Introduction
Osteoarthritis (OA) is a chronic, debilitating, and
degenerative joint disease, that affects ~250 million individuals
worldwide (1). Chronic pain is the
primary symptom of OA, and it reduces a patient's quality of life,
and is an important factor in the management of OA (2). Common pharmacological treatments for
OA are acetaminophen, non-steroidal anti-inflammatory drugs, and
opioids (3). Due to the uncertain
efficacies and overall safety of these agents, OA pain management
remains largely inadequate, it is becoming a major public health
concern (4). Therefore,
illustrating the mechanisms of OA pain may be useful for developing
novel treatments for the management of OA pain.
Joint destruction and disability trigger numerous
pain-producing agents and stimulate nociceptive signals, which are
transmitted to the spinal dorsal horn and brain, and finally cause
chronic pain (5). During this
process, the spinal cord undergoes central sensitization, resulting
in increased excitability and synaptic efficacy of neurons and
activation of glial cells (6). The
prevalence of central sensitization is observed in 35.3% of 150
inflammatory arthritis patients (7). Lower pain thresholds and punctate
hyperalgesia in the area of concern are thus often observed in
patients with OA (8). Patients
with advanced OA often experience widespread pain at the OA joint
and even in the whole leg (9). In
an OA animal model, mechanical hyperalgesia and allodynia are also
observed (5). Activated glial
cells, especially astrocytes, undergo morphological changes,
activating a neuroinflammatory response via the release of a
variety of pro-inflammatory cytokines, stimulating nociceptive
synaptic transmission, modulating pain signaling, and regulating
pain maintenance (10). In spinal
cord injury patients who experience neuropathic pain, the levels of
metabolites that regulate neuroinflammation are elevated based on
magnetic resonance spectroscopy (11). In patients suffering from a common
chronic pain disorder (lumbar radiculopathy), the levels of the
neuroinflammation marker 18 kDa translocator protein in the spinal
cord are also elevated (12). The
levels of IL-1α, IL-1β, TNF-α, IL-17, and other inflammatory
mediators are also significantly increased in the lumbar spinal
cord of the OA pain rat model (13). Additionally, NF-κB expression (a
transcription factor for inflammatory responses) and astrocyte
proliferation are also increased in the spinal dorsal horn
(14). Further, spinal inhibition
of NF-κB significantly alleviates mechanical hyperalgesia and
decreases the expression of the inflammatory cytokines IL-1β,
TNF-α, and IL-33 in the dorsal horn of OA animals (15). Inhibition of spinal inflammation
alleviates OA pain.
Oxidative stress, a result of an imbalance between
the production of reactive oxygen species (ROS) and their clearance
by the antioxidant defense system, is a major cause of chronic
inflammation and pain (16). In
the whole blood and in the monocytes of rheumatoid arthritis
patients, mitochondrial ROS production is increased five-fold,
compared with healthy subjects (17). Blood concentrations of a lipid
oxidation biomarker, malondialdehyde (MDA), in rheumatoid arthritis
patients are significantly increased (18). In an OA murine model, deletion of
the transcription factor nuclear factor (erythroid-derived 2)-like
2 (Nrf2) resulted in increased OA severity (19). Additionally, oxidative
stress-activated cellular signal transduction pathways, such as
NF-κB inflammatory signal and the caspase signaling pathway,
leading to chronic inflammation (20). In a monoarthritic rat model,
injection of methane-rich saline suppressed oxidative stress (MDA
and 8-OHDG), increased superoxide dismutase and catalase activity,
and reduced chronic inflammatory pain (21). Thus, targeting oxidative stress may
be an effective treatment for the management of spinal inflammation
and OA chronic pain.
Lycorine is a pyrrolo[de]phenanthridine ring-type
alkaloid isolated from the Amaryllidaceae family of plants
that possesses anti-tumor, anti-viral, and anti-inflammatory
properties (22). Lycorine works
as a potent anti-tumor compound against various types of cancer
cells, including gastric cancer, bladder cancer, colorectal cancer,
prostate cancer, and breast cancer, amongst others (23). Lycorine is effective in a very low,
single digit µM concentration and is well tolerated with minimal
toxicity. In tumor xenografted mouse models, 5-15 mg/kg/day
lycorine treatment did not induce any significant changes in the
mice, thus being indicative of very low to no toxicity (24). Additionally, lycorine possesses
significant anti-inflammatory and hepatoprotective effects on mice
at doses ranging from 1-2 mg/kg, decreases the percentages of
immature granular leukocytes, and may be useful in the management
of acute promyelocytic leukemia at 5-10 mg/kg (25). Lycorine also has analgesic effects.
In an acetic-acid and carrageenan-induced rat model of pain,
lycorine intraperitoneal administration (i.p) showed
antinociceptive and anti-inflammatory effects at doses of 1.0 and
1.5 mg/kg (26). In an
intervertebral disc degeneration model, 5 mg/kg lycorine (i.p)
inhibited NF-κB-mediated proinflammatory cytokine expression to
prevent degeneration (27). In a
model of pulmonary inflammation and fibrosis, lycorine inhibited
NOD-like receptor protein 3 (NLRP3) inflammasome activation and
pyroptosis to act as a therapeutic agent (28). In a model of cardiac dysfunction,
lycorine treatment inhibited inflammation and oxidative stress in
heart tissues (29). Thus, in the
present study, the role and pathological mechanism of lycorine on
neuroinflammation and arthritic pain were studied.
A complete Freund's adjuvant (CFA) induced arthritic
pain mouse model was established, which is a commonly used model
for research on chronic polyarthritis for the evaluation of the
anti-inflammatory and analgesic potential of drugs (30,31).
CFA inducement results in pathophysiological changes such as
synovial hyperplasia and cartilage degradation, which are similar
to clinical arthritis (32). Here,
lycorine was intraperitoneally administered in mice, and the
effects of lycorine on behavioral, morphological, and protein
expression changes were analyzed. The results of the present study
provide a theoretical basis for the development of lycorine as an
analgesic drug for the management of arthritic pain.
Materials and methods
Animal model and drug
administration
A total of 30 male C57BL/6J mice weighing 18-20 g
(6-8 weeks old) were purchased from Hubei Province Experimental
Animal Center. All animals were housed with a 12 h light/dark with
ad libitum access to standard mouse chow and water. All
efforts were made to minimize the number of animals used and their
suffering. The toe-pinch reflex and a loss of righting reflex were
used to determine the level of anesthesia in the present study
(33,34). Apnea and the cessation of the
heartbeat were used to confirm death (35). If mice became sick or injured, they
were euthanized using an overdose (150 mg/kg) of pentobarbital
sodium by intraperitoneal injection.
Mice were acclimatized to the environment for 5 days
prior to the experiments, and randomly divided into three groups:
Control, CFA, and CFA + lycorine (n=10 per group). A mouse model of
CFA was established by intra-articular injection with 10 µl CFA
into the left hind knee joint on days 0 and 7; the control group
was injected with the same volume of saline (36). Behavioral tests were performed on
days 0, 7, and 14. On days 15-17 after CFA injection, mice from the
CFA and CFA + lycorine groups were intraperitoneally injected with
vehicle or lycorine (10 mg/kg), respectively, for 3 consecutive
days (37). Lycorine (Shanghai
yuanye Bio-Technology) was dissolved in DMSO and diluted with 0.9%
NaCl before use. Subsequently, behavioral tests were performed at 4
h after lycorine administration. Following completion of the
behavioral tests, all animals were sacrificed for further
experimental analysis.
Antibodies and reagents
Anti-IL-1β rabbit polyclonal antibody (cat. no.
AF5103), glial fibrillary acidic protein (GFAP) rabbit polyclonal
antibody (cat. no. DF6040), p-GSK3β (S9) rabbit polyclonal antibody
(cat. no. AF2016), GSK3β rabbit polyclonal antibody (cat. no.
AF5016), Caspase 1 rabbit polyclonal antibody (cat. no. AF5418),
cleaved-Caspase 1 rabbit polyclonal antibody (cat. no. AF4005), and
β-actin rabbit antibody (cat. no. AF7018) were obtained from
Affinity Biosciences, Ltd. Nrf2 rabbit polyclonal antibody (cat.
no. A1244), NLRP3 rabbit polyclonal antibody (cat. no. A5652), and
NF-κB rabbit polyclonal antibody (cat. no. A19653) were purchased
from ABclonal Biotech Co., Ltd. Hematoxylin and eosin (H&E)
staining solution (cat. no. BL735B) was purchased from Biosharp
Life Sciences. Lycorine was purchased from Shanghai yuanye
Bio-Technology. The secondary antibody used for western blotting
was an HRP Goat anti-rabbit IgG (H+L) (cat. no. AS014, ABclonal
Biotech Co., Ltd.). The secondary antibody used for
immunofluorescence analysis was a Goat Anti-Rabbit IgG H&L
(FITC) (cat. no. ab6717, Abcam).
Mechanical threshold test
Mechanical threshold values, which are indicative of
mechanical pain sensitivity, were measured and presented as the paw
withdrawal threshold (38). Von
Frey filaments (Stoelting; ranging from 0.008 to 6.0 g) were used
to stimulate the left hind paw. Mice were placed in a 30x30x30 cm
plexiglass chamber and allowed to acclimatize for at least 30 min
before the behavioral experiments were performed. Filaments were
pressed vertically against the plantar surfaces until the filaments
were bent and held for 3-5 sec, and a brisk withdrawal and paw
flinching was considered a positive response. Once a positive
response was observed, the von Frey filament with the next lower
force was applied, and whenever a response was not observed, the
filament with the next higher force was applied. Then, the pattern
of positive and negative withdrawal responses was converted to the
mechanical threshold as described previously (39).
Spontaneous flinch test
The number of flinches representative of spontaneous
pain was recorded. Mice were placed in a 30x30x30 cm plexiglass
chamber and acclimatized for at least 30 min. The number of
flinches in 5 min was counted three times independently. The mean
of the total number of flinches was taken (40).
Rotarod test
An accelerating rotarod was used to assess motor
coordination and the balance of animals. Three days before the
experiments, the mice were trained at a fixed speed of 4
revolutions/min for 10 min and this was repeated 3 times at 10 min
intervals. At the beginning of the experiment, the rotation speed
was set at a fixed value of 10 revolutions/min for 10 sec,
accelerated for 10 sec to a working speed of 20 revolutions/min for
30 sec, and then accelerated again for 10 sec. This movement was
continuously carried out for 10 min. Experiments were repeated
three times with intervals of 10 mins. The latency to fall of rats
was recorded (41).
H&E staining
After behavioral tests were performed, 5 C57BL/6J
mice in each group were anesthetized using 60 mg/kg sodium
pentobarbital by intraperitoneal injection, perfused transcardially
with saline containing heparin, following perfusion with 4%
paraformaldehyde (PFA, 0.1 M phosphate buffer, pH 7.4) until the
animal body was stiff and rigid. After perfusion, spinal cords were
collected and post-fixed in 4% PFA for 12 h at 4˚C, embedded in
paraffin, and cut into 4 µm sections using a microtome (RM 2165;
Leica Microsystems GmbH). The sections were stained using the
standard H&E method. Briefly, after dewaxing, the sections were
dyed with hematoxylin solution for 20 min at 25˚C and washed with
tap water for 10 sec. Subsequently, the sections were stained with
eosin for 5 min at 25˚C and washed with tap water for 10 sec. The
dehydration and transparent treatment were conducted by placing the
slices in 70% ethanol (10 sec at 25˚C), 80% ethanol (10 sec at
25˚C), 90% ethanol (30 sec at 25˚C), 100% ethanol (1 min at 25˚C)
and xylene (1 min twice at 25˚C). Finally, the sections were sealed
with neutral balsam and observed using a fluorescence microscope
(Olympus IX73; Olympus Corporation). The images were analyzed using
ImageJ version 1.51j8 (National Institutes of Health). The scoring
criteria of inflammatory cell infiltration was: 0, normal; 1,
lymphocyte infiltration around meninges and blood vessels; 2, 1-10
lymphocytes in a field; 3, 11-100 lymphocytes in a field of view;
4, >100 lymphocytes in a field of view.
Immunofluorescence analysis
Spinal cord sections were dewaxed, antigen retrieval
was performed using Improved Citrate Antigen Retrieval Solution
(cat. no. P0083, Beyotime Institute of Biotechnology) according to
the manufacturer's protocol, treated with hydrogen peroxide,
blocked with immunofluorescence blocking solution (Beyotime
Institute of Biotechnology) at 25˚C for 1 h, incubated with a
primary antibody overnight at 4˚C, and subsequently incubated with
fluorescent secondary antibody at 25˚C for 1 h and observed under a
fluorescence microscope (Olympus IX73; Olympus Corporation). The
fluorescence intensities were analyzed using ImageJ. The following
primary antibodies were used: Anti-IL-1β (1:100), anti-Nrf2
(1:100), anti-GFAP (1:100), anti-Caspase 1 (1:100), anti-p-GSK3β
(S9) (1:100), and anti-NF-κB (1:100).
Western blotting
After behavioral tests, another 5 mice from each
group were euthanized with an overdose of pentobarbital sodium (150
mg/kg) by intraperitoneal injection and sacrificed through
decapitation. Lumbar spinal cord samples were collected,
homogenized in RIPA lysis buffer containing 1% protease inhibitors
(MilliporeSigma), centrifuged at 13,523 x g, 4 ˚C for 20 min. The
supernatant was collected, loaded on an 8-12% SDS gel, resolved
using SDS-PAGE, and transferred to PVDF membranes. Protein
concentrations were quantified using a BCA protein assay kit
(Beyotime Institute of Biotechnology). The membranes were blocked
with QuickBlock™ Blocking Buffer for Western Blot (Beyotime
Institute of Biotechnology) for 15 min at 25˚C, and incubated with
the appropriate primary antibodies overnight at 4˚C, followed by
HRP-conjugated secondary antibodies in TBST (1:50,000) at 25˚C for
1 h. Protein bands were visualized using Super-sensitive Enhanced
Chemiluminescence Substrate Kit (Biosharp Life Sciences) and
visualized using an iBright 1500 instrument (Invitrogen; Thermo
Fisher Scientific, Inc.). Densitometry analysis was performed using
ImageJ. β-actin was used as a loading control. The following
primary antibodies were used: Anti-IL-1β (1:1,000), anti-GFAP
(1:1,000), anti-NF-κB (1:1,000), anti-Cleaved-Caspase 1 (1:1,000),
anti-Nrf2 (1:1,000), anti-NLRP3 (1:1,000), anti-Phospho-GSK3β (S9)
(1:1,000), anti-GSK3β (1:1,000), and anti-β-actin (1:50,000).
Molecular docking
The X-ray crystal structure of GSK-3β was obtained
from the Protein Data Bank (PDB ID: 2o5k, https://www.rcsb.org/). The structure of lycorine was
downloaded from the PubChem database (https://www.pubchem.ncbi.nlm.nih.gov/compound) and
optimized using ChemBio3D Ultra 14.0 software (PerkinElmer
Informatics). Auto Dock Vina 1.2.0 software (Center for
Computational Structural Biology) was used for docking conformation
between GSK-3β and lycorine. PyMOL version 2.2.3 (DeLano
Scientific) was used to visualize the conformation.
Measurement of superoxide dismutase
(SOD) activity
For the determination of SOD enzyme activity, the
CuZn/Mn-SOD assay kit with WST-8 (cat. no. S0103; Beyotime
Institute of Biotechnology) was used. Briefly, spinal cords were
homogenized in ice-cold PBS buffer, centrifuged at 13,523 x g at
4˚C for 15 min, and the supernatant was collected and mixed with
WST-8 enzyme working solution for 20 min at 37˚C, the
OD450nm absorbance value of each well was measured. SOD
activity was expressed as units per mg of total protein (U/mg
protein).
Statistical analysis
All statistical analysis was performed using SPSS
21.0 statistics software (IBM Corp.). A paired samples t-test was
used to compare the means of knee width. A one-way ANOVA followed
by Tukey's post-hoc test was used to analyze the data for
behaviors, H&E staining, immunofluorescence analysis, and
western blotting. Data for behavioral tests are presented as the
mean ± SEM. Data for H&E staining, immunofluorescence, and
western blotting are presented as the mean ± SD. P<0.05 was
considered to indicate a statistically significant difference.
Results
Lycorine treatment relieves pain
hypersensitivity in the CFA mice
Behavioral tests were performed using the protocol
shown in Fig. 1A. As shown in
Fig. 1B, on day 14, CFA treatment
led to a swelling of the knee. The knee width of the mice in the
control group was 5.32±0.17 mm, while in the CFA group, it was
7.85±0.17 mm (P<0.05). Additionally, compared with the control
group, mechanical threshold values were significantly lower in the
CFA mice from 0.92±0.05 (day 0) to 0.49±0.08 (day 7, P<0.05),
0.30±0.07 (day 14, P<0.05) (Fig.
1C). The number of flinches was significantly increased in the
CFA mice from 2.78±0.49 (day 0) to 7±0.55 (day 7, P<0.05),
9.44±0.82 (day 14, P<0.05) (Fig.
1D). Latency to fall was reduced in the CFA mice from
518.34±28.34 (day 0) to 374.23±33.36 (day 7, P<0.05), and
252.91±26.48 (day 14, P<0.05) (Fig.
1E). These data showed that CFA treatment-induced pain
hypersensitivity and motor disability in the mice; that is, the
mouse model of arthritis had been successfully established. The
effect of lycorine on pain sensitivity and motor ability was next
assessed. After lycorine treatment on days 15-17, mechanical
threshold values of CFA + lycorine mice were significantly higher,
0.59±0.04, 0.76±0.06, and 0.74±0.04 on days 15-17, respectively
(P<0.05 vs. CFA group) (Fig.
1C). The number of flinches in the CFA + lycorine mice was
notably lower, 6.67±0.62, 6.56±0.78, and 6.11±0.61, on days 15-17,
respectively (P<0.05 vs. CFA group) (Fig. 1D). The latency to fall of CFA +
lycorine mice were increased to 396.97±34.35, 411.65±28.43 and
413.21±23.69, on days 15-17, respectively (P<0.05 vs. CFA group)
(Fig. 1E). Thus, lycorine
treatment increased mechanical pain sensitivity, suppressed
spontaneous pain, and promoted recovery of motor coordination in
the CFA-induced mice.
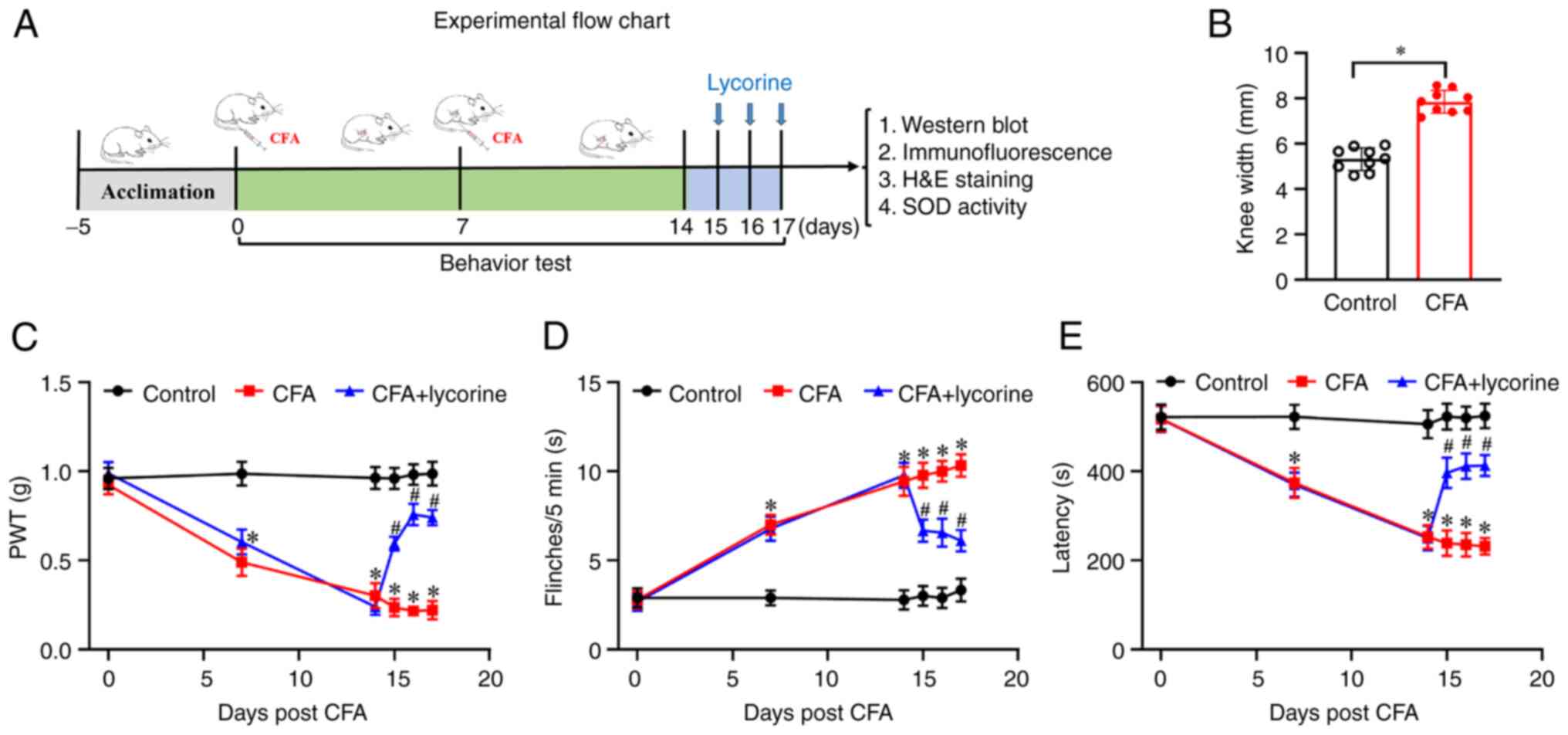 | Figure 1Effect of lycorine treatment on pain
response in the CFA mice. (A) Schematic diagram of the experimental
procedures. On days 0 and 7, CFA was intraarticularly injected into
the left knee joint of the mice, and behavioral tests were
performed on days 0, 7, and 14. Lycorine was intraperitoneally
injected in mice on days 15-17; 4 h after lycorine treatment,
behavioral tests were performed. Subsequently mice were sacrificed,
and the spinal cord tissues were collected for further analysis.
(B) Changes of knee width from control in the CFA mice on day 14
after CFA treatment. (C) Changes of PWT values, (D) spontaneous
flinches, and (E) latency to fall values in the mice. Data are
presented as the mean ± SEM (n=9). *P<0.05 vs.
control group; #P<0.05 vs. CFA group. CFA, complete
Freund's adjuvant; PWT, paw withdrawal threshold; SOD, superoxide
dismutase. |
Lycorine treatment decreases spinal
inflammation
Spinal inflammatory reactions were determined based
on inflammatory infiltration and IL-1β expression levels. Using
H&E staining, infiltration of inflammatory cells was increased
in the spinal dorsal horn of the CFA mice with the relative
inflammation score at 2.80±0.14. (P<0.05 vs. control group,
Fig. 2A); lycorine treatment
decreased the inflammatory response with a relative inflammation
score of 2.55±0.12 (P<0.05 vs. CFA group, Fig. 2B). Meanwhile the expression levels
of IL-1β were detected using immunofluorescence staining and
western blotting. Compared with the control group, the fluorescence
intensity of IL-1β in the spinal dorsal horn was significantly
higher in the CFA group (P<0.05), and lycorine treatment
decreased IL-1β intensity (P<0.05 vs. CFA group) (Fig. 2C). Relative intensity values of
IL-1β in the CFA and CFA + lycorine groups were 1.89±0.08 and
1.22±0.11, respectively (Fig. 2D).
Western blotting showed that the expression levels of spinal cord
IL-1β in the CFA mice were increased to 1.71±0.09 (P<0.05 vs.
control group). Lycorine treatment reduced IL-1β expression levels
to 1.06±0.11 (P<0.05 vs. CFA group) (Fig. 2E and F).
Lycorine treatment suppresses spinal
astrocytic activation
Astrocytic activation is an important source of
inflammatory cytokines and is assessed based on GFAP levels, which
is used as a marker of abnormal astrocyte activation and
proliferation (42). The intensity
of GFAP in the spinal dorsal horn of the CFA group was
significantly increased to 1.53±0.09 (P<0.05 vs. control group),
and lycorine treatment decreased the intensity to 1.10±0.11
(P<0.05 vs. CFA) (Fig. 3A and
B). Consistently, spinal GFAP
expression in the CFA group was increased (P<0.05 vs. control
group), and this was reversed by lycorine treatment (P<0.05 vs.
CFA group) (Fig. 3C). Relative
gray values of GFAP in the CFA and CFA + lycorine groups were
1.60±0.12 and 1.01±0.12, respectively (Fig. 3D).
Lycorine treatment inhibits spinal
NLPR3 inflammasome activity
NLRP3 inflammasome mediates Caspase 1 activation and
IL-1β secretion (43). The
fluorescence intensity of Caspase 1 in the spinal dorsal horn was
increased in the CFA group with a relative intensity value of
1.50±0.12 (P<0.05 vs. control group). Lycorine treatment reduced
the intensity of Caspase 1 to 1.03±0.09 (P<0.05 vs. CFA group)
(Fig. 4A and B). Western blotting showed that spinal
expression of NLRP3 and Cleaved-Caspase 1 were increased in the CFA
group (P<0.05 vs. control group; Fig. 4C), and the relative grey values
were 1.44±0.13 and 1.68±0.11, respectively. Lycorine treatment
decreased the expression levels of NLRP3 and Caspase 1 to 1.00±0.12
and 1.03±0.06, respectively (P<0.05 vs. CFA group; Fig. 4D).
Lycorine treatment decreases spinal
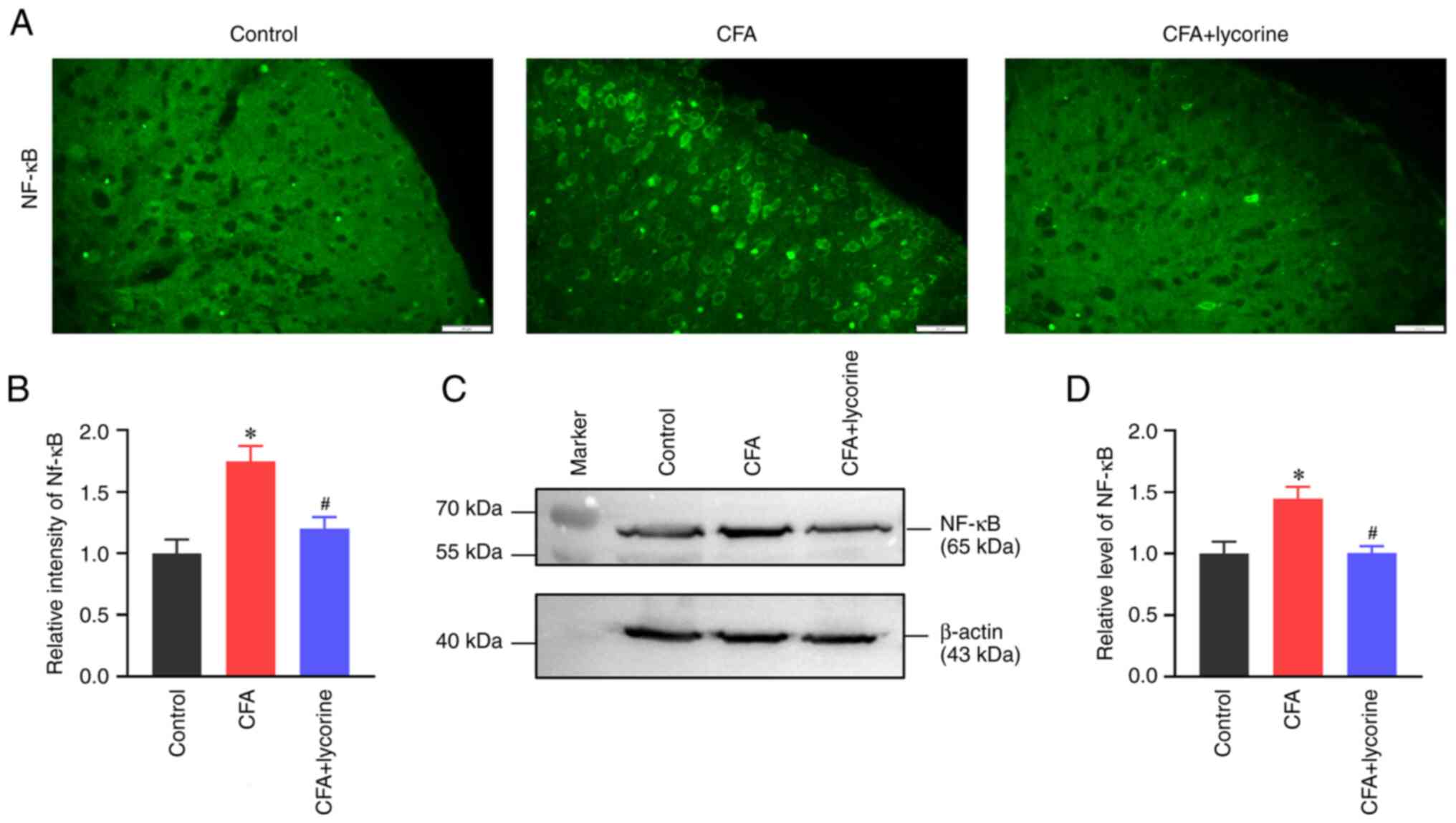
NF-κB levels
Distribution and expression of NF-κB in the spinal
cord were analyzed. The fluorescence intensity of NF-κB in the
spinal dorsal horn in the CFA group was significantly increased to
1.75±0.13 (P<0.05 vs. control group), and lycorine treatment
significantly reduced the intensity to 1.20±0.09 (P<0.05 vs. CFA
group) (Fig. 5A and B). NF-κB expression levels in the spinal
cord was increased in the CFA group with a grey value of 1.45±0.10
(P<0.05 vs. control group), and lycorine treatment significantly
reduced the grey value of spinal NF-κB expression to 1.00±0.06 in
the CFA + lycorine group (P<0.05 vs. CFA group) (Fig. 5C and D).
Lycorine treatment increases spinal
Nrf2 expression and SOD activity
Oxidative stress was determined based on the levels
of the antioxidant element Nrf2 and SOD activity. As shown in
Fig. 6A, the fluorescence
intensity of Nrf2 in the spinal dorsal horn was reduced in the CFA
group, with a relative intensity of 0.70±0.09 (P<0.05 vs.
control group). Lycorine treatment increased the intensity of Nrf2
to 0.89±0.10 (P<0.05 vs. CFA group) (Fig. 6B). Western blot analysis showed
that the spinal expression of Nrf2 was reduced in the CFA group
(P<0.05 vs. control group; Fig.
6C), with a relative grey value of 0.67±0.08. Lycorine
treatment increased the expression levels of Nrf2 to 0.90±0.06
(P<0.05 vs. CFA group; Fig.
6D).
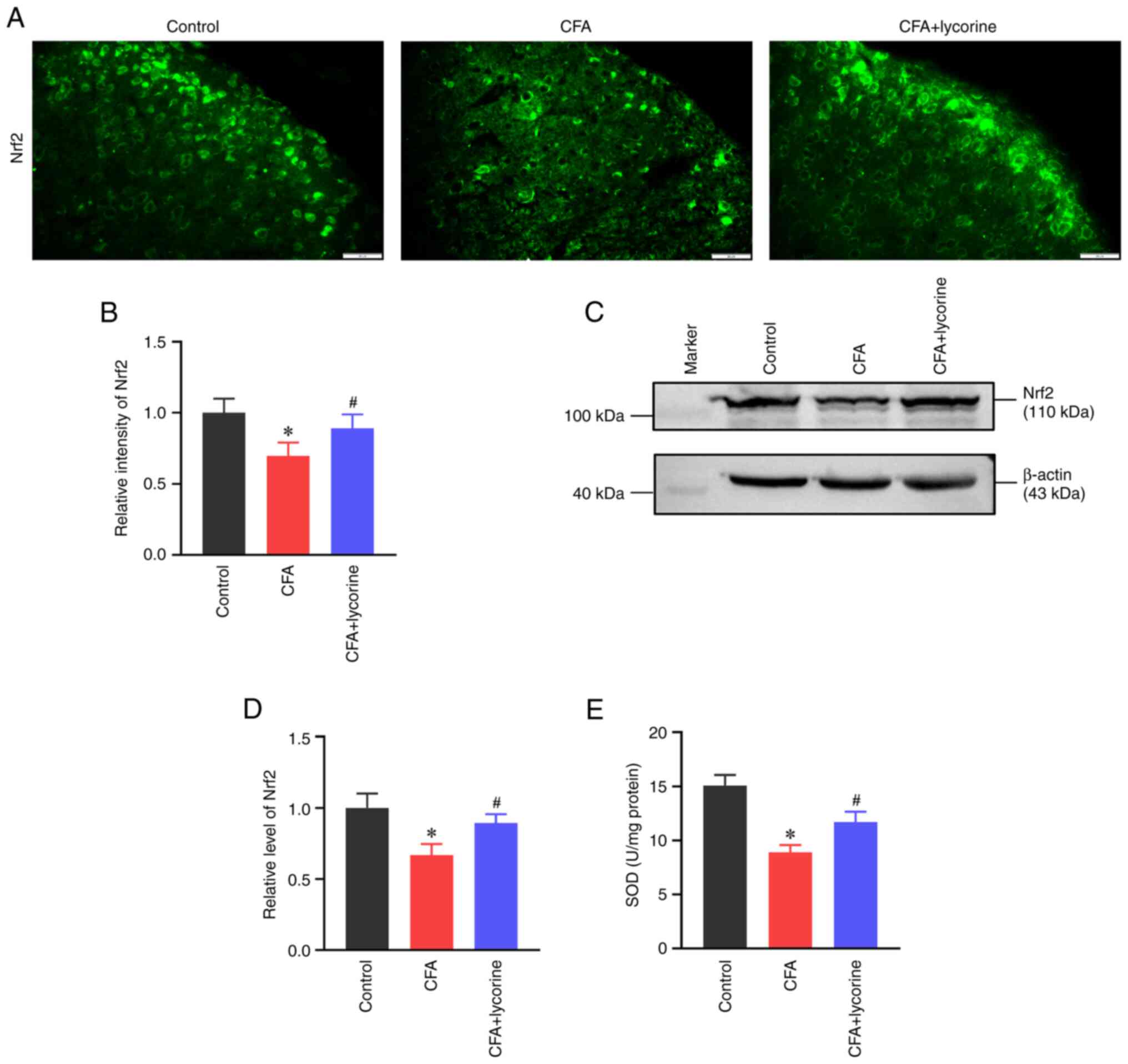 | Figure 6Effect of lycorine treatment on
oxidative stress. (A) Representative immunofluorescence staining
images and (B) quantitative intensity analysis of Nrf2 in the
spinal dorsal horns of the control, CFA, and CFA + lycorine groups.
Scale bar, 20 µm. (C) Representative western blots and (D) and
densitometry analysis of the Nrf2 expression levels in the spinal
cords of the control, CFA, and CFA + lycorine groups. β-actin was
used as the loading control. (E) Assay for SOD activity in the
spinal cord of the control, CFA, and CFA + lycorine groups. Data
are presented as the mean ± SD (n=5). *P<0.05 vs.
control group, #P<0.05 vs. CFA group. CFA, complete
Freund's adjuvant; SOD, superoxide dismutase; Nrf2, nuclear factor
(erythroid-derived 2)-like 2. |
SOD is important in controlling ROS levels and is a
significant inducer of Nrf2(44).
As shown in Fig. 6E, SOD activity
in the control group was 15.06±0.98 U/mg. However, CFA treatment
suppressed spinal SOD activity to 8.92±0.65 U/mg (P<0.05 vs.
control group) and lycorine treatment increased the activity to
11.72±0.94 U/mg (P<0.05 vs. CFA group).
Lycorine treatment inhibits spinal
GSK-3β activity
A molecular docking assay was performed on the X-ray
crystal structures of GSK-3β and the ligand lycorine (Fig. 7A-C); lycorine formed three
electrovalent bonds with GSK-3β at residues I85 and R141. The
electrovalent bond distances were measured to be 2.3 and 2.6
angstroms between the R141 residue and the lycorine, and 2.5
angstroms between the I62 residue and the lycorine. The binding
affinity was -7.0 kcal/mol.
 | Figure 7Effect of lycorine treatment on
GSK-3β activity. (A-C) The docking results of lycorine with GSK-3β.
(A) The modelled 3D structure of GSK-3β docked with lycorine. (B)
Enlarged view of the binding site is shown in the inset box. (C)
The interaction bonds of GSK-3β with lycorine. The GSK-3β protein
is shown in cyan, lycorine is colored green, the interacting
residues as red, and bonds are shown as yellow dotted lines, with
bond lengths presented as numbers. (D) Representative
immunofluorescence staining images and (E) quantitative intensity
analysis of p-GSK-3β(S9) in the spinal dorsal horns of the control,
CFA, and CFA + lycorine groups. Scale bar, 20 µm. (F)
Representative western blots and (G) densitometry analysis of
p-GSK-3β(S9) expression levels in the spinal cord. β-actin was used
as a loading control. Data are presented as the mean ± SD (n=5).
*P<0.05 vs. control group, #P<0.05 vs.
CFA group. CFA, complete Freund's adjuvant; GSK-3β, glycogen
synthase kinase 3β. |
Phosphorylation of GSK-3β at Ser-9 (p-GSK-3β-S9)
represents an inactive state of GSK-3β (45). The fluorescence intensity of
p-GSK-3β-S9 was lower in the spinal dorsal horn of the CFA group,
with a relative fluorescence intensity of 0.59±0.06 (P<0.05 vs.
control group). Lycorine treatment increased the intensity to
0.88±0.10 in the CFA + lycorine group (P<0.05 vs. CFA group)
(Fig. 7D and E). Western blot analysis showed that
p-GSK-3β-S9 levels were reduced in the CFA group (Fig. 7F), with a relative gray value of
0.58±0.10 (P<0.05 vs. control group; Fig. 7G). The p-GSK-3β-S9 expression was
increased following lycorine treatment in the CFA + lycorine group
(Fig. 7F), with a relative gray
value of 0.94±0.13 (P<0.05 vs. CFA group; Fig. 7G).
Discussion
In the present study, it was found that lycorine
inhibited GSK-3β activity and alleviated CFA-induced arthritic
pain. GSK-3β is a potential target for pain management (46,47).
In the rat model of spinal nerve ligation, mechanical allodynia and
thermal hyperalgesia were increased, and GSK-3β activity was also
increased (47). Additionally, the
GSK-3β selective inhibitor AR-A014418 or Thiadiazolidinone-8
(TDZD-8) administration decreased mechanical allodynia (48). In a neuropathic pain rat model of
chronic sciatic nerve constriction injury, intrathecal injection of
ghrelin suppressed the activation of GSK-3β in the spinal dorsal
horn and markedly alleviated neuropathic pain (49). In a rat model of cancer-induced
bone pain, injection of the GSK-3β inhibitor TDZD-8 suppressed the
NLRP3 inflammasome cascade and consequently decreased mechanical
pain sensitivity (50). In a model
of knee OA, pharmacological inhibitors of the GSK-3β/β-catenin
pathway attenuated apoptosis (51). In the present study, GSK-3β
activity was increased in the spinal cord of the CFA-induced mouse
model of arthritis. Lycorine binds to the I85 and R141 residues of
GSK-3β, which belong to the ATP-binding pocket and are known
targets for kinase inhibitors (52). Additionally, the binding affinity
between lycorine and GSK-3β was -7.0 kcal/mol, indicative of a
relatively stable docking result (53). Meanwhile, GSK-3β activity was
inhibited by lycorine in the spinal cord of CFA mice. Thus, the
results suggested that lycorine alleviated arthritic pain by
binding with GSK-3β and inhibiting its activity.
Lycorine suppressed neuroinflammation and oxidative
stress in the spinal cord via the GSK-3β pathway. NF-κB serves as a
pivotal mediator of inflammatory responses via inducing the
expression of various pro-inflammatory genes, such as cytokines and
chemokines, and by also participating in NLRP3 inflammasome
regulation (54). In a model of
inflammatory pain, intrathecal pretreatment with NF-κB inhibitors,
namely, NF-κB decoy or pyrrolidine dithiocarbamate, reduced
mechanical allodynia, and thermal hyperalgesia (55). Lycorine inhibited NF-κB signaling
activity, IκB-α phosphorylation/degradation and p65 phosphorylation
in prostate cancer cells and a mouse model (56). Lycorine decreased the levels of
inflammatory cytokines and MDA levels by attenuating the activity
of the high-mobility group box 1/Toll-like receptors/NF-κB pathway
in a model of lung injury that utilizes LPS (57). Lycorine also alleviated oxidative
stress by reducing total reactive oxygen species, based on the
lower MDA levels and higher SOD activity, significantly reduced the
levels of the inflammatory cytokines IL-1β, IL-6, and TNF-α, and
protected against cardiac dysfunction in a model of cardiac
dysfunction (29). In the present
study, it was found that lycorine reduced spinal inflammation and
increased antioxidant reactions in the spinal cords of CFA model
animals. Moreover, GSK-3β controls NF-κB recruitment and regulates
gene transcription. GSK-3β null cells or cells treated with a
GSK-3β pharmacological inhibitor exhibited reduced NF-κB DNA
binding activity (58). GSK-3β
also modulates the phosphorylation of the NF-κB essential modifier
NEMO at serine residues 8, 17, 31 and 43, and decreases NF-κB
signaling (59). Moreover, GSK-3β
serves a critical role in regulating and degrading Nrf2. In liver
cancer cells, inhibiting GSK-3β reduced nuclear export and
degradation of Nrf2(60). In brain
ischemia and reperfusion injury, GSK-3β downregulated the
expression levels of Nrf2 and its downstream genes (61). Based on the results of the present
study, it was shown that lycorine suppressed NF-κB mediated spinal
inflammation and enhanced the Nrf2-mediated antioxidant response
via inhibition of GSK-3β activity (Fig. 8).
The present study has some potential limitations.
First, central sensitization was assessed by specific experimental
proxies, such as widespread hyperalgesia, temporal summation, and
descending inhibition (62). In OA
pain processing, the spinal cord and brain undergo central nervous
sensitization (63,64). In the present study, only the
changes in the spinal cord and pain-related behaviors were
detected; the changes in the brain and descending pain-modulated
pathways were not analyzed. Secondly, the anti-inflammatory and
pain-relief effects of lycorine on the CFA model were estimated
based on three consecutive days of treatment. However, the
long-term effects of lycorine and its effects (if any) on the
affected joints are still not known. Finally, in this study, only
the status of spinal astrocytes was evaluated; however, the
microglia in the spinal cord also respond to injury and undergo
rapid proliferation. Thus, the functions of microglia in OA pain
should be assessed in the future. Of note, reactive astrocytes are
observed in different animal models of pain, but here a focus was
placed on the CFA mice. Thus, additional models should be evaluated
to elaborate the astrocyte-mediated central inflammatory
mechanisms.
In conclusion, during the processing of pain in OA,
spinal inflammatory reactions are stimulated, spinal oxidative
stress is increased, and neuropathic pain is induced. Lycorine
treatment inhibits spinal GSK-3β activity, suppresses spinal NF-κB
mediated inflammatory reactions, enhances spinal Nrf2-mediated
antioxidant responses, and alleviates arthritic pain. Additionally,
a preliminary mechanism by which lycorine alleviated neuropathic
pain was determined. These results highlight the potential
analgesic value of lycorine for the management of pain in patients
with OA.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81971066 and
81901149), and Hubei University of Science and Technology Program
(grant nos. 2020TD02 and 2020XZ40).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HLZ and MLC conceived and designed the study. YDH,
YFY, TC, ZDW, JQD, MX, DL and HLZ acquired, analyzed and
interpreted the data. YDH drafted and edited the manuscript. All
authors revised the manuscript. YDH, YFY, TC, HLZ and MLC confirm
the authenticity of all the raw data generated during the study.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures were performed according
to the local and international guidelines on the ethical use of
animals, and all efforts were made to minimize the number of
animals used and their suffering. Ethics approval was obtained from
the Laboratory Animal Ethics Committee of Hubei University of
Science and Technology (approval no. 2021-05-981; Xianning,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xie SH, Wang Q, Wang LQ, Wang L, Song KP
and He CQ: Effect of internet-based rehabilitation programs on
improvement of pain and physical function in patients with knee
osteoarthritis: Systematic review and meta-analysis of randomized
controlled trials. J Med Internet Res. 23(e21542)2021.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Malfait AM, Miller RE and Miller RJ: Basic
Mechanisms of pain in osteoarthritis: Experimental observations and
new perspectives. Rheum Dis Clin North Am. 47:165–180.
2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rajamäki TJ Jr, Puolakka PA, Hietaharju A,
Moilanen T and Jämsen E: Use of prescription analgesic drugs before
and after hip or knee replacement in patients with osteoarthritis.
BMC Musculoskelet Disord. 20(427)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
D'Arcy Y, Mantyh P, Yaksh T, Donevan S,
Hall J, Sadrarhami M and Viktrup L: Treating osteoarthritis pain:
Mechanisms of action of acetaminophen, nonsteroidal
anti-inflammatory drugs, opioids, and nerve growth factor
antibodies. Postgrad Med. 133:879–894. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Schaible HG, König C and Ebersberger A:
Spinal pain processing in arthritis: Neuron and glia
(inter)actions. J Neurochem: Dec 15, 2022 (Epub ahead of
print).
|
|
6
|
Leuchtweis J, Segond von Banchet G, Eitner
A, Ebbinghaus M and Schaible HG: Pain-related behaviors associated
with persistence of mechanical hyperalgesia after antigen-induced
arthritis in rats. Pain. 161:1571–1583. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Adami G, Gerratana E, Atzeni F, Benini C,
Vantaggiato E, Rotta D, Idolazzi L, Rossini M, Gatti D and Fassio
A: Is central sensitization an important determinant of functional
disability in patients with chronic inflammatory arthritides? Ther
Adv Musculoskelet Dis. 13(1759720X21993252)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Woolf CJ: Central sensitization:
Implications for the diagnosis and treatment of pain. Pain. 152
(Suppl 3):S2–S15. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Amodeo G, Franchi S, Galimberti G, Comi L,
D'Agnelli S, Baciarello M, Bignami EG and Sacerdote P:
Osteoarthritis pain in old mice aggravates neuroinflammation and
frailty: The positive effect of morphine treatment. Biomedicines.
10(2847)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kwon HS and Koh SH: Neuroinflammation in
neurodegenerative disorders: The roles of microglia and astrocytes.
Transl Neurodegener. 9(42)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pfyffer D, Wyss PO, Huber E, Curt A,
Henning A and Freund P: Metabolites of neuroinflammation relate to
neuropathic pain after spinal cord injury. Neurology. 95:e805–e814.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Albrecht DS, Ahmed SU, Kettner NW, Borra
RJH, Cohen-Adad J, Deng H, Houle TT, Opalacz A, Roth SA, Melo MFV,
et al: Neuroinflammation of the spinal cord and nerve roots in
chronic radicular pain patients. Pain. 159:968–977. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Im HJ, Kim JS, Li X, Kotwal N, Sumner DR,
van Wijnen AJ, Davis FJ, Yan D, Levine B, Henry JL, et al:
Alteration of sensory neurons and spinal response to an
experimental osteoarthritis pain model. Arthritis Rheum.
62:2995–3005. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Matsushita T, Otani K, Oto Y, Takahashi Y,
Kurosaka D and Kato F: Sustained microglial activation in the area
postrema of collagen-induced arthritis mice. Arthritis Res Ther.
23(273)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li Y, Yang Y, Guo J, Guo X, Feng Z and
Zhao X: Spinal NF-kB upregulation contributes to hyperalgesia in a
rat model of advanced osteoarthritis. Mol Pain.
16(1744806920905691)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ansari MY, Ahmad N and Haqqi TM: Oxidative
stress and inflammation in osteoarthritis pathogenesis: Role of
polyphenols. Biomed Pharmacother. 129(110452)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Quiñonez-Flores CM, González-Chávez SA,
Del Río Nájera D and Pacheco-Tena C: Oxidative stress relevance in
the pathogenesis of the rheumatoid arthritis: A systematic review.
Biomed Res Int. 2016(6097417)2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ediz L, Hiz O, Ozkol H, Gulcu E, Toprak M
and Ceylan MF: Relationship between anti-CCP antibodies and oxidant
and anti-oxidant activity in patients with rheumatoid arthritis.
Int J Med Sci. 8:139–147. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Poulet B and Beier F: Targeting oxidative
stress to reduce osteoarthritis. Arthritis Res Ther.
18(32)2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chen L, Tian Q, Shi Z, Qiu Y, Lu Q and Liu
C: Melatonin alleviates cardiac function in sepsis-caused
myocarditis via maintenance of mitochondrial function. Front Nutr.
8(754235)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dong D, Wu J, Sheng L, Gong X, Zhang Z and
Yu C: FUNDC1 induces apoptosis and autophagy under oxidative stress
via PI3K/Akt/mTOR pathway in cataract lens cells. Curr Eye Res.
47:547–554. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shen J, Zhang T, Cheng Z, Zhu N, Wang H,
Lin L, Wang Z, Yi H and Hu M: Lycorine inhibits glioblastoma
multiforme growth through EGFR suppression. J Exp Clin Cancer Res.
37(157)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Xiao H, Xu X, Du L, Li X, Zhao H, Wang Z,
Zhao L, Yang Z, Zhang S, Yang Y and Wang C: Lycorine and organ
protection: Review of its potential effects and molecular
mechanisms. Phytomedicine. 104(154266)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Roy M, Liang L, Xiao X, Feng P, Ye M and
Liu J: Lycorine: A prospective natural lead for anticancer drug
discovery. Biomed Pharmacother. 107:615–624. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu J, Li Y, Tang LJ, Zhang GP and Hu WX:
Treatment of lycorine on SCID mice model with human APL cells.
Biomed Pharmacother. 61:229–234. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Çitoğlu GS, Acikara OB, Yilmaz BS and
Ozbek H: Evaluation of analgesic, anti-inflammatory and
hepatoprotective effects of lycorine from Sternbergia fisheriana
(Herbert) Rupr. Fitoterapia. 83:81–87. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang G, Huang K, Dong Y, Chen S, Zhang J,
Wang J, Xie Z, Lin X, Fang X and Fan S: Lycorine suppresses
endplate-chondrocyte degeneration and prevents intervertebral disc
degeneration by inhibiting NF-κB signalling pathway. Cell Physiol
Biochem. 45:1252–1269. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liang Q, Cai W, Zhao Y, Xu H, Tang H, Chen
D, Qian F and Sun L: Lycorine ameliorates bleomycin-induced
pulmonary fibrosis via inhibiting NLRP3 inflammasome activation and
pyroptosis. Pharmacol Res. 158(104884)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wu J, Fu Y, Wu YX, Wu ZX, Wang ZH and Li
P: Lycorine ameliorates isoproterenol-induced cardiac dysfunction
mainly via inhibiting inflammation, fibrosis, oxidative stress and
apoptosis. Bioengineered. 12:5583–5594. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Parvathy SS and Masocha W: Gait analysis
of C57BL/6 mice with complete Freund's adjuvant-induced arthritis
using the CatWalk system. BMC Musculoskelet Disord.
14(14)2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen S, Gu Y, Dai Q, He Y and Wang J:
Spinal miR-34a regulates inflammatory pain by targeting SIRT1 in
complete Freund's adjuvant mice. Biochem Biophys Res Commun.
516:1196–1203. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kumar VL and Roy S: Calotropis procera
latex extract affords protection against inflammation and oxidative
stress in Freund's complete adjuvant-induced monoarthritis in rats.
Mediators Inflamm. 2007(47523)2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xu SF, Du GH, Abulikim K, Cao P and Tan
HB: Verification and defined dosage of sodium pentobarbital for a
urodynamic study in the possibility of survival experiments in
female rat. Biomed Res Int. 2020(6109497)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Laferriere CA, Leung VS and Pang DS:
Evaluating intrahepatic and intraperitoneal sodium pentobarbital or
ethanol for mouse euthanasia. J Am Assoc Lab Anim Sci. 59:264–268.
2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zatroch KK, Knight CG, Reimer JN and Pang
DS: Refinement of intraperitoneal injection of sodium pentobarbital
for euthanasia in laboratory rats (Rattus norvegicus). BMC Vet Res.
13(60)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Robledo-González LE, Martínez-Martínez A,
Vargas-Muñoz VM, Acosta-González RI, Plancarte-Sánchez R,
Anaya-Reyes M, Fernández Del Valle-Laisequilla C, Reyes-García JG
and Jiménez-Andrade JM: Repeated administration of mazindol reduces
spontaneous pain-related behaviors without modifying bone density
and microarchitecture in a mouse model of complete Freund's
adjuvant-induced knee arthritis. J Pain Res. 10:1777–1786.
2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chen S, Fang XQ, Zhang JF, Ma Y, Tang XZ,
Zhou ZJ, Wang JY, Qin A and Fan SW: Lycorine protects cartilage
through suppressing the expression of matrix metalloprotenases in
rat chondrocytes and in a mouse osteoarthritis model. Mol Med Rep.
14:3389–3396. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Luo H, Liu L, Zhao JJ, Mi XF, Wang QJ and
Yu M: Effects of oxaliplatin on inflammation and intestinal floras
in rats with colorectal cancer. Eur Rev Med Pharmacol Sci.
24:10542–10549. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hao M, Tang Q, Wang B, Li Y, Ding J, Li M,
Xie M and Zhu H: Resveratrol suppresses bone cancer pain in rats by
attenuating inflammatory responses through the AMPK/Drp1 signaling.
Acta Biochim Biophys Sin (Shanghai). 52:231–240. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Mao Y, Wang C, Tian X, Huang Y, Zhang Y,
Wu H, Yang S, Xu K, Liu Y, Zhang W, et al: Endoplasmic reticulum
stress contributes to nociception via neuroinflammation in a murine
bone cancer pain model. Anesthesiology. 132:357–372.
2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Shi X, Bai H, Wang J, Wang J, Huang L, He
M, Zheng X, Duan Z, Chen D, Zhang J, et al: Behavioral assessment
of sensory, motor, emotion, and cognition in rodent models of
intracerebral hemorrhage. Front Neurol. 12(667511)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chatterjee P, Pedrini S, Stoops E, Goozee
K, Villemagne VL, Asih PR, Verberk IMW, Dave P, Taddei K, Sohrabi
HR, et al: Plasma glial fibrillary acidic protein is elevated in
cognitively normal older adults at risk of Alzheimer's disease.
Transl Psychiatry. 11(27)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kelley N, Jeltema D, Duan Y and He Y: The
NLRP3 inflammasome: An overview of mechanisms of activation and
regulation. Int J Mol Sci. 20(3328)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Lee MJ, Agrahari G, Kim HY, An EJ, Chun
KH, Kang H, Kim YS, Bang CW, Tak LJ and Kim TY: Extracellular
superoxide dismutase prevents skin aging by promoting collagen
production through the activation of AMPK and Nrf2/HO-1 cascades. J
Invest Dermatol. 141:2344–2353.e7. 2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Chen Y, Maejima Y, Shirakabe A, Yamamoto
T, Ikeda Y, Sadoshima J and Zhai P: Ser9 phosphorylation of GSK-3β
promotes aging in the heart through suppression of autophagy. J
Cardiovasc Aging. 1(9)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Yuan L, Liu C, Wan Y, Yan H and Li T:
Effect of HDAC2/Inpp5f on neuropathic pain and cognitive function
through regulating PI3K/Akt/GSK-3β signal pathway in rats with
neuropathic pain. Exp Ther Med. 18:678–684. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Xu W, Zhu M, Yuan S and Yu W: Spinal CXCL5
contributes to nerve injury-induced neuropathic pain via modulating
GSK-3β phosphorylation and activity in rats. Neurosci Lett.
634:52–59. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Rashvand M, Danyali S and Manaheji H: The
potential role of glycogen synthase kinase-3β in neuropathy-induced
apoptosis in spinal cord. Basic Clin Neurosci. 11:15–30.
2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Peng Z, Zha L, Yang M, Li Y, Guo X and
Feng Z: Effects of ghrelin on pGSK-3β and β-catenin expression when
protects against neuropathic pain behavior in rats challenged with
chronic constriction injury. Sci Rep. 9(14664)2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Yang HY, Zhang F, Cheng ML, Wu J, Xie M,
Yu LZ, Liu L, Xiong J and Zhu HL: Glycogen synthase kinase-3β
inhibition decreases inflammation and relieves cancer induced bone
pain via reducing Drp1-mediated mitochondrial damage. J Cell Mol
Med. 26:3965–3976. 2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Shu Z, Miao X, Tang T, Zhan P, Zeng L and
Jiang Y: The GSK-3β/β-catenin signaling pathway is involved in
HMGB1-induced chondrocyte apoptosis and cartilage matrix
degradation. Int J Mol Med. 45:769–778. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Shin D, Lee SC, Heo YS, Lee WY, Cho YS,
Kim YE, Hyun YL, Cho JM, Lee YS and Ro S: Design and synthesis of
7-hydroxy-1H-benzoimidazole derivatives as novel inhibitors of
glycogen synthase kinase-3beta. Bioorg Med Chem Lett. 17:5686–5689.
2007.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Dutta M, Tareq AM, Rakib A, Mahmud S, Sami
SA, Mallick J, Islam MN, Majumder M, Uddin MZ, Alsubaie A, et al:
Phytochemicals from leucas zeylanica targeting main protease of
SARS-CoV-2: Chemical profiles, molecular docking, and molecular
dynamics simulations. Biology (Basel). 10(789)2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Li H, Zhang W, Lou Q, Chang Y, Lin Z and
Lou L: XueFu ZhuYu Decoction alleviates cardiopulmonary
bypass-induced NLRP3 inflammasome-dependent pyroptosis by
inhibiting IkB-α/NF-κB pathway in acute lung injury rats. Evid
Based Complement Alternat Med. 2022(6248870)2022.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Lee KM, Kang BS, Lee HL, Son SJ, Hwang SH,
Kim DS, Park JS and Cho HJ: Spinal NF-kB activation induces COX-2
upregulation and contributes to inflammatory pain hypersensitivity.
Eur J Neurosci. 19:3375–3381. 2004.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Liu J, Sun S, Zhou C, Sun Z, Wang Q and
Sun C: In vitro and in vivo anticancer activity of Lycorine in
prostate cancer by inhibiting NF-κB signaling pathway. J Cancer.
13:3151–3159. 2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Ge X, Meng X, Fei D, Kang K, Wang Q and
Zhao M: Lycorine attenuates lipopolysaccharide-induced acute lung
injury through the HMGB1/TLRs/NF-κB pathway. 3 Biotech.
10(369)2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Steinbrecher KA, Wilson W III, Cogswell PC
and Baldwin AS: Glycogen synthase kinase 3beta functions to specify
gene-specific, NF-kappaB-dependent transcription. Mol Cell Biol.
25:8444–8455. 2005.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Medunjanin S, Schleithoff L, Fiegehenn C,
Weinert S, Zuschratter W and Braun-Dullaeus RC: GSK-3β controls
NF-kappaB activity via IKKγ/NEMO. Sci Rep. 6(38553)2016.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Rada P, Rojo AI, Chowdhry S, McMahon M,
Hayes JD and Cuadrado A: SCF/{beta}-TrCP promotes glycogen synthase
kinase 3-dependent degradation of the Nrf2 transcription factor in
a Keap1-independent manner. Mol Cell Biol. 31:1121–1133.
2011.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Chen X, Liu Y, Zhu J, Lei S, Dong Y, Li L,
Jiang B, Tan L, Wu J, Yu S and Zhao Y: GSK-3β downregulates Nrf2 in
cultured cortical neurons and in a rat model of cerebral
ischemia-reperfusion. Sci Rep. 6(20196)2016.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Arendt-Nielsen L, Morlion B, Perrot S,
Dahan A, Dickenson A, Kress HG, Wells C, Bouhassira D and Drewes
AM: Assessment and manifestation of central sensitisation across
different chronic pain conditions. Eur J Pain. 22:216–241.
2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Lluch E, Nijs J, Courtney CA, Rebbeck T,
Wylde V, Baert I, Wideman TH, Howells N and Skou ST: Clinical
descriptors for the recognition of central sensitization pain in
patients with knee osteoarthritis. Disabil Rehabil. 40:2836–2845.
2018.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Pan TT, Pan F, Gao W, Hu SS and Wang D:
Involvement of macrophages and spinal microglia in osteoarthritis
pain. Curr Rheumatol Rep. 23(29)2021.PubMed/NCBI View Article : Google Scholar
|