Introduction
Hepatitis B virus (HBV) infection is a worldwide
epidemic. According to World Health Organization, there are ~257
million chronic HBV infections worldwide. China is a moderately
endemic country and there are currently ~70 million cases of
chronic HBV infection, including 20-30 million cases of chronic
hepatitis B (CHB), a notable proportion of which is accompanied by
liver fibrosis (1). Liver fibrosis
progresses to cirrhosis in 25-40% of patients if they do not
receive timely treatment. Globally, ~887,000 people died from HBV
infection-associated diseases in 2015, with cirrhosis and primary
hepatocellular carcinoma (HCC) accounting for 52 and 38% of deaths,
respectively. Hepatic fibrosis is a pathological process of
excessive deposition of diffuse extracellular matrix (ECM) in
hepatocytes during the injury repair process, and is a common
occurrence in the progression of various chronic hepatic disease to
cirrhosis (2). Liver fibrosis is a
dynamic and reversible process, whereas progression to intermediate
to advanced cirrhosis is considered irreversible. Therefore, early
intervention decreases risk of progression to end-stage liver
disease (3,4). Although almost all types of chronic
liver diseases (chemically toxic, infectious, genetic/metabolic,
autoimmune) can lead to liver fibrosis, the mechanisms of liver
fibrosis are still not fully understood (5,6). The
successful treatment of liver fibrosis remains unsatisfactory with
cytokine analogs, antioxidants and other drugs (7-11).
Investigation of the mechanism of liver fibrosis and
screening the targets for anti-fibrotic therapy are key in liver
disease research. Cyclooxygenase-2 (COX-2) inhibitor aspirin not
only has anti-inflammatory properties, but also decreases tissue
fibrosis via multiple pathways; studies have shown that aspirin can
exert anti-pulmonary fibrosis effects by inhibiting expression of
TGF-β1, TNF-α and IL-4 (12,13).
A large case-review study in the United States showed that aspirin
significantly decreased liver fibrosis index in adults (14). Another multicenter retrospective
study found that low-dose aspirin treatment may be associated with
a lower risk of liver fibrosis progression in patients with
hepatitis C virus (HCV) recurrence after liver transplantation
(15). Yoshida et al
(16) showed that platelets can
promote liver fibrosis through direct activation of hepatic
stellate cells in mice. Platelets are a promising target for
antifibrotic therapy. Platelet activation and degranulation are
important events of the physiological response to tissue injury,
which activates wound closure and repair (17). A previous study (18) showed that platelets undergo
significant qualitative and quantitative changes when liver
fibrosis occurs and confirmed that liver fibrosis is associated
with platelet alteration.
To the best of our knowledge, only Sun et al
(6) have reported the effect of
aspirin on liver fibrosis via inhibition of the TGFβ1/SMAD pathway.
Aspirin has become one of the most commonly used drugs, given its
role as an analgesic, antipyretic and agent for cardiovascular
prophylaxis (19,20). More studies are needed to determine
the protective effect of aspirin in liver fibrosis models and its
potential mechanisms (6). Here, to
explore the effect of aspirin on liver fibrosis, a hepatic fibrosis
rat model induced by CCl4 was used and evaluated
following aspirin treatment. In addition, the present study further
investigated whether aspirin attenuated hepatic fibrosis via the
TGFβ1 signaling pathway and the effect of aspirin on the
pro-inflammatory cytokine IL-1β, thus revealing the potential
molecular and inflammatory mechanisms underlying the protective
effect of aspirin on hepatic fibrosis.
Materials and methods
Chemicals
CCl4 was obtained from Jiangxi Gang
Instrument Technology Co., Ltd. Aspirin enteric-coated tablets were
purchased from Bayer Medical Health Co., Ltd. and prepared as a
suspension with saline. Olive oil was purchased from the local
market, sealed and stored at room temperature following high
temperature sterilization.
Animals
A total of 32 SPF male Sprague-Dawley rats (age, 6-8
weeks, mean weight, 250±20 g) were obtained from Jiangxi University
of Traditional Chinese Medicine (Jiangxi, China), with license SCXK
(Gan) 2018-0003. The rats were divided into groups (n=8/group) as
follows: Healthy and CCl4 control and low- and high-dose
aspirin group. The rats were housed under normal laboratory
conditions (21±2˚C, 12/12-h light/dark cycle, humidity 50-60%) with
free access to standard pellet diet and water. Body weight and
behavior of all animals were monitored every two days. Humane
endpoints were >20% weight loss, dehydration and loss of
locomotion. No animals reached the humane endpoints. Blood was
taken 1 h after the last administration. After the blood was taken,
the rats were euthanized by cervical dislocation and liver
specimens were collected immediately. Death was confirmed by
evaluating vital signs, including heartbeat, pupillary response and
respiratory pattern (lack of cardiac activity for 5 min through
cardiac palpation, unresponsiveness to light with dilated pupils
and lack of spontaneous breathing with a shallow and irregular
breathing pattern). Animal experiments were approved by the Animal
Ethics Committee of the Second Affiliated Hospital of Nanchang
University (Nanchang, China; approval no. 2017062). All efforts
were made to minimize suffering and reduce the number of animals
used.
Treatment
To induce hepatic fibrosis, animals in the
CCl4 control and low- and high-dose aspirin group were
subcutaneous (s.c.) administered 3 ml/kg body weight 40%
CCl4 (20% v/v CCl4 in olive oil) twice/week
for 8 weeks. The CCl4 control group was gavaged with
distilled water daily. The low- and high-dose aspirin group were
given 10 and 300 mg/kg aspirin suspension by gavage once daily for
8 weeks, respectively.
Serum biochemical analysis
The rats were fasted without water for 12 h, one
hour after the last administration, anesthetized by intraperitoneal
injection of 10% chloral hydrate (0.3 ml/100 g) and blood (3~4 ml)
was obtained from the abdominal aorta and centrifuged at 3,000 x g
for 10 min at 4˚C. After blood collection, the rats were euthanized
and liver specimens were collected immediately. Serum alanine
transaminase (ALT) and aspartate transaminase (AST) levels and
liver fiber indexes Hyaluronic acid (HA), laminin (LN) and type IV
collagen (IV.C) were measured at the Second Affiliated Hospital of
Nanchang University and Nuclear Medicine Department of Nanchang
University Hospital (Nanchang, China), respectively. Liver samples
were dissected and washed with ice-cold saline, then immediately
stored at -80˚C for further analysis. The largest right lobe of
each liver was excised and fixed in 4% formaldehyde solution for
24-48 h at room temperature for histopathological analysis.
Cytokine IL-1β measurement
Following centrifugation at 3,000 x g for 10 min at
4˚C, the supernatant was obtained and the IL-1β in the serum was
measured using specific anti-mouse ELISA from Elabscience
Biotechnology Co., Ltd. (cat. no. E-EL-M0037c). The kit was used
according to the manufacturer's instructions.
Protein quantification of TGF-β1
Liver tissue was lysed in RIPA lysis buffer (cat.
no. P0013B, Beyotime Institute of Biotechnology) and PMSF (cat. no.
P105539, Aladdin). Following grinding and centrifugation (4˚C,
12,000 x g, 20 min), the protein was extracted and the
concentration was measured using a BCA kit (cat. no. P0010,
Beyotime Institute of Biotechnology). Samples (30 µg/lane) were
separated by 12% SDS-PAGE and transferred onto polyvinylidene
difluoride membranes (cat. no. IPVH00010; Millipore). The membranes
were incubated in blocking buffer (5% skimmed milk powder) at room
temperature for 2 h prior to the addition of primary antibodies at
4˚C overnight. Primary antibodies were as follows: Anti-TGFβ
polyclonal (cat. no. Af1027; 1:1,000; Affinity Biosciences) and
β-actin monoclonal (cat. no. BM0627; 1:50; Wuhan Boster Biological
Technology Ltd.) as loading control. Peroxidase-conjugated goat
anti-rabbit (cat. no. BA1054) and anti-mouse secondary antibody
(cat. no. BA1051; both 1:10,000; both Boster Biological Technology
Ltd.) were incubated at room temperature for 2 h. Image-pro Plus
(IPP6.0; Media Cybernetics Corporation, USA) software was used to
analyze the gray value of each protein band.
Liver histopathology
Liver tissue was fixed in 4% formaldehyde for 24-48
h at room temperature, embedded in paraffin (58-60˚C, 10-30 sec)
and cut into 4-µm-thick sections. Paraffin sections were dewaxed,
stained with hematoxylin for 5 min at room temperature, dipped in
1% hydrochloric acid ethanol and returned to blue with 1% ammonia
for 2 min. Following rinsing with tap water, the sections were
stained with eosin for 1 min at room temperature. Sections were
dehydrated, made transparent, sealed and observed by light
microscopy at 100x magnification. In total, 10 fields of view were
randomly selected and observed from each section. For Masson's
staining. Paraffin sections were dewaxed with xylene, rehydrated in
gradient alcohol and washed with tap water for 1 min; oxidized with
potassium permanganate for 5 min, rinsed with distilled water;
bleached with 2% oxalic acid for 1-2 min, rinsed with distilled
water; stained with Ponceau staining for 5 min, rinsed with
distilled water; treated with phosphomolybdic acid for 5 min,
decanted to remove excess stain, stained with aniline blue for 5
min; the slices were treated with glacial acetic acid at 2% v/v for
1 min, quickly dehydrated, transparent with dimethylbenzene and
sealed with neutral gum. Fibrotic changes and collagen deposition
were observed under the microscope. Histopathological examination
of the liver was performed at the Department of Pathology, the
Ninth Hospital of Nanchang University (Nanchang, China). The degree
of liver injury and fibrosis was examined by specialized
pathologists blinded to the groups. Histopathological diagnosis,
grading of inflammatory necrosis of liver tissue and staging of the
degree of fibrosis were performed according to the pathological
diagnostic criteria in the grading criteria for chronic hepatitis
(21). Inflammatory necrosis was
graded as G0-G4, with G0-1 being mild inflammation, G2 being
moderate inflammation and G3-4 being severe inflammation; the
degree of fibrosis was graded as S0-S4, with S0-1 being mild
fibrosis, S2 being moderate fibrosis and S3-4 being severe
fibrosis.
Statistical analysis
All experiments were repeated three times. Data were
analyzed using IBM SPSS software version 25.0 (IBM Corp.). Data are
presented as the mean ± SD. Data normality was assessed by the
Shapiro-Wilk test. One-way ANOVA followed by post hoc Bonferroni's
correction was used to compare >2 groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Histopathological features suggest
aspirin attenuates CCl4-induced liver fibrosis and
inflammation
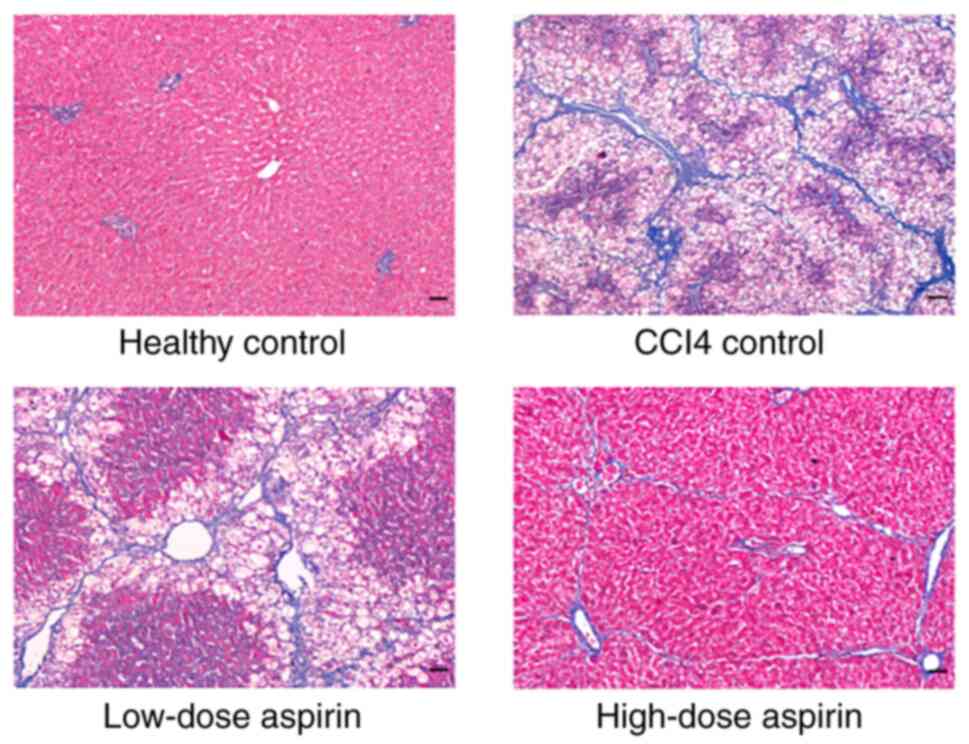
Hematoxylin-eosin staining for the healthy control
group showed normal architecture. However, large area steatosis was
observed in the liver tissue of rats in the CCl4 control
group and extensive infiltration of inflammatory cells was observed
in the portal area, showing spot, focal and clastic necrosis.
Fusion necrosis was observed, most of the nuclei disappeared and
fibrous hyperplasia was obvious. The low- and the high-dose aspirin
group exhibited improved liver morphology and structure with fewer
false lobes and inflammatory cell infiltrates compared with the
CCl4 control group. The high-dose aspirin group showed
better improvement than the low-dose aspirin group (Fig. 1). Masson's staining for the healthy
control group showed a small number of small blue collagen fibers
around the sink area and no fiber proliferation was observed.
However, in the CCl4 control group, fibrosis was notable
around the sink area, with a large number of collagen fibers and
fibrous septum formation. The hepatic lobules were divided into
false lobules of different sizes. Collagen fibrous hyperplasia of
liver tissue was notably decreased in the low-dose aspirin group
and high-dose aspirin group; this was more pronounced in the
high-dose aspirin group (Fig.
2).
Aspirin attenuates liver inflammation
indicators in a CCl4-induced liver fibrosis model in
rats
Compared with the healthy control group, serum
concentrations of ALT and AST were significantly increased in
CCl4 control group (Table
I). The high-dose aspirin group significantly attenuated the
increase of ALT and AST.
 | Table IEffects of aspirin on serum
concentrations of ALT and AST. |
Table I
Effects of aspirin on serum
concentrations of ALT and AST.
| Group | ALT, U/l | AST, U/l |
|---|
| Healthy
control | 33.51±2.15 | 162.62±40.22 |
| CCl4
control |
176.71±46.41a |
284.44±71.09a |
| Low-dose aspirin
(10 mg/kg + CCL4) | 145.92±19.55 | 208.86±50.21 |
| High-dose aspirin
(300 mg/kg + CCL4) |
55.61±19.33b,c |
182.11±44.90b |
Aspirin attenuates levels of
inflammatory cytokine IL-1β in CCl4-induced rat liver
fibrosis model
Compared with the healthy control group,
CCl4-induced toxicity caused a significant increase in
IL-1β levels. The high-dose aspirin group significantly attenuated
the increase of IL-1β (Fig.
3).
Aspirin decreases serum liver fibrosis
index levels in a CCl4-induced liver fibrosis model in
rats
Serum liver fibrosis index (HA and LN) levels were
significantly higher in the CCl4 control group compared
with the healthy control group (Table
II). Compared with the CCl4 control, the low-dose
and high-dose aspirin group significantly attenuated the increase
in HA, high-dose aspirin group significantly attenuated the
increase in LN, and the low-dose aspirin group significantly
attenuated the increase in IV.C. IV.C levels were almost
unmeasurable in the healthy control and high-dose aspirin groups.
High-dose aspirin group significantly attenuated the increase of LN
compared with the low-dose aspirin group.
 | Table IIEffect of aspirin on
CCl4-induced changes in serum indices for hepatic
fibrosis. |
Table II
Effect of aspirin on
CCl4-induced changes in serum indices for hepatic
fibrosis.
| Group | HA, ng/ml | LN, ng/ml | IV.C, ng/ml |
|---|
| Healthy
control | 112.23±30.88 | 38.93±18.09 | - |
| CCl4
control |
242.62±45.21a |
93.30±10.20a | 10.57±5.45 |
| Low-dose aspirin
(10 mg/kg + CCL4) |
154.99±17.91c |
82.11±12.13a |
5.01±3.79b |
| High-dose aspirin
(300 mg/kg + CCL4) |
146.36±21.43c |
50.97±11.75c,d | - |
Effect of aspirin on stage of liver
fibrosis in a CCl4-induced liver fibrosis model in
rats
Compared with the CCl4 control group,
aspirin intervention resulted in a decrease in liver fibrosis stage
S3 and an increase in stage S2 (Table III). However, there was no
significant difference between groups.
 | Table IIIEffect of aspirin on the staging of
liver fibrosis in rats. |
Table III
Effect of aspirin on the staging of
liver fibrosis in rats.
| Group | S0 | S1 | S2 | S3 | S4 |
|---|
| Healthy
control | 7 | 1 | 0 | 0 | 0 |
| CCl4
control | 0 | 0 | 1 | 6 | 1 |
| Low-dose (aspirin
10 mg/kg + CCL4) | 0 | 0 | 2 | 5 | 1 |
| High-dose aspirin
(300 mg/kg + CCL4) | 0 | 0 | 4 | 3 | 1 |
Aspirin decreases expression of TGFβ-1
protein in a CCl4-induced liver fibrosis model in
rats
Compared with the healthy control group, the TGFβ-1
protein levels in liver tissue were significantly increased in the
CCl4 control group (Fig.
4). TGFβ-1 protein expression level was significantly lower in
the high-dose aspirin group compared with the CCl4
control group.
Discussion
Liver fibrosis is characterized by progressive
accumulation of extracellular matrix (ECM), which destroys the
physiological architecture of the liver (22). Correlating with liver disease
progression, fibrosis is a key factor for liver disease outcome and
risk of hepatocellular carcinoma (HCC). Currently, there are no
safe and effective drugs for the treatment of liver cirrhosis.
Effective therapy to block or reverse liver fibrosis at an early
stage would improve treatment of liver fibrosis in the clinic
(23). The present study revealed
that aspirin attenuated liver fibrosis by suppressing TGF-β1
signaling and pro-inflammatory cytokine IL-1β.
CCl4 is a typical liver toxin that can
destroy hepatocyte function and induce lipid peroxidation to
destroy membrane structure and damage hepatocytes. Liver fibrosis
model established by with CCl4 is simple, inexpensive,
typical of lesions and widely used (24-27).
CCl4 destroys the hepatocyte membrane, thus causing
intracellular ALT and AST to leak into the blood; activity of ALT
and AST in the serum indicates the degree of liver damage, which is
a sensitive indicator of hepatocyte damage (28). In the present study, the serum ALT,
AST levels increased markedly after CCl4 administration,
but these increases were attenuated by aspirin. IL-1β is a
pleiotropic cytokine that exerts a range of inflammatory and
immunomodulatory effects and is involved in a host responses to
inflammation, immune regulation, tumor progression and microbial
invasion (29) and is synthesized
by a variety of cells, including macrophages, monocytes, and T,
natural killer and endothelial cells (30,31).
Serum IL-1β levels are significantly higher in patients with
chronic hepatitis B than in normal controls (32). Here, IL-1β expression in the
CCl4 control group was significantly higher than that in
the healthy control; the increase in IL-1β in the aspirin
intervention groups was lower than that in the CCl4
control group, especially in the high-dose aspirin group. All of
these results suggest that aspirin protected hepatocytes and
inhibited inflammatory damage in the liver. These phenomena were
also confirmed by histological observation.
The key to development of liver fibrosis is the
activation of hepatic stellate cells (HSC) and excessive deposition
of ECM. The activated HSCs proliferate and transform into
myofibroblasts, which produce large amounts of ECM (33). Serum indexes of liver fibrosis (HA,
LN, type III procollagen and IV.C) are associated with the degree
of liver damage and liver fibrosis. HA is the simplest proteoglycan
and important component of ECM; its levels reflect the function of
endothelial cells and the degree of cirrhosis (34). LN is a non-collagenous structural
glycoprotein; when liver fibrosis occurs, LN is deposited in the
liver sinusoids and released into the blood, which increases LN
content in serum (35). Here,
aspirin could inhibit the increase of HA and LN levels in the serum
of liver fibrosis rats. Serum levels of HA and LN in the high-dose
aspirin intervention group were significantly lower than those in
the CCl4 control group. In addition, IV.C levels were
significantly decreased in the low-dose aspirin group compared with
the CCl4 control. IV.C levels were almost unmeasurable
in the healthy control and high-dose aspirin groups. The
aforementioned results are consistent with the formation process of
liver fibrosis, which is dominated by elevated HA and LN secretion
in the early stages; in the late stage of liver fibrosis, the
secretion of PC III and IVC increases (36). TGFβ-1 is a key cytokine that
stimulates activation of HSCs; it also upregulates the expression
of α-smooth muscle actin and increase ECM deposition (37-39).
TGFβ-1 activates signaling pathways involved in the development of
liver fibrosis, such as SMAD, PI3K and MAPK signaling pathways,
which regulate activation, migration and apoptosis of HSCs
(40-42).
Here, aspirin significantly inhibited the elevation in TGFβ-1
protein levels following CCl4 administration, suggesting
that aspirin protected against CCl4-induced hepatic
fibrosis in rats.
The mechanisms of fibrosis occurring in each organ
are similar and specific; several studies have shown that aspirin
has an anti-pulmonary fibrosis effect (12,13).
To the best of our knowledge, the present study is the first to
demonstrate that aspirin has beneficial hepatoprotective effects in
CCl4-induced liver fibrosis primarily via inhibition of
the TGF-β-1 pathway and pro-inflammatory cytokines IL-1β. In
addition, COX-2 serves an important role in the progression of
liver fibrosis. Emerging evidence has suggested that COX-2 serves a
role in the development of fibrosis in the kidney, pancreas and
liver and that inhibition of COX-2 expression can have an
anti-fibrotic effect (43-45).
Therefore, the protective effect of aspirin against
CCl4-induced liver fibrosis in rats may also be mediated
by inhibition of COX-2. Further studies are required to determine
the specific mechanisms of COX-2 in the development of liver
fibrosis and the pathway involved in the regulation of TGF-β1.
Although further studies are required to elucidate its potential
clinical applications for hepatic fibrosis, the present study
demonstrated that aspirin may represent a promising new strategy
for the treatment of hepatic fibrosis.
The present study has limitations. TGFβ-1 activates
multiple signaling pathways involved in the development of liver
fibrosis, such as SMAD, PI3K and MAPK signaling pathways,
regulating the activation, proliferation, migration and apoptosis
of HSCs (46-48).
The present study provided preliminary evidence that aspirin
ameliorated liver fibrosis by inhibiting the TGFβ-1 pathway, but
its exact mechanism needs to be further explored. Second, aspirin
alleviated CCl4-induced liver fibrosis in rats. However,
there are other liver fibrosis models that may have different
underlying mechanisms. Third, the results showed that aspirin
alleviated CCl4-induced liver fibrosis via inhibition of
pro-inflammatory IL-1β. However, whether
NLRP3/Caspase-1/IL-1β/TGF-β1 signaling is involved in the
antifibrotic effects of aspirin should be further investigated.
Aspirin is a commonly used non-steroidal
anti-inflammatory drug with a range of applications, including
reducing fever, relieving pain and reducing inflammatory responses.
However, it is also used to prevent and treat cardiovascular
disease and even certain types of cancer (19,20).
Determining the protective effect of aspirin in patients with
hepatic fibrosis and its potential mechanism is key for patients
with liver, rheumatic and cardiovascular disease and cerebral
infarction. With further research, aspirin may be used to treat
liver disease in future. Therefore, future studies should
investigate the association between aspirin dose and the protective
effect against CCl4-induced hepatic fibrosis in rats and
patients and the potential mechanism of aspirin against hepatic
fibrosis.
Acknowledgements
The authors would like to thank Dr Zhang Ping (Ninth
Hospital of Nanchang) for assessing liver histology.
Funding
Funding: The present study was supported by the Natural Science
Foundation of Jiangxi province, China (grant no. 20192BAB205014)
and Jiangxi Provincial Health Commission Traditional Chinese
Medicine Science and Technology Project, China (grant no.
2016A109).
Availability of data and materials
The datasets used during the current study are
available from the corresponding author upon reasonable
request.
Authors' contributions
ZW, YW, SSu and BX conceived and designed the study.
WZ, XL, FQ and ZS performed animal experiments. QZ performed
Hematoxylin-eosin and Masson's staining. DJ and DY performed
western blot analysis. SR and SSo performed serum biochemical
analysis. MA, YL and ZG collected and analyzed the data. ZW and YW
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The animal study was reviewed and approved by the
Animal Ethics Committee of the Second Affiliated Hospital of
Nanchang University (Nanchang, China; approval no. 2017062).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu J, Liang W, Jing W and Liu M:
Countdown to 2030: Eliminating hepatitis B disease, China. Bull
World Health Organ. 97:230–238. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mormone E, George J and Nieto N: Molecular
pathogenesis of hepatic fibrosis and current therapeutic
approaches. Chem Biol Interact. 193:225–231. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Czaja AJ: Review article: The prevention
and reversal of hepatic fibrosis in autoimmune hepatitis. Aliment
Pharmacol Ther. 39:385–406. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Iredale J: Defining therapeutic targets
for liver fibrosis: Exploiting the biology of inflammation and
repair. Pharmacol Res. 58:129–136. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lee NY and Suk KT: The role of the gut
microbiome in liver cirrhosis treatment. Int J Mol Sci.
22(199)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sun Y, Liu B, Xie J, Jiang X, Xiao B, Hu X
and Xiang J: Aspirin attenuates liver fibrosis by suppressing
TGF-β1/Smad signaling. Mol Med Rep. 25(181)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhu H, Zhao H, Xu S, Zhang Y, Ding Y, Li
J, Huang C and Ma T: Sennoside A alleviates inflammatory responses
by inhibiting the hypermethylation of SOCS1 in
CCl4-induced liver fibrosis. Pharmacol Res.
174(105926)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fagone P, Mangano K, Pesce A, Portale TR,
Puleo S and Nicoletti F: Emerging therapeutic targets for the
treatment of hepatic fibrosis. Drug Discov Today. 21:369–375.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Adrian JE, Poelstra K, Scherphof GL,
Meijer DK, van Loenen-Weemaes AM, Reker-Smit C, Morselt HW, Zwiers
P and Kamps JA: Effects of a new bioactive lipid-based drug carrier
on cultured hepatic stellate cells and liver fibrosis in bile
duct-ligated rats. J Pharmacol Exp Ther. 321:536–543.
2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Prud'homme GJ: Pathobiology of
transforming growth factor beta in cancer, fibrosis and immunologic
disease, and therapeutic considerations. Lab Invest. 87:1077–1091.
2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Song M, Song Z, Barve S, Zhang J, Chen T,
Liu M, Arteel GE, Brewer GJ and McClain CJ: Tetrathiomolybdate
protects against bile duct ligation-induced cholestatic liver
injury and fibrosis. J Pharmacol Exp Ther. 325:409–416.
2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tao Z, Yuan Y, Gu JY, Shi DW, Yao CJ, Tong
CY, Shen H, Xue MM, Song Z and Cai YY: Inhibitory effect and
mechanism of aspirinin in the treatment of bleomycin-induced
pulmonary fibrosis in rats. Fudan Univ J Med Sci. 40:395–399.
2013.
|
|
13
|
Guilherme RF, Xisto DG, Kunkel SL,
Freire-de-Lima CG, Rocco PR, Neves JS, Fierro IM, Canetti C and
Benjamim CF: Pulmonary antifibrotic mechanisms aspirin-triggered
lipoxin A(4) synthetic analog. Am J Respir Cell Mol Biol.
49:1029–1037. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jiang ZG, Feldbrügge L, Tapper EB, Popov
Y, Ghaziani T, Afdhal N, Robson SC and Mukamal KJ: Aspirin use is
associated with lower indices of liver fibrosis among adults in the
United States. Aliment Pharmacol Ther. 43:734–743. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Poujol-Robert A, Boëlle PY, Conti F,
Durand F, Duvoux C, Wendum D, Paradis V, Mackiewicz V,
Chazouillères O, Corpechot C and Poupon R: Aspirin may reduce liver
fibrosis progression: Evidence from a multicenter retrospective
study of recurrent hepatitis C after liver transplantation. Clin
Res Hepatol Gastroenterol. 38:570–576. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yoshida S, Ikenaga N, Liu SB, Peng ZW,
Chung J, Sverdlov DY, Miyamoto M, Kim YO, Ogawa S, Arch RH, et al:
Extrahepatic platelet-derived growth factor-β, delivered by
platelets, promotes activation of hepatic stellate cells and
biliary fibrosis in mice. Gastroenterology. 147:1378–1392.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gawaz M and Vogel S: Platelets in tissue
repair: Control of apoptosis and interactions with regenerative
cells. Blood. 122:2550–2554. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Qing P, Feng T and Chang L: The
relationship between platelets and liver fibrosis. J Pract Hepatol.
14:315–317. 2011.
|
|
19
|
Drew DA, Cao Y and Chan AT: Aspirin and
colorectal cancer: The promise of precision chemoprevention. Nat
Rev Cancer. 16:173–186. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hua H, Zhang H, Kong Q, Wang J and Jiang
Y: Complex roles of the old drug aspirin in cancer chemoprevention
and therapy. Med Res Rev. 39:114–145. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sun Y, Zhou J, Wang L, Wu X, Chen Y, Piao
H, Lu L, Jiang W, Xu Y, Feng B, et al: New classification of liver
biopsy assessment for fibrosis in chronic hepatitis B patients
before and after treatment. Hepatology. 65:1438–1450.
2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Iredale JP: Models of liver fibrosis:
Exploring the dynamic nature of inflammation and repair in a solid
organ. J Clin Invest. 117:539–548. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Zhang W, Yi Z, Ye CG, Liu CY, Sun SL, Li
JM and Xi WN: Interferon α-2a reduces carbon tetrachloride-induced
hepatic fibrosis in rats. World Chin J Dig. 19:3207–3211. 2011.
|
|
24
|
lv Y, Wu S, Wang Z and Ye X: Research
progress of modeling methods for animal models of liver fibrosis.
Guangxi Med J. 42:875–878. 2020.
|
|
25
|
Mu M, Zuo S, Wu RM, Deng KS, Lu S, Zhu JJ,
Zou GL, Yang J, Cheng ML and Zhao XK: Ferulic acid attenuates liver
fibrosis and hepatic stellate cell activation via inhibition of
TGF-β/Smad signaling pathway. Drug Des Devel Ther. 12:4107–4115.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li J, Wang Y, Ma M, Jiang S, Zhang X,
Zhang Y, Yang X, Xu C, Tian G, Li Q, et al: Autocrine CTHRC1
activates hepatic stellate cells and promotes liver fibrosis by
activating TGF-β signaling. EBioMedicine. 40:43–55. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen X, Ying X, Zhang W, Chen Y, Shi C,
Hou Y and Zhang Y: The hepatoprotective effect of fraxetin on
carbon tetrachloride induced hepatic fibrosis by antioxidative
activities in rats. Int Immunopharmacol. 17:543–547.
2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Parsons CJ, Takashima M and Rippe RA:
Molecular mechanisms of hepatic fibrogenesis. J Gastroenterol
Hepatol. 22 (Suppl 1):S79–S84. 2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Oberholzer A, Oberholzer C and Moldawer
LL: Cytokine signaling-regulation of the immune response in normal
and critically ill states. Crit Care Med. 28 (4 Suppl):N3–N12.
2000.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wewers MD, Dare HA, Winnard AV, Parker JM
and Miller DK: IL-1 beta-converting enzyme (ICE) is present and
functional in human alveolar macrophages: Macrophage IL-1 beta
release limitation is ICE independent. J Immunol. 159:5964–5972.
1997.PubMed/NCBI
|
|
31
|
Dinarello CA: Biologic basis for
interleukin-1 in disease. Blood. 87:2095–2147. 1996.PubMed/NCBI
|
|
32
|
Xu W, Zhang G and Wang H: Detection and
significance of IL-1β, mIL-2R and IL-10 in peripheral blood of
patients with chronic hepatitis B. Chin J Nosocomiol. 19:742–744.
2009.
|
|
33
|
Zhang H, Zhang J and Deng W: Experimental
study of curcumin against hepatic fibrosis in schistosomiasis and
its mechanism. Chin Tradit Herbal Drugs. 40:1274–1277. 2009.
|
|
34
|
Mehta P, Ploutz-Snyder R, Nandi J, Rawlins
SR, Sanderson SO and Levine RA: Diagnostic accuracy of serum
hyaluronic acid, FIBROSpect II, and YKL-40 for discriminating
fibrosis stages in chronic hepatitis C. Am J Gastroenterol.
103:928–936. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nallagangula KS, Nagaraj SK, Venkataswamy
L and Chandrappa M: Liver fibrosis: A compilation on the biomarkers
status and their significance during disease progression. Future
Sci OA. 4(FSO250)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang H, Hui X, Li W, Xu Y, Fan H and Du D:
The relationship between serum level of PC III and the liver
fibrosis activity. J Mod Lab Med. 21:68–70. 2006.
|
|
37
|
Gressner AM, Weiskirchen R, Breitkopf K
and Dooley S: Roles of TGF-beta in hepatic fibrosis. Front Biosci.
7:d793–d807. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
38
|
De Minicis S, Seki E, Uchinami H, Kluwe J,
Zhang Y, Brenner DA and Schwabe RF: Gene expression profiles during
hepatic stellate cell activation in culture and in vivo.
Gastroenterology. 132:1937–1946. 2007.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Seki E, De Minicis S, Osterreicher CH,
Kluwe J, Osawa Y, Brenner DA and Schwabe RF: TLR4 enhances TGF-beta
signaling and hepatic fibrosis. Nat Med. 13:1324–1332.
2007.PubMed/NCBI View
Article : Google Scholar
|
|
40
|
Meng XM, Tang PM, Li J and Lan HY:
TGF-β/Smad signaling in renal fibrosis. Front Physiol.
6(82)2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wang Y, Jiang XY, Liu L and Jiang HQ:
Phosphatidylinositol 3-kinase/Akt pathway regulates hepatic
stellate cell apoptosis. World J Gastroenterol. 14:5186–5191.
2008.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gäbele E, Reif S, Tsukada S, Bataller R,
Yata Y, Morris T, Schrum LW, Brenner DA and Rippe RA: The role of
p70S6K in hepatic stellate cell collagen gene expression and cell
proliferation. J Biol Chem. 280:13374–13382. 2005.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yang H, Xuefeng Y, Shandong W and Jianhua
X: COX-2 in liver fibrosis. Clin Chim Acta. 506:196–203.
2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wei J, Deng X, Li Y, Li R, Yang Z, Li X,
Song S, Shi Y, Duan H and Wu H: PP2 ameliorates renal fibrosis by
regulating the NF-κB/COX-2 and PPARγ/UCP2 pathway in diabetic mice.
Oxid Med Cell Longev. 2021(7394344)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Xu XF, Fan JW, Xin JQ, Wu N, Gao H, Duan
LF, Zou WB, Zhang H and Li ZS: Aspirin ameliorates pancreatic
inflammation and fibrosis by inhibiting COX-2 expression in
experimental chronic pancreatitis. J Inflamm Res. 15:4737–4749.
2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Jiang N, Zhang J, Ping J and Xu L:
Salvianolic acid B inhibits autophagy and activation of hepatic
stellate cells induced by TGF-β1 by downregulating the MAPK
pathway. Front Pharmacol. 13(938856)2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zhao Y, Liu X, Ding C, Zheng Y, Zhu H,
Cheng Z, Zhao C and Liu W: Aronia melanocarpa polysaccharide
ameliorates liver fibrosis through TGF-β1-mediated the activation
of PI3K/AKT pathway and modulating gut microbiota. J Pharmacol Sci.
150:289–300. 2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Shang X and Li X: Advances in the
experimental research on traditional Chinese medicine against liver
fibrosis. J Clin Hepatol. 39:249–259. 2023.
|