Introduction
Lumbar interbody fusion (LIF) is used to treat
pathological spinal changes caused by lumbar degenerative disease,
trauma, infections, congenital malformations, tumors and other
diseases (1). LIF is one of the
most common types of spinal surgery used to provide support between
the vertebral bodies to stabilize the vertebral structure, restore
lordosis, correct deformity and provide indirect decompression of
compressed nerves, which is shown to have good clinical effects in
the treatment of spinal disease (2). Technological developments have
gradually evolved into a variety of intervertebral space treatment
methods and implant materials. Interbody fusions include anterior,
direct lateral, oblique lateral and transforaminal. The types of
implants used in fusion also vary. The most commonly used materials
for fusion grafts are Ti, PEEK and newer generation implants
(3). The material of the interbody
fusion cage is a key factor in interbody fusion. An ideal fusion
material must be sufficiently rigid to maintain stability and have
an elastic modulus similar to that of bone to prevent subsidence
and stress shielding (4). Titanium
(Ti) has been used in orthopedic surgery since the 1940s owing to
its excellent biocompatibility, low toxicity and good mechanical
properties (5). However, Ti has
poor radiation penetration and a large elastic modulus (70-100
GPa), which is higher than the 18.6 GPa of the cortical bone and
can easily lead to complications such as the sinking of the lumbar
interbody fusion apparatus (6).
With the development of medical-grade polyetheretherketone (PEEK),
PEEK has been used as a potential replacement material for Ti cages
in lumbar interbody fusion since the 1990s. Several studies showed
that the PEEK elastic modulus is close to that of human cortical
bone (7), which gives PEEK cages
advantages in spine load distribution and stress distribution
(8). PEEK was shown to have
excellent radiation penetration and could be used to evaluate the
progress of intervertebral bone fusion using X-rays (9). According to Setzer et al
(10), PEEK fusion devices are the
most commonly used because of their excellent elastic modulus and
effective fixation. However, PEEK also has a few hydrophilic groups
that do not provide sufficient space for cell adhesion (11). The biological inertness of the
material is related to its inability to integrate well with the
surrounding bone (12) and its
non-bone conductivity characteristics adversely affect bone fusion
(3,9). One of the techniques currently used
to improve the biological function of existing implants is to treat
cage surfaces with osteoconductive materials to increase fusion
rate and improve integration of the implant (3,9).
With the development of low-temperature coating technologies, Ti
metal has been combined with PEEK. Ti is coated on a PEEK surface
using a low-temperature coating process to enhance the
biocompatibility of the PEEK surface in a new type of interbody
fusion device, the Ti-PEEK cage, with the advantages of both
materials (7,13). Han et al (13) found through in vivo and
in vitro experiments that the biocompatibility of PEEK was
significantly improved after Ti coating and the wettability, cell
reactivity and bone conductivity of the PEEK cage also improved,
indicating that the Ti-PEEK cage had high application potential in
vertebral fusion surgery. Therefore, the present study aimed to
compare these differences using a meta-analysis to provide
theoretical guidance for clinical practice.
Materials and methods
Included data
The subjects were those included in published
controlled clinical studies. Non-case-control studies, case
reports, literature reviews, letters and repeated reports were
excluded. Literature that included cases which did not provide
enough relevant data was also excluded. Based on the patient
medical history, physical examination and imaging examination, all
patients underwent lumbar fusion. The intervention measures were
Ti-PEEK and PEEK cages. The outcome indicators were bone fusion
rate, cage settlement rate, postoperative Oswestry Disability Index
(ODI) score and postoperative lower back pain visual analog scale
(VAS) score (14-20).
Search strategy
A comprehensive search of the Embase (embase.com), PubMed (pubmed.ncbi.nlm.nih.gov/), Central Cochrane Library
(https://www.cochranelibrary.com/), China
National Knowledge Infrastructure (https://www.cnki.net/) and Wanfang databases
(https://www.wanfangdata.com.cn/) was
performed. Additionally, a manual search of journal catalogues and
bibliographical references was performed to find grey literature,
such as unpublished academic papers and chapters in monographs. All
relevant documents were searched in any language and translated if
necessary. No language restrictions were imposed. The English
search terms were ‘titanium-coated polyetheretherketone’,
‘Ti-PEEK’, ‘polyetheretherketone’, ‘PEEK’, ‘lumbar interbody
fusion’ and ‘LIF’.
Quality assessment of the
literature
The included studies were independently analyzed by
two authors and disagreements were resolved through discussion or
handed over to a third author to determine the quality of the
literature. This was done in strict accordance with the Cochrane
risk of bias assessment criteria (21) as follows: i) Experimental design
adopts randomization; ii) double blinding; iii) experimental data
is complete and reliable; iv) allocation concealment is used; v)
selective data reporting method; or vi) other bias. The quality of
the literature was also evaluated according to the Newcastle-Ottawa
scale (NOS) (22), with a total
score of nine points as follows: The representativeness of the
exposed group (true or representative of the community population),
1 point; choice of the non-exposed cohort (from the same community
as an exposed cohort), 1 point; determination of exposure factors
(reliable records or structured surveys), 1 point; no outcome to
observe at the start of the study (yes), 1 point; comparability
between groups, ≤2 points; double-blind principle, 1 point;
follow-up time length (≥2 years), 1 point and no loss to follow-up
(loss rate <15%), 1 point. A total score of ≥7 was considered
high-quality case study literature.
Statistical analysis
ReviewManager 5.4 software (training.cochrane.org/online-learning/core-software/revman)
was used to analyze the extracted data. Secondary variables are
expressed using Q-test, odds ratios (ORs) and 95% CIs. Continuous
variables are represented as mean or standardized mean differences
and 95% CIs. The I2 value was calculated to test the
heterogeneity between studies. When I2<50%, the
heterogeneity between the studies was considered small and a
fixed-effects model was used. If I²>50%, the heterogeneity
between studies was considered large and the cause of heterogeneity
was analyzed using a random-effects model. A sensitivity analysis
was conducted by removing some studies and creating funnel charts
to evaluate publication bias. P<0.05 was considered to indicate
a statistically significant difference.
Results
Search results
Using the aforementioned strategy, 84 relevant
studies were retrieved. After reading the titles and abstracts, 53
non-controlled studies, repeated publications and articles
irrelevant to the research purpose were excluded and 31 relevant
articles were screened. After reading the full text, the inclusion
and exclusion criteria were strictly followed for screening and
yielded seven studies (14-20).
All the included studies compared the baseline conditions of the
patients, such as age and disease duration, which were comparable
(P>0.05). A flowchart of the literature search strategy is shown
in Fig. 1; basic characteristics
of the included literature are shown in Table I.
 | Table IBaseline characteristics of the
studies included in the meta-analysis. |
Table I
Baseline characteristics of the
studies included in the meta-analysis.
| First author/s,
year | Study design | Country | Groups | Patients, n | Mean age,
years | Sex,
male/female | Outcomes | Newcastle- Ottawa
scale | (Refs.) |
|---|
| Hasegawa et
al, 2020 | Prospective | Japan | Ti-PEEK | 69 | 67.4±10.9 | 38/31 | (1) (2) (3) | 7 | (14) |
| | | | PEEK | 80 | 67.0±10.6 | 46/34 | | | |
| Rickert et
al, 2017 | RCT | Germany | Ti-PEEK | 20 | 67.7±12.5 | 6/14 | (1) (2) (3)
(4) | 8 | (15) |
| | | | PEEK | 20 | 68.3±10.5 | 6/14 | | | |
| Sakaura et
al, 2021 | Prospective | Japan | Ti-PEEK | 32 | 70.8±11.2 | 13/19 | (1) | 7 | (16) |
| | | | PEEK | 37 | 69.3±10.0 | 19/18 | | | |
| Schnake et
al, 2021 | RCT | Germany | Ti-PEEK | 28 | 52.9±32.7 | 13/15 | (1) (2) (3)
(4) | 9 | (17) |
| | | | PEEK | 27 | 50.6±33.3 | 6/21 | | | |
| Singhatanadgige
et al, 2022 | RCT | Thailand | Ti-PEEK | 41 | 62.73±9.5 | 15/26 | (1) (2) | 8 | (18) |
| | | | PEEK | 41 | 64.12±11.5 | 13/28 | | | |
| Willems et
al, 2019 | RCT | Belgium | Ti-PEEK | 44 | 50.0±9.7 | 17/27 | (1) (3) (4) | 8 | (19) |
| | | | PEEK | 37 | 51.5±8.4 | 21/16 | | | |
| Yao et al,
2023 | Retrospective | China | Ti-PEEK | 27 | 67.9±13.4 | 6/21 | (1) (2) (3)
(4) | 8 | (20) |
| | | | PEEK | 27 | 68.6±10.3 | 6/21 | | | |
Quality evaluation of the included
studies
The present study included four randomized
controlled trials, two prospective studies and one retrospective
study. NOS was used for quality evaluation. One trial scored 9
points, four trials scored 8 points, and two trials scored 7
points, equaling a total of seven high-quality and zero low-quality
articles. The methodological evaluation of the included studies was
performed using Cochrane Tools of Risk of Bias (training.cochrane.org/online-learning/core-software/revman)
and the evaluation items included random assignment method, hidden
grouping, blinding of participants, blinding of analysts,
completeness of outcome data, selective reporting of study results
and other sources of bias. For each entry, a judgment of low risk,
unclear or high risk was made. The risk of bias is shown in
Fig. S1. Although the number of
included studies was limited and there was some bias, the overall
quality was moderate.
Meta-analysis results
Comparison of postoperative bone fusion
rates. A total of five studies reported the postoperative bone
fusion rates at 6 months after surgery comparing Ti-PEEK and PEEK
(429 patients, 208 in the Ti-PEEK group and 221 in the PEEK group).
Heterogeneity tests showed heterogeneity between the five studies
(I2=65%; Q-test, P=0.02; Fig. 2). Effect sizes were combined using
the random-effects model and showed statistical significance
(Z=2.18; P=0.03; OR, 2.48; 95% CI, 1.09-5.60), suggesting that the
Ti-PEEK group had a superior lumbar interbody fusion rate compared
with that in the PEEK group 6 months postoperatively. A total of
six studies reported postoperative bone fusion rates (12 months)
comparing Ti-PEEK and PEEK (492 patients, 236 in the Ti-PEEK group
and 256 in the PEEK group). Heterogeneity tests were performed on
the six studies and the results showed no heterogeneity
(I2=43%; Q-test, P=0.12) and the fixed-effects model was
used to combine effect sizes. The results of the meta-analysis
showed no statistically significant difference between the two
groups (Z=0.60; P=0.55; OR, 1.22; 95% CI, 0.64-2.33), suggesting
that the Ti-PEEK and PEEK groups had similar rates of lumbar
interbody fusion at 12 months postoperatively. Combined with the
aforementioned results, this suggested the Ti-PEEK group exhibited
increased osteocyte growth compared with that of the PEEK group,
which in turn increased the rate of intervertebral fusion in the
short term (<6 months); however, the rate of vertebral fusion
was the same between the two groups in the long term (>12
months; Fig. 2).
Comparison of postoperative cage subsidence rates
(6-12 months after surgery). A total of five studies reported
postoperative cage subsidence rates comparing the Ti-PEEK and PEEK
groups (394 patients, 191 in the Ti-PEEK group and 203 in the PEEK
group). As the heterogeneity test showed no statistically
significant heterogeneity between the five studies
(I2=0%; Q-test, P=0.74), the fixed-effects model was
used for the effect size combination and the results showed no
statistically significant difference between the two groups
(Z=1.44, P=0.15; OR, 0.68; 95% CI, 0.40-1.15). This suggested that
the Ti-PEEK and PEEK groups had similar postoperative cage
sedimentation rates at 6-12 months postoperatively (Fig. 3).
Comparison of postoperative ODI scores (3, 6 and
12 months). A total of four studies reported the postoperative
ODI scores (3 months) between the Ti-PEEK and PEEK groups (229
patients, 118 in the Ti-PEEK group and 111 in the PEEK group). As
the heterogeneity test (I2=0%; Q-test, P=0.75) found
that the heterogeneity of these studies was not statistically
significant, the fixed-effects model was used for effect size
combination. The meta-analysis showed a statistically significant
difference between the two groups [Z=2.30; P=0.02; OR,-4.21; 95%
CI, (-7.80)-(-0.62)], suggesting that the Ti-PEEK group had
improved postoperative functional recovery compared with that in
the PEEK group at 3 months. A total of five studies reported the
postoperative ODI scores (6 months) between the Ti-PEEK and PEEK
groups (372 patients, 184 in the Ti-PEEK group and 188 in the PEEK
group). As the heterogeneity test (I2=49%; Q-test,
P=0.1) suggested that the heterogeneity was not statistically
significant, the fixed-effects model was used to combine the effect
sizes and the results showed no statistically significant
difference between the two groups [Z=0.86; P=0.39; OR, -1.25; 95%
CI, (-4.11)-1.61], indicating that functional recovery was
comparable between the two groups at 6 months postoperatively. A
total of five studies reported postoperative ODI scores (12 months)
between the Ti-PEEK and PEEK groups (366 patients, 181 in the
Ti-PEEK group and 185 in the PEEK group). As that the heterogeneity
test (I2=40%; Q-test, P=0.15) suggested no statistically
significant heterogeneity, the meta-analysis using the
fixed-effects model showed no statistically significant difference
between the two groups [Z=1.18; P=0.24; OR, 1.89; 95% CI,
(-1.25)-5.02)], suggesting that the functional recovery effect was
comparable between the two groups at 12 months postoperatively.
Combined with the aforementioned results, this suggested that the
Ti-PEEK group had improved spinal stability in the short term
(<3 months) and thus improved postoperative functional recovery,
while the two groups converged in terms of postoperative functional
recovery in the long term (>6 months; Fig. 4).
Comparison of postoperative VAS score for back
pain (3, 6 and 12 months). A total of four studies reported the
postoperative VAS scores for back pain (3 months) between the
Ti-PEEK and PEEK groups (229 patients, 118 in the Ti-PEEK group and
111 in the PEEK group). As the heterogeneity test
(I2=75%; Q-test, P=0.008) suggested that the
heterogeneity was statistically significant, meta-analysis using
the random effects model showed no statistically significant
difference between the two groups [Z=0.37; P=0.71; OR, -0.15; 95%
CI, (-0.96)-0.66)]. This suggested that the effect of lower back
pain relief at 3 months postoperatively was comparable between the
two groups. A total of four studies reported the postoperative VAS
scores for back pain (6 months) between the Ti-PEEK and PEEK groups
(223 patients, 115 in the Ti-PEEK group and 108 in the PEEK group).
As the heterogeneity test (I2=0%; Q-test, P=0.87)
suggested that the heterogeneity was not statistically significant,
meta-analysis using a fixed-effects model showed a statistically
significant difference between the two groups [Z=3.34, P=0.0008;
OR=-0.56; 95% CI, (-0.89)-(-0.23)], suggesting that the Ti-PEEK
group had improved postoperative lower back pain symptom relief
compared with that in the PEEK group at 6 months postoperatively. A
total of four studies reported the postoperative VAS scores for
back pain (12 months) between the Ti-PEEK and PEEK groups (221
patients, 116 in the Ti-PEEK group and 105 in the PEEK group). As
the heterogeneity test (I2=63%; Q-test, P=0.04)
suggested that the heterogeneity was statistically significant,
meta-analysis using the random effects model showed no
statistically significant difference between the two groups
[Z=0.11; P=0.91; OR, 0.04; 95% CI, (-0.63)-0.71)], suggesting that
the Ti-PEEK and PEEK groups were similar in terms of lower back
pain symptom relief at 12 months postoperatively (Fig. 5).
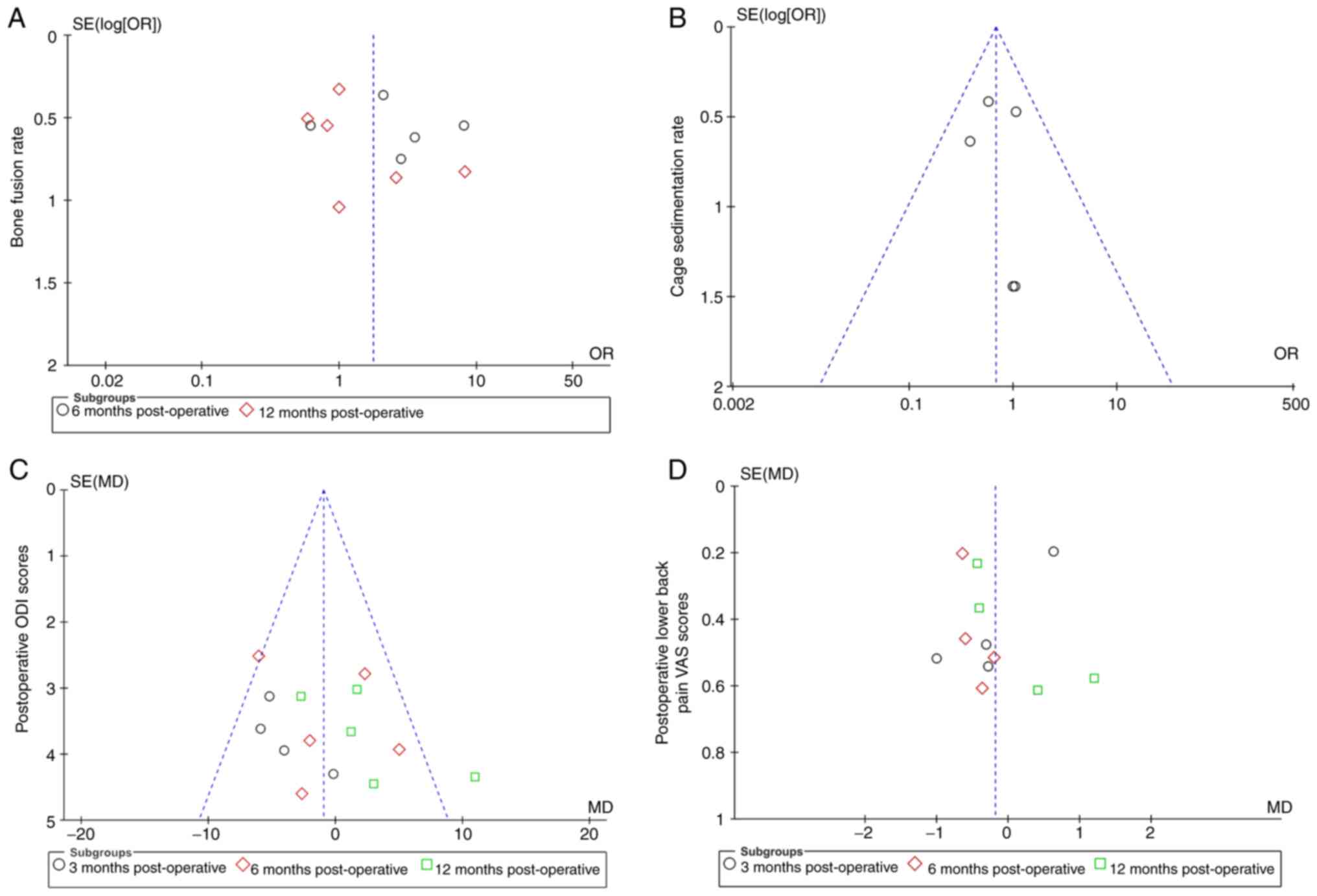
Publication bias and sensitivity
analysis
ReviewManager 5.4 statistical software provided by
the Cochrane Collaboration was used to analyze the publication bias
of the four outcome indicators: i) Bone fusion rate; ii) cage
subsidence rate; iii) postoperative ODI score; and iv)
postoperative lower back pain VAS score. A funnel plot is provided
in Fig. 6. The horizontal axis of
the funnel plot is the effect size, which is related to the OR
value; the smaller the OR value, the more the distribution is to
the left and vice versa. The y axis is the standard error, which is
related to the sample size; the larger the sample size, the higher
the accuracy, and the smaller the standard error, the more the
distribution is concentrated upward. The vertical median in the
middle, which is perpendicular to the horizontal axis, is the
combined OR value. Ideally, each study should be evenly distributed
on the left and right sides of the vertical midline, with large
samples concentrated at the top and small samples scattered at the
bottom of the graph. The results showed that the samples were
concentrated around the vertical midline and in the upper middle of
the graph, with symmetry between the left and right sides,
suggesting no significant publication bias (Fig. 6). After eliminating the samples
with a large bias one by one through sensitivity analysis, the
remaining samples were pooled for meta-analysis, and the results
did not show any changes; therefore, the data of this study were
considered to be relatively stable and reliable.
Discussion
The lumbar vertebrae often bear high pressure in the
human body, leading to degenerative vertebral disease in the lumbar
vertebrae. This occurs mainly in the lower L vertebrae (L4/5 and
L5/S1) (23,24) and is often accompanied by severe
degeneration and collapse of the intervertebral disc resulting in
smaller local lordosis, thus affecting overall balance of the spine
(25). LIF is the standard
procedure used by spinal surgeons to treat various lumbar diseases.
Intervertebral fusion aims to reconstruct the height and lordosis
angle of the intervertebral space and achieve radiological
interbody bone fusion (26).
Currently, PEEK and Ti-PEEK cages are the most commonly used fusion
devices in spine surgery (27).
PEEK cages have an elastic modulus similar to that of human
cortical bone and good radiation penetration, which enables them to
improve the evaluation of the progress of bone fusion. PEEK has
been widely used for vertebral fusion in spinal surgery and is
currently the most widely used fusion material (12). Based on the PEEK cage, the Ti-PEEK
cage is evenly coated on the surface using low-temperature coating
technology and has an elastic modulus similar to that of PEEK with
improved biocompatibility and bone conductivity; therefore, it has
been increasingly favored by spine surgeons (28). Therefore, the present study
compared the bone fusion rate, cage settlement rate, postoperative
ODI score and VAS score of lower back pain between Ti-PEEK and PEEK
cages.
At present, to the best of our knowledge, only a few
studies compared Ti-PEEK cages with PEEK cages and the follow-up
time is limited (4,29). In the present study, the bone
fusion rates were compared at 6 and 12 months after surgery. The
results showed that compared with the uncoated PEEK cage, the
Ti-PEEK cage showed more advantages in terms of the bone fusion
rate 6 months after the operation. This indicated that compared
with that achieved using uncoated PEEK cages, Ti-PEEK cages ensured
improved bone growth capacity in the short term (≤6 months), which
was consistent with the results of Massaad et al (30). Studies showed that PEEK cages form
a special biofilm that affected cortical bone growth, thereby
slowing down the rate of bone fusion (3,28).
Ti-PEEK has no such characteristics and the surface Ti coating can
provide more solid stability in the vertebral space by increasing
friction to limit micromovement, while its superior cell adhesion
characteristics provide a good environment for cell growth.
Simultaneously, Ti-PEEK stimulates osteoblasts activity and reduces
osteoclast activity, promoting early bone fusion in the surgical
segment (28,31). Kashii et al (32) and Welsh et al (33) also showed through in vitro
and in vivo experiments in animals that Ti or Ti-coated
cages promoted osteocyte growth and surface bone tissue growth of
Ti-coated cages was ~five times that of uncoated cages. However,
the present study showed that there was no statistically
significant difference in the bone fusion rate between the two
groups at 12 months after surgery. The present results indicated
that the two groups had a comparable rate of intervertebral bone
fusion at 12 months after lumbar interbody fusion and the
postoperative effect was similar.
The cage settlement rate, which is the opposite of
the bone fusion rate during LIF, poses a challenge for spine
surgeons. The elastic modulus of Ti cage material is much larger
than that of human cortical bone, which leads to a high
sedimentation rate, which is one of its main disadvantages. Medical
grade PEEK materials are regarded as promising alternatives because
they have the same elastic modulus as human cortical bone, which is
beneficial for reducing settlement rate in the cage. As a composite
material, it was unclear if the Ti-PEEK cage had the same low cage
settlement rate as the PEEK cage (3,32,34).
The current meta-analysis compared the cage settlement rate between
Ti-PEEK and PEEK cages 6 months after lumbar interbody fusion, and
the results showed that there was no statistically significant
difference in the settlement rate between the two groups. This
indicated that the Ti coating on the PEEK surface did not change
the elastic modulus of the PEEK. This result was consistent with
those of Lv et al (4) and
Massaad et al (30). Due to
the limited follow-up of the postoperative cage sedimentation rate
in the literature included in the present study, caution needs to
be exercised in the assumption of the cage sedimentation rate
results of the present meta-analysis.
The primary purpose of LIF is to relieve pain and
achieve good functional recovery and clinical efficacy.
Postoperative ODI and VAS scores are used to evaluate postoperative
functional recovery and pain relief. The results of the present
study showed that, compared with that of the PEEK cages, Ti-PEEK
cages had a significant advantage in the ODI score at 3 months
after the operation. However, there was no significant difference
in the ODI scores between the two groups at 6 and 12 months
postoperatively. Regarding the VAS score, Ti-PEEK cages had an
advantage over PEEK cages in relieving lower back pain 6 months
after surgery. However, there was no difference in the VAS scores
for lower back pain between 3 and 12 months after surgery.
Combining the postoperative ODI scores, lower back pain VAS scores
and bone fusion rates, the results indicated that the Ti-PEEK cage
could promote bone growth in the early period (≤6 months), which
nay achieve improved bone fusion, and thus provide improved relief
of lower back pain symptoms and postoperative functional recovery
in the early period. The clinical effect of the Ti-PEEK cage in the
early period (≤6 months) was improved compared with that in the
PEEK group. However, the two groups had similar bone fusion rates
in the long term after surgery (≥12 months), so it is reasonable
that there was no significant difference in later ODI and VAS
scores between the two groups.
In conclusion, both Ti-PEEK and PEEK cages had high
bone fusion rates, low cage settlement rates and good clinical
efficacy. However, Ti-PEEK cages provided the advantages of both Ti
and PEEK. Ti-PEEK has an elastic modulus close to that of human
cortical bone and promotes the growth of osteoid cells and
increases the cell adhesion space, enabling Ti-PEEK cages to
achieve a higher bone fusion rate and improved relief of lower back
pain in the early postoperative period without increasing the cage
settlement rate, thereby obtaining improved clinical outcomes.
This meta-analysis had some limitations. First,
spinal surgeons differed in their surgical methods and use of cage
fillings (autologous bone, allogeneic bone or mixed fillings).
Second, only seven articles were included, including four
randomized controlled trials, two prospective studies and one
retrospective study with a low level of evidence. Third, this
limited data restricted the ability to conduct further comparisons
and subgroup analyses. Fourth, the current study was based on a
secondary analysis of the original literature and the relative
follow-up duration of the outcome indicators adopted in the
original literature that met the inclusion criteria was limited.
For example, detailed data on the long-term follow-up results of
the cage sedimentation rate were not provided in the original
literature. Meta-analysis of the long-term postoperative cage
sedimentation rates could not be performed. However, with an
increase in study sample size, extension of the follow-up and an
improvement in the quality of the included samples, the conclusions
obtained from the present meta-analysis could be further validated
and supported.
Supplementary Material
Summary of risk of bias. (A) Risk of
bias graph. Each risk of bias item is presented as a percentage
across all included studies and indicates the proportional level
for each risk of bias item. (B) Risk of bias. The methodological
quality of the included studies according to a risk of bias tool
that assessed randomization (sequence generation and allocation
concealment), blinding (participants, personnel and outcome
assessors), completeness of outcome data, selection of outcomes
reported and other sources of bias.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Ethics approval and consent to
participate
Not applicable.
Authors' contributions
SL and XL participated in the design of this study
and performed the statistical analysis. XB and YW performed the
study and collected data. PH and HL conceived the study,
interpreted data and drafted the manuscript. All authors have read
and approved the final manuscript. SL and XL confirm the
authenticity of all the raw data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Resnick DK, Choudhri TF, Dailey AT, Groff
MW, Khoo L, Matz PG, Mummaneni P, Watters WC III, Wang J, Walters
BC, et al: Guidelines for the performance of fusion procedures for
degenerative disease of the lumbar spine. Part 12: Pedicle screw
fixation as an adjunct to posterolateral fusion for low-back pain.
J Neurosurg Spine. 2:700–706. 2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
de Kunder SL, Rijkers K, Caelers IJMH, de
Bie RA, Koehler PJ and van Santbrink H: Lumbar interbody fusion: A
historical overview and a future perspective. Spine (Phila Pa
1976). 43:1161–1168. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Verma R, Virk S and Qureshi S: Interbody
fusions in the lumbar spine: A review. HSS J. 16:162–167.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lv ZT, Xu Y, Cao B, Dai J, Zhang SY, Huang
JM, Liang S and Jiang FX: Titanium-coated PEEK versus uncoated PEEK
cages in lumbar interbody fusion: A systematic review and
meta-analysis of randomized controlled trial. Clin Spine Surg: Aug
24, 2022 (Epub ahead of print).
|
|
5
|
Su EP, Justin DF, Pratt CR, Sarin VK,
Nguyen VS, Oh S and Jin S: Effects of titanium nanotubes on the
osseointegration, cell differentiation, mineralisation and
antibacterial properties of orthopaedic implant surfaces. Bone
Joint J. 100-B:9–16. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Li C, Liao Z, Zhu J, et al: Structure and
materials of lumbar interbody fusion apparatus. Int J Orthop.
38:360–363. 2017.
|
|
7
|
Ma R and Tang T: Current strategies to
improve the bioactivity of PEEK. Int J Mol Sci. 15:5426–5445.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mobbs RJ, Phan K, Assem Y, Pelletier M and
Walsh WR: Combination Ti/PEEK ALIF cage for anterior lumbar
interbody fusion: Early clinical and radiological results. J Clin
Neurosci. 34:94–99. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Olivares-Navarrete R, Hyzy SL, Slosar PJ,
Schneider JM, Schwartz Z and Boyan BD: Implant materials generate
different peri-implant inflammatory factors:
Poly-ether-ether-ketone promotes fibrosis and microtextured
titanium promotes osteogenic factors. Spine (Phila Pa 1976).
40:399–404. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Setzer M, Eleraky M, Johnson WM, Aghayev
K, Tran ND and Vrionis FD: Biomechanical comparison of anterior
cervical spine instrumentation techniques with and without
supplemental posterior fusion after different corpectomy and
discectomy combinations: Laboratory investigation. J Neurosurg
Spine. 16:579–584. 2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nemoto O, Asazuma T, Yato Y, Imabayashi H,
Yasuoka H and Fujikawa A: Comparison of fusion rates following
transforaminal lumbar interbody fusion using polyetheretherketone
cages or titanium cages with transpedicular instrumentation. Eur
Spine J. 23:2150–2155. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Seaman S, Kerezoudis P, Bydon M, Torner JC
and Hitchon PW: Titanium vs polyetheretherketone (PEEK) interbody
fusion: Meta-analysis and review of the literature. J Clin
Neurosci. 44:23–29. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Han CM, Lee EJ, Kim HE, Koh YH, Kim KN, Ha
Y and Kuh SU: The electron beam deposition of titanium on
polyetheretherketone (PEEK) and the resulting enhanced biological
properties. Biomaterials. 31:3465–3470. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hasegawa T, Ushirozako H, Shigeto E, Ohba
T, Oba H, Mukaiyama K, Shimizu S, Yamato Y, Ide K, Shibata Y, et
al: The titanium-coated PEEK cage maintains better bone fusion with
the endplate than the PEEK cage 6 months after PLIF surgery: A
multicenter, prospective, randomized study. Spine (Phila Pa 1976).
45:E892–E902. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rickert M, Fleege C, Tarhan T, Schreiner
S, Makowski MR, Rauschmann M and Arabmotlagh M: Transforaminal
lumbar interbody fusion using polyetheretherketone oblique cages
with and without a titanium coating: A randomised clinical pilot
study. Bone Joint J. 99-B:1366–1372. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sakaura H, Ikegami D, Fujimori T, Sugiura
T, Yamada S and Mukai Y: Surgical outcomes after posterior lumbar
interbody fusion using traditional trajectory screw fixation or
cortical bone trajectory screw fixation: A comparative study
between the polyetheretherketone cage and the same shape
titanium-coated polyetheretherketone cage. Clin Neurol Neurosurg.
209(106945)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Schnake KJ, Fleiter N, Hoffmann C, Pingel
A, Scholz M, Langheinrich A and Kandziora F: PLIF surgery with
titanium-coated PEEK or uncoated PEEK cages: A prospective
randomised clinical and radiological study. Eur Spine J.
30:114–121. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Singhatanadgige W, Tangchitcharoen N, Kerr
SJ, Tanasansomboon T, Yingsakmongkol W, Kotheeranurak V and
Limthongkul W: Comparison of PEEK and TiPEEK in minimally invasive
transforaminal lumbar interbody fusion: A randomized clinical
trial. World Neurosurg. 68:2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Willems K, Lauweryns P, Verleye G and VAN
Goethem J: Randomized controlled trial of posterior lumbar
interbody fusion with Ti- and CaP-nanocoated polyetheretherketone
cages: Comparative study of the 1-year radiological and clinical
outcome. Int J Spine Surg. 13:575–587. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Yao YC, Chou PH, Lin HH, Wang ST and Chang
MC: Outcome of Ti/PEEK versus PEEK cages in minimally invasive
transforaminal lumbar interbody fusion. Global Spine J. 13:472–478.
2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Higgins JPT, Altman DG, Gøtzsche PC, et
al: The Cochrane Collaboration’s tool for assessing risk of bias in
randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rade M, Määttä JH, Freidin MB, Airaksinen
O, Karppinen J and Williams FMK: Vertebral endplate defect as
initiating factor in intervertebral disc degeneration: Strong
association between endplate defect and disc degeneration in the
general population. Spine (Phila Pa 1976). 43:412–419.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hey HWD, Lau ET, Tan KA, Lim JL, Choong D,
Lau LL, Liu KG and Wong HK: Lumbar spine alignment in six common
postures: An ROM analysis with implications for deformity
correction. Spine (Phila Pa 1976). 42:1447–1455. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Barrey C, Jund J, Noseda O and Roussouly
P: Sagittal balance of the pelvis-spine complex and lumbar
degenerative diseases. A comparative study about 85 cases. Eur
Spine J. 16:1459–1467. 2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lee JH, Lee JH, Yoon KS, Kang SB and Jo
CH: Comparative study of unilateral and bilateral cages with
respect to clinical outcomes and stability in instrumented
posterior lumbar interbody fusion. Neurosurgery. 63:109–114.
2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fang S and Zeng Z: Research progress of
bone grafting material in interbody fusion. J Spinal Surg.
16:57–61. 2018.
|
|
28
|
Wu X, Liu X, Wei J, Ma J, Deng F and Wei
S: Nano-TiO2/PEEK bioactive composite as a bone substitute
material: In vitro and in vivo studies. Int J Nanomedicine.
7:1215–1225. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Assem Y, Mobbs RJ, Pelletier MH, Phan K
and Walsh WR: Radiological and clinical outcomes of novel Ti/PEEK
combined spinal fusion cages: a systematic review and preclinical
evaluation. Eur Spine J. 26:593–605. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Massaad E, Fatima N, Kiapour A, Hadzipasic
M, Shankar GM and Shin JH: Polyetheretherketone versus titanium
cages for posterior lumbar interbody fusion: Meta-analysis and
review of the literature. Neurospine. 17:125–135. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gittens RA, McLachlan T,
Olivares-Navarrete R, et al: The effects of combined
micron-/submicron-scale surface roughness and nanoscale features on
cell proliferation and differentiation. Biomaterials. 32:3395–3403.
2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kashii M, Kitaguchi K, Makino T and Kaito
T: Comparison in the same intervertebral space between
titanium-coated and uncoated PEEK cages in lumbar interbody fusion
surgery. J Orthop Sci. 25:565–570. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Walsh WR, Pelletier MH, Christou C, He J,
Vizesi F and Boden SD: The in vivo response to a novel Ti coating
compared with polyether ether ketone: Evaluation of the periphery
and inner surfaces of an implant. Spine J. 18:1231–1240.
2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chong E, Mobbs RJ, Pelletier MH and Walsh
WR: Titanium/Polyetheretherketone Cages for Cervical Arthrodesis
with Degenerative and Traumatic Pathologies: Early Clinical
Outcomes and Fusion Rates. Orthop Surg. 8:19–26. 2016.PubMed/NCBI View
Article : Google Scholar
|