Introduction
Desmoid fibromatosis (DF) is prone to recurrence
following surgery, with a high local recurrence rate of 17.6-30.7%
(1-4).
Surgery is the preferred treatment for familial adenomatous
polyposis (FAP), and DF usually occurs after FAP surgery (5). The incidence of DF in patients with
FAP ranges between 10 and 30% and is 1,000 times higher than that
noted in the general population (5,6).
Based on the disease characteristics of DF, it may be stable for a
long time or even subside naturally (7). Meanwhile, mesenteric DF surgery can
easily cause short bowel syndrome, affecting the quality of life of
patients. After performing FAP radical surgery, it is therefore
feasible to choose a waiting strategy for DF observation. An
additional summary of clinical cases and findings of evidence-based
medicine is required in future for effective evaluation.
Case report
A 32-year-old male patient was admitted to Weihai
Central Hospital (Weihai, China) in October 2021 due to
experiencing abdominal pain and distension for 3 days. The
abdominal pain was paroxysmal dull pain around the umbilicus, which
was without other radiating pain. There was no history of surgery
or trauma. The family history revealed adenomatous polyposis coli
(APC) in both the mother and sister; the mother had passed away due
to colon cancer. Physical examination indicated a slightly
distended abdomen, an absence of gastrointestinal type or
peristaltic waves and the lack of abdominal wall varices. The
examination further revealed periumbilical tenderness, absence of
rebound tenderness and lack of abdominal muscle tension. No
significant mass was obvious in the whole abdomen. Both Murphy's
sign and shifting dullness were negative. The abdomen plain
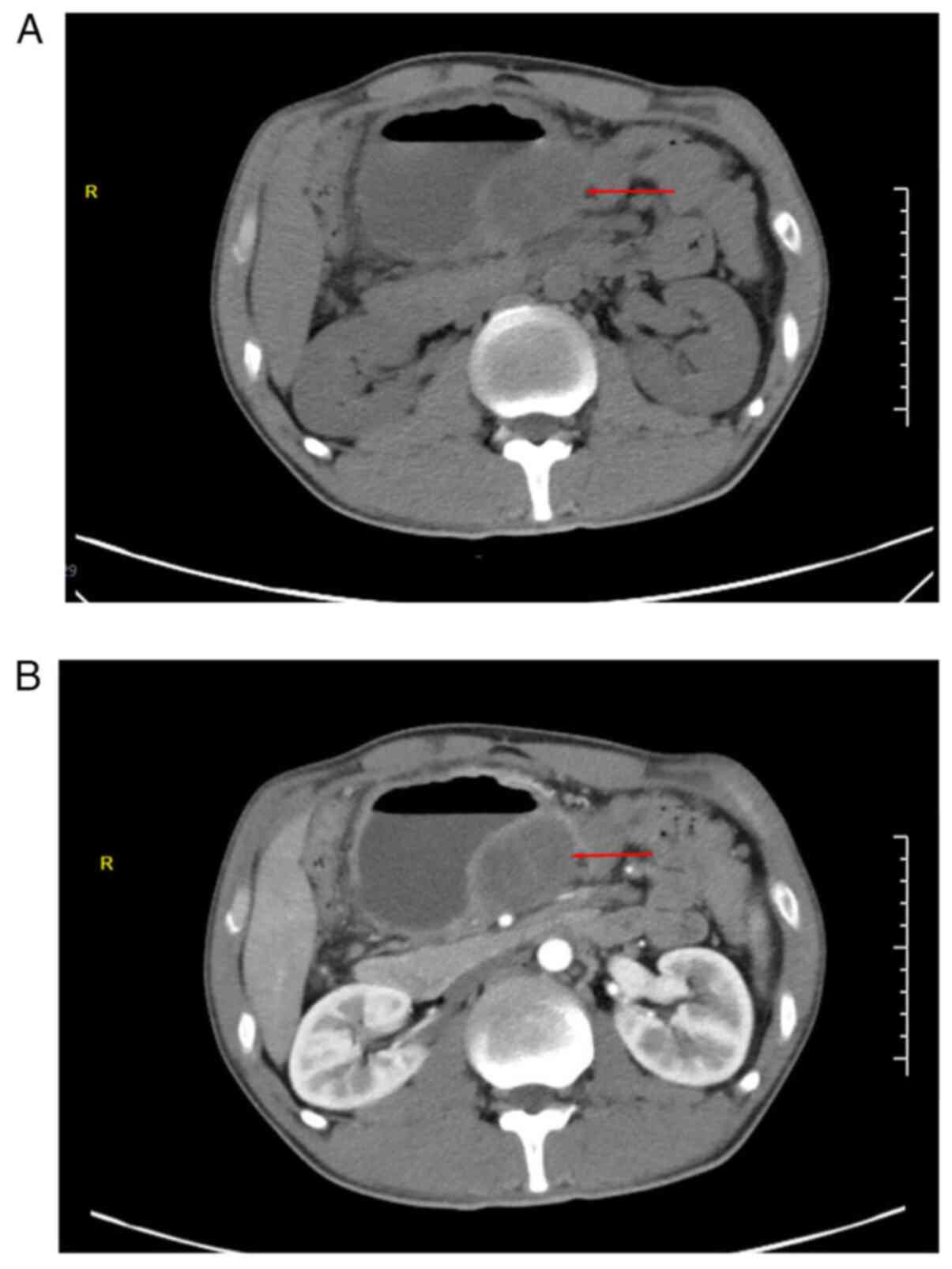
computed tomography (CT; Fig. 1A)
indicated a slightly low-density shadow of ~4.8x5.7 cm in the
abdominal cavity, surrounded by a dilated intestinal canal, and a
liquid-gas interface. In contrast-enhanced CT (Fig. 1B), the solid lesion exhibited
mild-to-moderate enhancement with fairly clear margins. In summary,
the patient was diagnosed with an abdominal space-occupying lesion
(considering the source of mesenchyme) with intestinal
obstruction.
The admission diagnosis was abdominal occupancy and
incomplete intestinal obstruction. Due to intestinal obstruction,
it was not possible to perform bowel preparation. The patient was
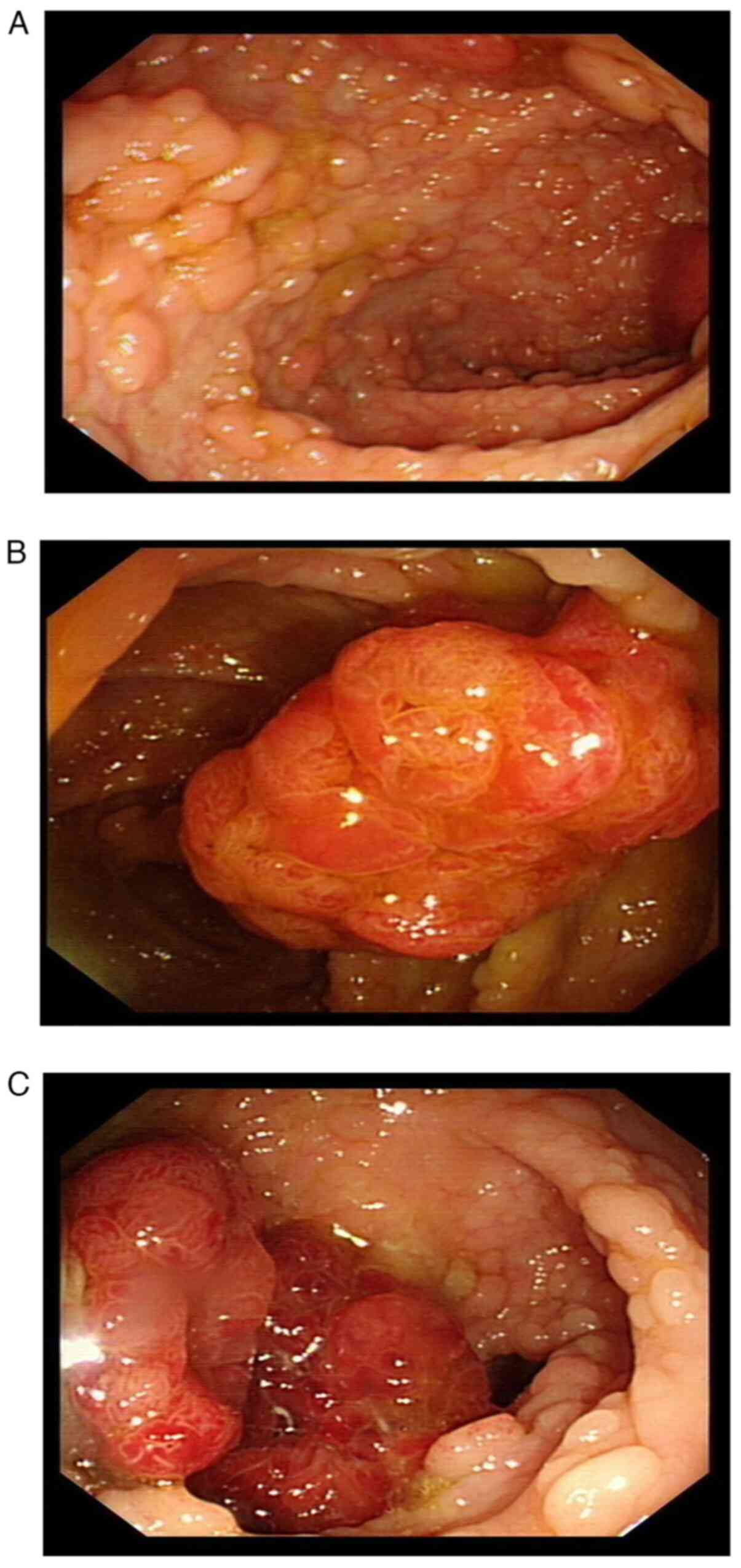
examined by gastroscopy. The mucosa of the gastric fundus, body and
sinuses were congested. Dozens of polyps were scattered at the
gastric mucosa, the largest one was ~6 mm in diameter, and the
surface was congested (Fig. 2A and
B). Chronic inflammation of the
mucosa with erosion and reactive hyperplasia of specific glands was
noted by gastroscopic biopsy (Fig.
2C).
Conservative treatment methods, including diet
prohibition, gastrointestinal decompression and parenteral
nutrition, did not relieve the intestinal obstruction. A
laparoscopic exploration was accordingly performed.
Intraoperatively, a coiled small intestinal canal was detected,
which was found to adhere to the tumor. Following loosening of the
adhesion, the tumor was located at the root of the small intestine
mesentery, surrounded by inflammatory exudation, without invasion
of the intestinal wall. Considering the family history of the
patient, FAP could not be excluded; therefore, the mesenteric mass
was biopsied. Microscopically, proliferating spindle cells arranged
in bundles, with mild cell morphology and interstitial
collagenization with mucus degeneration (Fig. 3A-C). The postoperative pathology
was invasive fibromatosis (also known as DF) with the following
immunohistochemical results noted: β-catenin (partial nuclear
positive), smooth muscle actin (partial positive), desmin
(scattered positive), CD117 (negative), Ki-67 (positive; 5%), CD34
(vascular positive), S-100 (negative) and SRY-box transcription
factor 10 (negative) (Fig.
3D-K).
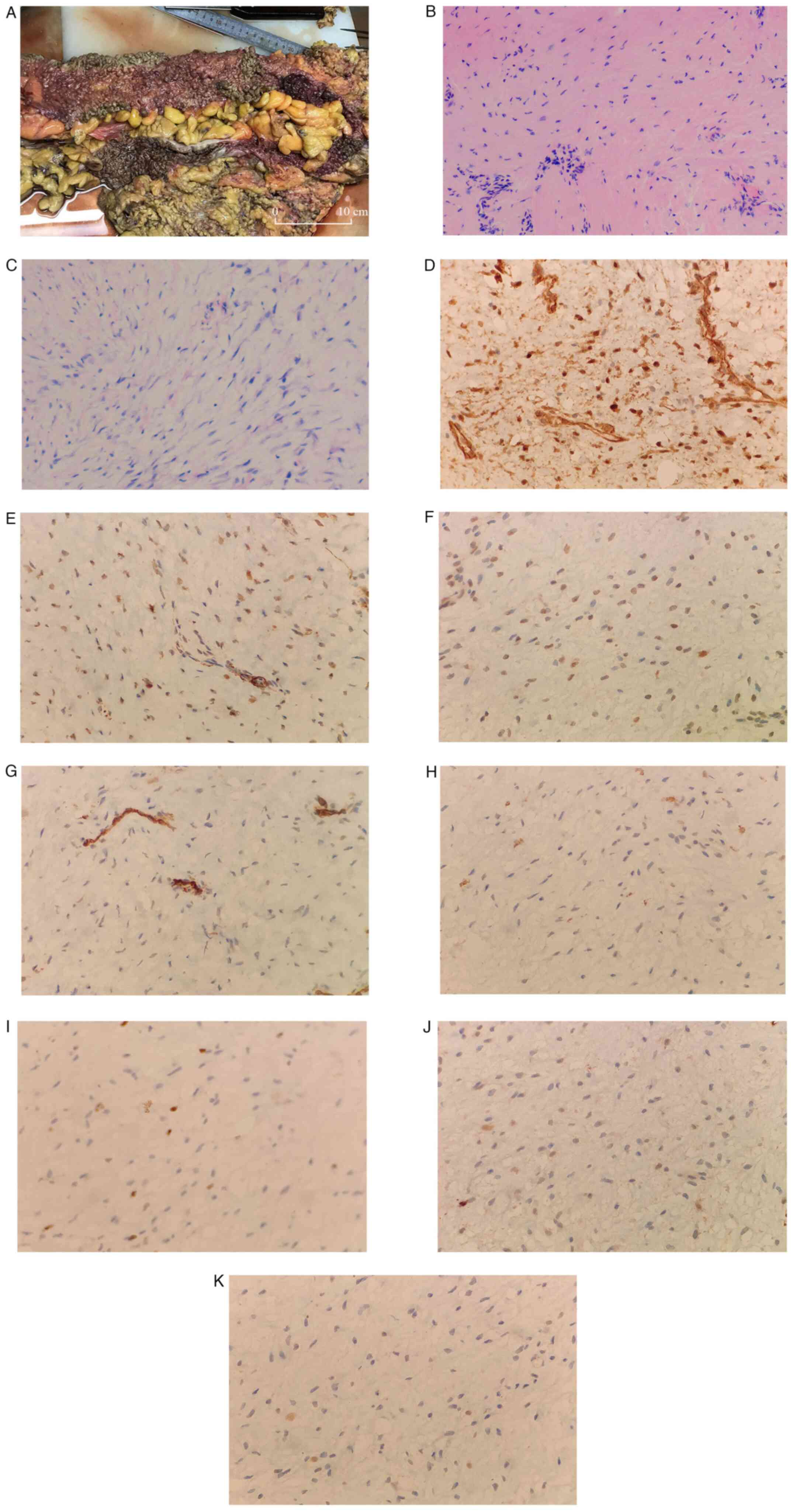 | Figure 3Continued. An excised portion of the
colon. (A) Mucosal surface of the large intestine was densely
covered with polyps. (B and C) Proliferating spindle cells arranged
in bundles, with mild cell morphology and interstitial
collagenization with mucus degeneration. (B) H&E
(magnification, x100). (C) H&E (magnification, x200).
Immunohistochemistry of the biopsy determined the sample to be (D)
β-catenin (partial nuclear positive), (E) smooth muscle actin
(partial positive), (F) desmin (scattered positive), (G) CD117
(negative), (H) Ki-67 (positive; 5%), (I) CD34 (vascular positive),
(J) S-100 (negative) and (K) SRY-box transcription factor 10
(negative). (D-K) Magnification, x400. |
IHC staining was performed on a BenchMark XT (Roche
Diagnostics) automatic IHC staining device. All procedures were
performed as per the manufacturer's protocols. The endogenous
peroxides and protein were blocked using the Endogenous Biotin
Blocking kit (cat. no. PV-6000; OriGene Technologies, Inc.) at 37˚C
for 4 min. The following primary antibodies were used:
Anti-β-catenin (cat. no. ZA-0646; working solution), anti-SMA (cat.
no. ZM-0003; working solution), anti-desmin (cat. no. ZA-0610;
working solution), anti-CD117 (cat. no. ZA-0523; working solution),
anti-Ki-67 (cat. no. ZM-0166; working solution), anti-CD34 (cat.
no. ZA-0550; working solution), anti-S-100 (cat. no. ZA-0225;
working solution) and anti-SOX10 (cat. no. ZA-0624; working
solution) (all OriGene Technologies, Inc.). Primary antibodies were
added and incubated for 15 min at 37˚C. A light microscope was used
for observation.
Following the operation, the intestinal obstruction
of the patient was relieved. Colonoscopy examination (Fig. 4A) indicated dense and flat polyps
3-10 mm in diameter in the total colon and rectum. A lobulated
polyp of ~25 mm in diameter with dilated surface ducts was detected
~40 cm from the anal verge (Fig.
4B). Multiple subpial lobulated polyps 10-15 mm in diameter
were detected ~27 cm from the anal verge (Fig. 4C). No abnormal signs were detected
in the anal canal. A confined operation of total proctocolectomy
(TPC) with ileal pouch-anal anastomosis (IPAA) was performed. Two
macroadenomas and hundreds of tubular adenomas were removed.
Postoperative pathology of the two large adenomas: Adenoma 1 was a
tubular adenoma (low-grade intraepithelial neoplasia, some
high-grade intraepithelial neoplasia; Fig. 5A); adenoma 2 was a tubular adenoma
(high-grade intraepithelial neoplasia; Fig. 5B) with focal adenocarcinoma.
Postoperative pathology of the tubular adenoma: Hundreds of pieces
(low-grade intraepithelial neoplasia), 0.3-1.0 cm in diameter,
consistent with familial adenomatous polyposis. No lesions were
found on the surgical line. No cancer was found in 11
peri-intestinal lymph nodes (0/11). The patient was followed up in
the outpatient clinic for 1 year, where CT scans were performed
every 6 months. The patient has since been stable up to the last
examination in June 2022.
Discussion
DF is a clinically rare clonal proliferative
disease, originating from the mesenchymal tissue, and is a
borderline tumor (8). Certain
cases of aggressive fibromatosis with variable clinical courses may
remain stable for a long time and some may grow invasively to
surround the organs. Other cases of DF may even regress
spontaneously (9). Although not
prone to distant metastasis, DF is prone to recurrence following
surgery with a high local recurrence rate of 17.6-30.7% (1-4).
Primary DF is rare but a common concomitant disease of FAP. The
incidence of DF in patients with FAP ranges between 10 and 30% and
is 1,000 times higher than that noted in the general population
(5,6).
FAP is an autosomal dominant inherited disease. It
is considered that FAP is mainly caused by mutations in the
oncogene APC located on the fifth chromosome. FAP can develop in
adolescence, accounting for ~1% of the total incidence of
precancerous colorectal cancer (10). FAP lesions present as widespread
adenomatous polyps in the colorectum and may also occur in the
upper gastrointestinal tract or even throughout the
gastrointestinal tract of patients (11). The development of FAP to colorectal
cancer is almost inevitable without treatment, and surgery is the
preferred treatment strategy in addition to regular monitoring
(7). Surgical interventions are
available, including for suspicious canceration, multiple >6-mm
adenomas, a significant increase in the number of adenomas after
multiple examinations, pathological indications of highly atypical
hyperplastic adenomas, obstruction or bleeding. There are three
surgery options for this disease: TPC with IPAA, total abdominal
colectomy with ileorectal anastomosis (IRA) and TPC with end
ileostomy (12). It has been
reported that IPAA and IRA are routinely used as preventive surgery
for FAP, while the risk of DF following IPAA surgery is
significantly higher than that following IRA. The probability of DF
was increased sequentially when comparing patients who underwent
small bowel rectal anastomosis, small bowel anal anastomosis and
small bowel stoma (13).
Nieuwenhuis et al (14)
followed up 62 cases of postoperative DF following FAP for a median
time of 8 years and reported no difference in progression-free
survival between patients treated with and without surgery (33% vs.
49%; P=0.163). A total of 9 out of the 36 patients (25%) who
underwent surgery succumbed to the disease due to recurrence.
Patients with DF have a high rate of local
recurrence following surgery. Rutenberg et al (15) reported a postoperative recurrence
rate of 24-77% for DF. Conventional treatment of DF includes
non-steroidal anti-inflammatory drugs, anti-estrogens, cytotoxic
chemotherapy and radiotherapy, in addition to local resection
(12). Research shows that
targeted drugs such as sorafenib have a good control effect on
patients with AF, with a total effective rate of treatment of 33%
(16). Considering the likely
complications associated with surgery and radiotherapy and the
unpredictable progression of DF, which may grow, stabilize over
time or even subside spontaneously, a watchful waiting treatment
strategy becomes reasonable and feasible (7). Tumor regression occurs in 20-30% of
patients during watchful waiting. This may occur in DF in any part
of the body but is more likely in intra-abdominal DF (17). Bonvalot et al (18) performed a survival analysis of
patients with DF, with one group treated with a watchful waiting
strategy and the other treated with microscopically completed
surgery and aggressive drug therapy. There was no difference
between the two groups in terms of sex, age, tumor size and other
baseline levels. The results indicated no difference in the 3-year
progression-free survival rate between the two groups after a
median follow-up time of 76 months (waiting group, 68% vs. surgery
group, 65%; P>0.05).
There have been clinical reports about DF secondary
to FAP. The present patient was diagnosed with DF and FAP at the
same time at the first visit and the symptoms were caused by DF.
Previous literature has reported additional retreatment strategies
for induction of new DF following FAP surgery and DF recurrence
following local excision (1-4).
In the present case, the patient had been diagnosed with DF
combined with FAP, with surgical indications including suspicious
canceration, multiple >6-mm adenomas and highly atypical
hyperplastic adenoma. However, since the DF tumor was located at
the root of the mesentery of the small intestine, the majority of
the small intestine had to be resected to remove the DF tumor,
which would be likely to cause short bowel syndrome following
surgery and seriously affect the quality of life of the patient.
Finally, the treatment option proposed for this patient was to
undergo an initial FAP surgery followed by a subsequent watchful
waiting strategy to monitor DF changes, whilst considering the
natural course of DF. In the present case, it was noted that the
inflammatory adhesions around the DF tumor body had been loosened
and the obstruction caused by the inflammatory adhesions had been
released. This suggested that DF had not yet caused tissue invasion
to the intestinal wall or other organs.
In conclusion, the present study demonstrated that
an individualized treatment strategy was important for the patient
when DF was combined with FAP. The surgical intervention should be
selected carefully according to the anatomical location of DF. A
consensus was established in the present study on the surgical
approach for FAP. It is considered that the retention of additional
functional organs, such as the intestine, is more important to
improve the quality of life of the patient when dealing with
intra-abdominal DF combined with FAP. Surgical resection may not be
the first option; non-surgical treatments, such as watchful waiting
and drug treatment, could also benefit patients with DF combined
with FAP.
The disadvantage of the current study is that no
radical treatment had been carried out for the DF tumor, and the
watchful-waiting strategy may lead to disease progression in the
future. Whether the proposed treatment strategy can benefit
patients requires a comprehensive evaluation by regular follow-up
examinations such as symptoms, signs and imaging examinations, and
a large amount of clinical trial data.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ and SZ contributed to the original conception of
the study. LZ, SZ and KY wrote the manuscript. LZ, YZ and XY
analyzed and interpreted the imaging findings. KY and YZ obtained
and analyzed pathological images. KY and SZ edited and reviewed the
prepublication version of the manuscript. LZ and SZ confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The studies involving human participants were
reviewed and approved by the Ethics Committee of Weihai Central
Hospital (Weihai, China; approval no. LL-2022-139).
Patient consent for publication
Written informed consent for the publication of the
patient's clinical information and images was obtained from the
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Crago AM, Denton B, Salas S, Dufresne A,
Mezhir JJ, Hameed M, Gonen M, Singer S and Brennan MF: A prognostic
nomogram for prediction of recurrence in desmoid fibromatosis. Ann
Surg. 258:347–353. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mullen JT, Delaney TF, Kobayashi WK,
Szymonifka J, Yeap BY, Chen YL, Rosenberg AE, Harmon DC, Choy E,
Yoon SS, et al: Desmoid tumor: Analysis of prognostic factors and
outcomes in a surgical series. Ann Surg Oncol. 19:4028–4035.
2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bonvalot S, Tzanis D and Bouhadiba T:
Desmoid tumors: Are there still any surgical indications? Bull
Cancer. 107:364–370. 2020.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
4
|
He XD, Zhang YB, Wang L, Tian ML, Liu W,
Qu Q, Li BL, Hong T, Li NC and Na YQ: Prognostic factors for the
recurrence of sporadic desmoid-type fibromatosis after
macroscopically complete resection: Analysis of 114 patients at a
single institution. Eur J Surg Oncol. 41:1013–1019. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Devata S and Chugh R: Desmoid tumors: A
comprehensive review of the evolving biology, unpredictable
behavior, and myriad of management options. Hematol Oncol Clin
North Am. 27:989–1005. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kummar S, O'Sullivan Coyne G, Do KT,
Turkbey B, Meltzer PS, Polley E, Choyke PL, Meehan R, Vilimas R,
Horneffer Y, et al: Clinical activity of the γ-secretase inhibitor
PF-03084014 in adults with desmoid tumors (aggressive
fibromatosis). Clin Oncol. 35:1561–1569. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Turner B, Alghamdi M, Henning JW, Kurien
E, Morris D, Bouchard-Fortier A, Schiller D, Puloski S, Monument M,
Itani D and Mack LA: Surgical excision versus observation as
initial management of desmoid tumors: A population based study. Eur
J Surg Oncol. 45:699–703. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kasper B, Baumgarten C, Garcia J, Bonvalot
S, Haas R, Haller F, Hohenberger P, Penel N, Messiou C, van der
Graaf WT, et al: An update on the management of sporadic
desmoid-type fibromatosis: A european consensus initiative between
sarcoma PAtients EuroNet (SPAEN) and european organization for
research and treatment of cancer (EORTC)/soft tissue and bone
sarcoma group (STBSG). Ann Oncol. 28:2399–2408. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lewis JJ, Boland PJ, Leung DH, Woodruff JM
and Brennan MF: The enigma of desmoid tumors. Ann Surg.
229:866–872. 1999.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Righetti AE, Jacomini C, Parra RS, de
Almeida AL, Rocha JJ and Féres O: Familial adenomatous polyposis
and desmoid tumors. Clinics (Sao Paulo). 66:1839–1842.
2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Samadder NJ, Baffy N, Giridhar KV, Couch
FJ and Riegert-Johnson D: Hereditary cancer syndromes-A primer on
diagnosis and management, part 2: Gastrointestinal cancer
syndromes. Mayo Clin Proc. 94:1099–1116. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Monahan KJ, Bradshaw N, Dolwani S, et al:
Guidelines for the management of hereditary colorectal cancer from
the British Society of Gastroenterology (BSG)/Association of
Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom
Cancer Genetics Group (UKCGG)[J]. Gut. 69:411–444. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhenzhen W and Yaozong Y: Risk factors of
invasive fibroma in familial adenomatous polyposis. Chin J Dig.
34:640–642. 2014.
|
|
14
|
Nieuwenhuis MH, Mathus-Vliegen EM, Baeten
CG, Nagengast FM, van der Bijl J, van Dalsen AD, Kleibeuker JH,
Dekker E, Langers AM, Vecht J, et al: Evaluation of management of
desmoid tumours associated with familial adenomatous polyposis in
Dutch patients. Br J Cancer. 104:37–42. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rutenberg MS, Indelicato DJ, Knapik JA,
Lagmay JP, Morris C, Zlotecki RA, Scarborough MT, Gibbs CP and
Marcus RB: External-beam radiotherapy for pediatric and young adult
desmoid tumors. Pediatr Blood Cancer. 57:435–442. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gounder MM, Mahoney MR, Van Tine BA, Ravi
V, Attia S, Deshpande HA, Gupta AA, Milhem MM, Conry RM, Movva S,
et al: Sorafenib for advanced and refractory desmoid tumors. N Engl
J Med. 379:2417–2428. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Roussin S, Mazouni C, Rimareix F, Honoré
C, Terrier P, Mir O, Dômont J, Le Péchoux C, Le Cesne A and
Bonvalot S: Toward a new strategy in desmoid of the breast? Eur J
Surg Oncol. 41:571–576. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bonvalot S, Eldweny H, Haddad V, Rimareix
F, Missenard G, Oberlin O, Vanel D, Terrier P, Blay JY, Le Cesne A
and Le Péchoux C: Extra-abdominal primary fibromatosis: Aggressive
management could be avoided in a subgroup of patients. Eur J Surg
Oncol. 34:462–468. 2008.PubMed/NCBI View Article : Google Scholar
|