality of life of cancer patients.
Previous studies have shown that approximately 55% of patients
receiving anticancer therapy, and 66% of patients with advanced and
metastatic malignant tumors experience pain (2). The goal of cancer pain management is
to reduce pain to an acceptable level. In 1986, the WHO recommended
a ‘three-step treatment principle of cancer pain’, which has played
a vital role in cancer pain control in the past 35 years. However,
because of the results of in-depth studies on cancer pain,
analgesics are no longer required to be strictly administered in a
step-by-step fashion. If patients present with moderate or severe
pain, opioid painkillers can be administered immediately to avoid
delay in treatment (3). Therefore,
opioids, as the cornerstone of analgesic drugs, are widely used in
clinical ‘painless wards.’ In this clinical setting, in order to
relieve pain as one of the common symptoms of malignant peritoneal
mesothelioma, morphine hydrochloride sustained-release tablets were
given to this patient.
Opioid drugs used by humans to treat pain can be
traced back to ancient Egypt (4).
The pharmacological effects of opioid analgesics come from their
complex interactions with three distinct opioid receptors (the µ,
κ, and δ receptors) (5). When
activated by opioid receptor agonists, these receptors indirectly
inhibit voltage-dependent calcium channels, reduce the level of
cAMP, and block pain neurotransmitters (such as glutamate and
substance P), resulting in an analgesic effect (6).
The opioid morphine is widely used in clinical
practice. Morphine can stimulate opioid receptors (mainly the µ
receptor) on central and peripheral neurons, neuroendocrine
(pituitary and adrenal), immune and ectodermal cells, to produce an
analgesic effect (4,7,8). µ
receptors are mainly expressed in the brainstem and medial thalamus
(9,10). The µ receptor is encoded by the
Oprm1 gene and can be the µ1,
µ2, or µ3 subtypes. µ1 is related
to analgesia, euphoria, and sedation; µ2 is related to
inhibition of breathing, itching, stimulation of prolactin release,
opioid dependence, pupil dilation, gastrointestinal motility
(constipation), and sedation; and µ3 is related to
vasodilation (4,10). Morphine can also act on the κ and δ
receptors. The κ receptor is mainly expressed in the marginal zone
and other diencephalic regions, the brainstem, and the spinal cord,
and is responsible for analgesia, sedation, diuresis, respiratory
depression, and opioid dependence (4,10,11).
The δ receptor is mainly expressed in the brain, and its effects
may be related to analgesia, anxiety, and reduction of
gastrointestinal motility (4,10).
The roles of these opioid receptors explain the analgesic effect of
morphine and its adverse reactions, such as respiratory depression,
orthostatic hypotension and syncope, endocrine abnormalities,
immune dysfunction, sleep and mood changes, SIADH, and addiction
(10,12-14).
This patient had obvious symptoms of the µ receptor.
Morphine is mainly metabolized by glucuronidation
and demethylation in the liver. Glucuronidation, which produces
morphine-6-glucuronide and morphine-3-glucuronide, is the main
metabolic process (4,15,16).
Morphine-6-glucuronide is thought to cause some of the analgesic
effects of morphine (15), whereas
morphine-3-glucuronide has no analgesic effect; some studies have
even found that a sufficiently high concentration of
morphine-3-glucuronide may lead to hyperalgesia (4,17).
The metabolites of morphine are mainly eliminated by the kidneys,
although small amounts are excreted in the bile and milk. With
regard to the route of administration, morphine is absorbed rapidly
after subcutaneous and intramuscular injection and absorbed through
the gastrointestinal tract after oral administration. It is then
rapidly metabolized by microsomal enzymes through the liver, so
that the blood concentration of morphine is relatively low. After
absorption, it is distributed to the lung, liver, spleen, kidney,
and other tissues. Only a small amount of morphine can pass through
the blood-brain barrier, but it produces an efficient analgesic
effect (4). This patient had
extensive abdominal metastasis and high tumor load (Fig. 1C and D), resulting in slower intestinal
peristalsis, prolonged drug retention time, increased absorption,
and drug concentration accumulation. Simultaneously, morphine can
also lead to the weakening of gastrointestinal peristalsis, which
further aggravates the above effects. Another noteworthy problem is
that this patient had normal renal function during initial
treatment but he later developed renal insufficiency, as evidenced
by a serum creatinine level of 136.1 µmol/l. This would reduce drug
excretion and further increase the concentration of morphine in the
blood.
Drug interactions must also be considered in
patients receiving morphine. Morphine can reduce the peak blood
concentration and efficacy of P2Y12 receptor antagonists by
inhibiting gastrointestinal peristalsis and digestive juice
secretion (18). Fine et al
(19) reported a case of
respiratory arrest, seizures, insanity, and general convulsions
caused by cimetidine combined with morphine. However, Mojaverian
et al (20) tested healthy
individuals and found that cimetidine did not affect the metabolism
and efficacy of morphine; no other adverse reactions were observed
during the study. Shirooie et al (21) reported that long-term
administration of metformin can improve morphine tolerance and
dependence by inhibiting microglial activation and mTOR signaling.
Okura et al (22) found
that quinidine enhances the blood concentration of morphine by
changing the activity of p-glycoprotein. Continuous oral
administration of morphine combined with etoposide increases the
blood concentration of etoposide, which may also be related to
changes in intestinal p-glycoprotein activity (23). Manara et al (24) reported that oral administration of
morphine and metoclopramide can accelerate the effect of morphine
and enhance its analgesic effect. Morphine also prolongs the
half-life of theophylline in rats and reduces the clearance rate by
competing with theophylline for receptor binding (25). Some studies have shown that
morphine may enhance the binding of benzodiazepines to GABAA
receptors by acting on opioid receptors, thereby enhancing the
efficacy of benzodiazepines (26).
This patient took morphine and estazolam tablets at the same time,
which may have led to poisoning due to drug interaction which
enhanced each drug's effects.
Benzodiazepines, such as diazepam, midazolam and
estazolam, are established first-line drugs for sedative-hypnotic,
anxiolytic and antiseizure. Benzodiazepines are a family of drugs
that exert their effects by allosterically modulating the activity
of the ionotropic gamma-aminobutyric acid (GABA)-A receptor in the
central nervous system . These drugs increase the probability that
GABA binding to the receptor will open the associated Cl- channel.
Thus, these drugs generally decrease neuronal excitation and
exhibit sedative-hypnotic, anxiolytic and antiseizure (27). Estazolam is an s-triazolo
benzodiazepine derivative whose structure is derived from the
introduction of a triazole ring in the 1,2 position of the
well-known diazepam structure. The plasma concentration of
estazolam reached its peak 3 h after oral administration. It has a
half-life of 10-24 h, which agree well with those reported by
Mancinelli et al who described the human elimination of
estazolam, determined in good agreement from the single- and
multiple-dose studies, averaged 19 h (28). Pierce et al reported that
estazolam 1.0 and 2.0 mg produce significant increases in total
sleep time. Estazolam 0.25 and 0.5 mg are also effective, but the
improvement in total sleep time may be too small to be clinically
significant for most patients (29). Therefore, oral 1-2 mg Estazolam can
play a good effect in the treatment of insomnia. The patient's oral
1 mg estazolam (safe dose) led to drug poisoning, so other factors
were considered such as drug interaction, liver and kidney
damage.
At present, the only drug approved by the FDA for
the prevention and treatment of opioid overdose is naloxone
(30). France et al
(30) recently documented several
new drugs or methods for treating opioid overdoses, including
intranasal nalmefene, a competitive, reversible opioid receptor
antagonist with a longer duration of action than naloxone;
methocinnamox, a novel opioid receptor antagonist; covalent
naloxone nanoparticles; serotonin1A receptor agonists;
fentanyl-binding cyclodextrin scaffolds; detoxifying biomimetic
‘nanosponge’ decoy receptors; and antibody-based strategies. Thus,
naloxone was administered to promote awakening.
In this case, the patient first received morphine
for moderate and severe pain caused by peritoneal malignant
mesothelioma. Morphine hydrochloride sustained-release tablets were
administered because of their convenient use, definite analgesic
effect, and long maintenance time. During the period of medication,
the patient suddenly appeared in a deep coma with respiratory
depression and pupil contraction (needle-like). Blood gas analysis
showed hypoxia and CO2 retention. A morphine component
of 3.5 mg/l and a diazepam component of 6.6 mg/l were detected by
blood sampling; therefore, we diagnosed respiratory failure caused
by drug poisoning. We believe the following factors contributed to
morphine poisoning in this patient: i) Both morphine and
benzodiazepines have respiratory depression, and the combination of
morphine and estazolam has an interaction, which enhances each
other's efficacy. ii) Extensive metastasis in the abdominal cavity
and high tumor burden lead to slow intestinal peristalsis and
prolonged the retention time of the drug; the absorption rate
increased, and the drug concentration accumulated. iii) Renal
insufficiency occurred during medication, which may have prolonged
the drug half-life and slowed down the excretion, thus further
increasing the content of morphine in the blood. This case reminds
us that it is very important to monitor the liver and kidney
function during treatment. This patient's condition was improved
after active treatment. The key to successful rescue lies in the
application of effective comprehensive measures, such as the
following: i) timely and rapid treatment, such as immediate
endotracheal intubation and ventilation to assist breathing,
through the most direct way to effectively supply oxygen and expel
excess CO2 from the body; ii) adequate intravenous
infusion to dilute the effective concentration of absorbed toxic
drugs in the blood and promote excretion; iii) timely
administration of naloxone to block and replace the binding of
morphine to opioid receptors, quickly reversing the toxic state,
promoting the recovery of spontaneous respiration, protecting the
stability of cell membranes, antagonizing the production of
inflammatory mediators, and reducing brain edema; and iv) timely
administration of flumazenil to block the effect of benzodiazepines
on the central nervous system and promoting awakening. Further,
flumazenil and naloxone have a synergistic effect and can
accelerate awakening.
In conclusion, for cancer pain, routine screening,
standardized evaluation, and effective pain control should be
performed, the indications and contraindications of drugs should be
strictly grasped, omni-directional and whole-process management
should be emphasized, and patients and their families should be
well educated. For patients with high abdominal tumor load,
physicians should consider the effect of the tumor on
gastrointestinal peristalsis and drug absorption, and if necessary,
when morphine is used in combination with benzodiazepines,
attention should be paid to the interaction between drugs,
resulting in enhanced respiratory depression. In addition, the
effect of patients' liver and kidney function on drug metabolism
and excretion should be fully evaluated before treatment, and the
dynamic changes of patients' liver and kidney function should be
monitored during treatment to adjust the dose in time. For this
kind of patient, clinicians should consider reducing the drug dose
at initial administration in order to avoid drug poisoning. At the
same time, in the process of drug treatment, the general state and
vital signs of patients should be monitored in time. If the patient
displays poisoning symptoms, timely measures should be taken to
reduce poison absorption, increase poison excretion, and administer
antagonists to reverse the poisoning state, so as to reduce the
damage caused by drug poisoning. Due to opioid dosage ranges
widely, varies widely between individuals and patients using
morphine and estazolam are both within the safe range, the
relationship between dosages of opioid and Estazolam cannot be well
discussed. Moreover, studies of the two drugs are rarely reported.
We look forward to more studies reporting on the interaction
between the two drug classes in the future.
Acknowledgements
Not applicable.
Funding
Funding: Funding support was provided by the Key R&D Project
of Hebei Province (grant no. 19277736D; Shijiazhuang, China).
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
Authors' contributions
CZ, JB and FZ collected the patient data, reviewed
relevant literature and wrote the original draft. XL and FZ
conceived and designed the study, and suggested revisions to the
manuscript. SJ, XZ, DG, DL, YW and SG reviewed the relevant
literature, analyzed the patient data, proposed manuscript
revisions and wrote the final version of the manuscript. All
authors contributed to the article, and read and approved the final
manuscript. CZ and FZ confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed written consent was obtained from
the patient for the publication of this case report and the
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
van den Beuken-Van Everdingen MH,
Hochstenbach LM, Joosten EA, Tjan-Heijnen VC and Janssen DJ: Update
on prevalence of pain in patients with cancer: Systematic review
and meta-analysis. J Pain Symptom Manage. 51:1070–1090.e9.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
WHO Guidelines for the Pharmacological and
Radiotherapeutic Management of Cancer Pain in Adults and
Adolescents. Geneva: World Health Organization; 2018.
|
|
4
|
Trescot AM, Datta S, Lee M and Hansen H:
Opioid pharmacology. Pain Phys. 11(2 Suppl):S133–S153.
2008.PubMed/NCBI
|
|
5
|
Stein C: New concepts in opioid analgesia.
Expert Opin Investig Drugs. 27:765–775. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mccleane G and Smith HS: Opioids for
persistent noncancer pain. Med Clin North Am. 91:177–197.
2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Stein C: Opioid receptors. Annu Rev Med.
67:433–451. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Stein C and Machelska H: Modulation of
peripheral sensory neurons by the immune system: Implications for
pain therapy. Pharmacol Rev. 63:860–881. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Roeckel LA, Utard V, Reiss D, Mouheiche J,
Maurin H, Robé A, Audouard E, Wood JN, Goumon Y, Simonin F and
Gaveriaux-Ruff C: Morphine-induced hyperalgesia involves mu opioid
receptors and the metabolite morphine-3-glucuronide. Sci Rep.
7(10406)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dhaliwal A and Gupta M: Physiology, Opioid
Receptor. In: StatPearls [Internet]. Treasure Island (FL):
StatPearls Publishing;2022.
|
|
11
|
Millan MJ: Kappa-opioid receptors and
analgesia. Trends Pharmacol Sci. 11:70–76. 1990.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Farmer AD, Holt CB, Downes TJ, Ruggeri E,
Del Vecchio S and De Giorgio R: Pathophysiology, diagnosis, and
management of opioid-induced constipation. Lancet Gastroenterol
Hepatol. 3:203–212. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Al-Hasani R and Bruchas MR: Molecular
mechanisms of opioid receptor-dependent signaling and behavior.
Anesthesiology. 115:1363–1381. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Karahan S, Karagöz H, Erden A, Avcı D and
Esmeray K: Codeine-induced syndrome of inappropriate antidiuretic
hormone: Case report. Balkan Med J. 31:107–109. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lötsch J and Geisslinger G:
Morphine-6-glucuronide: An analgesic of the future? Clin
Pharmacokinet. 40:485–499. 2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hasselström J and Säwe J: Morphine
pharmacokinetics and metabolism in humans. Enterohepatic cycling
and relative contribution of metabolites to active opioid
concentrations. Clin Pharmacokinet. 24:344–354. 1993.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Smith MT: Neuroexcitatory effects of
morphine and hydromorphone: Evidence implicating the 3-glucuronide
metabolites. Clin Exp Pharmacol Physiol. 27:524–528.
2000.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kubica J, Kubica A, Jilma B, Adamski P,
Hobl EL, Navarese EP, Siller-Matula JM, Dąbrowska A, Fabiszak T,
Koziński M and Gurbel PA: Impact of morphine on antiplatelet
effects of oral P2Y12 receptor inhibitors. Int J Cardiol.
215:201–208. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fine A and Churchill DN: Potentially
lethal interaction of cimetidine and morphine. Can Med Assoc J.
124:1434–1436. 1981.PubMed/NCBI
|
|
20
|
Mojaverian P, Fedder IL, Vlasses PH,
Rotmensch HH, Rocci ML Jr, Swanson BN and Ferguson RK: Cimetidine
does not alter morphine disposition in man. Br J Clin Pharmacol.
14:809–813. 1982.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shirooie S, Sahebgharani M, Esmaeili J and
Dehpour AR: In vitro evaluation of effects of metformin on morphine
and methadone tolerance through mammalian target of rapamycin
signaling pathway. J Cell Physiol. 234:3058–3066. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Okura T, Morita Y, Ito Y, Kagawa Y and
Yamada S: Effects of quinidine on antinociception and
pharmacokinetics of morphine in rats. J Pharm Pharmacol.
61:593–597. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Miyazaki M, Kawase T, Nishimura C,
Kitamura T, Iwanaga K and Kakemi M: Pharmacokinetics and toxicity
of repeated oral etoposide is altered by morphine coadministration
in rats. Eur J Drug Metab Pharmacokinet. 40:335–341.
2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Manara AR, Shelly MP, Quinn K and Park GR:
The effect of metoclopramide on the absorption of oral controlled
release morphine. Br J Clin Pharmacol. 25:518–521. 1988.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Rocci ML Jr, Mojaverian P and Saccar CL:
Morphine inhibition of theophylline clearance. Pharm Res.
1:231–233. 1984.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lopez F, Miller LG, Thompson ML, Schatzki
A, Chesley S, Greenblatt DJ and Shader RI: Chronic morphine
administration augments benzodiazepine binding and GABAA receptor
function. Psychopharmacol (Berl). 101:545–549. 1990.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kienitz R, Kay L, Beuchat I, Gelhard S,
von Brauchitsch S, Mann C, Lucaciu A, Schäfer JH, Siebenbrodt K,
Zöllner JP, et al: Benzodiazepines in the management of seizures
and status epilepticus: A review of routes of delivery,
pharmacokinetics, efficacy, and tolerability. CNS Drugs.
36:951–975. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mancinelli A, Guiso G, Garattini S, Urso R
and Caccia S: Kinetic and pharmacological studies on estazolam in
mice and man. Xenobiotica. 15:257–265. 1985.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pierce MW and Shu VS: Efficacy of
estazolam. The United States clinical experience. Am J Med.
88(3A):6S–11S. 1990.PubMed/NCBI View Article : Google Scholar
|
|
30
|
France CP, Ahern GP, Averick S, Disney A,
Enright HA, Esmaeli-Azad B, Federico A, Gerak LR, Husbands SM,
Kolber B, et al: Countermeasures for preventing and treating opioid
overdose. Clin Pharmacol Ther. 109:578–590. 2021.PubMed/NCBI View
Article : Google Scholar
|