Introduction
Lung ischemia-reperfusion (I/R) injury is a common
complication that occurs following lung transplantation,
cardiopulmonary bypass, pulmonary embolism thrombectomy, and other
surgical procedures (1,2). Despite improvements in medical
procedures, the incidence of lung I/R injury remains high as has
the clinical mortality rate following, especially in lung
transplantation (3).
During the lung I/R process, excessive levels of
inflammatory mediators and reactive oxygen species (ROS) are
released into the circulation, with a crucial role in the sequence
of events leading to lung failure (4). Indeed, both inflammatory response and
oxidative stress have been found to contribute to the pathogenesis
of multi-lung injuries and the inhibition of inflammation and
oxidative stress was shown to ameliorate lung injuries (5-7).
Sulforaphane (SFN) is an isothiocyanate derived by
the hydrolysis of glucosinolates via the enzyme myrosinase and is
enriched in cruciferous vegetables, particularly in broccoli
sprouts (8). SFN plays a vital
role in redox homeostasis, exerting a cytoprotective action against
oxidative stress via activation of Nrf2-related pathways (9-11).
Recent studies have shown that the beneficial effects of SFN in
I/R-related diseases are due to its antioxidant and
anti-inflammatory properties (12). For example, SFN could protect
against I/R injury in the liver by activating the Nrf2/ARE pathway
(13). Furthermore, the
cardioprotective effects of SFN against oxidative stress in
cardiomyocytes undergoing I/R were mediated by activation of the
Nrf2/HO-1 pathway (14).
Oxidative stress and inflammatory responses underlie
several pathological conditions, including I/R injury, and
metabolic and age-related diseases. Natural compounds such as SFN
may trigger a cellular self-defense mechanism that can effectively
mitigate oxidative stress commonly associated with several diseases
(15). During the lung I/R
process, excessive levels of inflammatory mediators and ROS are
released into the circulation, ultimately leading to lung failure;
however, the molecular mechanism of SFN on lung I/R injury remains
unclear. Therefore, here, the hypothesis that SFN may protect the
lung against I/R-induced oxidative stress and inflammation via
regulation of the Nrf2-related antioxidant pathway was assessed,
based on the following evidence: i) Lung I/R injury is closely
associated with oxidative stress and inflammation (6,16);
ii) oxidative stress and subsequent apoptosis are involved in lung
function disorder (17); iii) SFN
was shown to exert a protective effect against oxidative stress via
the Nrf2-related antioxidant pathway (18).
The present study aimed to explore the effects of
SFN against lung I/R-induced inflammation and oxidative stress and
the potential mechanisms involved.
Materials and methods
Animal management and ethical
statement
Male Wistar rats (200-250 g) aged 8-9 weeks were
obtained from the animal research center at Lvye Pharmaceutical
Co., Ltd. in Shandong, China (certificate number SYXK 2018-0028).
Rats were housed in a standard environment with a regular
light/dark cycle and free access to water and standard chow. The
project was approved by the Ethics Committee of Yantai Mountain
Hospital, Yantai, China (approval no. 2021-12.). The rats received
humane care and all efforts were made to alleviate suffering.
Establishment of the lung I/R
model
The rat model of lung I/R injury was induced as
described previously (19).
Pentobarbital sodium (50 mg/kg, i.p.) was used to fully anesthetize
rats, which were then placed on a homeothermic table to maintain
the core body temperature at 37˚C. The left pulmonary hilum was
clamped with a noninvasive arterial clip, resulting in complete
ischemia and hypoxia of the left lung for 1 h. Next, the vascular
clamp was released for 2 h to restore ventilation and perfusion to
the left lung. The rats were anesthetized with ether and sacrificed
by exsanguination at the end of the experiment. Death was confirmed
by a lack of autonomous respiration, no reflexive responses, and a
lack of a heartbeat.
SFN was obtained from MilliporeSigma (cat. no.
S4441), and a stock solution of 5 mM was prepared in DMSO (cat. no.
#D2650, MilliporeSigma; final concentration <0.1%). Stock
solutions were stored at -25˚C. The stock solution was diluted
using 0.9% NaCl solution into 30 mg/kg as previously reported
(20). The rats were divided into
three equal and random groups (n=10): Sham group, rats subjected to
the same thoracotomy procedure but without a hilar block; SFN
group, rats subjected to lung I/R injury given SFN (30 mg/kg/day)
by intraperitoneal injection for 7 consecutive days before the I/R
model was established; I/R group, rats subjected to lung I/R injury
were given the same volume of 0.9% NaCl solution. Fig. 1B shows a schematic diagram of the
grouping and interventions.
 | Figure 1SFN attenuates I/R-induced lung
lesions in rats. (A) Schematic representation of the chemical
structure of the isothiocyanate sulforaphane. (B) Schematic of
experimental design. (C) Representative images of H&E stained
lung sections. Scaler bar, 100 µm. H&E staining showed that the
infiltration of inflammatory cells was reduced and the alveolar
wall thinned gradually in the lungs of the SFN group compared with
those of the I/R group. (D) Quantification of lung injury scores.
(E) The W/D ratio, (F) PPI, and (G) MPO values of the lungs. Data
are presented as the mean ± SD. **P<0.01, Sham vs.
I/R group; #P<0.05, ##P<0.01, I/R vs.
SFN group. SFN, sulforaphane; I/R, ischemia/reperfusion; H&E,
hematoxylin and eosin; i.p. intraperitoneal; W/D, wet/dry; PPI,
pulmonary permeability index; MPO, myeloperoxidase. |
Blood samples were taken from the femoral artery
after reperfusion. Subsequently, the animals were sacrificed with
an intravenous overdose of pentobarbital sodium (100 mg/kg). The
left lungs were removed for further examination.
Hematoxylin-eosin staining
The middle of the left lung was immediately fixed in
10% formalin and maintained at 4˚C for 24 h. Tissues were
dehydrated after 24 h, embedded in paraffin, sectioned at 6 µm, and
stained with hematoxylin and eosin for 30 min at room temperature.
All images were taken using a Nikon Eclipse 80i microscope
(magnification, x400; Nikon Corporation). The extent of lung damage
was evaluated blindly using a histological scoring system as
described previously (21).
Blood gas analysis
Arterial blood samples were obtained for blood gas
analysis. A blood gas analyzer was used to record pH, the partial
pressure of oxygen (PaO2), and the partial pressure of
carbon dioxide (PaCO2) in a 0.5 ml sample of arterial
blood drawn from the abdominal aorta (RapidPoint 500, Siemens
AG).
Detection of lung tissue wet-to-dry
(W/D) weight ratio
Wet weight was determined by immediately weighing
freshly harvested left upper lung lobe samples. The lung tissue
sample was then dried until its weight remained constant. Finally,
the dry weight of the lung tissue sample was determined. The lung
tissue's W/D weight ratio was calculated by dividing the wet weight
by the dry weight.
Detection of pulmonary permeability
index
The pulmonary permeability index (PPI) was measured
as previously reported (22). To
determine total plasma protein, plasma supernatants were obtained
and stored at -70˚C. Bronchoalveolar lavage of the lung was
performed with 1 ml normal saline. The bronchoalveolar lavage fluid
(BALF) was centrifuged at 3,000 x g at 4˚C for 15 min. The BALF
supernatant was then stored at -70˚C for the Bradford assay to
detect total BALF protein concentration. PPI was calculated by
dividing the protein concentration in the BALF by the protein
concentration in the plasma.
Detection of myeloperoxidase
activity
A total of 100 mg lung tissue was mixed with 1 ml
RIPA lysate, homogenized with a glass homogenizer, placed on ice
for 30 min to fully lyse the cells, and then centrifuged at 13,000
x g at 4˚C for 15 mi to collect supernatants. The lung tissue
myeloperoxidase (MPO) activity was measured using a colorimetry
assay kit (cat. no. #A044-1-1, Nanjing Jiancheng Bioengineering
Institute) according to the manufacturer's protocol.
Detection of antioxidant capacity
The lung tissues were homogenized and centrifuged to
obtain the supernatant as above for the following experimental
detections. The activities of glutathione peroxidase (GSH-Px; cat.
no. #A005-1-2), superoxide dismutase (SOD; cat. no. A001-3-2), and
catalase (CAT; cat. no. #A007-1-1) in lung tissues were assessed by
colorimetry using commercial kits (all from Nanjing Jiancheng
Bioengineering Institute) as described previously (23).
Detection of oxidative stress
The lung tissues were homogenized and centrifuged to
obtain the supernatant for the following experimental detections.
ELISA was used to detect 8-OH-dG (cat. no. ab285254; Abcam Co.US)
in lung tissues as described previously (24). Briefly, 40 µl sample dilution
buffer was added to 10 µl samples in sample wells. A well was left
empty as blank control. 8-OH-dG Biotinylated Detection antibody (50
µl; 1:100) was added to each well and incubated for 45 min at 37˚C.
The plate sealer was removed and the wells aspirated and refilled
with the wash solution. The washing procedure was repeated three
times and the plates dried on absorbent filter papers. SABC working
solution (100 µl) was added to each well, the plate covered and
incubated at 37˚C for 30 min. Then, the solution was discarded and
the plate washed five times with 1X Wash Solution. TMB substrate
(90 µl) was added to each well and incubated at 37˚C in dark for
15-30 min. The shades of blue should be seen in the first 3-4 wells
by the end of the incubation. Stop solution (50 µl) was added to
each well to terminate the reaction. The color in the well changed
from blue to yellow. Absorbance at 450 nm was recorded using a
VersaMax ELISA Microplate Reader (Molecular Devices, LLC) within 15
min. after adding stop solution. A colorimetric method was used to
detect malondialdehyde (MDA; cat. no. A003-1-2; Nanjing Jiancheng
Bioengineering Institute) levels in the lung tissues as previously
described (25).
Detection of serum cytokines
Blood samples were collected from the femora artery
after reperfusion. The levels of IL-6, IL-1β, and TNF-α in serum
were then analyzed using the Pro™ Mouse Cytokine Panel kit (#5827,
Bio-Rad Laboratories, USA) according to the manufacturer's
instructions.
Western blot analysis
Total protein was extracted from lung tissues.
Briefly, 100 mg of lung tissues were homogenized in RIPA buffer for
10 min followed by centrifugation at 13,000 x g for 10 min at 4˚C.
The protein concentration was detected using a Bradford Assay kit
(cat. no. P0006; Beyotime Institute of Biotechnology). Western blot
analyses were performed as described previously (26). Briefly, SDS-PAGE was performed by
heating the samples for 8 min at 100˚C and loading 10 µg/lane of
the proteins onto a 5-15% linear acrylamide gradient gel. Following
transfer to PVDF membranes, the membranes were blocked in 5% BSA
(cat. no. SW3015, Beijing Solarbio Science & Technology Co.,
Ltd.) dissolved in TBST (20%Tween-20) for 2 h at room temperature,
and subsequently treated overnight at 4˚C with primary antibodies
against the following proteins: Nrf2 (1:3,000; cat. no. #12721),
HO-1 (1:3,000; cat. no. #43966), NQO1 (1:3,000; cat. no. #62262),
CAT (1:3,000; cat. no. #12980), Histone H3 (1:3,000; cat. no.
#4499), Bax (1:3,000; cat. no. #14796), Bcl-2 (1:3,000; cat. no.
#498), Cleaved Caspase-3 (1:3,000; cat. no. #9664), and β-actin
(1:5,000; cat. no. #4970). All primary antibodies were obtained
from Cell Signaling Technology, Inc. The membranes were then
incubated with a secondary antibody [1:5,000; anti-rabbit IgG
(H+L), cat. no. #14708; Cell Signaling Technology, Inc.) for 2 h at
room temperature, followed by TBST washes. Chemiluminescent
detection was performed using an ECL kit and a ChemiDoc Touch
imaging system (Bio-Rad Laboratories, Inc.). ImageJ (version 1.53;
National Institutes of Health was used for densitometry
analysis.
TUNEL assay
Apoptosis was determined using a TUNEL assay with a
TUNEL test kit (cat. no. T2190, Beijing Solarbio Science &
Technology Co., Ltd.) according to the manufacturer's instructions.
The lung tissues were placed in 10% formalin at room temperature
overnight for paraffin embedding and then were sectioned into 5 µm
thick slices and processed for TUNEL. Next, tissues were
dehydrated, incubated with 0.9% NaCl for 5 min, then rinsed with
PBS for 5 min, fixed in 4% paraformaldehyde at room temperature for
15 min, then rinsed twice with PBS (5 min per wash). The sections
were mixed with biotinylated nucleotides and terminal
deoxynucleotidyl transferase and incubated at 37˚C for 60 min.
Following PBS washes, the lung tissues were blocked with 0.3%
hydrogen peroxide and incubated with HRP-conjugated streptavidin at
room temperature for 30 min, then washed three times with PBS and
stained with hematoxylin at room temperature for 3 min. A total of
5 fields of view were automatically selected by Image-Pro Plus
version 5.1 (Media Cybernetics, Inc.). The percentage of apoptotic
cells was calculated for each field of view. The mean was
calculated to obtain the percentage of apoptotic cells and the
results are expressed as the apoptotic index (AI), calculated as:
AI (%) = (apoptotic nuclei count / total nucleus count) x100%.
Immunohistochemistry
The lung tissues were fixed in 10% formalin for 24 h
at 4˚C. Sections of paraffin-embedded specimens were sectioned into
5 µm thick slices and prepared as above. The sections were then
rinsed with PBS after being incubated in 3 percent
H2O2 for 10 min. Following DAB chromogen
incubation at room temperature for 5 min, the sections were
counterstained with hematoxylin. Images were taken using a Nikon
Eclipse 80i microscope (magnification, x400; Nikon
Corporation).
Statistical analysis
Statistical analysis was performed using SPSS
version 19.0 (IBM Corp.). Data are presented as the mean ± SD of
three independent repeats. A one-way ANOVA followed by a Tukey's
post hoc test or an unpaired Student's t-test were used for
comparisons between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
SFN attenuates I/R-induced lung
lesions in rats
To determine the effects of SFN on lung injury after
I/R, hematoxylin-eosin staining for lung histology was evaluated
(Fig. 1C). Notably, a disordered
alveolar structure was observed in the I/R group, with significant
pulmonary interstitial edema, and a large number of inflammatory
cells in the alveolar cavity. However, pretreatment with SFN
significantly attenuated I/R-induced lung injury
histopathologically. All of these changes were corroborated by the
histological scoring. Pretreatment with SFN significantly decreased
the lung injury score. (Fig.
1D).
An increase in lung permeability will promote the
occurrence of pulmonary edema (27). Here, the extent of pulmonary edema
based on the W/D ratio and PPI was assessed. As shown in Fig. 1E-F, the W/D ratio and PPI were
markedly increased after I/R compared with the Sham group. SFN
pretreatment significantly decreased the W/D ratio and PPI in the
I/R rats. MPO activity was next assessed to evaluate neutrophil
accumulation in the lung tissues. As shown in Fig. 1G, I/R treatment significantly
increased MPO activity, whereas MPO activity was significantly
reduced by SFN pretreatment.
Rat arterial blood gas analysis was performed to
further assess lung injury. As shown in Table I, the arterial blood pH value was
significantly decreased after I/R compared with the Sham group, and
the I/R-induced acidosis was significantly prevented by SFN
pretreatment. In addition, pretreatment with SFN also significantly
prevented the increase in PaCO2 and decrease in
PaO2 values in the I/R-induced rats. These results
indicate that SFN pretreatment attenuated I/R-induced lung lesions
in rats.
 | Table IArterial blood gases at the end of
reperfusion. |
Table I
Arterial blood gases at the end of
reperfusion.
| Group | pH | PaO2
(mmHg) | PaCO2
(mmHg) |
|---|
| Sham | 7.35±0.11 | 262.21±18.12 | 55.22±6.41 |
|
Ischemia-reperfusion |
7.12±0.08a |
167.35±21.21a |
76.61±6.71a |
| SFN |
7.22±0.09b |
214.10±23.54b |
62.24±7.43b |
SFN alleviates I/R-induced oxidative
stress and inflammation in the lungs of rats
The effect of SFN pretreatment on the redox
metabolism and inflammatory response in the lungs of I/R rats was
next evaluated. As shown in Fig.
2A and B, compared with the
Sham group, the levels of MDA and 8-OH-dG were significantly
increased in the I/R group. However, SFN pretreatment significantly
downregulated the levels of MDA and 8-OH-dG in the I/R-induced
rats. In addition, pretreatment with SFN markedly reversed the
decrease in the antioxidant enzyme activities of CAT, SOD, and
GSH-Px in the lung of I/R treated rats (Fig. 2C-E). Furthermore, serum IL-6,
IL-1β, and TNF-α levels in the I/R group were significantly
increased compared with those in the Sham group, and a significant
decrease was observed in the SFN pretreatment group (Fig. 2F-H). These results show that SFN
pretreatment alleviated the I/R-induced imbalance of redox
metabolism and the aggravation of inflammatory reactions in the
lungs of rats.
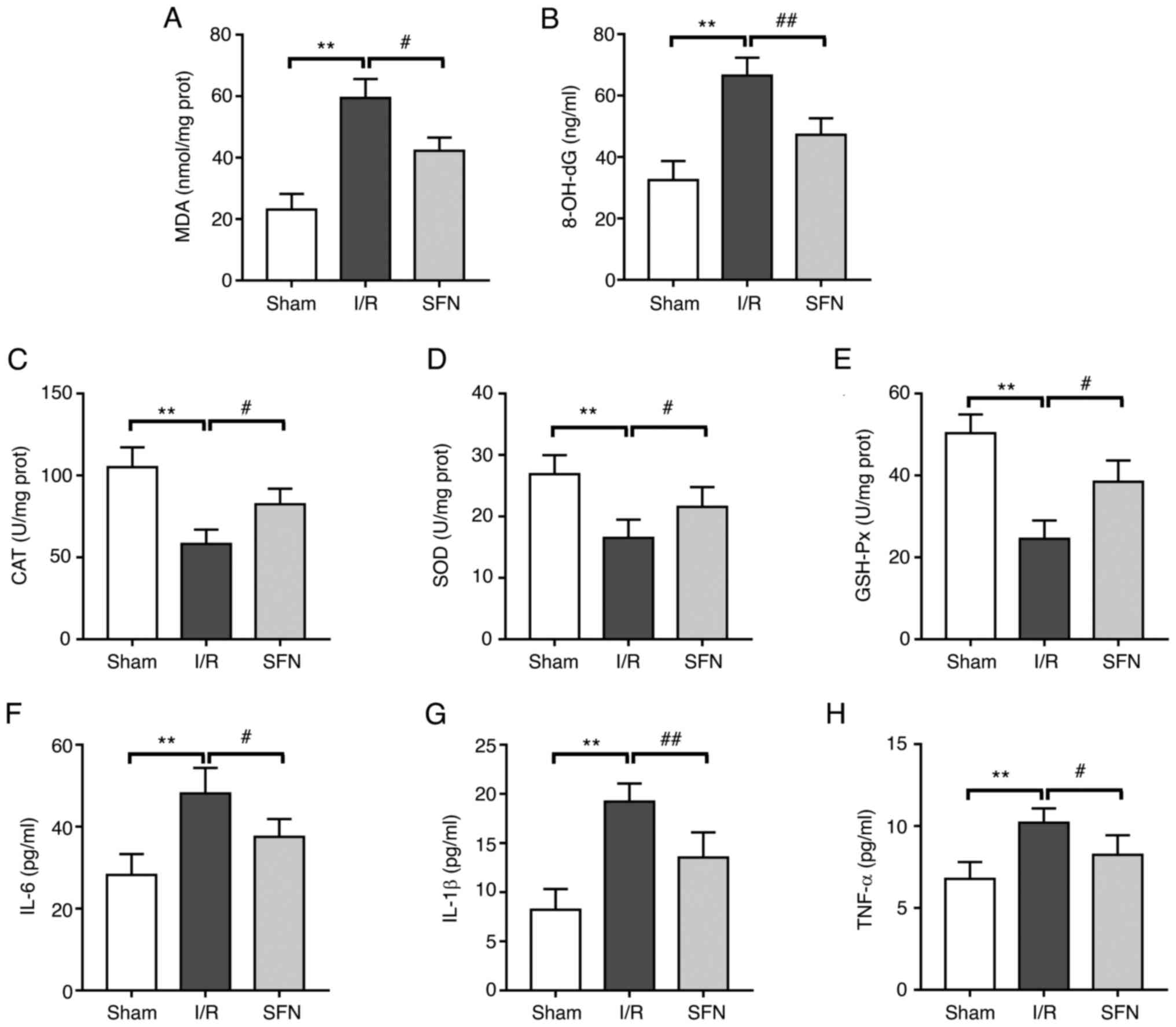 | Figure 2SFN alleviates I/R-induced lung
oxidative stress and inflammation in rats. The levels of (A) MDA
and (B) 8-OH-dG oxidative stress parameters. The antioxidant enzyme
activities of (C) CAT, (D) SOD, and (E) GSH-Px. The levels of serum
inflammatory cytokines (F) IL-6, (G) IL-1β, and (H) TNF-α. Data are
presented as the mean ± SD. **P<0.01, Sham vs. I/R
group; #P<0.05, ##P<0.01, I/R vs. SFN
group. SFN, sulforaphane; I/R, ischemia/reperfusion; MDA,
malondialdehyde; CAT, catalase; SOD, superoxide dismutase; GSH-Px,
glutathione peroxidase; prot, protein. |
SFN improves I/R-induced lung
apoptosis in rats
TUNEL staining was used to determine whether SFN
attenuated lung tissue apoptosis following I/R in rats. As shown in
Fig. 3A and B, the results of the TUNEL assays showed
a significant increase in the AI in the I/R group compared with the
Sham group, and SFN pretreatment significantly decreased the AI in
the lung tissues of the I/R rats.
To determine the potential molecular mechanism of
SFN in reducing lung apoptosis in I/R rats, the expression of
several apoptosis-associated proteins in the lung tissues was
measured (Fig. 3C). As shown in
Fig. 3D-F, the expression of the
pro-apoptotic proteins Bax and C-casp-3 were significantly
increased, whereas the expression of the anti-apoptotic protein
Bcl-2 was markedly decreased in the I/R group compared with the
Sham group. SFN pretreatment notably attenuated the decrease in
Bcl-2 expression and the increase in Bax and C-casp-3 expression
compared with the I/R group. These data showed that SFN improved
I/R-induced lung apoptosis in rats by suppressing the Bax/Bcl-2
pathway.
SFN protects against I/R-induced lung
lesions via activation of the Nrf2/HO-1 pathway
It has been shown that the Nrf2/HO-1 pathway plays
an important role in maintaining redox metabolism and inhibiting
apoptosis in lung tissues (28).
Thus, the expression of Nrf2 and its downstream antioxidant genes
in the lung tissues of I/R rats with or without SFN pretreatment
was assessed (Fig. 4A). As shown
in Fig. 4B, the nuclear transfer
of Nrf2 (n-Nrf2) was markedly increased in the I/R group compared
with the Sham group. Accordingly, the expression levels of Nrf2
target antioxidant genes HO-1, NQO1, and CAT were also markedly
increased in the I/R group compared with the Sham group (Fig. 4C-E). Interestingly, SFN
pretreatment further increased the expression of n-Nrf2 and its
downstream antioxidant genes compared with the I/R group (Fig. 4B-E). These results suggested that
SFN protected against I/R-induced lung lesions in rats via
activation of the Nrf2/HO-1 pathway.
Discussion
As lung I/R injury is a recognized fatal
complication following lung transplantation, there is an urgent
need to identify novel therapeutic targets for alleviating lung I/R
injury. I/R is directly related to the formation of ROS, increased
vascular permeability, the activation of neutrophils, and cytokine
release (29). The findings of the
present study demonstrate that SFN can protect the lung against
I/R-induced oxidative stress and inflammation, as evidenced by
reducing ROS production and the release of pro-inflammatory
cytokines. The beneficial effects of SFN on lung I/R injury
involved activation of the Nrf2-related antioxidant pathway.
Oxidative stress is the outcome of an imbalance
between the generation of ROS and the antioxidant defense systems,
which is characterized by increases in ROS and other free radicals,
leading to cellular injury (30).
Oxidative stress is closely associated with the initiation and
progression of lung I/R injury (31). The Nrf2-related pathway is
considered a defense system aimed to counteract oxidative stress
and preserve cellular homeostasis (32-34).
Under physiological conditions, Nrf2 binds to its negative
regulator Keap1 and is maintained in an inactive state. In the
presence of ROS, Nrf2 dissociates from Keap1 and translocates to
the nucleus, and binds to Maf (35,36).
The Nrf2-Maf heterodimers then bind to antioxidant response
elements in the promoters of key antioxidant genes (such as HO-1,
NQO1, and CAT) and activates their transcription (37,38).
HO-1 catalyzes the breakdown of heme to produce biliverdin, ferrous
ions, and carbon monoxide, all of which are essential components of
the inflammatory process (39).
The homodimeric luteinase NQO1 promotes the elimination of
hydrazine, which can produce harmful semihydroquinone radicals
through the redox cycle, by catalyzing the reduction of hydrazine
to hydroquinone (26,39). CAT promotes the synthesis of
intracellular catalase, which catalyzes the decomposition of
H2O2 into H2O and O2
(40). By scavenging excessive ROS
levels and restoring redox homeostasis, Nrf2 can prevent
I/R-related disorders (41). As
previously described, SFN is a bioactive molecule present in
broccoli, which exerts its cytoprotective effect by activating an
Nrf2-related pathway (42). In
this study, the expression of Nrf2 and its downstream target genes
HO-1 and NQO1 were significantly decreased in the I/R rats, and SFN
treatment significantly suppressed ROS generation and activated the
Nrf2 antioxidant pathway, thus exerting therapeutic effects on
IRI-induced injury by restoring cellular ROS homeostasis.
It is well established that the over-production of
pro-inflammatory cytokines is another crucial trigger of cellular
damage (43,44). Oxidative stress is closely
correlated with the inflammatory response, especially during the
I/R injury process. High levels of ROS produced during oxidative
stress stimulate the release of pro-inflammatory mediators and
increase inflammation, which may further aggravate I/R injury. In
addition, activation of Nrf2 not only inhibits oxidative stress
response but also contributes to the anti-inflammatory process by
regulating cytokine secretion (45). SFN is a natural product that exerts
its beneficial effects via the activation of the antioxidant
systems and suppression of pro-inflammatory responses through the
activation of Nrf2-related pathways (46,47).
In the present study, elevated levels of pro-inflammatory cytokines
were observed in the I/R rats, which were also effectively
attenuated by SFN treatment. In line with the results of the
present study, SFN was reported to ameliorate LPS-induced ROS,
reactive nitrogen species, pro-inflammatory cytokine production,
and cell death via Nrf2 activation (48).
Oxidative stress and inflammation are two major
factors involved in the pathogenesis of lung I/R injury, which
jointly contribute to the apoptosis of lung cells. Apoptosis is
closely associated with the pathological process of lung I/R
injury, according to earlier studies (49,50).
Bcl-2, an anti-apoptotic protein, can inhibit the production of
free radicals and endoplasmic reticulum Ca2+ as well as
prevent the formation of lipid peroxides (51). Bax is an endogenous antagonist of
Bcl-2; by physically attaching to the related protein homologs, it
inhibits Bcl-2, thus inducing apoptosis (52). Bcl-2 and Bax expression levels are
typically balanced in a healthy state (53). In the present study, it was found
that lung cell apoptosis was activated in rats with I/R lung injury
and that Bax protein expression increased while Bcl-2 protein
expression was decreased. An important biochemical aspect of
apoptosis is the activation of caspases. The beginning and
completion of mammalian apoptotic processes are regulated by the
caspase family of cysteine proteases (54). Caspase-3 is created from a 32 kDa
zymogen and cleaves to a 17 kDa active subunit via mitochondrial
and death ligand mechanisms (55).
This zymogen is a crucial caspase effector that starts the cell's
disintegration during the final stages of apoptosis (56). In the present study, it was found
that SFN effectively inhibited the expression level of the
pro-apoptotic proteins Bax and C-casp-3 in the I/R group rats, thus
reducing apoptosis. A previous study showed that Nrf2/HO-1
activation counteracts the inflammatory response and apoptosis in
contrast-induced renal injury (57). Herein, the beneficial effects of
SFN on I/R injury via antioxidative stress, anti-inflammation, and
anti-apoptosis were dependent on the activation of the Nrf2/HO-1
pathway. Conversely, as mitochondria are the primary source of ROS,
the destruction of mitochondria leads to the accumulation of
excessive ROS levels, resulting in increased mitochondrial
dysfunction (58). MDA, SOD, and
GSH are important indicators for detecting oxidative stress.
Through a number of signaling mechanisms, the generation of ROS can
lead to oxidative stress in cells and cause cells to undergo
apoptosis (34,59). When mitochondria undergo apoptosis,
the expression of Bcl-2 is inhibited (60). However, at present, whether SFN
alleviates I/R-induced oxidative stress in lung tissue by
alleviating mitochondrial damage remains to be further studied.
In summary, the present study provided evidence that
SFN protected against lung I/R injury-induced oxidative injury,
inflammation, and apoptosis via activation of the Nrf2/HO-1
pathway. These findings may provide novel insights into the
development of therapeutic applications of SFN for lung I/R
injury.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ and CY conceived and designed the experiments. SW
and YZ performed the experiments. FL analyzed the data. LZ wrote
the manuscript. All authors read and approved the final manuscript.
LZ and CY confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Yantai Mountain Hospital, Yantai, China (approval no.
2021-12.).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wei L, Li J, Han Z, Chen Z and Zhang Q:
Silencing of lncRNA MALAT1 prevents inflammatory Injury after lung
transplant ischemia-reperfusion by downregulation of IL-8 via p300.
Mol Ther Nucleic Acids. 18:285–297. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion-from mechanism to translation. Nat Med. 17:1391–1401.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Chen-Yoshikawa TF: Ischemia-reperfusion
injury in lung transplantation. Cells. 10(1333)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Qin J, Su X, Jin X and Zhao J: Parecoxib
mitigates lung ischemia-reperfusion injury in rats by reducing
oxidative stress and inflammation and up-regulating HO-1
expression. Acta Cir Bras. 36(e360901)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Guo Y, Liu Y, Zhao S, Xu W, Li Y, Zhao P,
Wang D, Cheng H, Ke Y and Zhang X: Oxidative stress-induced FABP5
S-glutathionylation protects against acute lung injury by
suppressing inflammation in macrophages. Nat Commun.
12(7094)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kong L, Deng J, Zhou X, Cai B, Zhang B,
Chen X, Chen Z and Wang W: Sitagliptin activates the p62-Keap1-Nrf2
signalling pathway to alleviate oxidative stress and excessive
autophagy in severe acute pancreatitis-related acute lung injury.
Cell Death Dis. 12(928)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liao WI, Wu SY, Tsai SH, Pao HP, Huang KL
and Chu SJ: 2-Methoxyestradiol protects against lung
ischemia/reperfusion injury by upregulating annexin A1 protein
expression. Front Immunol. 12(596376)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Vanduchova A, Anzenbacher P and
Anzenbacherova E: Isothiocyanate from Broccoli, Sulforaphane, and
its properties. J Med Food. 22:121–126. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Russo M, Spagnuolo C, Russo GL,
Skalicka-Woźniak K, Daglia M, Sobarzo-Sánchez E, Nabavi SF and
Nabavi SM: Nrf2 targeting by sulforaphane: A potential therapy for
cancer treatment. Crit Rev Food Sci Nutr. 58:1391–1405.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dana AH and Alejandro SP: Role of
sulforaphane in endoplasmic reticulum homeostasis through
regulation of the antioxidant response. Life Sci.
299(120554)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bajpai VK, Alam MB, Quan KT, Kwon KR, Ju
MK, Choi HJ, Lee JS, Yoon JI, Majumder R, Rather IA, et al:
Antioxidant efficacy and the upregulation of Nrf2-mediated HO-1
expression by (+)-lariciresinol, a lignan isolated from Rubia
philippinensis, through the activation of p38. Sci Rep.
7(46035)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Franke M, Bieber M, Kraft P, Weber ANR,
Stoll G and Schuhmann MK: The NLRP3 inflammasome drives
inflammation in ischemia/reperfusion injury after transient middle
cerebral artery occlusion in mice. Brain Behav Immun. 92:223–233.
2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhao HD, Zhang F, Shen G, Li YB, Li YH,
Jing HR, Ma LF, Yao JH and Tian XF: Sulforaphane protects liver
injury induced by intestinal ischemia reperfusion through Nrf2-ARE
pathway. World J Gastroenterol. 16:3002–3010. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Peng N, Jin L, He A, Deng C and Wang X:
Effect of sulphoraphane on newborn mouse cardiomyocytes undergoing
ischaemia/reperfusion injury. Pharm Biol. 57:753–759.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li D, Shao R, Wang N, Zhou N, Du K, Shi J,
Wang Y, Zhao Z, Ye X, Zhang X and Xu H: Sulforaphane activates a
lysosome-dependent transcriptional program to mitigate oxidative
stress. Autophagy. 17:872–887. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yan J, Li J, Zhang L, Sun Y, Jiang J,
Huang Y, Xu H, Jiang H and Hu R: Nrf2 protects against acute lung
injury and inflammation by modulating TLR4 and Akt signaling. Free
Radic Biol Med. 121:78–85. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Han YK, Kim JS, Jang G and Park KM:
Cisplatin induces lung cell cilia disruption and lung damage via
oxidative stress. Free Radic Biol Med. 177:270–277. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ishida K, Kaji K, Sato S, Ogawa H, Takagi
H, Takaya H, Kawaratani H, Moriya K, Namisaki T, Akahane T and
Yoshiji H: Sulforaphane ameliorates ethanol plus carbon
tetrachloride-induced liver fibrosis in mice through the
Nrf2-mediated antioxidant response and acetaldehyde metabolization
with inhibition of the LPS/TLR4 signaling pathway. J Nutr Biochem.
89(108573)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ding Y, Tu P, Chen Y, Huang Y, Pan X and
Chen W: CYP2J2 and EETs protect against pulmonary arterial
hypertension with lung ischemia-reperfusion injury in vivo and in
vitro. Respir Res. 22(291)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhou A, Hong Y and Lv Y: Sulforaphane
attenuates endometriosis in rat models through inhibiting PI3K/Akt
signaling pathway. Dose Response.
17(1559325819855538)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li D, Song LL, Wang J, Meng C and Cui XG:
Adiponectin protects against lung ischemia-reperfusion injury in
rats with type 2 diabetes mellitus. Mol Med Rep. 17:7191–7201.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li X, Jamal M, Guo P, Jin Z, Zheng F, Song
X, Zhan J and Wu H: Irisin alleviates pulmonary epithelial barrier
dysfunction in sepsis-induced acute lung injury via activation of
AMPK/SIRT1 pathways. Biomed Pharmacother.
118(109363)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ma D, Gao W, Liu J, Kong D, Zhang Y and
Qian M: Mechanism of oxidative stress and Keap-1/Nrf2 signaling
pathway in bronchopulmonary dysplasia. Medicine (Baltimore).
99(e20433)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhou Y, Zhang L, Guan J and Yin X:
Improvement of lung ischemia-reperfusion injury by inhibition of
microRNA-155 via reductions in neuroinflammation and oxidative
stress of vagal afferent nerve. Pulm Circ.
10(2045894020922125)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dong H, Qiang Z, Chai D, Peng J, Xia Y, Hu
R and Jiang H: Nrf2 inhibits ferroptosis and protects against acute
lung injury due to intestinal ischemia reperfusion via regulating
SLC7A11 and HO-1. Aging (Albany NY). 12:12943–12959.
2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang Y, Han K, Li Z, Tang X, Wang C, Zhao
Y, Zhang H, Geng Z, Kong J, Luan X and Xiong Y: Protective effect
of hydroxysafflor yellow A on renal ischemia-reperfusion injury by
targeting the Akt-Nrf2 axis in mice. Exp Ther Med.
24(741)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chepurnova DА, Samoilova ЕV, Verin АD,
Fesenko AG, Anisimov АА and Korotaeva AA: Inhibition of Meprins
Reduces Pulmonary Edema in LPS-Induced Acute Lung Damage. Bull Exp
Biol Med. 166:719–721. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Huang CY, Deng JS, Huang WC, Jiang WP and
Huang GJ: Attenuation of lipopolysaccharide-Induced acute lung
injury by hispolon in mice, Through Regulating the
TLR4/PI3K/Akt/mTOR and Keap1/Nrf2/HO-1 pathways, and suppressing
oxidative stress-mediated ER stress-induced apoptosis and
autophagy. Nutrients. 12(1742)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liu YY, Chiang CH, Hung SC, Chian CF, Tsai
CL, Chen WC and Zhang H: Hypoxia-preconditioned mesenchymal stem
cells ameliorate ischemia/reperfusion-induced lung injury. PLoS
One. 12(e0187637)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu Q, Gao Y and Ci X: Role of Nrf2 and
Its activators in respiratory diseases. Oxid Med Cell Longev.
2019(7090534)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ferrari RS and Andrade CF: Oxidative
stress and lung ischemia-reperfusion injury. Oxid Med Cell Longev.
2015(590987)2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bellezza I, Giambanco I, Minelli A and
Donato R: Nrf2-Keap1 signaling in oxidative and reductive stress.
Biochim Biophys Acta Mol Cell Res. 1865:721–733. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Han B, Li S, Lv Y, Yang D, Li J, Yang Q,
Wu P and Lv Zand Zhang Z: Dietary melatonin attenuates
chromium-induced lung injury via activating the Sirt1/Pgc-1α/Nrf2
pathway. Food Funct. 10:5555–5565. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhang H, Tan X, Yang D, Lu J, Liu B,
Baiyun R and Zhang Z: Dietary luteolin attenuates chronic liver
injury induced by mercuric chloride via the Nrf2/NF-κB/P53
signaling pathway in rats. Oncotarget. 8:40982–40993.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nguyen T, Nioi P and Pickett CB: The
Nrf2-antioxidant response element signaling pathway and its
activation by oxidative stress. J Biol Chem. 284:13291–13295.
2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yang D, Lv Z, Zhang H, Liu B, Jiang H, Tan
X, Lu J, Baiyun R and Zhang Z: Activation of the Nrf2 signaling
pathway involving KLF9 plays a critical role in allicin resisting
against arsenic trioxide-induced hepatotoxicity in rats. Biol Trace
Elem Res. 176:192–200. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Shaw P and Chattopadhyay A: Nrf2-ARE
signaling in cellular protection: Mechanism of action and the
regulatory mechanisms. J Cell Physiol. 235:3119–3130.
2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Baird L, Swift S, Lleres D and
Dinkova-Kostova AT: Monitoring Keap1-Nrf2 interactions in single
live cells. Biotechnol Adv. 32:1133–1144. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Xiang Q, Zhao Y, Lin J, Jiang S and Li W:
The Nrf2 antioxidant defense system in intervertebral disc
degeneration: Molecular insights. Exp Mol Med. 54:1067–1075.
2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Xiong Y, Xiong Y, Zhang H, Zhao Y, Han K,
Zhang J, Zhao D, Yu Z, Geng Z, Wang L, et al: hPMSCs-derived
exosomal miRNA-21 protects against aging-related oxidative damage
of CD4(+) T cells by targeting the PTEN/PI3K-Nrf2 axis. Front
Immunol. 12(780897)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Cadenas S: ROS and redox signaling in
myocardial ischemia-reperfusion injury and cardioprotection. Free
Radic Biol Med. 117:76–89. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Liebman SE and Le TH: Eat Your Broccoli:
Oxidative stress, NRF2, and sulforaphane in chronic kidney disease.
Nutrients. 13(266)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Dolivo D, Rodrigues A, Sun L, Galiano R,
Mustoe T and Hong SJ: Reduced hydration regulates pro-inflammatory
cytokines via CD14 in barrier function-impaired skin. Biochim
Biophys Acta Mol Basis Dis. 1868(166482)2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ijaz S, Mohammed I, Gholaminejhad M,
Mokhtari T, Akbari M and Hassanzadeh G: Modulating pro-inflammatory
cytokines, tissue damage magnitude, and motor deficit in spinal
cord injury with subventricular zone-derived extracellular
vesicles. J Mol Neurosci. 70:458–466. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ahmed SM, Luo L, Namani A, Wang XJ and
Tang X: Nrf2 signaling pathway: Pivotal roles in inflammation.
Biochim Biophys Acta Mol Basis Dis. 1863:585–597. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Juge N, Mithen RF and Traka M: Molecular
basis for chemoprevention by sulforaphane: A comprehensive review.
Cell Mol Life Sci. 64:1105–1127. 2007.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Ruhee RT and Suzuki K: The integrative
role of sulforaphane in preventing inflammation, oxidative stress
and fatigue: A review of a potential protective phytochemical.
Antioxidants (Basel). 9(521)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Eren E, Tufekci KU, Isci KB, Tastan B,
Genc K and Genc S: Sulforaphane Inhibits lipopolysaccharide-induced
inflammation, cytotoxicity, oxidative stress, and miR-155
expression and switches to mox phenotype through activating
extracellular signal-regulated kinase 1/2-nuclear factor erythroid
2-related factor 2/antioxidant response element pathway in murine
microglial cells. Front Immunol. 9(36)2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Sancho-Martinez SM, Lopez-Novoa JM and
Lopez-Hernandez FJ: Pathophysiological role of different tubular
epithelial cell death modes in acute kidney injury. Clin Kidney J.
8:548–559. 2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Li Y, Cao Y, Xiao J, Shang J, Tan Q, Ping
F, Huang W, Wu F, Zhang H and Zhang X: Inhibitor of
apoptosis-stimulating protein of p53 inhibits ferroptosis and
alleviates intestinal ischemia/reperfusion-induced acute lung
injury. Cell Death Differ. 27:2635–2650. 2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Saitoh Y, Ouchida R, Kayasuga A and Miwa
N: Anti-apoptotic defense of bcl-2 gene against
hydroperoxide-induced cytotoxicity together with suppressed lipid
peroxidation, enhanced ascorbate uptake, and upregulated Bcl-2
protein. J Cell Biochem. 89:321–334. 2003.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Renault TT and Chipuk JE: Death upon a
kiss: Mitochondrial outer membrane composition and organelle
communication govern sensitivity to BAK/BAX-dependent apoptosis.
Chem Biol. 21:114–123. 2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Ye Q, Zhu YI, Ye S, Liu H, She X, Niu Y
and Ming Y: Gypenoside attenuates renal ischemia/reperfusion injury
in mice by inhibition of ERK signaling. Exp Ther Med. 11:1499–1505.
2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Hu L, Chen L, Yang G, Li L, Sun H, Chang
Y, Tu Q, Wu M and Wang H: HBx sensitizes cells to oxidative
stress-induced apoptosis by accelerating the loss of Mcl-1 protein
via caspase-3 cascade. Mol Cancer. 10(43)2011.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Yang D, Han B, Baiyun R, Lv Z, Wang X, Li
S, Lv Y, Xue J, Liu Y and Zhang Z: Sulforaphane attenuates
hexavalent chromium-induced cardiotoxicity via the activation of
the Sesn2/AMPK/Nrf2 signaling pathway. Metallomics. 12:2009–2020.
2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Yang X, Fang Y, Hou J, Wang X, Li J, Li S,
Zheng X, Liu Y and Zhang Z: The heart as a target for deltamethrin
toxicity: Inhibition of Nrf2/HO-1 pathway induces oxidative stress
and results in inflammation and apoptosis. Chemosphere.
300(134479)2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Gao Z, Han Y, Hu Y, Wu X, Wang Y, Zhang X,
Fu J, Zou X, Zhang J, Chen X, et al: Targeting HO-1 by
epigallocatechin-3-gallate reduces contrast-induced renal injury
via Anti-oxidative stress and anti-inflammation pathways. PLoS One.
11(e0149032)2016.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Patel J, Baptiste BA, Kim E, Hussain M,
Croteau DL and Bohr VA: DNA damage and mitochondria in cancer and
aging. Carcinogenesis. 41:1625–1634. 2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Han B, Lv Z, Han X, Li S, Han B, Yang Q,
Wang X, Wu P, Li J, Deng N and Zhang Z: Harmful effects of
inorganic mercury exposure on kidney cells: Mitochondrial dynamics
disorder and excessive oxidative stress. Biol Trace Elem Res.
200:1591–1597. 2022.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Zhang L, Huang X, Guo T, Wang H, Fan H and
Fang L: Study of cinobufagin as a promising anticancer agent in
uveal melanoma through intrinsic apoptosis pathway. Front Oncol.
10(325)2020.PubMed/NCBI View Article : Google Scholar
|


















